Abstract
Oral transmission of Trypanosoma cruzi has gained relevance because of its association with high morbidity and lethality rates. This transmission route is responsible for maintaining the infection of the parasite in sylvatic cycles, and human cases have been associated mainly with the consumption of food contaminated with triatomine feces or didelphid secretions. Several ecological changes allow the intrusion of sylvatic reservoirs and triatomines to the domestic environments with subsequent food contamination. Here, high-resolution molecular tools were used to detect and genotype T. cruzi across humans, reservoirs, and insect vectors in 2 acute outbreaks of presumptive oral transmission in eastern Colombia.
Keywords: Chagas disease, oral transmission, outbreaks, triatomines, Trypanosoma cruzi, opossums, canines, food
Oral transmission of Trypanosoma cruzi is considered ancient, allowing the circulation and maintenance of the parasite in the sylvatic cycle of transmission. T. cruzi has been classified into 6 discrete typing units (DTUs), TcI-TcVI, and, within TcI, into 2 genotypes (domestic TcI [TcIDom] and sylvatic TcI). In humans, this mechanism has been described in >1000 cases corresponding to 138 outbreaks, with a fatality rate of 8%–35% across Latin America [1, 2]. In Colombia, between 1992 and 2009, 11 outbreaks were reported, with a lethality rate of 16.0% [3]. In 2014, 2 outbreaks occurred in the Colombian Orinoco region. The aim of this study was to determine the possible sources of oral transmission, by using molecular tools, during the outbreaks that occurred in the municipalities of Restrepo, Meta Department, and Paz de Ariporo, Casanare Department.
METHODS
A total of 70 patients with suspected T. cruzi oral infection, along with 39 samples from reservoir organisms (opossums and canines) and 23 samples from triatomines (Panstrongylus geniculatus, Rhodnius pictipes, and Rhodnius prolixus) during the 2 outbreaks were included. Patients were confirmed as infected, using direct parasitological tests (thick blood smears), serological tests (trypomastigote excreted-secreted antigen [TESA] blot, enzyme-linked immunosorbent assay [ELISA], indirect immunofluorescence assay [IFA], or the hemagglutination inhibition test [HAI]), quantitative polymerase chain reaction (qPCR) for detection and quantification of T. cruzi DNA, clinical manifestations, and/or epidemiological links [4]. T. cruzi genotyping was performed as previously reported [5, 6]. Finally, in all qPCR-positive samples, amplification and analysis by Microsat (a program for microsatellite analysis) was performed using 7 microsatellite markers [7].
RESULTS
Restrepo Outbreak
This outbreak occurred in a family comprising 4 adults and 1 child, who visited Restrepo, including a farm, which contains forests and savannas, as well as some restaurants located along the road in Cumaral municipality, between February 13 and 16, 2014. The family returned to Bogota (the capital of Colombia) afterward, where the 4 adults were hospitalized for fever and edema. Thick blood smears were positive in 2 of 4 symptomatic patients. Results of conventional serological analyses, TESA blots, and molecular tests were positive for the 4 symptomatic patients. The median parasite load was 6.2 equivalent parasites/mL. The child was asymptomatic and had negative results of all diagnostic tests (Supplementary Table 1).
The fieldwork was focused on identification of food consumed by the family. Diagnostic tests were performed on specimens obtained from 15 human contacts, including farm workers, all of which yielded negative results. In addition, samples from 3 opossums, 29 canines, and 20 triatomines from the same house and neighboring farms were collected. T. cruzi DNA was detected in both types of samples collected (blood samples and samples from odoriferous secretion glands) from 3 opossums, in 27.5% of canines (8 of 29), and 85.0% of triatomines (17 of 20; 15 were P. geniculatus, and 2 were R. pictipes). Genotyping revealed sylvatic TcI across all symptomatic patients and insects, 50.0% of canines, and 33.3% of opossums. Additionally, TcIV was detected in reservoirs (33.3% of opossums and 50.0% of canines); mixed infection with sylvatic TcI/TcIII was detected in all P. geniculatus organisms, and mixed infection with TcIDOM was detected in 33.3% of opossums.
Paz de Ariporo Outbreak
Thirty-one samples from suspected acute cases, consisting of workers at an oil well that is approximately 6 hours by car from Paz de Ariporo, were collected. The oil well infrastructure includes a camp of temporary facilities for workers' accommodation and a restaurant that all suspected cases used to visit on a daily basis. The possible period of exposure via oral consumption of infected food was estimated to be between February 15th and March 30th, 2014.
On April 2nd, 2014, the first case was confirmed. This patient was an employee who had spent 1 month living in the camp before the symptoms started. He presented with an approximately 20-day history of febrile illness and had positive results of a blood smear and serological assays. Overall, we confirmed 30 symptomatic cases and that 2 patients died of acute myocarditis (Supplementary Table S1). Thick blood smear was performed for 23 patients, of whom 56.5% (13 of 23) had positive results. Conventional serological tests were positive in 100.0% of cases (30 of 30), and TESA blotting was conducted for 14 patients, all of whom were positive for SAPA antigen (a serological marker of acute infection). qPCR results were positive among 26 of 31 patients, and confirmation of T. cruzi infection among deceased patients (2 of 31) was confirmed by detection of T. cruzi DNA in cardiac tissue obtained after death. In addition, 54 suspected cases among workers from the camp and their contacts had negative results of serological tests and qPCR. Blood samples from 5 dogs, 2 opossums (in addition to odoriferous secretions), and 3 R. prolixus organisms (collected under the mattresses of the beds in the accommodation facilities) were collected. qPCR results were positive among all samples from reservoirs and insects. Sylvatic TcI was detected in 96.5% of the confirmed cases, 1 opossum, and 3 of 5 canines. TcIDOM genotype was found in all R. prolixus, 1 canine, and one of the patients with confirmed infection. Additionally, TcIV was detected in 1 opossum, and TcIII was detected in 1 canine.
Microsatellite Analyses
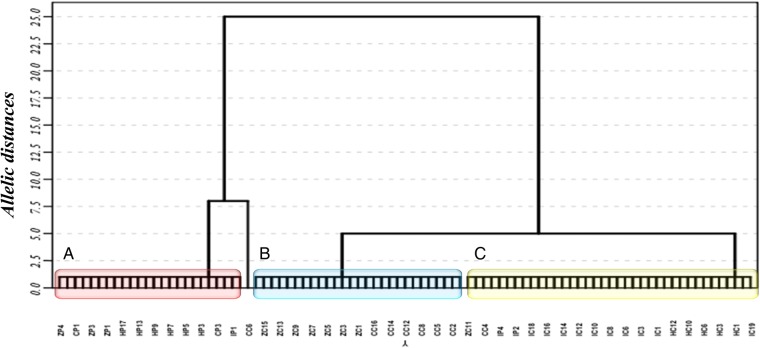
Amplification of 7 loci in positive samples of the 2 outbreaks was completed, and 19 alleles were found. The 2 loci that showed greater variability were AAAT6 and TAT20, each with 4 different alleles. Ten homozygous alleles and 9 heterozygous loci were detected across the 7 loci. Tree genetic distances based on the neighbor-joining model showed a grouping trend of the 2 outbreaks in different clusters. For the Restrepo outbreak, the grouping of human isolates was observed along with some vectors, whereas for the Paz De Ariporo outbreak, the grouping of human isolates was observed along with opossums and canines (Figure 1).
Figure 1.
Microsatellite genetic distances tree obtained from the samples analyzed of the outbreaks studied. The red cluster (A) shows samples from opossums, canines, and humans associated with the outbreak in Paz de Ariporo. The blue cluster (B) shows samples from opossums and canines involved in the outbreak in Restrepo. The yellow cluster (C) shows samples from triatomines and humans associated with the Restrepo outbreak. The identifiers in the x-axis are as defined as follows: among the first letters, “Z” denotes opossums, “C” denotes canines, “I” denotes triatomines, and “H” denotes humans; and among the second letters, “P” denotes the Paz de Ariporo outbreak, and “C” denotes the Restrepo outbreak.
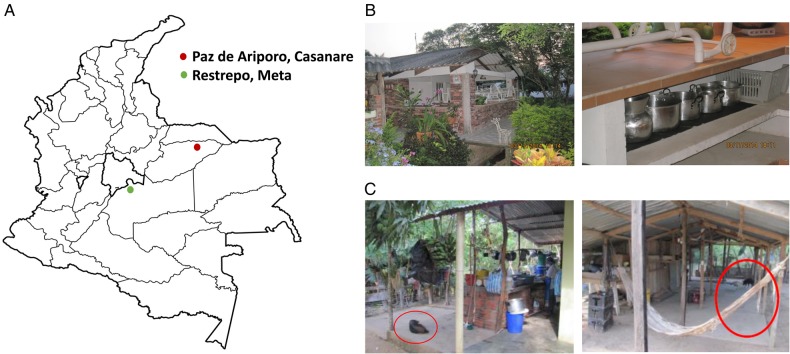
The results of this analysis allow us to infer that the Restrepo outbreak was possibly caused by the intrusion of triatomines into the kitchen at the farm and subsequent contamination of food with triatomine feces, while the Paz de Ariporo outbreak could be caused either by contamination of food with odoriferous secretions from opossums or by contamination with saliva from canines. Interestingly, both places had evidence of food preparation in open environments. In the farm where the Restrepo outbreak took place, the utensils for food preparation in the kitchen were exposed, favoring the contamination with triatomine feces and/or secretions from didelphids. In Paz de Ariporo outbreak, the presence of reservoirs near the restaurant and oil well facilities was identified (Figure 2).
Figure 2.
Geographical location of the outbreaks, and photographs of the places where the outbreaks occurred. A, Geographical location of the outbreaks in Paz de Ariporo and Restrepo. B, Photographs of the setting of the Restrepo outbreak, showing the kitchen and food preparation containers exposed outdoors on the farm. C, Photographs of the setting of the Paz de Ariporo outbreak, showing the areas of food preparation and food and beverage consumption near the oil well and in the presence of reservoirs (circled in red).
DISCUSSION
Acute infection was confirmed by observing the bands corresponding to SAPA antigen on TESA blots. Additionally, qPCR detected the parasite in reservoirs, insects, and heart tissue from both patients who died, providing more information for analysis of possible sources of infection. Regarding the reservoirs, it is important that T. cruzi DNA was detected in all samples of blood and odoriferous gland secretions from the opossums, revealing didelphids as a potential source of infection; this reservoir has been described elsewhere as one of the main sources of T. cruzi infection in outbreaks related to oral transmission [1, 4, 8]. In addition, it is important to emphasize that, in both outbreaks, specimens from dogs tested positive by qPCR, indicating active transmission of the parasite from the sylvatic to the domestic environment. The high positivity rate found among triatomines, especially among secondary vectors (P. geniculatus and R. pictipes), in Colombia and their participation in the Restrepo outbreak reaffirm the role of secondary vectors in oral transmission and the higher risk of vector transmission in areas where outbreaks occurred.
Genotyping revealed a similarity between the 2 outbreaks regarding the presence of the sylvatic TcI genotype, the genotype most likely associated with oral transmission. In the Restrepo outbreak, this genotype was present in vectors, reservoirs, and humans. In the outbreak in Paz de Ariporo, the genotype was observed only in reservoirs and humans, ruling it out as a possible source of infection. Additionally, detection of TcIV in opossums and TcIII in P. geniculatus is consistent with previous findings described in the literature [9, 10]. All DTUs detected are from the sylvatic cycle of transmission, reaffirming the role of these DTUs in oral transmission of the disease, because infection in humans is accidental, owing to the intrusion of the parasite into the domestic environment from the sylvatic cycle, as described previously in other outbreaks in Colombia [10], Venezuela, French Guyana, and Brazil [9, 11].
Microsatellite analysis, along with epidemiological information, allows us to infer that the outbreak in Restrepo was caused by possible contamination of food or beverages by triatomine feces. Although it was not a case-control study, it is interesting that the 4 confirmed cases and not the rest—the farm inhabitants and the child—remembered having eaten arepa (corn cake) and pineapple juice during the estimated exposure time. Several outbreaks have been associated with juices potentially contaminated with triatomines feces, such as açai and sugar cane in Brazil, guava in Venezuela, palm wine in French Guyana, and palm wine and tangerine juice in Colombia [11], together with in vitro tests that demonstrated survival of the parasite for up to 240 hours in a variety of beverages containing glucose [12]. Also, the vectors involved have been sylvatic and mostly infected. In particular, P. geniculatus has been previously associated with oral outbreaks in Colombia and Venezuela [10, 13]. Recent construction of housing in the forest in Restrepo has potentially altered the habitat of P. geniculatus and led to its intrusion into domestic settings. In this case, P. geniculatus is the vector most likely incriminated, because the specimens were collected in the peridomiciliary environment, unlike R. pictipes, specimens of which were collected in the extradomiciliary environment. Additionally, opossums collected in this outbreak were found 300 m from dwellings, decreasing the likelihood of food contamination.
On the other hand, microsatellite analysis allowed us to infer that the Paz de Ariporo outbreak probably occurred because of the contamination of food or beverages by the reservoirs' secretions. The vectors in this outbreak were collected in bedrooms, and TcIDOM was the predominant genotype. Canines have not been previously involved in outbreaks of orally transmitted infection; therefore, it is likely that the canine involved in this outbreak was in the acute phase of infection, as they are found infected in nature, and the infection source is the same as in humans [14]. This, coupled with the presence of a few triatomines in the area, allowed us to infer that infection could have involved glandular secretions by opossums in the wellbore, as microsatellite analyses of blood samples and odoriferous secretions yielded positive results. It is believed that the increased presence of opossums near the oil well by the time of the outbreak was due to a period of extreme drought in the department of Casanare and specifically in the municipality of Paz de Ariporo. This drought killed about 20 000 animals in the area and caused many of them to move from the sylvatic environment to the oil well surroundings in search of food and water, given that these were the only sources of water available in the area. This, coupled with the facts that didelphids are considered synanthropic reservoirs and that their presence in domestic habitats is considered a marker of environmental changes [1], explains the presence of opossums around the restaurant and specifically at the site where water and food were stored (Figure 2). A high rate of parasite infection among anal secretions from didelphids in Brazil and Colombia has been described elsewhere, implicating these secretions as a source of infection in several outbreaks of orally transmitted infection [1, 4, 15].
Molecular tools, such as the microsatellite genotyping used to analyze these 2 outbreaks, have demonstrated their value for understanding the possible sources and mechanisms of T. cruzi infection in such complex transmission cycles. The results indicate that food contamination was very likely in both events, suggesting triatomine feces in the Restrepo outbreak and opossums in the Paz de Ariporo outbreak as the potential sources of infection. These findings also lead us to hypothesize that the intensive environmental changes that have taken place in these regions increased the likelihood of these outbreaks.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the Ministry of Health and Social Protection of Colombia; the Department of Public Health laboratories in Meta and Casanare; the Vector-borne Diseases Group, Directorate of Vigilance and Risk Analysis in Public Health, National Institute of Health, Colombia; and the AT research group at the University Corpas.
Financial support. This work was supported by the Departamento Administrativo Nacional de Ciencia y Tecnología de Colombia Francisco José de Caldas–COLCIENCIAS and the Unión Temporal Programa Nacional de Investigación para la prevención, control y tratamiento integral de la enfermedad de Chagas en Colombia (grant 380- 2011; code 5014-537-30398).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Roque ALR, Xavier SCC, Da Rocha MG, Duarte ACM, D'Andrea PS, Jansen AM. Trypanosoma cruzi transmission cycle among wild and domestic mammals in three areas of orally transmitted chagas disease outbreaks. Am J Trop Med Hyg 2008; 79:742–9. [PubMed] [Google Scholar]
- 2.Sánchez LV, Ramírez JD. Congenital and oral transmission of American trypanosomiasis: an overview of physiopathogenic aspects. Parasitology 2013; 140:147–59. [DOI] [PubMed] [Google Scholar]
- 3.Instituto Nacional de Salud. Protocolo de vigilancia en salud publica Chagas. http://www.ins.gov.co/temas-de-interes/Paginas/chagas.aspx Accessed 27 April 2016.
- 4.Shikanai-Yasuda MA, Carvalho NB. Oral transmission of chagas disease. Clin Infect Dis 2012; 54:845–52. [DOI] [PubMed] [Google Scholar]
- 5.Ramírez JD, Guhl F, Rendón LM, Rosas F, Marin-Neto JA, Morillo CA. Chagas cardiomyopathy manifestations and Trypanosoma cruzi genotypes circulating in chronic chagasic patients. PLoS Negl Trop Dis 2010; 4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgos JM, Diez M, Vigliano C et al. Molecular identification of Trypanosoma cruzi discrete typing units in end‐stage chronic chagas heart disease and reactivation after heart transplantation. Clin Infect Dis 2010; 51:485–95. [DOI] [PubMed] [Google Scholar]
- 7.Duque M, Ramírez JD, Rendón L, Guhl F. Evaluación de la variabilidad genética de aislamientos colombianos de Trypanosoma cruzi mediante marcadores microsatélites. Infectio 2011; 15:227–34. [Google Scholar]
- 8.Coura JR. The main sceneries of Chagas disease transmission. The vectors, blood and oral transmissions - A comprehensive review. Mem Inst Oswaldo Cruz 2015; 110:277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messenger LA, Miles MA, Bern C. Between a bug and a hard place: Trypanosoma cruzi genetic diversity and the clinical outcomes of Chagas disease. Expert Rev Anti Infect Ther 2015; 13:995–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramírez JD, Montilla M, Cucunubá ZM, Floréz AC, Zambrano P, Guhl F. Molecular epidemiology of human oral Chagas disease outbreaks in Colombia. PLoS Negl Trop Dis 2013; 7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rueda K, Trujillo JE, Carranza JC, Vallejo GA. [Oral transmission of Trypanosoma cruzi : a new epidemiological scenario for Chagas' disease in Colombia and other South American countries]. Biomedica 2014; 34:631–41. [DOI] [PubMed] [Google Scholar]
- 12.Suárez DC, Rey ÁP, Orduz ML, Prada RL, Tarazona Z. Supervivencia de Trypanosoma cruzi en bebidas experimentalmente contaminadas. Biomédica, Rev del Inst Nac Salud 2012; 32:134–8. [DOI] [PubMed] [Google Scholar]
- 13.Alarcón De Noya B, Díaz-Bello Z, Colmenares C et al. Update on oral Chagas disease outbreaks in Venezuela: Epidemiological, clinical and diagnostic approaches. Mem Inst Oswaldo Cruz 2015; 110:377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocha FL, Roque AL, Arrais RC et al. Trypanosoma cruzi TcI and TcII transmission among wild carnivores, small mammals and dogs in a conservation unit and surrounding areas, Brazil. Parasitology 2013; 140:160–70. [DOI] [PubMed] [Google Scholar]
- 15.Deane MP, Lenzi HL, Jansen A. Trypanosoma cruzi: vertebrate and invertebrate cycles in the same mammal host, the opossum Didelphis marsupialis. Mem Inst Oswaldo Cruz 1984; 79:513–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.