Abstract
Marek’s disease (MD), caused by Marek’s disease virus (MDV), a poultry-borne alphaherpesvirus, is a devastating disease of poultry causing an estimated annual loss of one billion dollars to poultry producers, worldwide. Despite decades of control through vaccination, MDV field strains continue to emerge having increased virulence. The evolutionary mechanism driving the emergence of this continuum of strains to increased MDV virulence, however, remains largely enigmatic. Increase in MDV virulence has been associated with specific amino acid changes within the C-terminus domain of Mareks’s EcoRI-Q (meq)-encoded oncoprotein. In this study, we sought to determine whether the meq gene has evolved adaptively and whether past vaccination efforts have had any significant effect on the reduction or increase of MDV diversity over time. Our analysis suggests that meq is estimated to be evolving at a much faster rate than most dsDNA viruses, and is comparable with the evolutionary rate of RNA viruses. Interestingly, most of the polymorphisms in meq gene appear to have evolved under positive selection and the time of divergence at the meq locus coincides with the period during which the poultry industry had undergone transitions in management practices including the introduction and widespread use of live attenuated vaccines. Our study has revealed that the decades-long use of vaccines did not reduce MDV diversity, but rather had a stimulating effect on the emergence of field strains with increased genetic diversity until the early 2000s. During the years 2004–2005, there was an abrupt decline in the genetic diversity of field isolates followed by a recovery from this bottleneck in the year 2010. Collectively, these data suggest that vaccination seems to not have had any effect on MDV eradication, but rather had a stimulating effect on MDV emergence through adaptation.
Introduction
Despite the fact that live attenuated vaccines have widely been used to protect host populations against a circulating viral strain, there are several instances where viruses are capable of evolving rapidly in the face of vaccine-elicited protection [1, 2]. As a result, viral strains with new genetic makeup and distinct phenotypic traits have been found to emerge in the population. Mutations in viral proteins that are important to the emergence of the new viral strains with greater fitness are highly unlikely to be non-adaptive or neutral. Knowledge of the viral proteins that have evolved adaptively in vaccinated host populations is therefore crucial for surveillance and control of viral infections.
Marek’s disease (MD), caused by the Marek’s disease virus (MDV), a poultry-borne double-stranded DNA virus, belongs to the family Herpesviridae (subfamily: Alphaherpesvirinae; genus: Mardivirus), and is one of the unique examples among the known DNA viruses, where new viral strains with greater fitness have continued to emerge in the host population despite widespread vaccination [3–6]. The poultry industries in the United States, China, Australia, India, Japan, the United Kingdom, Poland, and many other European countries [4, 7–12] are reported to be affected by MD and suffer from significant economic loss. Although domestic chickens are the principal host of MDV [4, 13], recent reports indicate the prevalence of this poultry-borne herpesvirus in other wild birds, suggesting cross-species transmission and successful viral adaptation to a wide range of avian host species inhabiting temperate and tropical climates [14, 15]. These findings suggest that MD not only poses a continuing threat to the poultry industry, but also poses a potential concern to wildlife.
MD was initially described by a Hungarian veterinary pathologist, József Marek, in 1907 [16]. Since this time, the clinical features of MD have changed drastically from its initial description [4, 7]. Based on its pathogenicity, MDV is classified into four pathotypes: (1) classical MDV, having mild virulence (mMDVs), which was mostly assumed to have occurred before 1950s, (2) virulent or acute strains (vMDVs) that emerged during the 1950s through the 1960s, the period during which the broiler industry had undergone radical transition to mixed-age farms with a drastic increase in the density of chickens per square meter, (3) very virulent strains (vvMDVs), which emerged during the late 1970s, after the introduction and widespread adoption of the turkey herpesvirus (HVT) vaccine, and the fourth pathotype, designated as very virulent plus (vv+MDVs) emerged after the introduction of the bivalent vaccination (HVT + SB-1) in the early 1990s [4]. After the emergence of vv+MDVs in the US, an attenuated MDV-1 strain, Rispens-CVI988 [17, 18] was introduced in mid-1990s; however, no significant increase in virulence has been noted since this introduction [4, 7]. Despite widespread vaccination over the last four decades, MDV remains as one of the most devastating poultry diseases, worldwide. Despite control through vaccination, the mechanism mediating this evolution of MDV strains to increased virulence remains largely unknown. Interestingly, Schat and Baranowski [19] reported stepwise evolution of virulence of MDV isolates since 1940s till 2000s. According to Schat and Baranowski [19], MDV isolates appeared to be mild (m) during 1940s, virulent (v) during 1960s, very virulent (vv) during 1980s, and vv+ during 2000s. This stepwise increase in virulence seems positively correlated with the introduction of new MDV vaccine each decade. A recent study has also reported an increased pathogenecity of MDV, despite the decades of vaccinations [13]
In the early 2000’s, Shamblin et al., [3], reported the association between MDV virulence and patterns of genetic polymorphisms at the C-terminus domain of Mareks’s EcoRI-Q (meq)-encoded oncoprotein. Meq is a basic leucine zipper (b-ZIP) protein having characteristics of several viral oncoproteins including v-Jun [20], HBZ of HTLV-I [21], Tat of HIV [22, 23], and EBNA-3C of Epstein- Barr virus (EBV) [24, 25]. Meq is encoded as a 339-amino-acid unspliced open reading frame in vvMDV and vv+ pathotypes of MDV. Mild and virulent MDVs (m/vMDVs) from the 1960s and 1970s encode a larger form of meq (398+-amino acids) having multiple duplications of a C-terminal, proline-rich repeat (PRR) domain [3].
Although certain non-synonymous polymorphisms at the meq-gene are associated with the MDV virulence [3], it is unclear as to what forces have elicited such evolutionary changes in the vaccinated chicken populations. Given the relatively slow evolutionary rates of the dsDNA viruses [26–28], the meq gene, would be expected to evolve at a similar rate. Alternatively, most of these polymorphisms are likely to be adaptive and have evolved under strong, positive selection pressure. In such circumstance, the mutation rate of the meq gene would be expected to be significantly higher than the usual rate that has been reported for dsDNA viruses. Given the ongoing risk posed by MDV, it is crucial to estimate the rates of evolution and the pervasive role of positive selective pressures on MD viral emergence. Here, we sought to determine whether the meq gene has evolved adaptively and whether past vaccination efforts have had any significant effect on the reduction of MD viral diversity over time.
By performing a Bayesian coalescent analysis [29–31] using serially sampled meq-gene sequence data and site-specific selection analyses [32], we report the evolutionary parameters and gain valuable insight into the epidemiology of this highly contagious poultry herpesvirus, as well as the underlying genetic mechanisms that maintain polymorphisms in the meq gene. Understanding the past population dynamics and determining the evolutionary rate of change for meq may provide important insight into the vaccination, management and factors driving the selection for and fixation of particular mutations.
Materials and Methods
Phylogenetic analyses
A total of 84 meq gene complete nucleotide coding sequences, including 44 sequences of known MDV pathotypes that were previously reported by several authors [3, 4, 8–10, 12, 15] were retrieved from GenBank (S1 Table). Most of these sequences were from the flocks that were vaccinated either with HVT, bivalent vaccine (HVT + SB-1), Rispens-CVI988, or by 814-based-vaccines [3, 4, 9, 10, 12]. Sequences were aligned using MEGA, version 4 [33]. To infer the phylogenetic relationships among the known pathotypes, maximum likelihood (ML) tree under the appropriate nucleotide substitution model was reconstructed using PhYML, version 3 [34], and nodal supports were estimated with 1000 bootstrap replicates. To assess the robustness of ML tree topologies, we generated posterior probabilities for each node by performing BMCMC (Bayesian Markov Chain Monte Carlo) analyses implemented in MrBayes, version 3.1.2 [35].
Estimation of evolutionary parameters and population dynamics
We evaluated the clock-like behavior of the meq gene sequence data set (n = 69) using a conservative assessment of root-to-tip genetic distance regression analyses implemented in Path-O-Gen ver. 1.3 (http://tree.bio.ed.ac.uk/software/pathogen/; [36]). The coefficient of correlation was determined by fitting a regression of the year-of-sampling against the root-to-tip genetic distance of each sample, measured from an ML tree. We then used a Bayesian Markov Chain Monte Carlo (MCMC) approach to estimate the overall substitution rate (measured in substitutions per site per year) for the meq gene under the strict (constant molecular clock) and relaxed (uncorrelated lognormal) molecular clocks [29, 31] implemented in BEAST version, 1.7.1 [30].
To estimate the evolutionary rates and infer the population dynamics, we used all the complete nucleotide coding sequences of meq gene (n = 69) whose year of isolation were available. The MCMC chains were run for sufficient time to achieve convergence. Phylogenies were evaluated using a chain length of 40 million states under a General Time Reversible (GTR) model with proportion of invariable sites (I) and gamma distribution shape parameter (G). Uncertainty in the data was described by the 95% high-posterior density (HPD) intervals. Convergence of trees was checked using Tracer ver. 1.5 (available at: http://beast.bio.ed.ac.uk/Tracer). The inferred trees were visualized using FigTree ver. 1.3.1 (available at: http://tree.bio.ed.ac.uk/software/figtree/). We utilized the Bayesian skyline plot (BSP) [31] as a coalescent prior to inferring the population history of MDV. The BSP estimates changes in effective population size of MDV through time, measured in terms of relative genetic diversity. The best-fit clock model was evaluated from the coefficient of variation (CoV) values. The Maximum Clade Credibility (MCC) tree was generated using the TreeAnnotator software program implemented in the BEAST package.
Tests for positive selection
Prior to selection analyses, a recombination detection program (RDP) implemented in the RDP3 software package [37] was used to determine if any of the meq genes showed evidence of recombination. The analyses revealed no evidence of recombination, thus allowing us to test for positive selection. We performed ML-based selection analysis to determine whether the meq gene is evolving under positive selection. Several codon-specific models (M1a, M2a, M7, M8, and M8a) implemented in the codeml program of PAML [32] were used to determine which residues are evolving under positive selection. Sites models allow the rate of nonsynonymous (dN) to the synonymous (dS) ratio (dN/dS = ω) to vary among residues. The input trees for selection analyses were reconstructed using the PhyML program. The likelihood ratio test (LRT’s) was used to compare M1a, M7, and M8a models that assume no positive selection (ω < 1) with the M2a and M8 models that assume positive selection (ω > 1). Sites with Bayes Emperical Bayes (BEB) posterior probability ≥ 0.95 were considered to be evolved under strong positive selection. Positively selected sites were also detected using random effect likelihood (REL) and Fast Unbiased Bayesian Approximation (FUBAR) methods [38–40] via the Datamonkey website [39]. Bayes factor greater than 50 was used as thresholds for strong evidence of selection in REL.
Results and Discussion
Phylogenetic relationships of the pathotypes with vaccine strains
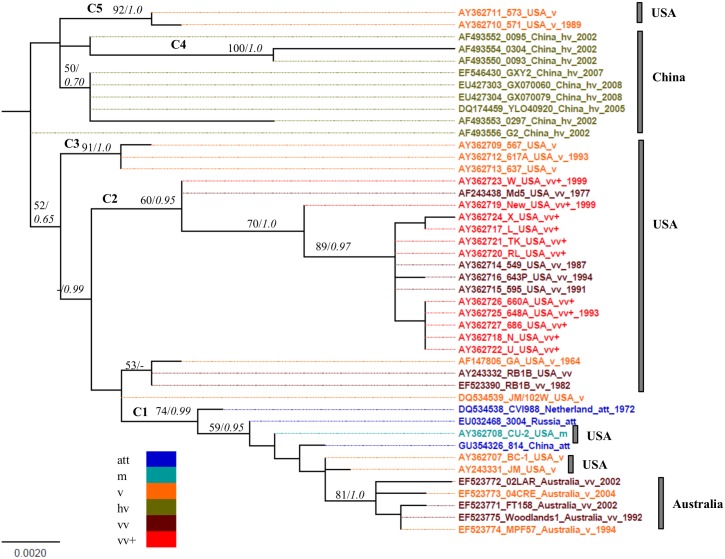
Phylogenetic relationships among the 44 pathotyped MDV strains, including three vaccines were inferred from the meq gene sequences, using ML and Bayesian MCMC approaches with bootstrapping and posterior probability analyses to assess clade robustness (Fig 1). Five clusters (C1 –C5) were defined with bootstrap supports ≥ 60 and posterior probability > 0.9 (Fig 1). MDV pathotypes from the USA appear to be on the multiple clusters (i.e. C1, C2, C3, and C5), whereas cluster 5 (C5) is represented by samples from China. All the five viral strains from Australia, along with a few strains from the USA, tended to cluster with the three vaccine strains, whereas all the vv+MDV pathotypes from the USA that were sampled between the years 1987–1995 formed a unique cluster (Fig 1). Although most of the viral samples with known pathotypes from China seem to form a cluster, the cluster is not strongly supported (Fig 1). Nevertheless, our analyses have revealed that all the viral strains with known pathotypes are not phylogenetically closely related to the vaccine strains.
Fig 1. Maximum likelihood tree of MDV meq sequences.
Maximum likelihood tree inferred from meq gene complete nucleotide sequence data of 44 MDV isolates with known pathotypes are shown. Bars at right identify the phylogenetic clustering of pathotypes from different geographic regions. Bootstrap supports/posterior probabilities are mentioned at the base of the nodes. Nodes with high bootstraps/posterior probabilities (> 60/0.95) are indicated by clusters, C1-C5. Abbreviations: m: mild, att: attenuated, hv: high virulence, vv: very virulence, v: virulent, vv+: very virulent plus.
Evolutionary rates and past population dynamics
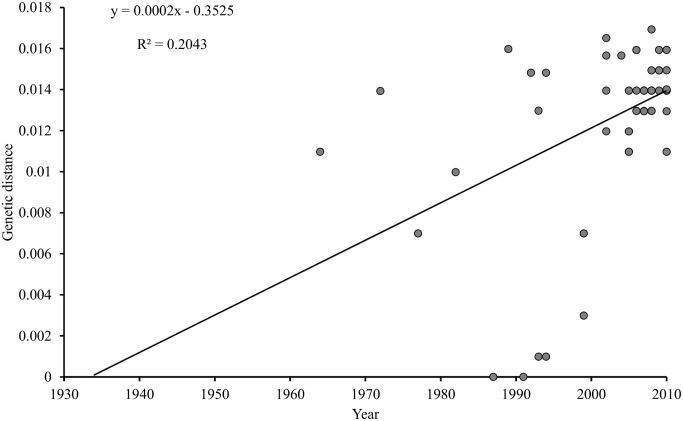
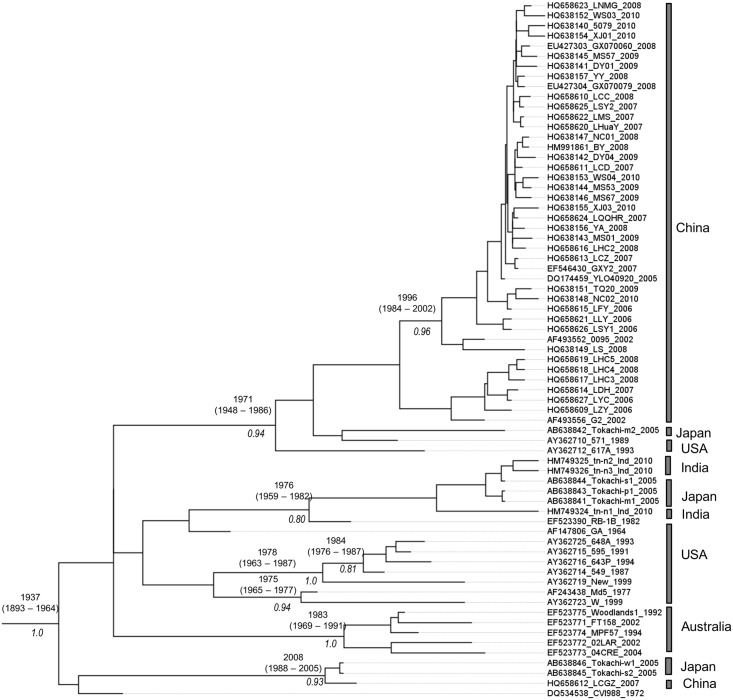
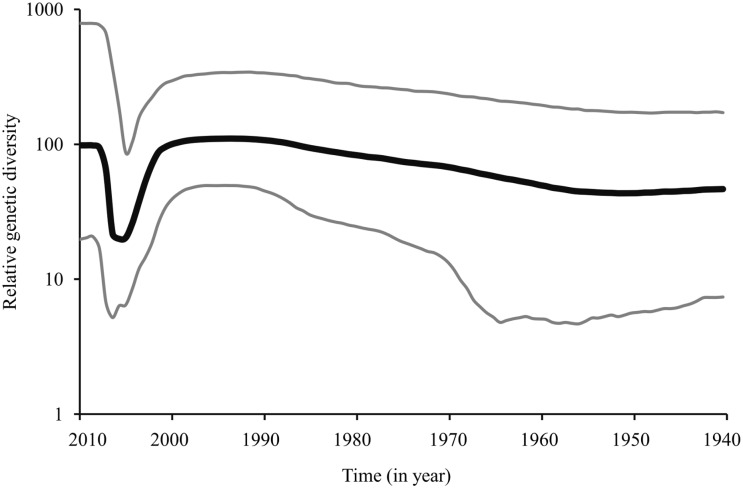
The clock-like behavior of the meq gene was assessed by plotting the root-to-tip genetic distance from a ML tree against the sampling-year of each MDV isolate. The resultant coefficient of correlation (R2) weakly suggested the presence of clock-like evolution of the meq gene in MDV (Fig 2). Although weak (i.e., R2 = 0.204), yet the dataset exhibits a positive correlation between genetic divergence and sampling time, thus indicating an extent of temporal structure in the data, and therefore, warrants further molecular clock-based phylogenetic analysis. We estimated the evolutionary parameters under strict and relaxed clock models implemented in the BEAST program [29, 30]. We inferred the evolutionary rates and past population dynamics of MDV using a Bayesian coalescent approach [29, 31]. This analysis was based on all the complete meq gene sequences (n = 69) in the dataset that had a date of isolation. Under the relaxed clock model, although higher mean CoV value was observed for meq, the lower 95% HPD is close to zero, indicating that the strict molecular clock could not be rejected (Table 1). The mean rate and TMRCAs estimated by both clocks are comparable and showed a narrow range of posterior distributions around the estimated mean posterior values in respective clock models (Table 1). The mean evolutionary rate for meq is estimated to be 1.02 (95% HPD: 0.50–1.60) ×10−4 substitutions/site/year and the estimated mean TMRCA is approximately around the year 1935 (95% HPD: 1893–1964) (Table 1). Most of the internal nodes were estimated to have diverged during the period of 1950s to early 2000s (Fig 3). Bayesian skyline plot (BSP) inferred from the meq gene sequence data was used to estimate the genetic diversity over time (Fig 4). The genetic diversity appeared to be relatively constant until the late-1950s, followed by a steady increase until early-2000. During 2004–2005, there was an abrupt decline in the genetic diversity followed by the recovery from this bottleneck in 2010 (Fig 4).
Fig 2. The root-to-tip genetic distance based on meq gene versus year of MDV isolation.
The regression coefficient (R2) estimates the fit of the data to a strict molecular clock by testing the degree of influence sampling time has over the amount of pairwise diversity in the data. This analysis suggests the presence of temporal structure for meq gene of MDV.
Table 1. Bayesian estimates of the evolutionary rate and TMRCAs (in year) inferred from the MDV meq gene.
| N | Year range | Clock Model | Mean substitution rate (95% HPD) | TMRCA (95% HPD) | CoV (95% HPD) |
|---|---|---|---|---|---|
| (in × 10−4 subs/site/ year) | (in year) | ||||
| 69 | 1964–2010 | Strict | 0.90 (0.50–1.38) | 1934 (1896–1959) | NA |
| Relaxed | 1.02 (0.55–1.60) | 1937 (1893–1964) | 0.58 (0.000089–1.36) |
N: Number of sequences.
HPD: Highest Posterior Density.
TMRCA: Time to the Most Recent Common Ancestor.
NA: Not Applicable.
CoV: Coefficient of Variation.
Fig 3. Maximum Clade Credibility (MCC) tree inferred from the Bayesian analysis of the MDV meq gene sequences.
The mean TMRCAs with confident intervals (above the nodes) and the posterior probabilities (below the nodes) are mentioned.
Fig 4. Bayesian skyline plot (BSP) inferred from the meq gene sequences.
The BSP above depicts the relative genetic diversity of MDV over time. The plot depicting MDV population had recovered from a recent bottleneck (~2005–2008).
Tests for positive selection
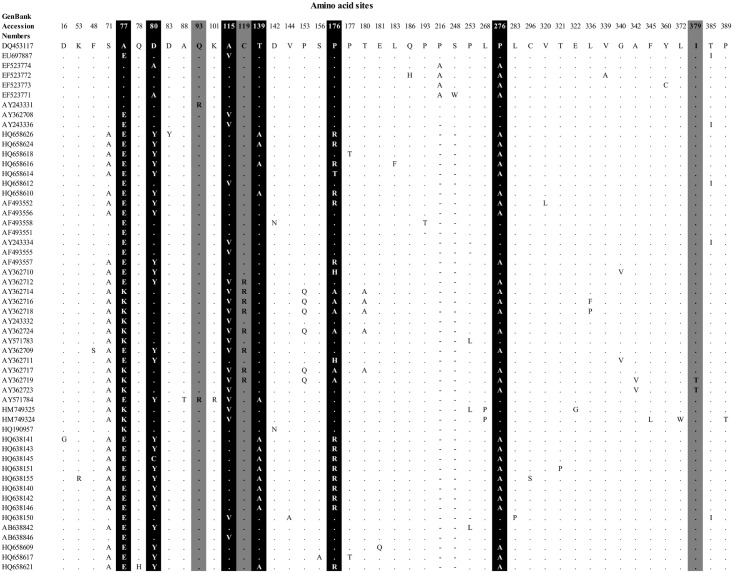
To determine the importance of natural selection in the evolution and divergence of meq, we performed codon-specific selection analyses using several models implemented in PAML program [32, 41] and FUBAR, REL methods implemented in the Datamonkey web server [39]. We performed the analyses on two datasets: (1) all the datasets and (2) exclusively on the known pathotypes. For both datasets, the overall dN/dS for meq is greater than one (Table 2), indicating most of the polymorphisms in meq have evolved under strong positive selection. To know which residues in meq have evolved under positive selection pressure, we have performed codon-by-codon selection analyses. For both datasets, the null models that assume neutral evolution were rejected (p < 0.00001) by the likelihood ratio tests (LRTs) (Table 2), thus indicating the pervasive role of positive selection in the evolution and divergence of meq gene in MDV. Notably, codons -77, 80, 115, 119, 139, 176, 276, 379, and 385 in both datasets have evolved under strong positive selection with BEB > 0.90 (Table 2; Fig 5). Most of these sites were detected to be under positive selection by FUBAR and REL methods (Table 3). We also performed selection analyses on gycoprotein B (gB); however, none of the sites were detected to be under positive selection. In contrast to meq, gB is appeared to be highly conserved. Amino acid alignments of gB8 are shown in S1 Fig.
Table 2. Sites under positive selection detected by PAML methods.
Likelihood ratio tests for positive selection for the MDV meq gene and positively selected sites detected by M2a and M8 selection model.
| Group | Overall dN/dS | Model Comparison | 2Δl | df | p-value | Postively-selected sitesa |
|---|---|---|---|---|---|---|
| All | 1.566 | M1a vs M2a | 42.623 | 2 | 7.464 x 10−8 | 77, 80, 93, 115, 119, 139, 176, 276, 342, 379 |
| M7 vs M8 | 42.621 | 2 | 1.936 x 10−8 | 77,80, 93, 119, 115, 139, 176, 253, 268, 276, 336, 342, 379, 385 | ||
| M8 vs M8a | 38.307 | 1 | 1.010 x 10−8 | |||
| Pathotype | 1.835 | M1a vs M2a | 35.589 | 2 | 1.869 x 10−8 | 77, 80, 115, 119, 139, 176, 276, 303, 336, 342, 379, 385 |
| M7 vs M8 | 35.589 | 2 | 1.869 x 10−8 | 77, 80, 115, 119, 139, 176, 276, 303, 336, 342, 379, 385 | ||
| M8 vs M8a | 34.045 | 1 | 5.385 x 10−9 |
dN/dS: Rate of nonsynonymous (dN) to the synonymous (dS) substitutions.
Null model (Neutral): M1a, M7, M8a.
Alternative model (Selection): M2a, M8.
Δl: Difference between the likelihood scores.
df: Degrees of freedom.
a: Sites with posterior probability > 0.95 are in bold, > 0.90 and < 0.95 underlined.
Fig 5. Variable amino acid sites in meq genes.
Positively selected with posterior probability > 0.95 and sites with posterior probability between 0.90–0.95 are highlighted in black and grey colors, respectively.
Table 3. Positively selected sites are shown as detected by REL and FUBAR methods.
| REL | FUBAR | ||
|---|---|---|---|
| Codon | Posterior Probability | Codon | Posterior Probability |
| 77 | 0.981 | 77 | 0.9520 |
| 80 | 0.986 | 80 | 0.9640 |
| 93 | 0.915 | 93 | 0.9159 |
| 115 | 0.989 | 115 | 0.9765 |
| 139 | 0.914 | 139 | 0.9377 |
| 176 | 0.992 | 176 | 0.9873 |
| 253 | 0.990 | 253 | 0.9816 |
| 276 | 0.993 | 276 | 0.9879 |
| 336 | 0.892 | 336 | 0.9247 |
| 342 | 0.916 | 342 | 0.9323 |
| 385 | 0.910 | 385 | 0.9322 |
REL: Random Effects Likelihood.
FUBAR: Fast Unconstrained Bayesian AppRoximation.
Understanding the evolutionary basis of viral emergence is crucial for effective vaccine development. Despite decades of vaccination efforts, the continuum of increasing virulence of MDV has been a serious concern [4, 13, 19, 42, 43]. The increased genetic polymorphisms in meq together with the reports of the association between MDV virulence and specific nonsynonymus mutations at the C-terminus domain of meq indicate the putative role of meq in the evolution of MDV virulence [3, 12]. In this study, we report that (1) meq is evolving at a much faster rate than most dsDNA viruses, (2) most of the polymorphisms in the meq gene have evolved under positive selection, (3) the time of divergence at the meq locus coincides with the period during which the poultry industry had undergone transitions in management practices including the introduction and widespread use of live attenuated vaccines, and (4) the decades-long use of vaccines did not reduce MDV diversity, but rather had a stimulating effect on the emergence of field strains with increased genetic diversity.
Most of the dsDNA viruses, including herpsviruses, have evolved at slower rates, mostly within a range of 10−7 to 10−5 substitutions per site per year [26, 28, 44–49]. In contrast, the meq gene of MDV is evolving at a rate that is comparable with the rates of RNA viruses [50]. Although several factors could be associated with the extremely slow evolutionary rate of most dsDNA viruses [26, 28, 49, 51], the assumption of host-pathogen co-divergence, where most of the dsDNA viruses are thought to evolve with their respective hosts, is a plausible explanation for this observation [27]. Utilizing the gene sequence data within a Bayesian framework, and without considering the assumption of host-virus co-divergence, Fitch et al., [27] reported a large-scale variation in the substitution rate of dsDNA viruses (in the order of 10−3 to 10−6 substitutions/site/year) and interestingly, substitution rates of some of the dsDNA viruses are comparable with that of RNA viruses. Although substitution rates of dsDNA viruses are likely to be gene-specific, genes that have evolved under positive selection have elevated substitution rates [27]. To determine the putative role of positive selection in the evolution and divergence of the MDV meq gene, we performed the ML-based positive selection analyses. The overall dN/dS for meq was 1.54, indicating the evidence of adaptive evolution of meq driven by positive selection. Thus, positive selection is the most likely contributing factor for such inflated substitution rate of meq.
From the results of our present study, although it is apparent that positive selection appears to be a cause of the rapid evolution of the meq gene of MDV, it is unclear as to what factors drive this gene to be under positive selection. Positive selection largely reflects the viral adaptation against host immune defenses, with adaptive changes providing beneficial consequences for the virus to evade the host’s acquired/intrinsic immunity. Although vaccines may provide protection against the viruses, viruses have the ability to adapt rapidly to the changing environment by modification of key amino acid residues in viral proteins that are involved in the host-pathogen interaction process. In the case of meq, however, it is unclear what adaptive advantage these substitutions would confer and why in particular they would be selected through the use of vaccines that lack a meq homolog (HVT, SB-1).
Nevertheless, such adaptive changes through positive selection pressures (i.e., accumulation of an excess of nonsynonymous changes relative to synonymous changes over time), may cause the emergence of new viral strains with greater fitness in the vaccinated populations. Under these circumstances, birds vaccinated with the old vaccines are unlikely to overcome the re-infection with the new viral strains. One might also expect the existence of multiple viral lineages of MDV with distinct genetic fitness. Human influenza A viruses are the classic example of such adaptation through the rapid evolutionary changes at antigenic sites (HA, NA). Because of this adaptation, vaccines for influenza viruses are seasonal and require continuous monitoring, surveillance, and determination of the pattern of genetic polymorphisms at the antigenic sites for the effective design of vaccines [1, 2]. This does not appear to be the case for MDV-1 strains and meq, as meq is a nuclear/nucleolar protein, and is not encoded by HVT and MDV-2 (SB-1) vaccine strains. Additionally, prior work has shown that the major structural genes of MDV-1 field strains do not appear to be under the level of selection that meq appears to be [52, 53].
Several mutations at the C-terminus region of meq gene (codons-80, 115, 176, 276) that were previously reported to be associated with MDV virulence [3] have apparently evolved under positive selection (see Fig 5). Recently, Australian isolates were found to increase in virulence with particular mutations in the tandem direct repeats of proline residues [54]. The model that they put forth was that the fewer prolines present in the C-terminus of meq, the greater the level of virulence. These adaptive evolutionary changes at the meq oncogene are likely to have some functional consequences and MDV evolution. Therefore, further experiments on the putative role of these specific mutations would provide a better understanding on the MD viral emergence and genetic basis of its adaptation. A detailed understanding on the geographic dominance of different MD viral strains is also required for effective vaccine development. Biochemically, changes in meq have been associated with increased transcriptional activation [55, 56], increased invasiveness of cells expressing meq isoforms, and most recently, changes in tumor composition [57]. How these particular mutations directly contribute to MDV changes in virulence await further mutant construction and characterization.
Prior to the 1950s, MDV was less virulent; however, the virus became a concern after the introduction of vaccines and significant changes in brooding practices [4]. Such changes might have a significant impact on the viral diversity, and the virus may have evolved much faster rate through adaptation. The meq gene based Bayesian analyses have revealed that the MDV is estimated to have originated sometime between the year 1893–1964; however, a rapid divergence of MDV leads to multiple lineages arising between 1950 to the early 2000s. Despite vaccination efforts, the Bayesian skyline plots revealed a steady increase in viral diversity from the 1950s until the mid-2000s. This pattern clearly indicates that vaccination did not reduce viral diversity, but increased it. The sharp decline in viral diversity during the early 2000s could possibly be the effect of the introduction of Rispens (CVI988) in the US and 814-vaccines in China. However, the recovery of the diversity of viral populations suggests vaccine failure.
Conclusions
Collectively, although our analyses revealed an interesting evolutionary dynamics of MDV, it is possible that the geographic origin of the viral strains may have profound influence on the rates of evolution and population dynamics of MDV. However, due to limited sample size, we could not perform the geographic-based analyses. Therefore, upon availability of large cohorts of samples representing different geographic regions, future study can be carried out to unravel the phylogeographic structure, distinct selection profiles, as well as to know whether there are significant differences in evolutionary rates among the geographic regions. Additionally, future studies should also focus on the evolutionary dynamics of MDV using other genes such as oncogenicity-associated phosphoproteins pp38 and pp24, lipase homologue, a CxC chemokine, and unique proteins of unknown function MDV087 and MDV097 and MDV093 that contribute towards virulence of MDV.
Supporting Information
Amino variable sites are highlighted in gray color. Those strains having identical sequence are:
AY510475 is Identical to: AF243438, KT833851, and KT833852. JX844666 is Identical to: EU499381, JQ809692, JQ836662, JQ809691, EF523390, U39846, DQ530348, JQ806362, JQ806361, AF147806, JQ820250, JF742597, AY129966, and D13713.
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank two anonymous reviewers for insightful comments, which greatly improved the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Dormitzer PR, Galli G, Castellino F, Golding H, Khurana S, Del Giudice G, et al. Influenza vaccine immunology. Immunological reviews. 2011;239(1):167–77. Epub 2011/01/05. 10.1111/j.1600-065X.2010.00974.x . [DOI] [PubMed] [Google Scholar]
- 2.Tusche C, Steinbruck L, McHardy AC. Detecting Patches of Protein Sites of Influenza A Viruses under Positive Selection. Molecular biology and evolution. 2012;29(8):2063–71. Epub 2012/03/20. 10.1093/molbev/mss095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shamblin CE, Greene N, Arumugaswami V, Dienglewicz RL, Parcells MS. Comparative analysis of Marek's disease virus (MDV) glycoprotein-, lytic antigen pp38- and transformation antigen Meq-encoding genes: association of meq mutations with MDVs of high virulence. Veterinary microbiology. 2004;102(3–4):147–67. Epub 2004/08/26. 10.1016/j.vetmic.2004.06.007 . [DOI] [PubMed] [Google Scholar]
- 4.Witter RL. Increased virulence of Marek's disease virus field isolates. Avian diseases. 1997;41(1):149–63. Epub 1997/01/01. . [PubMed] [Google Scholar]
- 5.Witter RL. Control strategies for Marek's disease: a perspective for the future. Poultry science. 1998;77(8):1197–203. Epub 1998/08/26. . [DOI] [PubMed] [Google Scholar]
- 6.Witter RL, Calnek BW, Buscaglia C, Gimeno IM, Schat KA. Classification of Marek's disease viruses according to pathotype: philosophy and methodology. Avian pathology: journal of the WVPA. 2005;34(2):75–90. Epub 2005/09/30. 10.1080/03079450500059255 . [DOI] [PubMed] [Google Scholar]
- 7.Osterrieder N, Kamil JP, Schumacher D, Tischer BK, Trapp S. Marek's disease virus: from miasma to model. Nature reviews Microbiology. 2006;4(4):283–94. Epub 2006/03/17. 10.1038/nrmicro1382 . [DOI] [PubMed] [Google Scholar]
- 8.Renz KG, Cooke J, Clarke N, Cheetham BF, Hussain Z, Fakhrul Islam AF, et al. Pathotyping of Australian isolates of Marek's disease virus and association of pathogenicity with meq gene polymorphism. Avian pathology: journal of the WVPA. 2012;41(2):161–76. Epub 2012/04/21. 10.1080/03079457.2012.656077 . [DOI] [PubMed] [Google Scholar]
- 9.Teng LQ, Wei P, Song ZB, He JJ, Cui ZZ. Molecular epidemiological investigation of Marek's disease virus from Guangxi, China. Archives of virology. 2011;156(2):203–6. Epub 2010/11/06. 10.1007/s00705-010-0840-8 . [DOI] [PubMed] [Google Scholar]
- 10.Tian M, Zhao Y, Lin Y, Zou N, Liu C, Liu P, et al. Comparative analysis of oncogenic genes revealed unique evolutionary features of field Marek's disease virus prevalent in recent years in China. Virology journal. 2011;8:121 Epub 2011/03/17. 10.1186/1743-422X-8-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wozniakowski G, Samorek-Salamonowicz E, Kozdrun W. Molecular characteristics of Polish field strains of Marek's disease herpesvirus isolated from vaccinated chickens. Acta veterinaria Scandinavica. 2011;53:10 Epub 2011/02/16. 10.1186/1751-0147-53-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang YP, Liu CJ, Zhang F, Shi W, Li J. Sequence analysis of the Meq gene in the predominant Marek's disease virus strains isolated in China during 2006–2008. Virus genes. 2011;43(3):353–7. Epub 2011/07/27. 10.1007/s11262-011-0645-1 . [DOI] [PubMed] [Google Scholar]
- 13.Wozniakowski G, Samorek-Salamonowicz AE. Molecular evolution of Marek's disease virus (MDV) field strains in a 40-year time period. Avian diseases. 2014;58(4):550–7. Epub 2015/01/27. 10.1637/10812-030614-Reg.1 . [DOI] [PubMed] [Google Scholar]
- 14.Murata S, Chang KS, Yamamoto Y, Okada T, Lee SI, Konnai S, et al. Detection of the virulent Marek's disease virus genome from feather tips of wild geese in Japan and the Far East region of Russia. Archives of virology. 2007;152(8):1523–6. Epub 2007/05/15. 10.1007/s00705-007-0982-5 . [DOI] [PubMed] [Google Scholar]
- 15.Murata S, Hayashi Y, Kato A, Isezaki M, Takasaki S, Onuma M, et al. Surveillance of Marek's disease virus in migratory and sedentary birds in Hokkaido, Japan. Veterinary journal. 2012;192(3):538–40. Epub 2011/09/13. 10.1016/j.tvjl.2011.07.006 . [DOI] [PubMed] [Google Scholar]
- 16.Marek J. Multiple Nervenentzündung (Polyneuritis) bei Hühnern. Dtsch Tierärztl Wochenschr. 1907;15:417–21. [Google Scholar]
- 17.Rispens BH, van Vloten H, Mastenbroek N, Maas HJ, Schat KA. Control of Marek's disease in the Netherlands. I. Isolation of an avirulent Marek's disease virus (strain CVI 988) and its use in laboratory vaccination trials. Avian diseases. 1972;16(1):108–25. Epub 1972/04/01. . [PubMed] [Google Scholar]
- 18.Rispens BH, van Vloten H, Mastenbroek N, Maas JL, Schat KA. Control of Marek's disease in the Netherlands. II. Field trials on vaccination with an avirulent strain (CVI 988) of Marek's disease virus. Avian diseases. 1972;16(1):126–38. Epub 1972/04/01. . [PubMed] [Google Scholar]
- 19.Schat KA, Baranowski E. Animal vaccination and the evolution of viral pathogens. Revue scientifique et technique. 2007;26(2):327–38. Epub 2007/09/26. . [PubMed] [Google Scholar]
- 20.Levy AM, Gilad O, Xia L, Izumiya Y, Choi J, Tsalenko A, et al. Marek's disease virus Meq transforms chicken cells via the v-Jun transcriptional cascade: a converging transforming pathway for avian oncoviruses. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(41):14831–6. Epub 2005/10/06. 10.1073/pnas.0506849102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinke AW, Grigoryan G, Keating AE. Identification of bZIP interaction partners of viral proteins HBZ, MEQ, BZLF1, and K-bZIP using coiled-coil arrays. Biochemistry. 2010;49(9):1985–97. Epub 2010/01/28. 10.1021/bi902065k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kung HJ, Xia L, Brunovskis P, Li D, Liu JL, Lee LF. Meq: an MDV-specific bZIP transactivator with transforming properties. Current topics in microbiology and immunology. 2001;255:245–60. Epub 2001/02/24. . [DOI] [PubMed] [Google Scholar]
- 23.Liu JL, Kung HJ. Marek's disease herpesvirus transforming protein MEQ: a c-Jun analogue with an alternative life style. Virus genes. 2000;21(1–2):51–64. Epub 2000/10/07. . [PubMed] [Google Scholar]
- 24.Brown AC, Baigent SJ, Smith LP, Chattoo JP, Petherbridge LJ, Hawes P, et al. Interaction of MEQ protein and C-terminal-binding protein is critical for induction of lymphomas by Marek's disease virus. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(6):1687–92. Epub 2006/02/01. 10.1073/pnas.0507595103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickabottom M, Parker GA, Freemont P, Crook T, Allday MJ. Two nonconsensus sites in the Epstein-Barr virus oncoprotein EBNA3A cooperate to bind the co-repressor carboxyl-terminal-binding protein (CtBP). The Journal of biological chemistry. 2002;277(49):47197–204. Epub 2002/10/10. 10.1074/jbc.M208116200 . [DOI] [PubMed] [Google Scholar]
- 26.Duffy S, Shackelton LA, Holmes EC. Rates of evolutionary change in viruses: patterns and determinants. Nature reviews Genetics. 2008;9(4):267–76. Epub 2008/03/06. 10.1038/nrg2323 . [DOI] [PubMed] [Google Scholar]
- 27.Firth C, Kitchen A, Shapiro B, Suchard MA, Holmes EC, Rambaut A. Using time-structured data to estimate evolutionary rates of double-stranded DNA viruses. Molecular biology and evolution. 2010;27(9):2038–51. Epub 2010/04/07. 10.1093/molbev/msq088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmes EC. What does virus evolution tell us about virus origins? Journal of virology. 2011;85(11):5247–51. Epub 2011/04/01. 10.1128/JVI.02203-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drummond AJ, Ho SY, Phillips MJ, Rambaut A. Relaxed phylogenetics and dating with confidence. PLoS biology. 2006;4(5):e88 Epub 2006/05/11. 10.1371/journal.pbio.0040088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC evolutionary biology. 2007;7:214 Epub 2007/11/13. 10.1186/1471-2148-7-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drummond AJ, Rambaut A, Shapiro B, Pybus OG. Bayesian coalescent inference of past population dynamics from molecular sequences. Molecular biology and evolution. 2005;22(5):1185–92. Epub 2005/02/11. 10.1093/molbev/msi103 . [DOI] [PubMed] [Google Scholar]
- 32.Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Computer applications in the biosciences: CABIOS. 1997;13(5):555–6. Epub 1997/11/21. . [DOI] [PubMed] [Google Scholar]
- 33.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular biology and evolution. 2007;24(8):1596–9. Epub 2007/05/10. 10.1093/molbev/msm092 . [DOI] [PubMed] [Google Scholar]
- 34.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic biology. 2003;52(5):696–704. Epub 2003/10/08. . [DOI] [PubMed] [Google Scholar]
- 35.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17(8):754–5. Epub 2001/08/29. . [DOI] [PubMed] [Google Scholar]
- 36.Drummond A, Pybus OG, Rambaut A. Inference of viral evolutionary rates from molecular sequences. Advances in parasitology. 2003;54:331–58. Epub 2004/01/09. . [DOI] [PubMed] [Google Scholar]
- 37.Martin DP, Lemey P, Lott M, Moulton V, Posada D, Lefeuvre P. RDP3: a flexible and fast computer program for analyzing recombination. Bioinformatics. 2010;26(19):2462–3. Epub 2010/08/28. 10.1093/bioinformatics/btq467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murrell B, Moola S, Mabona A, Weighill T, Sheward D, Kosakovsky Pond SL, et al. FUBAR: a fast, unconstrained bayesian approximation for inferring selection. Molecular biology and evolution. 2013;30(5):1196–205. Epub 2013/02/20. 10.1093/molbev/mst030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pond SL, Frost SD, Muse SV. HyPhy: hypothesis testing using phylogenies. Bioinformatics. 2005;21(5):676–9. Epub 2004/10/29. 10.1093/bioinformatics/bti079 . [DOI] [PubMed] [Google Scholar]
- 40.Pond SK, Muse SV. Site-to-site variation of synonymous substitution rates. Molecular biology and evolution. 2005;22(12):2375–85. Epub 2005/08/19. 10.1093/molbev/msi232 . [DOI] [PubMed] [Google Scholar]
- 41.Yang Z, Nielsen R, Goldman N, Pedersen AM. Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics. 2000;155(1):431–49. Epub 2000/05/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gimeno IM. Marek's disease vaccines: a solution for today but a worry for tomorrow? Vaccine. 2008;26 Suppl 3:C31–41. Epub 2008/09/06. . [DOI] [PubMed] [Google Scholar]
- 43.Schat KA, Calnek BW, Fabricant J, Graham DL. Pathogenesis of infection with attenuated Marek's disease virus strains. Avian pathology: journal of the WVPA. 1985;14(1):127–46. Epub 1985/01/01. 10.1080/03079458508436213 . [DOI] [PubMed] [Google Scholar]
- 44.Drake JW. A constant rate of spontaneous mutation in DNA-based microbes. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(16):7160–4. Epub 1991/08/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Q, Hwang YT, Hwang CB. Mutation spectra of herpes simplex virus type 1 thymidine kinase mutants. Journal of virology. 2002;76(11):5822–8. Epub 2002/05/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakaoka H, Kurita K, Iida Y, Takada S, Umene K, Kim YT, et al. Quantitative analysis of genomic polymorphism of herpes simplex virus type 1 strains from six countries: studies of molecular evolution and molecular epidemiology of the virus. The Journal of general virology. 1994;75 (Pt 3):513–27. Epub 1994/03/01. . [DOI] [PubMed] [Google Scholar]
- 47.Shackelton LA, Rambaut A, Pybus OG, Holmes EC. JC virus evolution and its association with human populations. Journal of virology. 2006;80(20):9928–33. Epub 2006/09/29. 10.1128/JVI.00441-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gago S, Elena SF, Flores R, Sanjuan R. Extremely high mutation rate of a hammerhead viroid. Science. 2009;323(5919):1308 Epub 2009/03/07. 10.1126/science.1169202 . [DOI] [PubMed] [Google Scholar]
- 49.Holmes EC. The comparative genomics of viral emergence. Proceedings of the National Academy of Sciences of the United States of America. 2010;107 Suppl 1:1742–6. Epub 2009/10/28. 10.1073/pnas.0906193106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jenkins GM, Rambaut A, Pybus OG, Holmes EC. Rates of molecular evolution in RNA viruses: a quantitative phylogenetic analysis. Journal of molecular evolution. 2002;54(2):156–65. Epub 2002/02/01. 10.1007/s00239-001-0064-3 . [DOI] [PubMed] [Google Scholar]
- 51.Sanjuan R. From molecular genetics to phylodynamics: evolutionary relevance of mutation rates across viruses. PLoS pathogens. 2012;8(5):e1002685 Epub 2012/05/10. 10.1371/journal.ppat.1002685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spatz SJ, Petherbridge L, Zhao Y, Nair V. Comparative full-length sequence analysis of oncogenic and vaccine (Rispens) strains of Marek's disease virus. J Gen Virol. 2007;88(Pt 4):1080–96. . [DOI] [PubMed] [Google Scholar]
- 53.Spatz SJ, Rue C, Schumacher D, Osterrieder N. Clustering of mutations within the inverted repeat regions of a serially passaged attenuated gallid herpesvirus type 2 strain. Virus Genes. 2008;37(1):69–80. Epub 2008/06/03. 10.1007/s11262-008-0242-0 . [DOI] [PubMed] [Google Scholar]
- 54.Renz KG, Cooke J, Clarke N, Cheetham BF, Hussain Z, Fakhrul Islam AF, et al. Pathotyping of Australian isolates of Marek's disease virus and association of pathogenicity with meq gene polymorphism. Avian pathology: journal of the WVPA. 2012;41(2):161–76. Epub 2012/04/21. 10.1080/03079457.2012.656077 . [DOI] [PubMed] [Google Scholar]
- 55.Murata S, Okada T, Kano R, Hayashi Y, Hashiguchi T, Onuma M, et al. Analysis of transcriptional activities of the Meq proteins present in highly virulent Marek's disease virus strains, RB1B and Md5. Virus Genes. 2011. Epub 2011/04/20. 10.1007/s11262-011-0612-x . [DOI] [PubMed] [Google Scholar]
- 56.Chang KS, Ohashi K, Onuma M. Suppression of transcription activity of the MEQ protein of oncogenic Marek's disease virus serotype 1 (MDV1) by L-MEQ of non-oncogenic MDV1. J Vet Med Sci. 2002;64(12):1091–5. [DOI] [PubMed] [Google Scholar]
- 57.Kumar P, Dong H, Lenihan D, Gaddamanugu S, Katneni U, Shaikh S, et al. Selection of a recombinant Marek's disease virus in vivo through expression of the Marek's EcoRI-Q (Meq)-encoded oncoprotein: characterization of an rMd5-based mutant expressing the Meq of strain RB-1B. Avian Diseases. 2012;56(2):328–40. Epub 2012/08/04. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Amino variable sites are highlighted in gray color. Those strains having identical sequence are:
AY510475 is Identical to: AF243438, KT833851, and KT833852. JX844666 is Identical to: EU499381, JQ809692, JQ836662, JQ809691, EF523390, U39846, DQ530348, JQ806362, JQ806361, AF147806, JQ820250, JF742597, AY129966, and D13713.
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.