Abstract
Introduction:
Homocysteine is process-product of methionine demethylation. It has proatherogenic, prothrombotic, prooxidative, proapoptotic, osteoporotic, neurotoxic, neuroinflamatory, and neurodegenerative effects. Hyperhomocysteinemia correlates with C667T MTHFR mutation, decrease of folic acid and vitamin B, as well as prolonged use of certain medications.
Materials and Methods:
We measured levels of homocysteine in thirty patients (15::15) with “de novo” Parkinson’s disease, with average age 64.17 ± 13.19 (28-82) years (Department of Neurology, University Clinical Center Tuzla). Normal level of homocysteine for women was 3.36-20.44 micromole/l and 5.9-16 micromole/l for men. We followed the effects of medicament approach (folic acid) every six months for next five years.
Results:
20% of patients with “de novo” Parkinson’s disease exhibited hyperhomocysteinemia. An average level of homocysteine was 13.85 ± 5.82 micromole/l. Differences due to age and homocysteine levels, regardless of sex, were not concluded. For the next five years intake of folic acid (periodically, 1-2 months, 5 mg per day, orally) was effective to normalized levels of homocysteine in all.
Conclusion:
Hyperhomocysteinemia is present in every fifth patient with “de novo” Parkinson’s disease. Folic acid is medication of choice in treatment of hyperhomocysteinemia coexisting with Parkinson’s disease.
Keywords: Homocysteine, Parkinson’s disease, Folic acid
1. INTRODUCTION
Homocysteine is a product of intracellular demethylation of amino acid methionine. It is metabolized through the process of remethylation and transsulphuration during which folic (pteroylglutamic) acid and vitamins B6 and B12 are utilized as cofactors (1). Nearly half a century ago, McCully was inferring to the existence of changes in the blood vessels of children with homocystinuria (2).
In the meantime, it was discovered that homocysteine has proatherogenic and prothrombotic effect, it multiplies the risk for vascular diseases and has significant neurotoxic effect through neurodegeneration (3, 4). Increased level of this metabolite is directly connected to the decreasing levels of folic acid, cobalamin and pyridoxine (5). Prospective and „post mortem” research have indicated that oxidative stress has unavoidable role in creating Parkinson’s disease (PD) and Alzheimer’s disease (AD).
Some studies done using experimental animals have confirmed these statements (6, 7). Homocysteine is one of the indicators of oxidative stress. Also, it is an important factor in initiation and progress of neurodegenerative diseases. Today, it is known that hyperhomocysteinemia in older patients with subcortical atherosclerotic encephalopathy represents more significant risk factor for dementia in comparison with smoking, diabetes and hypertension. Following this, the possibility of AD is elevated by 40% with every 5 micromole/l increase of homocysteine level. It is noticeable the connection between homocysteine level, brain atrophy and severity of cognitive dysfunction (8). Furthermore, homocystinuria and consequent osteoporosis are risk factors for hip fracture in patients with PD that are on levodopa therapy (9, 10).
Following the above mentioned, hyperhomocysteinemia is completely unfavorable. Its existence is not sufficiently considered or thought of and regardless of its significant side effects it is left unrecognized.
2. AIM
Reveal “de novo” patients with Parkinson’s disease and hyperhomocysteinemia,
Follow the effects of therapeutic approach (folic acid) on the lowering levels of homocysteine during the next five years.
3. MATERIAL AND METHODS
First part of the research was conducted during the initial hospitalization of participants with “de novo” Parkinson’s disease at the Department of Neurology in University Clinical Center (UCC) Tuzla (2006-2007). The approval for conducting this research was obtained from Ethical Committee of UCC Tuzla. Confirmation of PD presence was done in accordance with clinical criteria: tremor, bradykinesia, rigidity and postural abnormalities. Selected participants where those not taking medications that increase homocysteine level (anticonvulsants, contraceptives, methotrexate, levodopa, trimethoprim, sulphasalazine), medications that lower homocysteine level (COMT-inhibitors, folic acid, B-vitamin) and those who haven’t been diagnosed with homocystinuria. The group of participants was created by regular admissions and the doctor specialist announced the formal diagnosis to the patients in a usual way, common for all participants. Basic characteristics, process and possible prognosis of the disease has been explained to them, followed by introduction of research goals. They have been informed that the research is being conducted under supervision of professionals and the methods of the studies have been presented to them as well. Risk for negative effect on their health did not exist. With regards to recommendations of Helsinki’s declaration a high respect was given to life, health and human dignity with guaranteed individual autonomy. The signed consent could have been abolished at any given time and those who did not agree to participate have been excluded from the group regardless of satisfying clinical criteria. Homocysteine level test was performed in the Department of Biochemistry at the Polyclinic for laboratory diagnostics (UCC Tuzla). Blood samples were obtained via venipuncture in the early morning hours. Same samples were placed onto iced surfaces, which were later placed in the area of 2-8 degrees of Celsius so they can undergo the process of centrifugation. For optimal results it was necessary that the samples were free of fibrin or erythrocytes. The machine used for investigation was Abbott-AXSYM System® utilizing FPIA (fluorescence polarization immunoassay) method. According to recommendations, normal homocysteine level for women was 3.36-20.44 micromole/l and 5.9-16 micromole/l for men (11). Second part of research – following the effects of therapeutic approach with folic acid was conducted from 2007 until 2012, during follow up appointments with the specialists. The effects of this approach were tested twice a year. Patients with PD were not recommended to take B-vitamins (due to their effect on reabsorption of levodopa agents) or COMT (Catechol-O-Methyl-Transferase)–inhibitors that are known to reduce homocysteine levels (due to the fact that their market price in Bosnia and Herzegovina was extremely high and that it was possible to reduce homocysteine level with other, cheaper agents/products). During the analysis of acquired results standard statistical parameters were applied: arithmetic mean, minimal and maximal value, standard deviation and T-test. Differences were considered important for p < 0.05
4. RESULTS
Research was conducted with 30 participants who were hospitalized due to newly discovered Parkinson’s disease (PD), with average age 64.17 ± 13.19 (28-82) years. Average age of female patients (n=15) was 62.73 ± 10.69 (48-77), and male patients (n=15) 65.6 ± 15.54 (28-82). The average level of homocysteine was 13.85 ± 5.82 micromole/l, in females 13.43 ± 7.28 (6.6-34.6) micromole/l and in men was slightly higher 14.27 ± 4.11 (9.9-25.9) micromole/l. significantly, 20% of participants had hyperhomocysteinemia (13.3% female and 26.7% male patients). Differences due to age (p=0.561) and homocysteine levels (p=0.701) between male and female participants with PD were not concluded (Figure 1).
Figure 1.
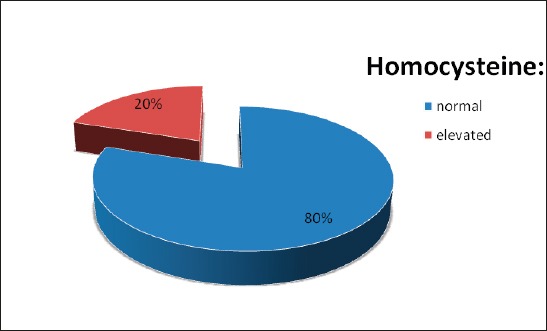
Distribution of PD patients considering homocysteine levels
Following the 5-years effects of therapeutic approach, it has been shown that all participants with hyperhomocysteinemia, regardless of sex, have achieved normal levels of metabolite as a result of periodically taking of folic acid (FOLIK®, ZADA Pharmaceuticals) for 1-2 months, 5 mg per day, orally.
5. DISCUSSION
Every 5th participant (20%) with “de novo” Parkinson’s disease had hyperhomocysteinemia. Earlier, it was considered that homocysteine through oxidative stress was predominantly risk factor for vascular diseases. However, the elevation was seen in patients with PD, AD, Huntington’s disease, amyotrophic lateral sclerosis and some dystonia. Elevation of this metabolite and consumption of highly caloric food makes human brain very vulnerable to neurodegenerative diseases caused by aging. The outcome of one laboratory study has indicated that by applying homocysteine into the striatum or substantia nigra results in fast motoric dysfunction (12). It is presumed that homocysteine by stressing out and increasing neurotoxic effect could cause pathological changes to the structures of basal ganglia and be one of the mediators in development of dyskinesia in patients on levodopa therapy, so as be the specific biologic marker which will indicate neurodegeneration (13). Furthermore, in the study of Blandini et al., significant average values were noted in patients with PD with 80% higher comparing values of controlled group (14). Hyperhomocysteinemia was present in the cerebrospinal fluid of patients with PD (15). According to study undertaken by O’Suilleabhain even the values above 14 micromole/l were considered as elevated and were in relation with the increased mortality rates (16). Patients who were taking levodopa agents and had consequential hyperhomocysteinemia have had a substantial cognitive deficit developed. Significantly, those not treated with levodopa agents also had homocysteine level above normal range. This refers us to consider the importance of investigating levels of homocysteine in all patients with PD, and not only those treated with levodopa medications. It is possible that levodopa therapy, not solely presence of PD, contributes to hyperhomocysteinemia development and following increase of metabolite level is in correlation with significant risk for substantia nigra degeneration in PD sufferers. In the studies that considered levels above 15 micromole/l as hyperhomocysteinemia, a direct link between homocysteine level and daily intake of levodopa was established (17). During the process of methylation a product S-adenosylhomocysteine is produced, which is later hydrolyzed into homocysteine. Therefore, adding COMT- inhibitors into the therapy of patients already on levodopa efficiently reduces the level of homocysteine. Harmful effects of oxidative stress, before all, are linked to inability of central nervous system to self prevents them in the first place. Understanding of connection between the progress of the disease, free radicals created by oxidative stress, neuroinflamation and neurotoxicity is important for disclosure of mechanism of the disease and attempts to develop new therapeutic possibilities/options.
Pharmacotherapy of PD patients with hyperhomocysteinemia
COMT-inhibitors (entacapone, tolcapone and tropolone) reduce the level of homocysteine (18, 19). One of the rationales is that at times of their use accumulation of homocysteine in astrocytes is reduced. By referring to some studies it might be one of the reasons for reduced dyskinesia in patients treated with COMT- inhibitors (13).
Folates could protect blood vessels and prevent DNA damage caused by oxidative stress (3,4). However, research undertaken on embryonic cortical neuron’s culture and human neuroblasts has shown that in the median there is an increase in products of oxidation and apoptosis without the presence of folate. Outcomes of some studies have indicated that the values of vitamin B12 and folic acid are in negative correlation with hyperhomocysteinemia of patients with PD (20). Vitamins B2, B6, B12 have antiatherogenic effect by reducing homocysteine level. It seems that diet approach and an increase of folic acid level, due to the effect on homocysteine metabolism, should have a positive influence on the fast aging process and degenerative diseases. It is considered that sole increased intake of B-vitamins also lowers the possibility of PD development by some mechanisms that are not utterly linked with the metabolism of homocysteine (21).
The importance of hyperhomocysteinemia is supported by the views of some associations involved in the research of this problem. Therefore, the view of ASHG/ASMG (American Society of Human Genetics) is such that a normal range of homocysteine should be between 5-15 micromole/l (22). According to DACH-LIGA Homocysteine (German, Austrian and Swiss Homocysteine Society) a targeted level of value of homocysteine should be less than 10 micromole/l (23). Furthermore, if we compare these values with the initial ones from our research it is noticeable that even an average level (13.85 micromole/l) in our participants indicated hyperhomocysteinemia. This kind of an “epidemic character of hyperhomocysteinemia” demands regular testing of homocysteine level in patients with risk factors for diseases characterized by this biochemical imbalance, as well as frequent and more energetic supplementation. That is why an early intake of vitamins and diet control should be established as earlier as possible. Regular practitioner should control the levels of folic acid in patients at risk, advise them and introduce therapeutic approach. In our study, we recommended folic acid according to laboratory test results. We didn’t have completely clear indications for how long it should be taken for. The intake was stopped once the normal range was established or it was commenced on six monthly test result that indicated hyperhomocysteinemia (possibly due to long time of not taking folates).
During our research, results of one interesting study have been published and it has concluded that a risk of side effects and damage is minimal if the daily dose of folic acid is 1-5 mg (24). Therefore, taking 5 mg of folic acid daily, for a month, had normalized homocysteine levels of patients with PD. After making a two monthly break from its intake, almost 90% of patients had normal levels, while following a four monthly break hyperhomocysteinemia was present in all patients. According to this, we started recommending to our participants to take folic acid for two months and make a two months break. We had similar positive experiences when it comes to control of hyperhomocysteinemia in patients after ischemic stroke (25). It could be speculated that patients with hyperhomocysteinemia not caused by levodopa or other drugs, could take a longer break from folic acid.
6. CONCLUSION
Homocysteine is a metabolite of methionine demethylation. It has proatherogenic, prothrombotic, prooxidative, proapoptotic, neurotoxic, neuroinflamatory, and neurodegenerative effects. Hyperhomocysteinemia is linked with the existence of C667T MTHFR mutation, reduced levels of folic acid and B-vitamins, but also with the consumption of different medications such as levodopa. Furthermore, a significant number of Parkinson’s disease sufferers have elevated levels of homocysteine. It is clear that prevention and treatment are equally significant. Therefore, folic acid can, ad hoc, become the medication of choice in treatment of hyperhomocysteinemia coexisting with Parkinson’s disease.
Footnotes
Conflict of interest: none
• Statement: All authors were included in preparing of this article, including final proof reading. declared.
REFERENCES
- 1.Sinischalchi A. Hyperhomocysteinemia in neurologic diseases. Recent Prog Med. 2004;95(7-8):371–5. [PubMed] [Google Scholar]
- 2.McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–28. [PMC free article] [PubMed] [Google Scholar]
- 3.Mattson MP, Haberman F. Folate and homocysteine metabolism: therapeutic targets in cardiovascular and neurodegenerative disiorders. Curr Med Chem. 2003;10(19):1923–9. doi: 10.2174/0929867033456864. [DOI] [PubMed] [Google Scholar]
- 4.Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26(3):137–46. doi: 10.1016/S0166-2236(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 5.Kocer A, Ince N, Canbulat CE, Sargin M. Serum vitamin B12 and folic acid levels in acute cerebral atherothrombotic infarction. Tohoku J Exp Med. 2004;204(2):155–61. doi: 10.1620/tjem.204.155. [DOI] [PubMed] [Google Scholar]
- 6.Serra JA, Dominguez RO, de Lustig ES, Guareschi EM, Famulari AL, Bartolome EL, et al. Parkinson's disease is associated with oxidative stress: comparison of peripheral antioxidant profiles in living Parkinson's, Alzheimer's and vascular dementia patients. J Neural Transm. 2001;108(10):1135–48. doi: 10.1007/s007020170003. [DOI] [PubMed] [Google Scholar]
- 7.Giasson BI, Ischiropoulos H, Lee VM, Trojanowski JQ. The relationship between oxidative /nitrative stress and pathological inclusions in Alzheimer's and Parkinson's diseases. Free Radic Biol Med. 2002;32(12):1264–75. doi: 10.1016/s0891-5849(02)00804-3. [DOI] [PubMed] [Google Scholar]
- 8.Tay SY, Ampil ER, Chen CP, Auchus AP. The relationship between homocysteine, cognition and stroke subtypes in acute stroke. J Neurol Sci. 2006;250(1-2):58–61. doi: 10.1016/j.jns.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 9.Sato Y, Iwamoto J, Kanoko T, Satoh K. Homocysteine as a predictive factor for hip fracture in elderly women with Parkinson's disease. Am J Med. 2005;118(11):1250–5. doi: 10.1016/j.amjmed.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Kim MJ, Kim BJ, Kim SR, Chun S, Ryu JS, et al. Homocysteine-lowering therapy or antioxidant therapy for bone loss in Parkinson's disease. Mov Disord. 2010;25(3):332–40. doi: 10.1002/mds.22866. [DOI] [PubMed] [Google Scholar]
- 11.Anonymous. Homocysteine (Direction for use) Dundee, UK: Axis-Shield; 2003. [Google Scholar]
- 12.Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson's disease. J Neurochem. 2002;80(1):101–10. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 13.Zoccolella S, Martino D, Defazio G, Lamberti P, Livrea P. Hyperhomocysteinemia in movement disorders: Current evidence and hypotheses. Curr Vasc Pharmacol. 2006;4(3):237–43. doi: 10.2174/157016106777698414. [DOI] [PubMed] [Google Scholar]
- 14.Blandini F, Fancellu R, Martignoni E, Mangiagalli A, Pacchetti C, Samuele A, et al. Plasma homocysteine and L-dopa metabolism in patients with Parkinson disease. Clin Chem. 2001;47(6):1102–4. [PubMed] [Google Scholar]
- 15.Isobe C, Abe T, Terayama Y. L-Dopa therapy increases homocysteine concentration in cerebrospinal fluid from patients with Parkinson's disease. J Clin Neurosci. 2010;17(6):717–21. doi: 10.1016/j.jocn.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 16.O'Suilleabhain PE, Oberle R, Bartis C, Dewey RB, Jr, Bottiglieri T, Diaz-Arrastia R. Clinical course in Parkinson's disease with elevated homocysteine. Parkinsonism Related Disord. 2006;12(2):103–7. doi: 10.1016/j.parkreldis.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Hassin-Baer S, Cohen O, Vakil E, Sela BA, Nitsan Z, Schwartz R, et al. Plasma homocysteine levels and Parkinson disease: disease progression, carotid intima-media thickness and neuropsychiatric complications. Clin Neuropharmacol. 2006;29(6):305–11. doi: 10.1097/01.WNF.0000236763.16032.60. [DOI] [PubMed] [Google Scholar]
- 18.Nevrly M, Kanowsky P, Vranova H, Langova K, Hlustik P. Effect of entacapone on plasma homocysteine levels in Parkinson's disease patients. Neurol Sci. 2010;31(5):565–9. doi: 10.1007/s10072-010-0262-0. [DOI] [PubMed] [Google Scholar]
- 19.Solla P, Cannas A, Marrosu F, Marrosu MG. Therapeutic interventions and adjustments in the management of Parkinson disease: role of combined carbidopa/levodopa/entacapone (Stalevo) Neuropsychiatr Dis Treat. 2010;6:483–90. doi: 10.2147/ndt.s5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozer F, Meral H, Hanoglu L, Aydemir T, Yilsen M, Cetin S, et al. Plasma homocysteine levels in patients treated with levodopa: motor and cognitive disorders. Neurol Res. 2006;28(8):853–8. doi: 10.1179/016164106X110445. [DOI] [PubMed] [Google Scholar]
- 21.Murakami K, Miyake Y, Sasaki S, Tanaka K, Fukushima W, Kiyohara C, et al. for Fukuoka Kinki Parkinson's Disease Study Group. Dietary intake of folate, vitamin B6, vitamin B12 and riboflavin and risk of Parkinson's disease: A case-control study in Japan. Br J Nutr. 2010;104(5):757–64. doi: 10.1017/S0007114510001005. [DOI] [PubMed] [Google Scholar]
- 22.Anonymous. American Society of Human Genetics/American College of Medical Genetics Test and Technology Transfer Committee Working Group (ASHG/ACMG) statement: Measurement and use of total plasma homocysteine. Am J Hum Genet. 1998;63:1541–3. [PMC free article] [PubMed] [Google Scholar]
- 23.Stanger O, Herrmann W, Pietrzik K, Fowler B, Geisel J, Dierkes J, et al. for DACH-LIGA Homocystein / DACH-LIGA homocystein (German, Austrian and Swiss homocysteine society): consensus paper on the rational clinic use of homocysteine, folic acid and B-vitamines in cardiovascular and thrombotic diseases: guidelines and recommendations. Clin Chem Lab. 2004;41(11):1392–1403. doi: 10.1515/CCLM.2003.214. [DOI] [PubMed] [Google Scholar]
- 24.Belcastro V, Pierguidi L, Castrioto A, Menichetti C, Gorgone G, Ientile R, et al. Hyperhomocysteinemia recurrence in levodopa-treated Parkinson's disease patients. Eur J Neurol. 2010;17(5):661–5. doi: 10.1111/j.1468-1331.2009.02894.x. [DOI] [PubMed] [Google Scholar]
- 25.IbrahimagićOĆSmajlović Dž, Dostović Z, Pašić Z, Šehanović A, Hodžić R. Hiperhomocisteinemija u pacijenata sa ishemijskim cerebrovaskularnim inzultom i njezino liječenje. Medicus. 2012;21(2):267–72. [Google Scholar]


