INTRODUCTION
The rapid increase of non-medical prescription opioid (PO) use among youth has been described as the most alarming drug use trend in North America (Compton and Volkow, 2006, McCabe et al., 2008 and Okie, 2010). After cannabis, youth consistently identify POs as the most commonly misused drug (Johnston, O’Malley, Bachman, & Schulenberg, 2012) and the 2013 US Centre for Disease Control national survey of American high schools found that over 20% of grade 12 students have misused prescription drugs (Kann et al., 2014). In addition to concerns regarding increased risk of morbidity and mortality associated with PO use (Frank et al., 2015, Hall et al., 2008, Office of the National Drug Control Strategy, 2011, Okie, 2010, USCDCP, 2011, USCDCP, 2012, Roy et al., 2004, Silva et al., 2013, Warner et al., 2011 and Zosel et al., 2013), there are also signals from qualitative, retrospective and cross-sectional studies that PO use may facilitate transitions to injection drug use; however, the impact of PO misuse on the incidence of initiation into injecting has not been characterized (Lankenau et al., 2012, Mars et al., 2014, Peavy et al., 2012, Rigg and Murphy, 2013, Young et al., 2012 and Young and Havens, 2012). The objective of this longitudinal study was to examine the relationship between PO misuse and time to injection initiation within an open prospective cohort of street-involved youth in Vancouver, Canada.
METHODS
Data for this study was obtained from the At-Risk Youth Study (ARYS) which is an open prospective cohort that began in 2005 and has been described in detail (Wood, Stoltz, Montaner, & Kerr, 2006). In brief, study recruitment is open and undertaken using snowball sampling and extensive street-based outreach methods. Study eligibility is restricted to participants who are aged 14–26 years at enrolment, use illicit drugs other than cannabis in the past 30 days, are “street-involved” defined as having been homeless in the past six months or recently having used a service for street-involved youth (e.g., housing or nutrition support), and provide written informed consent. At baseline participants complete an interviewer-administered questionnaire, are examined by a nurse for stigmata of injecting, and provide blood samples for infectious disease testing. All participants are subsequently eligible to complete follow-up study visits on a bi-annual basis that involve completing an interviewer-administered questionnaire, and an examination and blood draw from a study nurse. At each study visit participants are provided with a stipend ($30 CDN) for their time. The study is approved by the University of British Columbia/Providence Health Care Research Ethics Board.
To examine the potential relationship between non-medical PO use and initiation into injection drug use, all participants who had never injected drugs at baseline and had completed at least one follow-up visit during the study period (September 2005 to May 2014) were included in the analysis. The main outcome of interest was time to injection initiation, which was defined as the midpoint between the last report of remaining injection naïve and the first report of having used a needle to chip, fix or muscle drugs. This approach is consistent with prior investigations of injection initiation (Ahamad et al., 2014, Chami et al., 2013, Feng et al., 2013 and Richardson et al., 2014). Participants were also asked to specify the type of drug they used at their first injection initiation event.
The primary explanatory variable of interest was having ever used non-injection POs without a prescription at baseline. Categories of POs were dilaudid, morphine, codeine, street methadone, oxycontin, talwin, and ‘other’. As a sub analysis, each category of PO use was also examined independently with respect to injection initiation.
To determine whether there was a significant relationship between our main outcome of interest and our primary explanatory variable (PO use) we a priori selected a range of secondary explanatory variables, which we hypothesized might be associated with both injection initiation and baseline history of non-medical PO use. Secondary explanatory factors of interest included basic demographic variables and drug use patterns which, based on the literature, are known to be associated with drug use trajectories (Baldwin et al., 2013, Lankenau et al., 2012 and Mars et al., 2014) and were: age (per additional year); gender (female vs. male); ethnicity (Caucasian vs. other); non-injection cocaine use (yes vs. no); crack cocaine smoking (yes vs. no); non-injection crystal methamphetamine use (yes vs. no); and non-injection heroin use (yes vs. no). All drug use variables referred to the previous six months and were treated as time-updated covariates on the basis of semiannual follow-up data. In addition, all drug use variables were lagged to the previous available observation to protect against reverse causation–as done in prior studies of injection initiation in this setting (Ahamad et al., 2014, Chami et al., 2013, Feng et al., 2013 and Richardson et al., 2014).
As a first step, baseline characteristics of the study sample stratified by subsequent injection initiation were examined using Pearson’s chi-square test for dichotomous variables and the Wilcoxon rank sum test for continuous variables. The incidence density of injection initiation was then calculated using a Poisson model. The cumulative incidence of injection initiation from the time of study enrolment stratified by baseline history of PO use was calculated using a Kaplan–Meier estimate. Lastly, using Cox regression, we estimated the unadjusted relative hazards and 95% confidence intervals for factors associated with injection initiation. To fit our multivariable Cox model, we used a previously described backwards selection process (Maldonado and Greenland, 1993 and Rothman et al., 2008). We began with all explanatory variables of interest in a full model, then generated a series of reduced models by removing each secondary explanatory variable one at a time. For each of these models we assessed the relative change in the coefficient for our primary explanatory variable of interest (history of non-medical PO use at baseline). The secondary explanatory variable of interest that resulted in the smallest absolute relative change in the coefficient for baseline non-medical PO use was then removed. Secondary variables continued to be removed through this process until the smallest relative change exceeded 5%. Remaining variables were considered confounders and were included in the final multivariable model. To determine if our results were robust, we also ran a fixed multivariable model where all variables of interest were forced into a single model. All statistical analyses were performed using SAS software version 9.3 (SAS, Cary, NC, USA). All tests of significance were two-sided.
RESULTS
Between September 2005 and May 2014, 1157 youth were recruited into the ARYS open prospective cohort. At enrolment 659 (57%) participants reported being injection naïve. The average yearly lost to follow up rate during the study period among these participants was 3.15%. By the time this study was conducted, a total of 462 (70%) youth completed at least one study follow-up and were therefore eligible for the current analysis. There were no significant differences with respect to gender (p = 0.943), ethnicity (p = 0.117), or baseline PO use (p = 0.087) between the 462 youth who were included in the analysis vs. the 197 injecting naïve youth who were excluded because they either did not have a follow-up visit at the time this study was conducted or were not enrolled in the cohort long enough to be due for a study follow-up visit.
Among the study sample of 462 participants, 142 (31%) were female and the median age was 21.5 years (interquartile range [IQR] = 19.6–23.2). The median number of study visits completed per participant was 4 (IQR = 2–6), and the median follow up time per participant was 22.4 (IQR = 11.9–43.2) months. Baseline characteristics of the study sample are presented in Table 1.
Table 1.
Baseline characteristics and Cox regression analysis for factors associated with injection initiation among street-involved youth (n = 462).
| Baseline Characteristics | Bivariable and Multivariable Cox Regression Analysis | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Injection Initiation
|
Unadjusted HRa (95% CI)b |
p-value | Adjusted HR (95% CI) |
p-value | ||
| Characteristic | Yes (n=97) n (%) |
No (n=365) n(%) |
||||
| Prescription Opiate Usec,d | ||||||
| Yes | 49 (50.5) | 129 (35.3) | 1.82 (1.22 – 2.70) | 0.003 | 1.70 (1.12 – 2.58) | 0.012 |
| No | 48 (49.5) | 236 (64.7) | ||||
| Age (per year older) | ||||||
| Median IQR | 21.3 (19.6, 22.6) | 21.6 (19.6, 23.4) | 0.96 (0.90 – 1.03) | 0.288 | ||
| Caucasian Ethnicity | ||||||
| Yes | 68 (70.1) | 219 (60.0) | 1.50 (0.97 – 2.31) | 0.069 | ||
| No | 29 (29.9) | 146 (40.0) | ||||
| Female Gender | ||||||
| Yes | 28 (28.9) | 114 (31.2) | 0.96 (0.62 – 1.50) | 0.872 | ||
| No | 69 (71.1) | 251 (68.8) | ||||
| Heroin Used,e,f | ||||||
| Yes | 24 (24.7) | 52 (14.2) | 2.12 (1.34 – 3.36) | 0.001 | 1.66 (1.02 – 2.72) | 0.043 |
| No | 70 (72.2) | 307 (84.1) | ||||
| Cocaine Used,e,f | ||||||
| Yes | 43 (44.3) | 186 (51.0) | 1.17 (0.77 – 1.78) | 0.449 | ||
| No | 52 (53.6) | 176 (48.2) | ||||
| Crack Smokinge,f | ||||||
| Yes | 68 (70.1) | 190 (52.1) | 1.71 (1.11 – 2.63) | 0.015 | ||
| No | 27 (27.8) | 171 (46.8) | ||||
| Crystal Meth Used,e,f | ||||||
| Yes | 51 (52.6) | 122 (33.4) | 2.31 (1.53 – 3.47) | <0.001 | 2.08 (1.37 – 3.17) | <0.001 |
| No | 43 (44.3) | 238 (65.2) | ||||
HR: hazard ratio;
CI: confidence interval;
denotes activities ever;
denotes non-injection use;
denotes activities in the six months prior to follow-up interview;
refers to the activities lagged to the pervious available study follow-up
At baseline, 178 (39%) participants reported having ever misused a PO via non-injection. The types of POs that youth reported having ever misused were: codeine (n = 98, 55%); oxycontin (n = 82, 46%); morphine (n = 74, 42%); dilaudid (n = 42, 24%); street methadone (n = 23, 13%), talwin (n = 9, 5%) and ‘other’ (n = 11, 6%), specifically percocet (n = 10, 6%) and demerol (n = 1, 0.6%).
Over study follow-up, 97 (21%) injection initiation events were observed for an incidence density of 8.6 cases per 100 person years [95% confidence interval (CI): 7.0–10.6]. The median time to injection initiation from study enrolment was 11.2 months (IQR: 3.9–23.9), and the median number of years between initiation of non-medical PO use and initiation of injection drug use was 4.8 years (IQR = 2.7–7.5). The Kaplan–Meier estimates of the cumulative incidence of injection initiation stratified by baseline PO use indicate that youth who reported PO use at baseline were at significantly higher risk of injection initiation after five years of study follow-up (39.1% vs. 26.2%, log rank p = 0.002) (Fig. 1).
Figure 1.
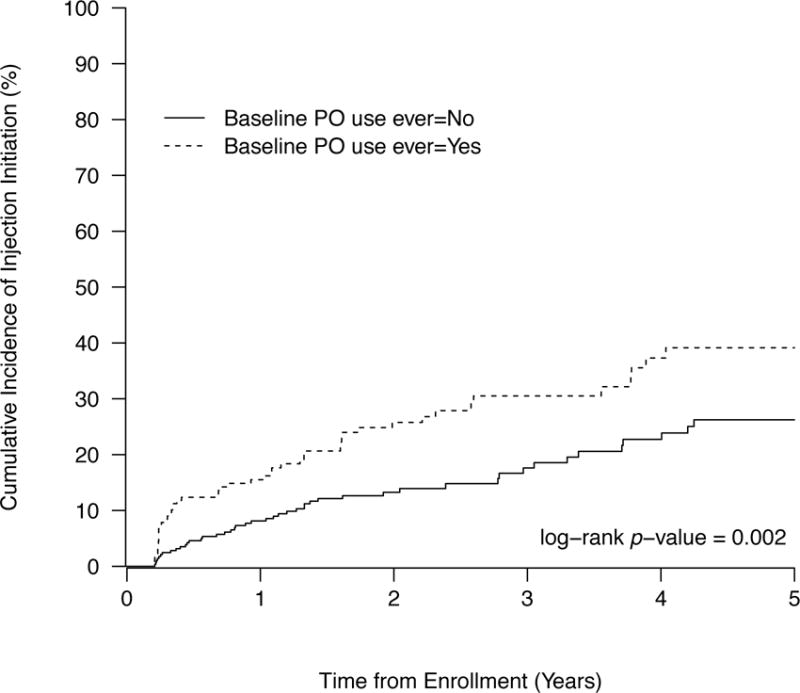
Time to initiation of injection drug use among a cohort of street-involved youth in Vancouver stratified by baseline history of prescription opiate use (n=462).
The type of drug that participants reported injecting at their first injection initiation event is presented in Table 2. As displayed, heroin was the most common drug (n = 57, 59%), followed closely by crystal meth (n = 52, 54%). Participants with a history of non-injection PO use at baseline were more likely to initiate injection drug use with heroin and POs, but the difference was only marginally significant (p = 0.083 and p = 0.070 respectively).
Table 2.
Type of drug injected at first injection initiation event (n = 97).
| Count (% out of 97 initiation events) |
Ever used non-injection PO at baseline
|
p-value | ||
|---|---|---|---|---|
| Category names | Yes (% out of 49 participants) |
No (% out of 48 participants) |
||
| Heroin | 57 (58.8) | 33 (67.4) | 24 (50.0) | 0.083 |
| Cocaine | 18 (18.6) | 11 (22.5) | 7 (14.6) | 0.319 |
| Crystal Meth | 52 (53.6) | 26 (53.1) | 26 (54.2) | 0.913 |
| Prescription opiates | 12 (12.4) | 9 (18.4) | 3 (6.3) | 0.070 |
| Ploy-drug | 3 (3.1) | 3 (6.1) | 0 | 0.242£ |
| Other | 4 (4.1) | 2 (4.1) | 2 (4.2) | 1.000£ |
P-value is from Fisher’s exact test.
Table 1 shows the unadjusted and adjusted relative hazards of injection initiation. Baseline PO use was significantly associated with time to first injection drug use in both bivariable [relative hazards = 1.82, 95% CI: 1.22–2.70] and multivariable Cox regression [adjusted relative hazards (ARH) = 1.70, 95% CI: 1.12–2.58] after adjustment for the effect of non-injection crystal methamphetamine and heroin use. The association between PO use and injection initiation was also significant in the fixed multivariable Cox model that adjusted for all variables of interest (ARH = 1.68, 95% CI: 1.11–2.54) (not shown in Table 1).
In sub analysis assessing specific categories of baseline non-injection PO use and injection initiation, we observed at the bivariable level that dilaudid (HR = 2.55, 95% CI: 1.43–4.53; p-value = 0.002) and oxycontin (HR = 1.66, 95% CI: 1.05–2.62; p-value = 0.029) were significantly associated with injection initiation. Conversely, codeine (HR = 1.20, 95% CI: 0.75–1.93; p-value = 0.457), and morphine (HR = 1.28, 95% CI: 0.76–2.18; p-value = 0.352) were not significantly associated, and a category comprised of street methadone, talwin, percocet, demerol, and ‘other’ was only marginally associated with injection initiation (HR = 1.88, 95% CI: 1.00–3.55; p-value = 0.051). When considered in multivariable models, dilaudid remained independently associated with injection initiation (AHR = 1.94, 95% CI: 1.03–3.63; p-value = 0.039) while oxycontin only approached marginal statistical significance (AHR = 1.56, 95% CI: 0.98–2.50; p-value = 0.061) (not shown in a table).
DISCUSSION
Non-medical prescription opiate use predicted initiation into injection drug use among our cohort of street-involved youth. Although this is the first longitudinal study to demonstrate that non-medical PO use is an independent risk factor for injection initiation, these findings are consistent with prior qualitative, retrospective and cross-sectional studies suggesting that non-medical PO use may facilitate transitions into injection drug use (Lankenau et al., 2012, Mars et al., 2014, Peavy et al., 2012, Rigg and Murphy, 2013 and Young and Havens, 2012). It is noteworthy that in sub analysis, certain categories of POs, specifically dilaudid and oxycontin in bivariable analysis and dilaudid in multivariable analysis, were significantly associated with injection initiation while other categories were not. However, limited statistical power may account for the absence of a statistically significant relationship between injection initiation and some categories of PO. Further investigation of the risks associated with specific categories of POs and injection initiation is warranted.
These findings are concerning, as they indicate that the dramatic increase in non-medical PO use observed in North America (Johnston et al., 2012 and Kann et al., 2014) may spur a new epidemic of injection drug use among youth. Given that young injectors are more likely to engage in behaviors that place them at increased risk of experiencing drug related harm, including drug overdose and HIV infection (Baldwin et al., 2013, Golub et al., 2007, Roy et al., 2004, Silva et al., 2013, Thiede et al., 2007 and Zosel et al., 2013), interventions to prevent and address non-medical PO use among vulnerable youth are urgently required. Changing current prescribing practices to reduce the availability of POs and improving addiction treatment options, including increasing access to evidence-based treatments for opiate addiction among youth, have been identified as priority areas for intervention (Bohnert et al., 2011, Fiellin, 2008, Greenfield et al., 2014, Jones et al., 2014, Kuehn, 2010, Pecoraro et al., 2013, Rogers and Copley, 2009 and Woody et al., 2008). In addition, our data indicate that evidence-based harm reduction services, such needle exchange programs and overdose prevention interventions, will be critical for limiting preventable drug related harms among PO using youth, including infectious disease acquisition. This may require tailoring existing services as emerging evidence indicates that young PO users may have unique needs that programs are not currently meeting (Frank et al., 2015, Roy et al., 2011 and Wu et al., 2011).
Our study has limitations. First, as with other studies of street-involved youth, the ARYS cohort is not a random sample and therefore these findings may not generalize to other street youth populations. Second, this study includes self-reported information that is susceptible to response biases. Third, as with any non-randomized study, the association between PO use and injection initiation could be influenced by unmeasured confounders.
In conclusion, our longitudinal study demonstrated that a history of PO use independently predicted the incidence of injection initiation among at-risk youth. These findings highlight the urgent need to implement interventions to prevent and treat non-medical PO use, as well as tailor and optimize public health and harm reduction programs for youth who use prescription opioids.
Acknowledgments
The authors thank the ARYS study participants for their contribution to the research, as well as current and past researchers and staff. We would specifically like to thank Cody Callon, Jennifer Matthews, Deborah Graham, Peter Vann, Steve Kain, Tricia Collingham, Kristie Starr and Carmen Rock for their research and administrative assistance.
Funding
The study was supported by the US National Institutes of Health (R01DA028532, U01DA038886) and the Canadian Institutes of Health Research (MOP-286532). KD is supported by a MSFHR/St. Paul’s Hospital-Providence Health Care Career Scholar Award and a Canadian Institutes of Health Research New Investigator Award. This research was undertaken, in part, thanks to funding from the Canada Research Chairs program through a Tier 1 Canada Research Chair in Inner City Medicine, which supports EW. KH is supported by Canadian Institutes of Health Research. JM is supported with grants paid to his institution by the British Columbia Ministry of Health and by the US National Institutes of Health (R01DA036307). Funding sources had no role in the: design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author contributions
KD, TK, and EW designed the study and wrote the protocol, KD managed the literature search and prepared the first draft of the analysis; HD conducted the statistical analyses with input from KD, TK and SD; all authors contributed to the main content and provided critical comments on the final draft. All authors approved the final manuscript. KD had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest
JM has received limited unrestricted funding, paid to his institution, from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. All other authors declare that they have no conflicts of interest.
References
- Ahamad K, Debeck K, Feng C, Sakakibara T, Kerr T, Wood E. Gender influences on initiation of injecting drug use. American Journal of Drug and Alcohol Abuse. 2014;40:151–156. doi: 10.3109/00952990.2013.860983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin P, Shrestha R, Potrepka J, Copenhaver M. The age of initiation of drug use and sexual behavior may influence subsequent HIV risk behavior: A systematic review. ISRN AIDS. 2013;2013:976035. doi: 10.1155/2013/976035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, et al. Association between opioid prescribing patterns and opioid overdoserelated deaths. JAMA. 2011;305:1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- Chami G, Werb D, Feng C, DeBeck K, Kerr T, Wood E. Neighborhood of residence and risk of initiation into injection drug use among street-involved youth in a Canadian setting. Drug and Alcohol Dependence. 2013;132:486–490. doi: 10.1016/j.drugalcdep.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: Concerns and strategies. Drug and Alcohol Dependence. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Feng C, DeBeck K, Kerr T, Mathias S, Montaner J, Wood E. Homelessness independently predicts injection drug use initiation among street-involved youth in a Canadian setting. Journal of Adolescent Health. 2013;52:499–501. doi: 10.1016/j.jadohealth.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA. Treatment of adolescent opioid dependence: No quick fix. JAMA. 2008;300:2057–2059. doi: 10.1001/jama.2008.567. [DOI] [PubMed] [Google Scholar]
- Frank D, Mateu-Gelabert P, Guarino H, Bennett A, Wendel T, Jessell L, et al. High risk and little knowledge: Overdose experiences and knowledge among young adult nonmedical prescription opioid users. International Journal of Drug Policy. 2015;26:84–91. doi: 10.1016/j.drugpo.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub ET, Strathdee SA, Bailey SL, Hagan H, Latka MH, Hudson SM, et al. Distributive syringe sharing among young adult injection drug users in five U.S. cities. Drug and Alcohol Dependence. 2007;91(Suppl. 1):S30–S38. doi: 10.1016/j.drugalcdep.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Greenfield BL, Owens MD, Ley D. Opioid use in Albuquerque New Mexico: A needs assessment of recent changes and treatment availability. Addiction Science & Clinical Practice. 2014;9:10. doi: 10.1186/1940-0640-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AJ, Logan JE, Toblin RL, Kaplan JA, Kraner JC, Bixler D, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on adolescent drug use: Overview of key findings 2011. Ann Arbor, USA: The University of Michigan; 2012. [Google Scholar]
- Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use United States, 2008–2011. JAMA Internal Medicine. 2014;174:802–803. doi: 10.1001/jamainternmed.2013.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann L, Kinchen S, Shanklin SL, Flint KH, Kawkins J, Harris WA, et al. Youth risk behavior surveillance—United States, 2013. MMWR Surveillance Summaries. 2014;63:1–168. [PubMed] [Google Scholar]
- Kuehn BM. Medication helps make therapy work for teens addicted to prescription opioids. JAMA. 2010;303:2343–2345. doi: 10.1001/jama.2010.789. [DOI] [PubMed] [Google Scholar]
- Lankenau SE, Teti M, Silva K, Bloom JJ, Harocopos A, Treese M. Initiation into prescription opioid misuse amongst young injection drug users. International Journal of Drug Policy. 2012;23:37–44. doi: 10.1016/j.drugpo.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado G, Greenland S. Simulation study of confounder-selection strategies. American Journal of Epidemiology. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- Mars SG, Bourgois P, Karandinos G, Montero F, Ciccarone D. “Every ‘never’ I ever said came true”: Transitions from opioid pills to heroin injecting. International Journal of Drug Policy. 2014;25:257–266. doi: 10.1016/j.drugpo.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Cranford JA, West BT. Trends in prescription drug abuse and dependence, co-occurrence with other substance use disorders, and treatment utilization: Results from two national surveys. Addictive Behaviors. 2008;33:1297–1305. doi: 10.1016/j.addbeh.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of the National Drug Control Strategy, & Executive Office of the President of the United States. EPIDEMIC: Responding to America’s prescription drug abuse crisis. 2011 Available at: http://www.whitehouse.gov/sites/default/files/ondcp/policy-and-research/rx_abuse_plan.pdf.
- Okie S. A flood of opioids, a rising tide of deaths. The New England Journal of Medicine. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- Peavy KM, Banta-Green CJ, Kingston S, Hanrahan M, Merrill JO, Coffin PO. “Hooked on” prescription-type opiates prior to using heroin: Results from a survey of syringe exchange clients. Journal of Psychoactive Drugs. 2012;44:259–265. doi: 10.1080/02791072.2012.704591. [DOI] [PubMed] [Google Scholar]
- Pecoraro A, Fishman M, Ma M, Piralishvili G, Woody GE. Pharmacologically assisted treatment of opioid-dependent youth. Paediatric Drugs. 2013;15:449–458. doi: 10.1007/s40272-013-0041-5. [DOI] [PubMed] [Google Scholar]
- Richardson L, DeBeck K, Feng C, Kerr T, Wood E. Employment and risk of injection drug use initiation among street involved youth in Canadian setting. Preventive Medicine. 2014;66:56–59. doi: 10.1016/j.ypmed.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigg KK, Murphy JW. Understanding the etiology of prescription opioid abuse: Implications for prevention and treatment. Qualitative Health Research. 2013;23:963–975. doi: 10.1177/1049732313488837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers PD, Copley L. The nonmedical use of prescription drugs by adolescents. Adolescent Medicine: State of the Art Reviews. 2009;20:1–8. vii. [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Lippincott Williams & Wilkins; 2008. [Google Scholar]
- Roy E, Arruda N, Bourgois P. The growing popularity of prescription opioid injection in downtown Montreal: New challenges for harm reduction. Substance Use & Misuse. 2011;46:1142–1150. doi: 10.3109/10826084.2011.552932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy E, Haley N, Leclerc P, Sochanski B, Boudreau JF, Boivin JF. Mortality in a cohort of street youth in Montreal. JAMA. 2004;292:569–574. doi: 10.1001/jama.292.5.569. [DOI] [PubMed] [Google Scholar]
- Silva K, Schrager SM, Kecojevic A, Lankenau SE. Factors associated with history of non-fatal overdose among young nonmedical users of prescription drugs. Drug and Alcohol Dependence. 2013;128:104–110. doi: 10.1016/j.drugalcdep.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiede H, Hagan H, Campbell JV, Strathdee SA, Bailey SL, Hudson SM, et al. Prevalence and correlates of indirect sharing practices among young adult injection drug users in five U.S. cities. Drug and Alcohol Dependence. 2007;91(Suppl. 1):S39–S47. doi: 10.1016/j.drugalcdep.2007.03.001. [DOI] [PubMed] [Google Scholar]
- US Centers for Disease Control and Prevention. Vital Signs. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. Prescription painkiller overdoses in the US. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6043a4.htm?s_cid=mm6043a4_w. [Google Scholar]
- US Centre for Disease Control and Prevention. Research update: State prescription drug monitoring programs and drug overdose deaths. 2012 Jun 29; Available at: http://www.cdc.gov/HomeandRecreationalSafety/Poisoning/ru_monitoring.html.
- Warner M, Chen LH, Makuc DM, Anderson RN, Mininõ AM. Drug poisoning deaths in the United States, 1980–2008. Hyattsville, MD: National Center for Health Statistics; 2011. (NCHS data brief no. 81). [PubMed] [Google Scholar]
- Wood E, Stoltz JA, Montaner JS, Kerr T. Evaluating methamphetamine use and risks of injection initiation among street youth: The ARYS study. Harm Reduction Journal. 2006;3:18. doi: 10.1186/1477-7517-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody GE, Poole SA, Subramaniam G, Dugosh K, Bogenschutz M, Abbott P, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: A randomized trial. JAMA. 2008;300:2003–2011. doi: 10.1001/jama.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Blazer DG, Li TK, Woody GE. Treatment use and barriers among adolescents with prescription opioid use disorders. Addictive Behaviors. 2011;36:1233–1239. doi: 10.1016/j.addbeh.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AM, Glover N, Havens JR. Nonmedical use of prescription medications among adolescents in the United States: A systematic review. Journal of Adolescent Health. 2012;51:6–17. doi: 10.1016/j.jadohealth.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Young AM, Havens JR. Transition from first illicit drug use to first injection drug use among rural Appalachian drug users: A cross-sectional comparison and retrospective survival analysis. Addiction. 2012;107:587–596. doi: 10.1111/j.1360-0443.2011.03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zosel A, Bartelson BB, Bailey E, Lowenstein S, Dart R. Characterization of adolescent prescription drug abuse and misuse using the Researched Abuse Diversion and Addiction-related Surveillance (RADARS((R))) System. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:196–204.e192. doi: 10.1016/j.jaac.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


