Abstract
Background:
Several studies have reported that an elevation in neutrophils/lymphocyte ratio (NLR) is correlated with poor survival in patients with colorectal cancer, but in rectal cancer (RC), it has been reported only in a few studies. It is necessary to separate colon cancer and rectal cancer to clarify the prognostic significance of NLR, especially in patients who received chemoradiotherapy.
Methods:
It is a comparative, observational retrospective study of a cohort of 175 patients. We grouped the patients into two based on their NLR (0-3 vs. > 3) to correlate with disease-specific survival (DSS) and pathologic complete response (pCR).
Results:
The average NLR was 2.65 + 1.32 (range 0.58-6.89), and 144 (82.3%) patients had an NLR of 0-3. The median follow-up was 33.53 months. There were no differences in pCR between the two groups. The 5-year DSS was 78.8%. NLR did not correlate with survival. Mesorectal quality, pT3-4 tumors, lymph node metastasis, lymphovascular invasion, perineural invasion, positive margins and recurrence were statistically significant predictors of increased mortality in univariate analysis. In multivariate analysis, only overall recurrence correlated with poor survival. The analysis of the association of NLR with outcomes with different cut points (2.0, 2.5, 4 and 5) did not show differences in DSS and pCR.
Conclusion:
In our cohort, the NLR did not serve as a prognostic marker in patients with locally advanced rectal cancer and who received chemoradiotherapy and did not correlate with pCR as well.
Keywords: colorectal, cancer, rectal, neutrophils, lymphocyte, survival
1. INTRODUCTION
Rectal cancer (RC) is one of the most prevalent cancers worldwide and it is an important cause of cancer mortality in developed countries for both sexes (1). Cumulative evidence have shown that inflammatory response is associated with tumorigenesis and tumor progression (2, 3). The interactions between tumor and inflammation are complex and involve different mechanisms. Inflammation plays an important role in carcinogenesis, involving tumor initiation, angiogenesis promotion, apoptosis inhibition and tumor metastasis (4). The changes in the systemic inflammatory response can be reflected by the measurement of various blood-based parameters.
A variety of blood-based indexes including C-reactive protein, modified Glasgow Prognostic Score, platelet count and neutrophil-to-lymphocyte ratio (NLR) have been explored to predict prognosis of cancer patients (5, 6). Several studies have reported that an elevation of NLR is associated with poor clinical outcomes in several cancers (7, 8), including poor survival in patients with colorectal cancer (9, 10). However, the clinical features, treatment options, surgical complexity and pathologic analysis are extremely diverse between colon cancer and rectal cancer; and these two neoplasms are merged in one unique group in majority of published studies.
The NLR has been evaluated in rectal cancer independent of colon cancer in very few studies but it is necessary to systematically separate these two entities and clarify the prognostic significance of NLR in patients with rectal cancer (11-14). Our objective was to evaluate the prognostic value of elevated NLR for disease-specific survival (DSS), recurrence-free survival (RFS) and its correlation with treatment outcomes in patients with rectal cancer treated with preoperative chemoradiotherapy (CRT).
2. MATERIAL AND METHOD
All consecutive patients undergoing rectal resection with curative intent for RC following preoperative CRT, from 2010 to 2014 at the Instituto Nacional de Cancerología (INCan, Mexico City, Mexico) were included in the study (n=175). Clinicopathologic data were prospectively collected. Demographic, preoperative, intra-operative, pathological and outcome variables were recorded. Blood samples were obtained within 7 days before CRT. White blood count, neutrophils and lymphocyte counts were recorded. NLR was calculated as the neutrophils count divided by the lymphocyte count using preoperative blood test results. An NLR > 3 was considered elevated according to a published study 16 because we failed to identify an appropriate cut-off value based on a receiver operating characteristic (ROC) (Figure 1).
Figure 1.
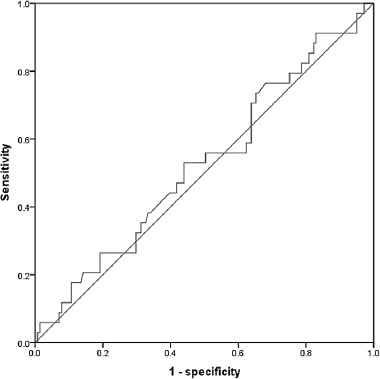
Receiver operating characteristic curve analysis of the neutrophil-to-lymphocyte ratio in patients with colorectal cancer. Area under the curve=0.528, 95% Confidence interval=0.419-0.637, p=0.615.
Figure 2.
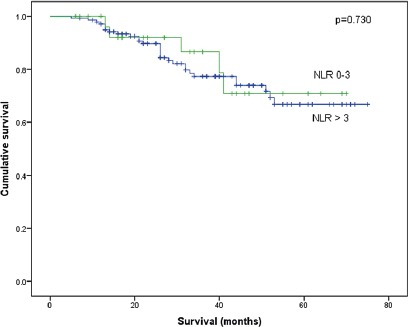
Five year-Overall survival of 175 patients with rectal carcinoma separated with neutrophil/lymphocyte ratio.
Preoperative staging was done by abdominopelvic computed tomography and with transendoscopic ultrasound. Under a multidisciplinary team consensus, patients with T3 and T4 tumors and with lymph node metastasis (LNM) underwent preoperative CRT to downsize and downstage the tumor, and then subsequent surgery was performed. Standardized routine pathological examination was carried out according to the protocol described by Quirke (15) and Nagtegaal (16). The quality of the mesorectum was scored in two grades: “adequate” and “inadequate”, according to a published study (17). The circumferential resection margin was evaluated and considered positive if the tumor was present to within 1 mm of the inked mesorectal margin.
In the postoperative setting, a multidisciplinary review of the case was made to define adequate patient follow-up. All patients were examined every 3 months for the first 2 years, and every 6 months thereafter. The patients were evaluated according to clinical conditions by clinical examination, rigid sigmoidoscopy, carcinoembryonic antigen estimation, colonoscopy, and by thoracic, abdominopelvic computer tomography. Patients categorized as T3 and T4 or with LNM underwent postoperative chemotherapy if appropriate.
The primary objective of this study was to evaluate the correlation of pretreatment NLR with DSS. The secondary endpoints were the association with RFS and pathologic complete response (pCR). DSS was defined as the time from the date of surgery to death from cancer. Recurrence free survival (RFS) was defined as the time from the date of surgery to the date of tumor relapse (local recurrence and/or distant metastases) or death. We classified tumor response pathologically in two categories, pCR and no-pCR.
Data were analyzed using the SPSS package 10.0 for Windows (SPSS, Chicago, Illinois, USA). The relationship between clinicopathological variables was analyzed using the Chi-square test and the U-Mann-Whitney test. Univariate survival analyses of time to recurrence, metastasis or death were performed using the Kaplan–Meier method, with the time of the surgery as the entry date. Differences in observed survival between groups were tested for statistical significance using log-rank tests. Multivariate analysis was performed using the Cox regression model, with a p value < 0.05 was considered statistically significant.
The study was approved by the ethical and scientific committee of our institution.
3. RESULTS
The clinical and pathological characteristics of the population (n=175) are summarized in Table 1. The median age was 56.45 + 12.78 years (23–82 years), and 98 (56%) patients were men. The average NLR was 2.65 + 1.32 (range 0.58-6.89) and 144 (82.3%) had an NLR of 0-3. One hundred and nine patients (62.3%) received low anterior resection (LAR) as treatment, 55 (31.4%) received abdomino-perineal resection (APR), and 11 (6.3%) underwent posterior exenteration. Laparoscopic resection was performed in 31 cases (30%). Major complications, such as anastomotic leakage, septicemia, or dehiscence, occurred in 31 patients (17.1%). The quality of the mesorectum was reported as adequate in 136 (77.7%) specimens and inadequate in 39 (22.3%).
Table 1.
Clinicopathologic data of the 175 patients
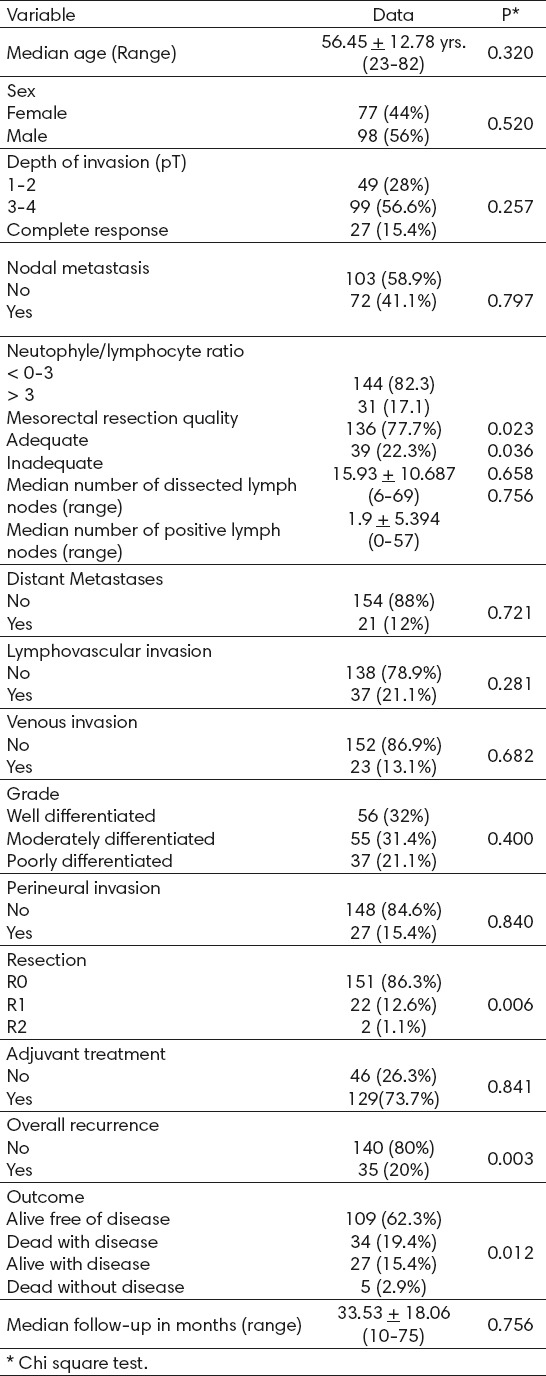
Overall, 27 patients (15.4%) presented complete pathologic responses, whereas 13 presented with T1 tumors, 36 with T2 tumors, 85 with T3 tumors and 14 with T4 tumors. In the series, only 16 (9.14%) cases had less than 10 lymph nodes resected. However, all of these cases received CRT and had at least six lymph nodes, so all patients could be properly staged. Regarding other relevant pathologic findings, 13 (7.4%) patients presented metastasis, 37 (21.1%) presented lymphovascular invasion (LVI), and 23 (13.1%) presented venous invasion and 27 (15.4%) presented perineural invasion. The R0 resection was achieved in 86.3%.
Outcome
The median follow-up of the study group was 33.53 months, with a range of 10-75 months. At the end of the study, 35 (20%) patients had a recurrence: 17 with distal recurrence and 3 with local recurrence. The median time for recurrence was 12 months, with a range of 4-23 months.
Of the patients studied, 62.3% are alive and free of the disease, 19.4% died from the disease, 15.4% are alive with the disease, and five patients died from other causes not related to RC. There were no differences in complete pathologic response between the two NLR groups.
Survival analysis
The correlations between cancer-specific survival and various clinicopathological factors are shown in Table 2. The 5-year DSS of the series was 78.8%. In univariate analysis, mesorectal quality, pT3-4 tumors, LNM, LVI, perineural invasion, positive margins and recurrence were statistically significant predictors of increased mortality (Table 2).
Table 2.
Univariate analysis of 175 Rectal carcinomas*
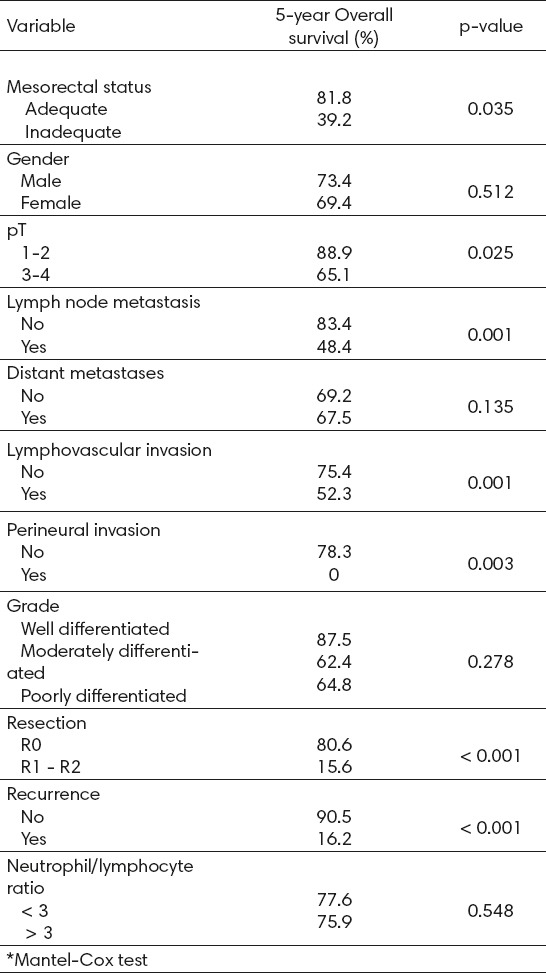
Patients (64.1%) with pT3-4 tumors showed a 5-year DSS, compared with 88.9% of patients with pT1-2 tumors (p=0.022). Forty-nine patients (28%) presented with LNM, with a 5-year DSS of 48.4% versus 84.4% of patients without metastasis (p=0.001). Twenty-five patients (14.3%) presented with LVI, and these showed poor prognosis, with a 5-year DSS of 52% versus 75% for patients without LVI (p=0.001). Only 27 (15.4%) patients presented with perineural invasion. However, the 5-year DSS of these patients was 0% versus 77.3% of patients without perineural invasion. Patients with R0 resections showed a 5-year DSS of 80.5% versus 15.6% for R1-R2 resections (p<0.001). Finally, recurrence correlated with a decreased 5-year DSS of 16.2%, compared with 92.5% for patients without recurrence (p<0.001).
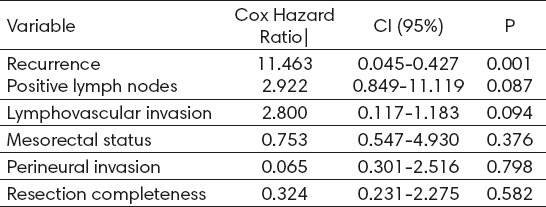
In the multivariate analysis, the only statistically independent adverse prognostic factor was overall recurrence. The presence of PLN and LVI showed statistically significant trends as independent prognostic factors, while NLR status, perineural invasion and resection completeness did not show any prognostic influence (Table 3).
Table 3.
Multivariate Analysis of 175 Rectal Adenocarcinomas
Finally, we repeated the analysis of the association of NLR with outcomes with different cut points (2.0, 2.5, 4 and 5) and did not find differences in DSS, RFS and complete pathologic response.
4. DISCUSSION
Systemic inflammatory response measured using the surrogate neutrophil-lymphocyte ratio (NLR) has been proposed as a marker to predict cancer patient survival. In patients with locally advanced rectal cancer, some studies have demonstrated that an elevated lymphocyte count is associated with increased downstaging following preoperative CRT (18), while elevated NLR is associated with short time to local recurrence and worse overall survival and DFS (19). However, with regard to the response of preoperative CRT and prognosis in patients with locally advanced rectal cancer, there are only a few studies with a small number of patients (9).
Another controversial question is the appropriate cut-off value for the NLR because in published studies, it varies from 2.59, 2.8 (11), 3.0 (14), 4.0 (20) to 5.0 (21). However, in our study, we did not find any difference in DSS, RFS and pCR in the NLR independent of the cut-off value. Kitayama et al. retrospectively analyzed 73 patients with locally advanced rectal cancer, and the results showed that NLR tended to be lower in a pCR group (P = 0.099). Krauthamer et al. reported a significantly higher probability of pCR in patients with clinical stage III who had an NLR <5 (OR = 2.54; P = 0.04), but note that only 10 patients achieved pCR (22). A further validation of these differences is necessary.
Our study had several strengths such as the homogeneity of the treatment, the adequate evaluation of the mesorectum and a balanced proportion of patients with T stages 1-2 vs. 3-4, and similar male/female relationships. The NLR average in our study group is consistent with most published series; however, we did not find an NLR > 7, contrary to published series with higher upper limits (8.9-23.5) (9, 11).
Although NLR is easy to measure, its utility as a marker of systemic inflammation may be affected by many conditions, including coronary disease, metabolic syndrome, inflammatory diseases and any medication related to inflammatory conditions in the patients (23, 24). These limitations must be considered and could explain the low NLR in our study. We cannot correct this because it is a retrospective study, and no specific data were available about comorbidities in all patients. These variables will influence the specificity of NLR as a prognostic factor in our cohort of patients with rectal cancer. Even considering these limitations, our data indicate that NLR does not serve as a prognostic marker for risk stratification in patients with locally advanced rectal cancer and who received CRT.
5. CONCLUSIONS
The NLR does not serve as a prognostic marker in patients with locally advanced rectal cancer and who received CRT in our cohort and that does not correlate with pCR as well.
Footnotes
• Ethic statement: The manuscript had been approved by the appropriate Ethics committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Due to the nature of the study, informed consent is not necessary, however, the confidentiality of the patient’s data is guaranteed.
• The manuscript has not been submitted to more than one journal for simultaneous consideration, it has not been published previously (partly or in full), no data have been fabricated or manipulated (including images) to support our sentences and there is not any form of plagiarism.
• Conflict of interest. We are not conflict of interest and none disclosure.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–6. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashimoto K, Ikeda Y, Korenaga D, Tanoue K, Hamatake M, Kawasaki K, et al. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer. 2005;103:1856–64. doi: 10.1002/cncr.20976. [DOI] [PubMed] [Google Scholar]
- 6.Gu L, Li H, Gao Y, Ma X, Chen L, Li X, et al. The association of platelet count with clinicopathological significance and prognosis in renal cell carcinoma: a systematic review and meta-analysis. PloS one. 2015;10:e0125538. doi: 10.1371/journal.pone.0125538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Chen X, Gao P, Song Y, Huang X, Yang Y, et al. Can the Neutrophils to Lymphocyte Ratio Be Used to Determine Gastric Cancer Treatment Outcomes? A Systematic Review and Meta-Analysis. Dis Markers. 2016;2016 doi: 10.1155/2016/7862469. 7862469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibutani M, Maeda K, Nagahara H, Noda E, Ohtani H, Nishiguchi Y, et al. A high preoperative neutrophil-to-lymphocyte ratio is associated with poor survival in patients with colorectal cancer. Anticancer Res. 2013;33(8):3291–4. [PubMed] [Google Scholar]
- 10.Jankova L, Dent OF, Chan C, Chapuis P, Clarke SJ. Preoperative neutrophils/lymphocyte ratio predicts overall survival but does not predict recurrence or cancer-specific survival after curative resection of node-positive colorectal cancer. BMC Cancer. 2013;13:442. doi: 10.1186/1471-2407-13-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen L, Zhang H, Liang L, Li G, Fan M, Wu Y, et al. Baseline neutrophil-lymphocyte ratio (≥2.8) as a prognostic factor for patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation. Radiat Oncol. 2014;9:295. doi: 10.1186/s13014-014-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim IY, You SH, Kim YW. Neutrophil-lymphocyte ratio predicts pathologic tumor response and survival after preoperative chemoradiation for rectal cancer. BMC Surg. 2014;14:94. doi: 10.1186/1471-2482-14-94. doi: 10.1186/1471- 2482-14-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagasaki T, Akiyoshi T, Fujimoto Y, Konishi T, Nagayama S, Fukunaga Y, et al. Prognostic Impact of Neutrophil-to-Lymphocyte Ratio in Patients with Advanced Low Rectal Cancer Treated with Preoperative Chemoradiotherapy. Dig Surg. 2015;32(6):496–503. doi: 10.1159/000441396. [DOI] [PubMed] [Google Scholar]
- 14.Chiang SF, Hung HY, Tang R, Changchien CR, Chen JS, You YT, et al. Can neutrophil-to-lymphocyte ratio predict the survival of colorectal cancer patients who have received curative surgery electively? Int J Colorectal Dis. 2012;27(10):1347–57. doi: 10.1007/s00384-012-1459-x. [DOI] [PubMed] [Google Scholar]
- 15.Quirke P, Durdey P, Dixon MF, Willliams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathologic study of lateral tumour spread and surgical excision. Lancet. 1986;2:996–9. doi: 10.1016/s0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 16.Nagtegaal ID, van de Velde CJ, van der Worp E, Kapiteijn E, Quirke P, van Krieken JH. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20(7):1729–34. doi: 10.1200/JCO.2002.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Lino-Silva LS, García-Gómez MA, Aguilar-Romero JM, Domínguez-Rodríguez JA, Salcedo-Hernández RA, Loaeza-Belmont R, et al. Mesorectal pathologic assessment in two grades predicts accurately recurrence, positive circumferential margin, and correlates with survival. J Surg Oncol. 2015;112(8):900–6. doi: 10.1002/jso.24076. [DOI] [PubMed] [Google Scholar]
- 18.Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat Oncol. 2010;5:47. doi: 10.1186/1748-717X-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carruthers R, Tho LM, Brown J, Kakumanu S, McCartney E, McDonald AC. Systemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancer. Colorectal Dis. 2012;14:e701–707. doi: 10.1111/j.1463-1318.2012.03147.x. [DOI] [PubMed] [Google Scholar]
- 20.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncology. 2005;91:181–4. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 21.Mallapa S, Sinha A, Gupta S, Chadwick SJ. Preoperative neutrophils lymphocyte ratio>5 is a prognostic factor for recurrent colorectal cancer. Colorectal Dis. 2013;15:323–8. doi: 10.1111/codi.12008. [DOI] [PubMed] [Google Scholar]
- 22.Krauthamer M, Rouvinov K, Ariad S, Man S, Walfish S, Pinsk I, et al. A study of inflammation-based predictors of tumor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Oncology. 2013;85:27–32. doi: 10.1159/000348385. [DOI] [PubMed] [Google Scholar]
- 23.Adamsson ES, Smith JG, Melander O, Hedblad B, Engstrom G. Incidence of coronary events and case fatality rate in relation to blood lymphocyte and neutrophils counts. Arterioscler Thromb Vasc Biol. 2012;32:533–9. doi: 10.1161/ATVBAHA.111.240416. [DOI] [PubMed] [Google Scholar]
- 24.Balta S, Cakar M, Demirkol S, Arslan Z, Akhan M. Higher neutrophils to lymhocyte ratio in patients with metabolic syndrome. Clin Appl Thromb Hemost. 2013;19:579. doi: 10.1177/1076029612475023. [DOI] [PubMed] [Google Scholar]


