Abstract
Introduction:
The term masked hypertension (MH) should be used for untreated individuals who have normal office blood pressure but elevated ambulatory blood pressure. For treated patients, this condition should be termed masked uncontrolled hypertension (MUCH).
Research Objectives:
Masked uncontrolled hypertension (MUCH) has gone unrecognized because few studies have used 24-h ABPM to determine the prevalence of suboptimal BP control in seemingly well-treated patients, and there are few such studies in large cohorts of treated patients attending usual clinical practice. This is important because masked hypertension is associated with a high risk of cardiovascular events. This study was conducted to obtain more information about the association between hypertension and other CV risk factors, about office and ambulatory blood pressure (BP) control as well as on cardiovascular (CV) risk profile in treated hypertensive patients, also to define the prevalence and characteristics of masked uncontrolled hypertension (MUCH) among treated hypertensive patients in routine clinical practice.
Patients and methods:
In this study 2514 male and female patients were included during a period of 5 years follow up. All patients have ambulatory blood pressure monitoring (ABPM) for at least 24h. We identified patients with treated and controlled BP according to current international guidelines (clinic BP, 140/90mmHg). Cardiovascular risk assessment was based on personal history, clinic BP values, as well as target organ damage evaluation. Masked uncontrolled hypertension (MUCH) was diagnosed in these patients if despite controlled clinic BP, the mean 24-h ABPM average remained elevated (24-h systolic BP ≥130mmHg and/or 24-h diastolic BP ≥80mmHg).
Results:
Patients had a mean age of 60.2+10 years, and the majority of them (94.6%) were followed by specialist physicians. Average clinic BP was 150.4+16/89.9+12 mmHg. About 70% of patients displayed a very high-risk profile. Ambulatory blood pressure monitoring (ABPM) was performed in all recruited patients for at least 24h. Despite the combined medical treatment (78% of the patients), clinic control (<140/90 mmHg) was achieved in only 26.2% of patients, the corresponding control rate for ambulatory BP (<130/80 mmHg) being 32.7%. From 2514 patients with treated BP, we identified 803 with treated and controlled office BP control (<140/90 mmHg), of whom 258 patients (32.1%) had MUCH according to 24-h ABPM criteria (mean age 57.2 years, 54.7% men). The prevalence of MUCH was slightly higher in males, patients with borderline clinic and office BP (130–139/80–89 mmHg), and patients at high cardiovascular risk (smokers, diabetes, obesity). Masked uncontrolled hypertension (MUCH) was most often due to poor control of nocturnal BP, with the proportion of patients in whom MUCH was solely attributable to an elevated nocturnal BP almost double that solely attributable to daytime BP elevation (22.3 vs. 10.1%, P 0.001).
Conclusion:
The prevalence of masked suboptimal BP control in patients with treated and well-controlled clinic BP is high. The characteristics of patients with MUCH (male, longer duration of hypertension, obesity, smoking history, and diabetes) indicate that this is a higher-risk group with most to gain from improved BP.
Keywords: Arterial hypertension, Cardiovascular risk factor, Ambulatory blood pressure monitoring, (ABPM), Masked uncontrolled hypertension (MUCH)
1. INTRODUCTION
Arterial hypertension remains the leading cause of mortality (1-10). Hypertension causes a large direct and indirect economic burden (11-20). The positive relationship between arterial hypertension and cardiovascular disease (CVD) risk has been observed in male and female patients of all ages, races, ethnic groups and countries (1-5, 21-30). Hypertension is the most common condition seen in primary care and leads to myocardial infarction, stroke, renal failure, and death if not detected early and treated appropriately (31-35). The term masked hypertension (MH) should be used for untreated individuals who have normal office blood pressure but elevated ambulatory blood pressure. For treated patients, this condition should be termed masked uncontrolled hypertension (MUCH). Despite major advances in our understanding of its pathophysiology and the availability of many drugs that can effectively reduce BP the available data shows that approximately 70% of hypertensive patients do not reach BP goals. Hypertension continues to be the most important modifiable factor for cardiovascular disease (CVD). Masked hypertension is defined as a normal seated blood pressure (BP) in the clinic or office (<140/90 mmHg), but an elevated BP out of clinic or office BP, as determined by ambulatory BP monitoring (ABPM) or home BP monitoring (HBPM). Adults with masked hypertension have increased risk of target organ damage and high risk of cardiovascular morbidity because they often remain undetected and untreated. The vast majority of the body of knowledge about hypertension has been built on the assessment of BP by means of the traditional auscultatory measurement at an office or clinic. To improve the assessment of actual 24-h BP levels, techniques for obtaining automated BP profiles over 24 h and BP measurements at home have been developed. Ambulatory BP monitoring (ABPM) is now the gold standard method for evaluating true BP levels, providing a more accurate estimation of true individual BP.
2. RESEARCH OBJECTIVES
Masked uncontrolled hypertension (MUCH) has gone unrecognized because few studies have used 24-h ABPM to determine the prevalence of suboptimal BP control in seemingly well-treated patients. There are few and limited data on the quality of treated blood pressure (BP) control during normal daily life, and in particular, the prevalence of “masked uncontrolled hypertension” (MUCH) in people with treated and seemingly well-controlled BP is almost still unknown. This is important because masked hypertension is associated with a high risk of cardiovascular events. This study was conducted to obtain more information about the association between hypertension and other CV risk factors, about office and ambulatory blood pressure (BP) control as well as on cardiovascular (CV) risk profile in treated hypertensive patients and define the prevalence and characteristics of masked uncontrolled hypertension (MUCH) among hypertensive patients in routine clinical practice in whom BP was treated and controlled to recommended BP goals.
3. PATIENTS AND METHODS
In this study 2514 male and female patients were included during a period of 5 years follow up. All patients have ambulatory blood pressure monitoring (ABPM) for at least 24h. We identified patients with treated and controlled BP according to current international guidelines (clinic BP <140/90mmHg). The collected study variables include: age, gender, weight and height [obesity defined as body mass index (weight in kg/height in meters squared) ≥30 kg/m2], duration of hypertension, known cardiovascular risk factors such as tobacco smoking and diabetes mellitus (American Diabetes Association criteria), biochemical values of creatinine and lipid profile, target organ damage(TOD) including urinary albumin excretion (UAE), left ventricular hypertrophy (LVH) (electrocardiographic Sokolow-Lyon voltage and/or Cornell duration/voltage index 2440 mm/ms), and radiological evidence of carotid plaque, and clinical CVD (coronary heart disease, congestive heart failure, or cerebrovascular disease). Renal disease was diagnosed when serum creatinine was 132.6 umol/L in men and 123.8 umol/L in women and/or when proteinuria was present. Details of anti-hypertensive treatment (e.g. drug class, number of drugs, and time of administration) were also recorded. Cardiovascular risk was stratified using the 2016 European Guidelines on cardiovascular disease prevention in clinical practice – European Society of Cardiology/European Association for Cardiovascular Prevention & Rehabilitation (EACPR), based on clinical BP category, the presence of other risk factors, TOD, or previous CVD for patients with well-controlled office BP. Masked uncontrolled hypertension (MUCH) was diagnosed in these patients if despite controlled clinic BP, the mean 24-h ABPM average remained elevated (24-h systolic BP ≥130mmHg and/or 24-h diastolic BP ≥80mmHg). Ambulatory Blood Pressure Monitoring all patients underwent one ambulatory blood pressure monitoring session using the Boso TM 2430 PC2. The Boso PC2 has satisfies the recommended British Hypertension Society (BHS) and Association for Medical Instrumentation accuracy levels. The monitors were programmed to measure BP at 30 minute intervals from 7am to 10pm and at 1 hour intervals from 10pm to 7am. For all patients we incorporated a participant diary and used it to define sleep and awake periods. Maximum BP measurement time was limited to less than 140 seconds, and the monitors were set for a maximum pressure of 220 mm Hg. Participants were given verbal instructions on wearing the monitor, including that they should try to leave the cuff on during the entire monitoring period, that they should try to hold their cuffed arm as still as possible during a reading to ensure that the monitor would get an accurate reading and that faulty readings would trigger a repeat measurement. The minimum number of readings we accepted as an adequate ABPM session was 18 for awake and 7 for sleep.
4. RESULTS
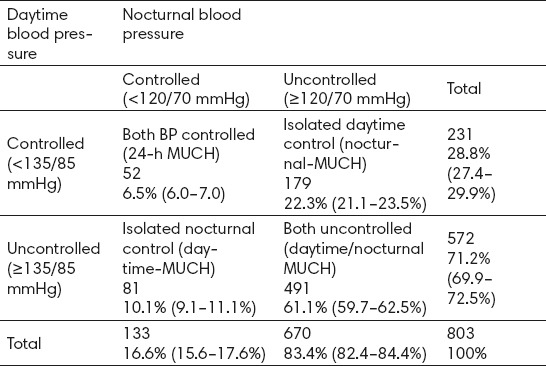
Patients had a mean age of 60.2+10 years, and the majority of them (94.6%) were followed by specialist physicians. Average clinic BP was 150.4+16/89.9+12 mmHg. About 70% of patients displayed a very high-risk profile. Electrocardiogram (ECG) was performed in 99% of patients, echocardiography was performed in 79%, carotid ultrasound and measuring of IMT in 36%, ophthalmological exam and fundoscopy in 66%, and search for microalbuminuria in 21%. Ambulatory blood pressure monitoring (ABPM) was performed in all recruited patients for at least 24h. Despite the combined medical treatment (78% of the patients), clinic control (<140/90 mmHg) was achieved in only 26.2% of patients, the corresponding control rate for ambulatory BP (<130/80 mmHg) being 32.7%. From 2514 patients with treated BP, we identified 803 with treated and controlled office BP control (<140/90 mmHg), of whom 258 patients (32.1%) had MUCH according to 24-h ABPM criteria (mean age 57.2 years, 54.7% men). The prevalence of MUCH was slightly higher in males, patients with borderline clinic and office BP (130-139/80-89 mmHg), and patients at high cardiovascular risk (smokers, diabetes, obesity). MUCH was most often due to poor control of nocturnal BP, with the proportion of patients in whom MUCH was solely attributable to an elevated nocturnal BP almost double that solely attributable to daytime BP elevation (22.3 vs. 10.1%, P<0.001).
Clinic and 24-h BP controlled hypertensive patients: clinic BP<140/90 mmHg and 24-h BP<130/80 mmHg. MUCH: clinic BP<140/90 mmHg and 24-h BP ≥130/80 mmHg. LVH, left ventricular hypertrophy; UAE, urinary albumin excretion; CVD cardiovascular disease; LDL, low-density lipoprotein; HDL, high-density lipoprotein. Values are mean ± SD or median (inter-quartile range), or n (%). The prevalence of MUCH was significantly higher in males, patients with borderline clinic BP (130-139/80-89mmHg), and patients at high cardiovascular risk (smokers, diabetes, obesity). Masked uncontrolled hypertension was often because of poor control of nocturnal BP, with the proportion of patients in whom MUCH was solely attributable to an elevated nocturnal BP almost double that solely attributable to daytime BP elevation (22.3 vs. 10.1%, P <0.001). The most often subtype of MUCH among subjects was both uncontrolled daytime/nocturnal MUCH (61%).
Figure 1.
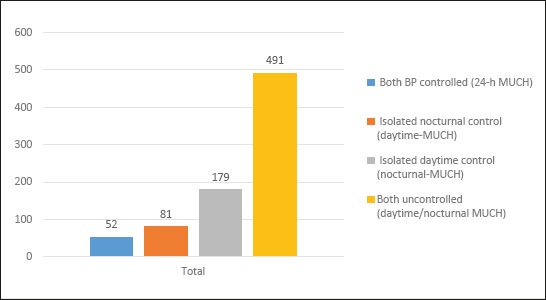
Patients with subtypes of masked uncontrolled hypertension. Masked uncontrolled hypertension: clinic blood pressure <140/90 mmHg and 24-h blood pressure ≥130/80 mmHg. Only-24-h masked uncontrolled hypertension: daytime blood pressure <135/85 mmHg, nocturnal blood pressure <120/70 mmHg, and 24-h blood pressure ≥130/80 mmHg. Only-nocturnal masked uncontrolled hypertension: nocturnal blood pressure ≥120/70 mmHg and daytime blood pressure <135/85 mmHg. Only-daytime masked uncontrolled hypertension: daytime blood pressure ≥135/85 mmHg and nocturnal blood pressure <120/70 mmHg. Daytime–nocturnal masked uncontrolled hypertension: daytime blood pressure ≥135/85 mmHg and nocturnal blood pressure ≥120/70 mmHg.
Figure 2.
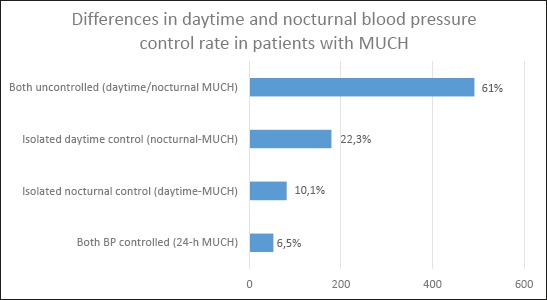
Percentage distribution of subtypes of masked uncontrolled hypertension (MUCH) in a sample of treated and controlled hypertensive patients during ambulatory blood pressure monitoring (ABPM).
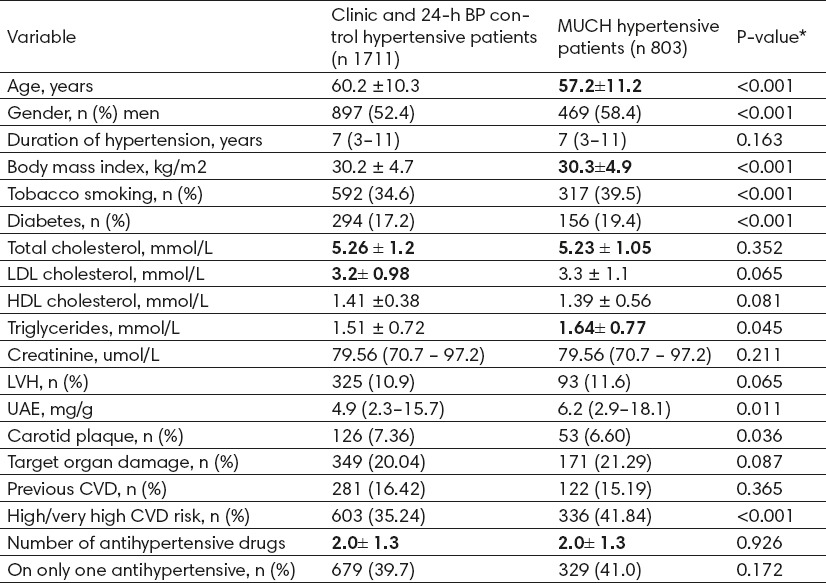
There were 803 patients with MUCH despite optimal clinic BP control (mean age 57.2 years, 52.4% men), and 1711 patients were identified as having optimal BP control, i.e. with both office and 24-h BP controlled. When compared with the optimal BP control group, MUCH patients were more likely to be male and had a worse CVD risk profile, including higher proportion of smokers, diabetes, higher levels of triglycerides, greater proportion of high estimated CVD risk, and marginally but not significantly higher levels of LDL cholesterol (P =0.065) and higher proportion of TOD (P =0.087) (Table 1).
Table 1.
Clinical features in treated clinically controlled hypertensive patients with and without masked uncontrolled hypertension. *P-values for association between MUCH patients and patients with both clinic and 24-h BP controlled.
The percentage of MUCH receiving monotherapy did not significantly differ from those optimally controlled (Table 1). Most MUCH patients took their anti-hypertensive medication only in the morning (55.4% vs. 55.1% in those optimally controlled), 11% only in the evening/night, and 39% in both the morning and the evening/night. The percentage of MUCH vs. optimal control patients taking specific drug classes were diuretics 8.3 and 8.1%, respectively (P = 0.594), beta-blockers 14.3 and 18.5% (P<0.01), angiotensin-converting enzyme inhibitors (ACEi) 27.3 and 26.5% (P = 0.544), angiotensin-receptor blockers (ARB) 20.9 and 20.7% (P = 0.932), calcium-channel blockers 8.3 and 5.1% (P <0.01), and alpha blockers 1.1 and 1.2% (P = 0.331). Mean daytime and nocturnal ambulatory BP was higher in those with MUCH when compared with the optimal control group (Table 3). The absolute difference in nocturnal SBP between both groups was 17.6 mmHg (127.2 vs. 109.6 mmHg, respectively), and 18.1 mmHg in daytime SBP (137.5 vs. 119.4 mmHg, respectively).
Table 3.
Differences in office, daytime, and nocturnal BP, as well as circadian pattern distribution, in treated well-controlled hypertensive patients with and without masked uncontrolled hypertension
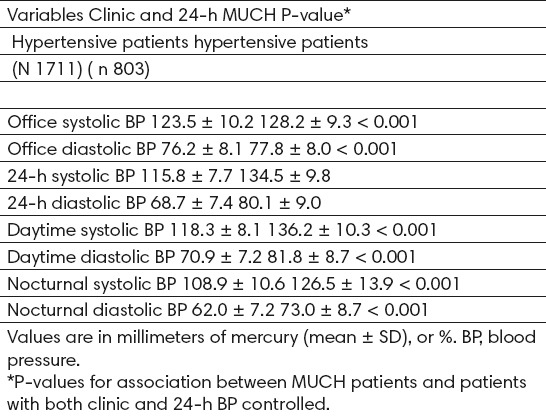
Table 2.
Differences in daytime and nocturnal blood pressure control rate in patients with masked uncontrolled hypertension (MUCH)
Adults with masked hypertension have increased risk of target organ damage and cardiovascular morbidity. The first study to look at the issue of target organ damage was published by Pickering et al. (6) in 1999, in which we showed that a group of patients with masked hypertension had a higher left ventricular mass and more carotid atherosclerosis than true normotensives, and thus were similar to true hypertensives (3, 6). In adolescents, masked hypertension has been shown to be present in nearly 40% of individuals and these were more than twice as likely to have a parental history of hypertension, and to have a higher ambulatory pulse rate, BMI, and greater prevalence of left ventricular hypertrophy as normotensive individuals. The proportion of MUCH among treated hypertensive patients well controlled in the clinic was 31.9% (95% confidence interval 30.7–32.2%). Possible characteristics of individuals with masked uncontrolled hypertension are: male patients, age 65 years, male sex, stress during the daytime, higher levels of cholesterol or triglycerides and smoking or drinking habits. The prevalence of MUCH was significantly higher in those patients. However, the difference in the prevalence of MUCH according to obesity status, TOD, or previous CVD were only marginally significant or not clinically relevant (absolute differences, 3 mmHg) (29). The prevalence of MUCH was not significantly different between patients on one drug vs. those on ≥2 drugs (31.5 vs. 30.4%, P =0.148), and either according to the time of drug administration. Lastly, the prevalence of MUCH was significantly lower in patients taking only beta-blockers (25.3%) and higher in those on only calcium-channel blockers (37.5%) or only alpha-blockers (35.4%). In studies (Obara et al. (1), Borbie et al. (2), Ungar et al. (3) and Mancia et al. (4) which evaluating the factors predictive of masked hypertension in multivariate analyses, being male, smoking and high BMI are frequently identified as risk factors.
5. DISCUSSION
Worldwide surveys of blood pressure control to targets recommended by national and international guidelines have consistently revealed that in clinical practice the conventional goal of a blood pressure <140/90 mmHg is reached by only a minority of patients (27). Arterial hypertension is associated with a high prevalence of metabolic risk factors, the combination drug treatment is frequently used. Many patients present with a stress reaction when visiting a doctor or nurse, or even when performing a self-automated BP measurement in a medical environment, and show an office BP that may be significantly higher than their BP levels during normal daily activities. Pickering et al. (6) proposed the new term relates to masked hypertension (elevated BP at home but not in the office), in preference to the term of “reverse white-coat hypertension” or “isolated home hypertension.” Data from 51573 hypertensive patients included in the Spanish ABPM Registry showed that daytime BPs were ≈16/8mmHg lower than office BPs, and this difference reached ≈20/10mmHg when comparing office and 24h BPs. The higher the BP or global cardiovascular risk level, the greater the difference between office and ambulatory BP values, as shown by our group in the comparison between patients with high-risk hypertension and patients with low-to-moderate-risk hypertension (7, 8, 9). The results of this study suggest that almost one-third of patients who are considered to have adequate BP control by conventional clinic criteria do not have their BP controlled when assessed by ABPM. Importantly, over one in three patients with borderline clinic BP have MUCH and therefore have a BP that is not adequately controlled. The frequency of MUCH was especially high in patients with major cardiometabolic risk factors or who smoke, all of which identify people who are at higher CVD risk who would benefit most from optimal BP control. The patients with normal office blood pressure but elevated ambulatory blood pressure (defined as “masked hypertensives”) clearly have a greater cardiovascular risk, higher than that of patients with “White Coat Hypertension” (10). The risks of organ damage and cardiovascular events in patients with masked hypertension are significantly higher than in those with a normal-range blood pressure level or white coat hypertension, being similar to those in patients with persistent hypertension (5).
These findings were observed in a large European population of people cared for in usual clinical practice, and the prevalence of MUCH was consistent across the status of cardiovascular risk factors, TOD, CVD, and anti-hypertensive medication. The results suggest that based on the currently recommended use of clinic BP to monitor BP control, physicians will substantially overestimate the number of patients who are truly controlled, leaving many higher-risk patients at excess risk (12, 13, 15).
The prevalence of masked suboptimal BP control in patients with treated and well-controlled clinic BP is high. The frequency of MH in HBPM studies ranges from 9 to 37%, and 9 to 21% based on ABPM (11). However, HBPM cannot properly assess BP during sleep, and nocturnal BP is a stronger risk factor for TOD and CVD (12-14). The fact remains that daytime BP measurements alone are insufficient to detect all MH cases. In untreated hypertensive patients, the prevalence of MH ranges from 9 to 14% (11). Perloff et al. (15) and Verdecchia et al. (16) demonstrated the better prognostic value of ambulatory BP monitoring than office measurement in a general untreated population, and Clement et al. (17) did so in patients being treated for hypertension.
Interestingly, in our untreated patients the prevalence of MH was 32.7%, a quite similar proportion to that of MUCH. But in general in patients with treated hypertension, the prevalence is less known. The present study thus adds new evidence on the importance of MUCH, particularly nocturnal-MUCH in population of already treated hypertensive patients attended in clinical practice. In our study there is no significant association between the number of drugs taken and the prevalence of MUCH, consistent with some studies (18). Also no statistically significant or clinically relevant associations between MUCH and time of drug administration either. Table 5 shows the prevalence of MH and MUCH in several studies from Spanish APBM Registry, so we can conclude that the prevalence of MUCH in our study 31.9% is similar to the previous published studies.
The demographic and clinical characteristics of patients with MH and MUCH are poorly defined (19) indeed, few large studies has previously focused on patients with MUCH. Available 24-h ABPM-based studies have identified high-normal clinic BP, age, smoking, obesity, diabetes, proteinuria, and high CVD risk associated with MH (21-23). In our study we have identified the clinical profile of MUCH patients as more likely to be male or obese, smokers, or those with diabetes, high cholesterol and triglycerides. Unfortunately, pathophysiological mechanisms responsible for MH are still unknown. Nevertheless, it is notable that clinic heart rate was marginally higher in MUCH patients than in controlled patients (74.4 and 72.3 b.p.m., respectively, P =0.009), and in particular there was a statistical trend in MUCH patients with diabetes (75.4 vs. 74.3, P = 0.08). This may suggest an increased sympathetic activity in some patients with MUCH, consistent with findings reported in detail by Grassi et al. (24). However, further research is needed. The MUCH is most often because of nocturnal hypertension is important because nocturnal BP has been strongly linked to CVD morbidity and mortality and nocturnal hypertension can only be detected by ABPM (25-28, 39)
6. CONCLUSION
Arterial hypertension remains the leading cause of mortality. Hypertension causes a large direct and indirect economic burden. The prevalence of masked uncontrolled hypertension (MUCH) in patients with treated and well-controlled clinic BP is high. Clinic BP monitoring alone is not adequate to optimize BP control because many patients have an elevated nocturnal BP. The characteristics of patients with MUCH (male, longer duration of hypertension, obesity, smoking history, and diabetes) indicate that this is a higher-risk group with most to gain from improved BP. An important determinant of MUCH is poorer control of nocturnal BP. Moreover, nocturnal BP is increasingly recognized as a strong predictor of risk in many studies of ABPM.
Footnotes
• Conflict of interest: none declared.
REFERENCES
- 1.Obara T, Ohkubo T, Funahashi J, Kikuyu M, Sasayama K, Motoko H, Oikawa T, Hashimoto J, Tonsure K, Imai Y. The J-HOME study group. Isolated uncontrolled hypertension at home and in the office among treated hypertensive patients from the J-HOME study. J Hypertens. 2005;23:1653–60. doi: 10.1097/01.hjh.0000178334.33352.56. [DOI] [PubMed] [Google Scholar]
- 2.Borbie G, Clerson P, Cuchet A, Mahmoudi A, Postel-Vinay N, Chatellier G. Prevalence and mechanism of masked hypertension: the Ol'mesures survey. Arch Mal Coeur Vaiss. 2006;99:760–3. [PubMed] [Google Scholar]
- 3.Ungar A, Pepe G, Monami M, Lambertucci L, Torrini M, Baldasseroni S, et al. Isolated ambulatory hypertension is common in outpatients referred to a hypertension centre. J Hum Hypertens. 2004;18:897–903. doi: 10.1038/sj.jhh.1001756. [DOI] [PubMed] [Google Scholar]
- 4.Mancia G. Reversed white-coat hypertension: definition, mechanisms and prognostic implications. J Hypertens. 2002;20:579–81. doi: 10.1097/00004872-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014) Hypertension Research. 2014;37:253–92. doi: 10.1038/hr.2014.20. [DOI] [PubMed] [Google Scholar]
- 6.Pickering TG, Davidson K, Gerin W, Schwartz JE. Masked hypertension. Hypertension. 2002;40:795–6. doi: 10.1161/01.hyp.0000038733.08436.98. [DOI] [PubMed] [Google Scholar]
- 7.Andalib A, Akhtari S, Rigal R, Curnew G, Leclerc JM, Vaillancourt M, Tardiff JC. Determinants of masked hypertension in hypertensive patients treated in a primary care setting. Intern Med J. 2012;42:260–6. doi: 10.1111/j.1445-5994.2010.02407.x. [DOI] [PubMed] [Google Scholar]
- 8.Verberk WJ, Kessels AG, de Leeuw PW. Prevalence, causes, and consequences of masked hypertension: a meta-analysis. Am J Hypertens. 2008;21:969–75. doi: 10.1038/ajh.2008.221. [DOI] [PubMed] [Google Scholar]
- 9.Banegas JR, Ruilope LM, de la Sierra A, de la Cruz JJ, Gorostidi M, Segura J, Martell N, García-Puig J, Deanfield J, Williams B. High prevalence of masked uncontrolled hypertension in people with treated hypertension. Eur Heart J. 2014;35(46):3304–12. doi: 10.1093/eurheartj/ehu016. [DOI] [PubMed] [Google Scholar]
- 10.Mancia G, Hachette R, Bomb Elli M, Grassi G, Sega R. Long-term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension. 2006;47:846–53. doi: 10.1161/01.HYP.0000215363.69793.bb. [DOI] [PubMed] [Google Scholar]
- 11.Borbie G, Clerson P, Me´nard J, Postel-Vinay N, Chatellier G, Plouin PF. Masked hypertension: a systematic review. J Hypertens. 2008;26:1715–25. doi: 10.1097/HJH.0b013e3282fbcedf. [DOI] [PubMed] [Google Scholar]
- 12.Verdecchia P, Angeli F, Mazzotta G, Garofoli M, Ramundo E, Gentile G, Ambrosio G, Reboldi G. Day-night and early-morning surge in blood pressure in hypertension. Prognostic implications. Hypertension. 2012;60:34–42. doi: 10.1161/HYPERTENSIONAHA.112.191858. [DOI] [PubMed] [Google Scholar]
- 13.De la Sierra A, Banegas JR, Segura J, Gorostidi M, Ruilope LM CARDIORISC Event Investigators. Ambulatory blood pressure monitoring and development of cardiovascular events in high-risk patients included in the Spanish ABPM registry: the CARDIORISC Event study. J Hypertens. 2012;30:713–9. doi: 10.1097/HJH.0b013e328350bb40. [DOI] [PubMed] [Google Scholar]
- 14.Banegas JR, Ruilope LM, de la Sierra A, de la Cruz JJ, Gorostidi M, Segura J, Martell N, García-Puig J, Deanfield J, Williams B. High prevalence of masked uncontrolled hypertension in people with treated hypertension. Eur Heart J. 2014;35(46):3304–12. doi: 10.1093/eurheartj/ehu016. [DOI] [PubMed] [Google Scholar]
- 15.Perloff D, Sokolow M, Cowan RM, Juster RP. Prognostic value of ambulatory blood pressure measurements: further analyses. J Hypertens Suppl. 1989;7:S3–S10. [PubMed] [Google Scholar]
- 16.Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure: an independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 17.Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–15. doi: 10.1056/NEJMoa022273. [DOI] [PubMed] [Google Scholar]
- 18.Andalib A, Akhtari S, Rigal R, Curnew G, Leclerc JM, Vaillancourt M, Tardiff JC. Determinants of masked hypertension in hypertensive patients treated in a primary care setting. Intern Med J. 2012;42:260–6. doi: 10.1111/j.1445-5994.2010.02407.x. [DOI] [PubMed] [Google Scholar]
- 19.Mancia G, Bomb Elli M, Servile G, Grassi G. Diagnosis and management of patients with white-coat and masked hypertension. Nat Rev Cardiol. 2011;8:686–93. doi: 10.1038/nrcardio.2011.115. [DOI] [PubMed] [Google Scholar]
- 20.Longo D, Derogate F, Palatine P. Masked hypertension in adults. Blood Press Monti. 2005;10:307–10. doi: 10.1097/00126097-200512000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Mancia G, Hachette R, Bomb Elli M, Grassi G, Sega R. Long-term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension. 2006;47:846–53. doi: 10.1161/01.HYP.0000215363.69793.bb. [DOI] [PubMed] [Google Scholar]
- 22.Pickering TG, Euchar K, Kari K. Masked hypertension: a review. Hypertens Res. 2007;30:479–88. doi: 10.1291/hypres.30.479. [DOI] [PubMed] [Google Scholar]
- 23.Mallion JM, Clerson P, Borbie G, Genes N, Vaisse B, Chatellier G. Predictive factors for masked hypertension within a population of controlled hypertensives. J Hypertens. 2006;24:2365–70. doi: 10.1097/01.hjh.0000251895.55249.82. [DOI] [PubMed] [Google Scholar]
- 24.Grassi G, Servile G, Trevano FQ, Dell'oro R, Bolla G, Cuspidi C, Arenare F, Mancia G. Neurogenic abnormalities in masked hypertension. Hypertension. 2007;50:537–42. doi: 10.1161/HYPERTENSIONAHA.107.092528. [DOI] [PubMed] [Google Scholar]
- 25.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. ESH/ESC Guidelines for the management of arterial hypertension. The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, Pickering T, Redon J, Stassen J, Stergiou G, Verdecchia P. On behalf of the European Society of Hypertension Working Group on Blood Pressure Monitoring. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self-blood pressure measurement. J Hypertens. 2005;23:697–701. doi: 10.1097/01.hjh.0000163132.84890.c4. [DOI] [PubMed] [Google Scholar]
- 27.Ohkubo T, Imai Y, Tsuji I, Nagai K, Ito S, Satoh H, Hisamichi S. Reference values for24-hour ambulatory blood pressure monitoring based on a prognostic criterion: the Osama Study. Hypertension. 1998;32:255–9. doi: 10.1161/01.hyp.32.2.255. [DOI] [PubMed] [Google Scholar]
- 28.Wolf-Maier K, Cooper RS, Kramer H, Banegas JR, Giampaoli S, Joffres MR, Poulter NR, Primatesta P, Stegmayr B, Thamm M. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004;43:10–17. doi: 10.1161/01.HYP.0000103630.72812.10. [DOI] [PubMed] [Google Scholar]
- 29.Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, Li Y, Dolan E, Tikhonoff V, Seidlerová J, Kuznetsova T, Stolarz K, Bianchi M, Richart T, Casiglia E, Malyutina S, Filipovsky J, Kawecka-Jaszcz K, Nikitin Y, Ohkubo T, Sandoya E, Wang J, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Staessen JA, O'Brien E IDACO Investigators. The International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Press Monit. 2007;12:255–62. doi: 10.1097/mbp.0b013e3280f813bc. [DOI] [PubMed] [Google Scholar]
- 30.Gorostidi M, Sobrino J, Segura J, Sierra C, De la Sierra A, Herna´ndez del Rey R, Vinyoles E, Galcera´n JM, Lo´pez-Eady MD, Marı´n R, Banegas JR, Sarrı´a A, Coca A, Ruilope LM. on behalf of the Spanish Society of Hypertension ABPM Registry investigators. Ambulatory blood pressure monitoring in hypertensive patients with high cardiovascular risk: a cross-sectional analysis of a 20,000-patient database in Spain. J Hypertens. 2007;25:977–84. doi: 10.1097/HJH.0b013e32809874a2. [DOI] [PubMed] [Google Scholar]
- 31.Sega R, Hachette R, Bomb Elli M, Cesana G, Corrao G, Grassi G, Mancia G, Prognostic De la Sierra A, Banegas JR, Segura J, Gorostidi M, Ruilope LM. CARDIORISC Event Investigators. Ambulatory blood pressure monitoring and development of cardiovascular events in high-risk patients included in the Spanish ABPM registry: the CARDIORISC Event study. J Hypertens. 2012;30:713–9. doi: 10.1097/HJH.0b013e328350bb40. [DOI] [PubMed] [Google Scholar]
- 32.Sega R, Hachette R, Bomb Elli M, Cesana G, Corrao G, Grassi G, Mancia G. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–83. doi: 10.1161/01.CIR.0000160923.04524.5B. [DOI] [PubMed] [Google Scholar]
- 33.Gorostidi M, de la Sierra A, González-Albarrán O, Segura J, de la Cruz JJ, Vinyoles E, Llisterri JL, Aranda P, Ruilope LM, Banegas JR. On behalf of the Spanish Society of Hypertension ABPM Registry investigators Abnormalities in ambulatory blood pressure monitoring in hypertensive patients with diabetes. Hypertens Res. 2011;34:1185–9. doi: 10.1038/hr.2011.100. [DOI] [PubMed] [Google Scholar]
- 34.Pierdomenico SD, Pannarale G, Rabbia F, Lapenna D, Licitra R, Zito M, Campanella M, Gaudio C, Veglio F, Cuccurullo F. Prognostic relevance of masked hypertension in subjects with prehypertension. Am J Hypertens. 2008;21:879–83. doi: 10.1038/ajh.2008.196. [DOI] [PubMed] [Google Scholar]
- 35.Dolan E, Stanton A, Thijs L, Hindi K, Atkins N, McCrory S, Den Hand E, McCormack P, Stassen JA, O'Brien E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin Outcome Study. Hypertension. 2005;46:156–61. doi: 10.1161/01.HYP.0000170138.56903.7a. [DOI] [PubMed] [Google Scholar]
- 36.Liu JE, Roman MJ, Pini R, Schwartz JE, Pickering TG, Devereux RB. Cardiac and arterial target organ damage in adults with elevated ambulatory and normal office blood pressure. Ann Intern Med. 1999;131:564–72. doi: 10.7326/0003-4819-131-8-199910190-00003. [DOI] [PubMed] [Google Scholar]
- 37.Sehestedt T, Jeppesen J, Hansen TW, Wachtell K, Ibsen H, Torp-Pedersen C, Hildebrandt P, Olsen MH. Risk prediction is improved by adding markers of subclinical organ damage to SCORE. Eur Heart J. 2010;31:883–91. doi: 10.1093/eurheartj/ehp546. [DOI] [PubMed] [Google Scholar]
- 38.Hansen TW, Kikuyu M, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, Richart T, Torp-Pedersen C, Lind L, Jeppesen J, Ibsen H, Imai Y, Stassen JA. IDACO Investigators Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7,030 individuals. J Hypertens. 2007;25:1554–64. doi: 10.1097/HJH.0b013e3281c49da5. [DOI] [PubMed] [Google Scholar]
- 39.Masic I, Dilic M, Raljevic E, Vulic D, Mott D. Trends in cardiovascular diseases in Bosnia and Herzegovina and perspectives with HeartScore Programme. Med Arh. 2010;64(5):260–3. doi: 10.5455/medarh.2010.64.260-263. [DOI] [PubMed] [Google Scholar]


