Abstract
C57BL/6 (B6; I-E-, Mls-2b) nude mice, reconstituted at birth with thymic epithelium (TE) from BALB/c (BA; I-E+, Mls-2a) day 10 embryos (E10), permanently accepted BALB/c skin, when grafted as adults. T-cell receptor repertoire analyses in the periphery of these mice revealed no difference in frequencies of I-E/superantigen-reactive T-cell receptor V beta families, as compared to chimeras constructed with syngeneic B6 E10 TE. T lymphocytes bearing V beta 3, V beta 5, and V beta 11 T-cell receptors, from either allogeneic or syngeneic TE chimeras, responded equally well to in vitro receptor-dependent stimulation. Similar results were obtained with nude mice reconstituted at birth with E14 thymuses, already colonized by hemopoietic cells. These observations indicate that neither TE cells nor the progenies of hemopoietic precursors that colonize the thymus up to E14 express or functionally present the superantigens addressed here; it follows that tolerance to skin grafts and superantigen-related T-cell deletions are unrelated phenomena.
Full text
PDF
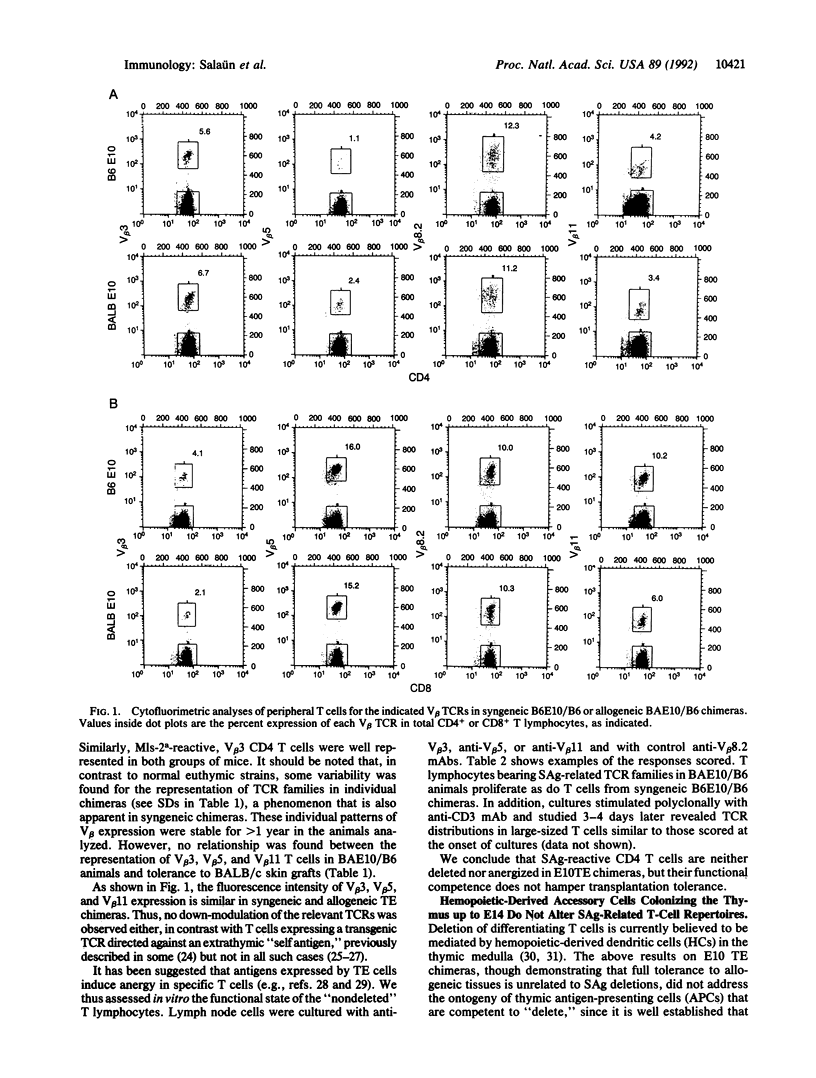
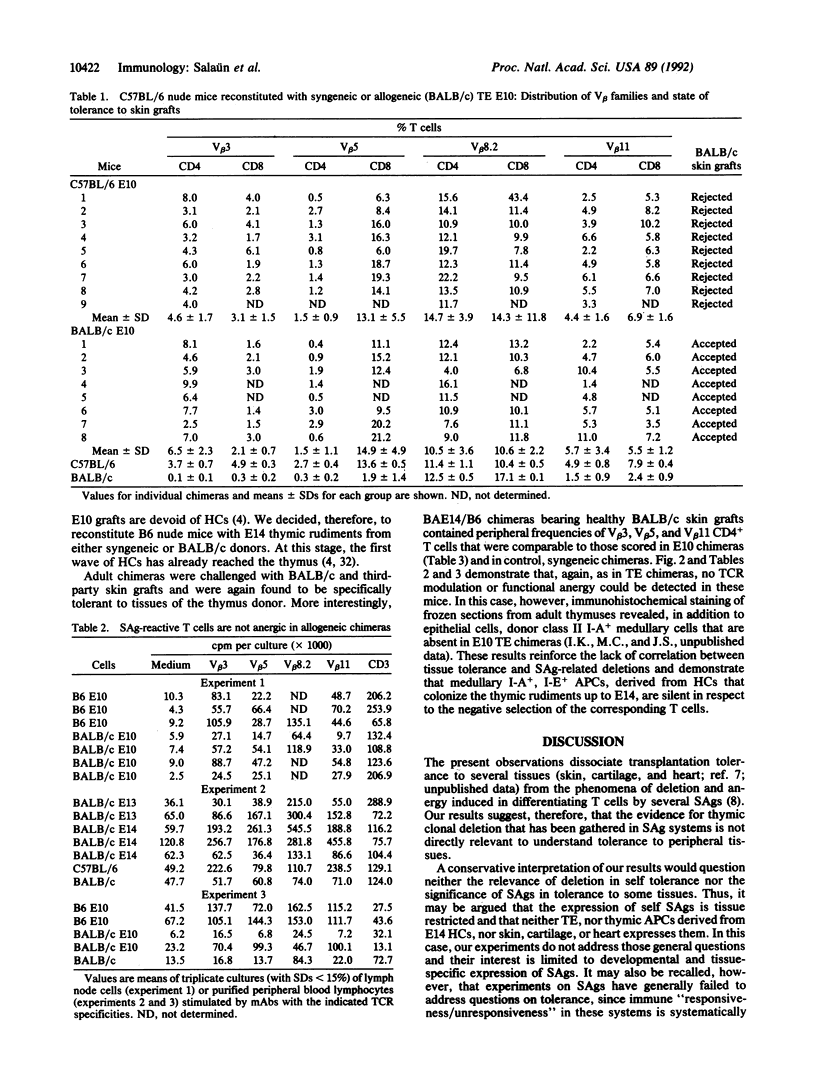
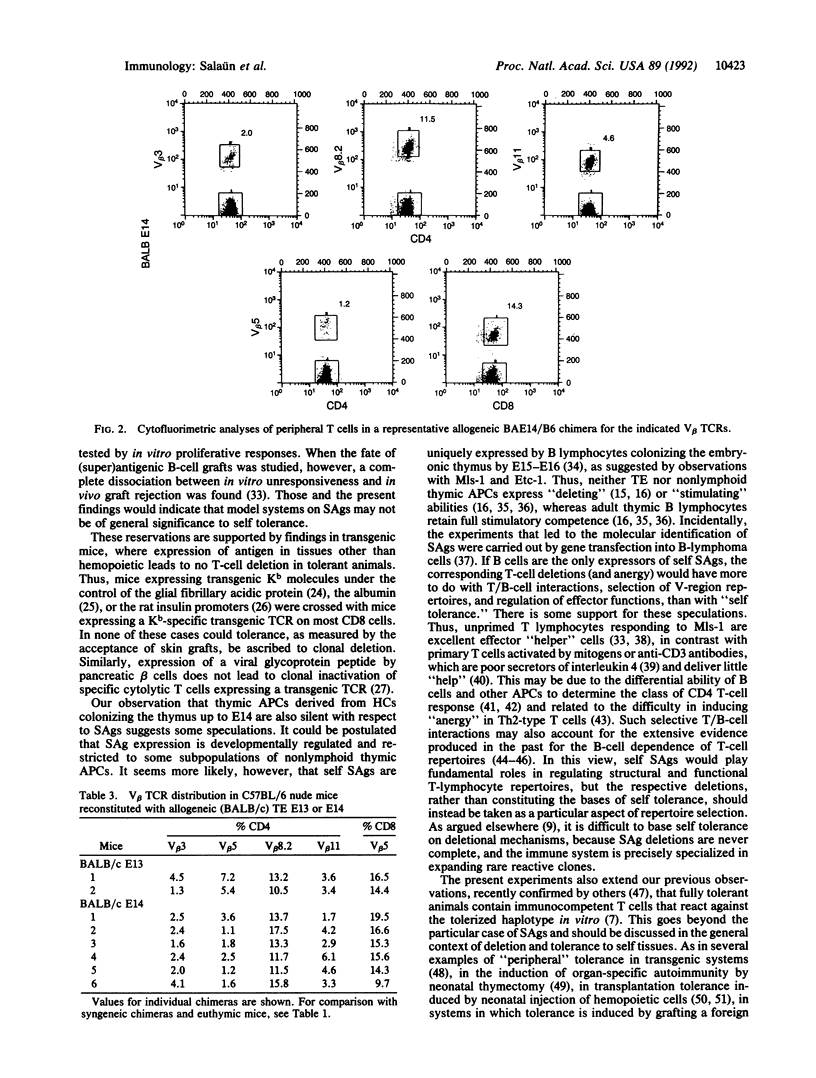

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustin A. A., Coutinho A. Specific T helper cells that activate B cells polyclonally. In vitro enrichment and cooperative function. J Exp Med. 1980 Mar 1;151(3):587–601. doi: 10.1084/jem.151.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira A., Coutinho A., Burlen-Defranoux O., Khazaal I., Coltey M., Jacquemart F., Le Douarin N., Salaün J. Thymic epithelium induces neither clonal deletion nor anergy to Mls 1a antigens. Eur J Immunol. 1992 Jun;22(6):1397–1404. doi: 10.1002/eji.1830220611. [DOI] [PubMed] [Google Scholar]
- Bandeira A., Coutinho A., Carnaud C., Jacquemart F., Forni L. Transplantation tolerance correlates with high levels of T- and B-lymphocyte activity. Proc Natl Acad Sci U S A. 1989 Jan;86(1):272–276. doi: 10.1073/pnas.86.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira A., Larsson E. L., Forni L., Pereira P., Coutinho A. "In vivo" activated splenic T cells are refractory to interleukin 2 growth "in vitro". Eur J Immunol. 1987 Jul;17(7):901–908. doi: 10.1002/eji.1830170702. [DOI] [PubMed] [Google Scholar]
- Bandeira A., Mengel J., Burlen-Defranoux O., Coutinho A. Proliferative T cell anergy to MIs-1a does not correlate with in vivo tolerance. Int Immunol. 1991 Sep;3(9):923–931. doi: 10.1093/intimm/3.9.923. [DOI] [PubMed] [Google Scholar]
- Belo M., Corbel C., Martin C., Le Douarin N. M. Thymic epithelium tolerizes chickens to embryonic graft of quail bursa of Fabricius. Int Immunol. 1989;1(2):105–112. doi: 10.1093/intimm/1.2.105. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson S. Z., Le Gros G., Conrad D. H., Finkelman F. D., Paul W. E. IL-4 production by T cells from naive donors. IL-2 is required for IL-4 production. J Immunol. 1990 Aug 15;145(4):1127–1136. [PubMed] [Google Scholar]
- Bill J., Kanagawa O., Woodland D. L., Palmer E. The MHC molecule I-E is necessary but not sufficient for the clonal deletion of V beta 11-bearing T cells. J Exp Med. 1989 Apr 1;169(4):1405–1419. doi: 10.1084/jem.169.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman M. A., Burgert H. G., Gerhard-Burgert H., Woodland D. L., Palmer E., Kappler J. W., Marrack P. A role for clonal inactivation in T cell tolerance to Mls-1a. Nature. 1990 Jun 7;345(6275):540–542. doi: 10.1038/345540a0. [DOI] [PubMed] [Google Scholar]
- Choi Y., Kappler J. W., Marrack P. A superantigen encoded in the open reading frame of the 3' long terminal repeat of mouse mammary tumour virus. Nature. 1991 Mar 21;350(6315):203–207. doi: 10.1038/350203a0. [DOI] [PubMed] [Google Scholar]
- Corbel C., Belo M., Martin C., Le Douarin N. M. Grafts of the bursal primordium in quail--chick chimeras are tolerated after implantation of thymic epithelium. Prog Clin Biol Res. 1989;307:31–43. [PubMed] [Google Scholar]
- Coutinho A., Bandeira A. Tolerize one, tolerize them all: tolerance is self-assertion. Immunol Today. 1989 Aug;10(8):264–266. doi: 10.1016/0167-5699(89)90138-2. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Coutinho G., Grandien A., Marcos M. A., Bandeira A. Some reasons why deletion and anergy do not satisfactorily account for natural tolerance. Res Immunol. 1992 Mar-Apr;143(3):345–354. doi: 10.1016/s0923-2494(92)80135-8. [DOI] [PubMed] [Google Scholar]
- Day M. J., Tse A. G., Puklavec M., Simmonds S. J., Mason D. W. Targeting autoantigen to B cells prevents the induction of a cell-mediated autoimmune disease in rats. J Exp Med. 1992 Mar 1;175(3):655–659. doi: 10.1084/jem.175.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Faro J., Marcos M. A., Andreu J. L., Martinez-A C., Coutinho A. Inside the thymus, Mls antigen is exclusively presented by B lymphocytes. Res Immunol. 1990 Oct;141(8):723–737. doi: 10.1016/0923-2494(90)90003-h. [DOI] [PubMed] [Google Scholar]
- Flajnik M. F., Du Pasquier L., Cohen N. Immune responses of thymus/lymphocyte embryonic chimeras: studies on tolerance and major histocompatibility complex restriction in Xenopus. Eur J Immunol. 1985 Jun;15(6):540–547. doi: 10.1002/eji.1830150603. [DOI] [PubMed] [Google Scholar]
- Fontaine-Perus J. C., Calman F. M., Kaplan C., Le Douarin N. M. Seeding of the 10-day mouse embryo thymic rudiment by lymphocyte precursors in vitro. J Immunol. 1981 Jun;126(6):2310–2316. [PubMed] [Google Scholar]
- Gollob K. J., Palmer E. Physiologic expression of two superantigens in the BDF1 mouse. J Immunol. 1991 Oct 15;147(8):2447–2454. [PubMed] [Google Scholar]
- Gullberg M., Pobor G., Bandeira A., Larsson E. L., Coutinho A. Differential requirements for activation and growth of unprimed cytotoxic and helper T lymphocytes. Eur J Immunol. 1983 Sep;13(9):719–725. doi: 10.1002/eji.1830130906. [DOI] [PubMed] [Google Scholar]
- Hoffmann M. W., Allison J., Miller J. F. Tolerance induction by thymic medullary epithelium. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2526–2530. doi: 10.1073/pnas.89.7.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling G. J., Schönrich G., Momburg F., Auphan N., Malissen M., Malissen B., Schmitt-Verhulst A. M., Arnold B. Non-deletional mechanisms of peripheral and central tolerance: studies with transgenic mice with tissue-specific expression of a foreign MHC class I antigen. Immunol Rev. 1991 Aug;122:47–67. doi: 10.1111/j.1600-065x.1991.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Inaba M., Inaba K., Hosono M., Kumamoto T., Ishida T., Muramatsu S., Masuda T., Ikehara S. Distinct mechanisms of neonatal tolerance induced by dendritic cells and thymic B cells. J Exp Med. 1991 Mar 1;173(3):549–559. doi: 10.1084/jem.173.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Murgita R. A., Weinbaum F. I., Asofsky R., Wigzell H. Evidence for an immunoglobulin-dependent antigen-specific helper T cell. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4582–4586. doi: 10.1073/pnas.74.10.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jotereau F., Heuze F., Salomon-Vie V., Gascan H. Cell kinetics in the fetal mouse thymus: precursor cell input, proliferation, and emigration. J Immunol. 1987 Feb 15;138(4):1026–1030. [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Khazaal I., Salaün J., Coltey M., Calman F., Le Douarin N. Restoration of T-cell function in nude mice by grafting the epitheliomesenchymal thymic rudiment from 10-day-old euthymic embryos. Cell Differ Dev. 1989 May;26(3):211–220. doi: 10.1016/0922-3371(89)90752-1. [DOI] [PubMed] [Google Scholar]
- Kimoto H., Shirasawa T., Taniguchi M., Takemori T. B cell precursors are present in the thymus during early development. Eur J Immunol. 1989 Jan;19(1):97–104. doi: 10.1002/eji.1830190116. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kushnir E., Kappler J. A maternally inherited superantigen encoded by a mammary tumour virus. Nature. 1991 Feb 7;349(6309):524–526. doi: 10.1038/349524a0. [DOI] [PubMed] [Google Scholar]
- Marrack P., McCormack J., Kappler J. Presentation of antigen, foreign major histocompatibility complex proteins and self by thymus cortical epithelium. Nature. 1989 Apr 6;338(6215):503–505. doi: 10.1038/338503a0. [DOI] [PubMed] [Google Scholar]
- Martin C., Ohki-Hamazaki H., Corbel C., Coltey M., Le Douarin N. M. Successful xenogeneic transplantation in embryos: induction of tolerance by extrathymic chick tissue grafted into quail. Dev Immunol. 1991;1(4):265–277. doi: 10.1155/1991/57259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C., Pereira P., Bernabé R., Bandeira A., Larsson E. L., Cazenave P. A., Coutinho A. Internal complementarities in the immune system: regulation of the expression of helper T-cell idiotypes. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4520–4523. doi: 10.1073/pnas.81.14.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Morahan G., Allison J., Hoffmann M. A transgenic approach to the study of peripheral T-cell tolerance. Immunol Rev. 1991 Aug;122:103–116. doi: 10.1111/j.1600-065x.1991.tb00599.x. [DOI] [PubMed] [Google Scholar]
- Morrissey P. J., Bradley D., Sharrow S. O., Singer A. T cell tolerance to non-H-2-encoded stimulatory alloantigens is induced intrathymically but not prethymically. J Exp Med. 1983 Aug 1;158(2):365–377. doi: 10.1084/jem.158.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Oehen S., Buerki K., Pircher H., Ohashi C. T., Odermatt B., Malissen B., Zinkernagel R. M., Hengartner H. Ablation of "tolerance" and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991 Apr 19;65(2):305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- Ohki H., Martin C., Corbel C., Coltey M., Le Douarin N. M. Tolerance induced by thymic epithelial grafts in birds. Science. 1987 Aug 28;237(4818):1032–1035. doi: 10.1126/science.3616623. [DOI] [PubMed] [Google Scholar]
- Powrie F., Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J Exp Med. 1990 Dec 1;172(6):1701–1708. doi: 10.1084/jem.172.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen A. M., Marrack P., Kappler J. W. The T-cell repertoire is heavily influenced by tolerance to polymorphic self-antigens. Nature. 1988 Oct 27;335(6193):796–801. doi: 10.1038/335796a0. [DOI] [PubMed] [Google Scholar]
- Ramsdell F., Lantz T., Fowlkes B. J. A nondeletional mechanism of thymic self tolerance. Science. 1989 Nov 24;246(4933):1038–1041. doi: 10.1126/science.2511629. [DOI] [PubMed] [Google Scholar]
- Roser B. J. Cellular mechanisms in neonatal and adult tolerance. Immunol Rev. 1989 Feb;107:179–202. doi: 10.1111/j.1600-065x.1989.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Takahashi T., Nishizuka Y. Study on cellular events in post-thymectomy autoimmune oophoritis in mice. II. Requirement of Lyt-1 cells in normal female mice for the prevention of oophoritis. J Exp Med. 1982 Dec 1;156(6):1577–1586. doi: 10.1084/jem.156.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaün J., Bandeira A., Khazaal I., Calman F., Coltey M., Coutinho A., Le Douarin N. M. Thymic epithelium tolerizes for histocompatibility antigens. Science. 1990 Mar 23;247(4949 Pt 1):1471–1474. doi: 10.1126/science.247.4949.1471. [DOI] [PubMed] [Google Scholar]
- Salaün J., Calman F., Coltey M., Le Douarin N. M. Construction of chimeric thymuses in the mouse fetus by in utero surgery. Eur J Immunol. 1986 May;16(5):523–530. doi: 10.1002/eji.1830160511. [DOI] [PubMed] [Google Scholar]
- Schönrich G., Kalinke U., Momburg F., Malissen M., Schmitt-Verhulst A. M., Malissen B., Hämmerling G. J., Arnold B. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991 Apr 19;65(2):293–304. doi: 10.1016/0092-8674(91)90163-s. [DOI] [PubMed] [Google Scholar]
- Speiser D. E., Schneider R., Hengartner H., MacDonald H. R., Zinkernagel R. M. Clonal deletion of self-reactive T cells in irradiation bone marrow chimeras and neonatally tolerant mice. Evidence for intercellular transfer of Mlsa. J Exp Med. 1989 Aug 1;170(2):595–600. doi: 10.1084/jem.170.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Lo D., Gao E. K., Ron Y. T cell selection in the thymus. Immunol Rev. 1988 Jan;101:173–190. doi: 10.1111/j.1600-065x.1988.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Sy M. S., Lowy A., HayGlass K., Janeway C. A., Jr, Gurish M., Greene M. I., Benacerraf B. Chronic treatment with rabbit anti-mouse mu-chain antibody alters the characteristic immunoglobulin heavy-chain restriction of murine suppressor T-cell factors. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3846–3850. doi: 10.1073/pnas.81.12.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S. R., Sprent J. Induction of neonatal tolerance to Mlsa antigens by CD8+ T cells. Science. 1990 Jun 29;248(4963):1643–1646. doi: 10.1126/science.1973003. [DOI] [PubMed] [Google Scholar]
- Woodland D., Happ M. P., Bill J., Palmer E. Requirement for cotolerogenic gene products in the clonal deletion of I-E reactive T cells. Science. 1990 Feb 23;247(4945):964–967. doi: 10.1126/science.1968289. [DOI] [PubMed] [Google Scholar]
- Zamoyska R., Waldmann H., Matzinger P. Peripheral tolerance mechanisms prevent the development of autoreactive T cells in chimeras grafted with two minor incompatible thymuses. Eur J Immunol. 1989 Jan;19(1):111–117. doi: 10.1002/eji.1830190118. [DOI] [PubMed] [Google Scholar]



