Abstract
The continuous synthesis of biodegradable photothermal copper sulfide nanoparticles has been carried out with the aid of a microfluidic platform. A comparative physicochemical characterization of the resulting products from the microreactor and from a conventional batch reactor has been performed. The microreactor is able to operate in a continuous manner and with a 4-fold reduction in the synthesis times compared to that of the conventional batch reactor producing nanoparticles with the same physicochemical requirements. Biodegradation subproducts obtained under simulated physiological conditions have been identified, and a complete cytotoxicological analysis on different cell lines was performed. The photothermal effect of those nanomaterials has been demonstrated in vitro as well as their ability to generate reactive oxygen species.
Keywords: CuS nanoparticles, microfluidic synthesis, photothermal, biodegradable nanomaterial, cells viability
Introduction
Copper(II) sulfide nanoparticles are semiconductor chalcogenides with unique electronic and optical properties. As semiconductor, the bandgap of copper sulfide varies depending on its atomic composition between 1.2 eV for Cu2S to 2.0 eV for CuS, which allows it to absorb a large fraction of the solar spectrum.1 As plasmonic material, it absorbs near-infrared (NIR) light converting it into heat due to the excitation of direct (band-to-band) transitions, indirect transitions, and plasmonic photoexcitation.2 Those electro-optical properties make copper sulfide nanoparticles useful in not only photovoltaic cells but also a wide variety of biomedical applications including electrochemical sensing,3 photothermal therapy,4,5 diagnosis,6 theragnosis,7,8 and combination therapies.9,10
In the biomedical field, photothermal therapy using plasmonic nanoparticles has reached clinical trials. Thus, PEGylated gold nanoparticles surface functionalized with TNF-target molecules and containing a therapeutic payload is going to be used in a phase II clinical trial for the treatment of non-small-cell lung cancer patients in combination with standard-of-care second-line therapy.11 Plasmonic gold nanoshells (SiO2/Au) are also undergoing different clinical trials in patients with refractory and/or recurrent tumors of the head and neck, with primary and/or metastatic lung tumors, and in the treatment of prostate disease.12 However, in those applications, tissue light penetration is reduced. Short-wave visible light penetrates typical biological tissues between 0.5 and 2.5 mm where upon it undergoes an exponential decrease of intensity,13 whereas NIR light can penetrate deeper into the tissues.14 But even in the water window, where water, melanin, and hemoglobin show a reduced light absorption, the scattering of the light passing through regions having different refractive indexes (i.e., cell membranes, vessel walls, etc.) limits light penetration into the biological tissues; consequently, in most of those clinical applications, the activating light is conducted to the tumoral mass guided by an optical fiber in an invasive manner. Therefore, the design of nanoparticles with enhanced absorption and with response at wavelengths with reduced photon scattering14 can potentially be applied deeper in the tissues.
Biodegradability and higher photothermal conversion efficiencies (∼22–60%)2 are some of the main advantages of CuS nanoparticles (NPs) when used in vivo compared to gold-based plasmonic nanoparticles. Thus, Guo et al.15 demonstrated that using similar injected doses plasmonic hollow gold nanoparticles remained in the body one month after injection in BALB/c mice at high levels (more than 96% of the injected dose) whereas during the same time period only 10% of the injected dose remained in the animals when using CuS NPs being mostly excreted following the hepatobiliary route. The same authors demonstrated that the polycrystalline CuS NPs disintegrate from CuS shells into single CuS crystals after laser treatment, but in those works, the biodegradation subproducts were not evaluated.9 Other carbon-based nanoparticles used in photothermal therapy such as carbon nanotubes or graphene oxide have shown large physiological persistence16,17 despite the identification of some biodegradation cellular routes.18 Upconversion nanoparticles have also been used in photothermal therapy,19 but again, biopersistence in the mononuclear phagocyte system is the major hurdle for their clinical translation unless they are produced in reduced sizes allowing renal excretion.20 In addition, Goodman et al.21 demonstrated how hollow gold plasmonic nanoparticles show instability and fragmentation in vivo, which could be potentially attributable to the remaining silver on the surface of the gold nanoparticles used for their synthesis, and concluded that new biocompatible plasmonic nanoparticles appropriate for nanomedicine are required. In addition, it has been demonstrated that the combination of CuS nanoparticles with other plasmonic metal nanoparticles can enhance the photothermal effect.22
Polydispersity, low yield, and batch-to-batch inconsistencies are the main shortcomings when synthesizing nanoparticles, which can be overcome by using continuous-flow microfluidic reactors. Comparative syntheses of polymers, metals, and oxides using conventional batch reactors or microreactors have demonstrated a superior performance of the latter thanks to the use of confined growth in the microchannels driven by molecular diffusion under reduced concentration and temperature gradients. Combinatorial synthesis,23 multistep microfluidic platforms,24 and on-line monitoring with feedback control to render a product with specific properties25 are also possible using microfluidics. Some of the nanoparticles produced using microfluidics are obtained in passive or active micromixers where no chemical reaction is needed, just controlling the self-assembly of precursors under the presence of surfactants or stabilizers to form polymeric nanoemulsions,26 nanoprecipitated particles,27 liposomal formulations,28 solid–lipid nanoparticles,29 micelles,30 niosomes,31 and so on.
Photothermal nanoparticles have also been produced in microfluidic reactors. In this regard, redox reactions using sacrificial templates in a galvanic replacement have been used to produce hollow gold nanoparticles.24 Seeded or unseeded synthesis of anisotropic gold nanoparticles have also been reported by using capping agents and surface passivation components (i.e., halides), which adsorb preferentially on specific crystal facets directing the formation of gold nanorods during the growth.32,33 Gold nanoshells can also be prepared using sequential microfluidic platforms with significant time savings and with an improved control over the product properties compared to a conventional batch processing operation.34 Recently, Cheung et al.35 have described the millifluidic synthesis of copper sulfide nanoparticles using organic solvents and surfactants to control the geometry and crystalline phase. In this work, we have developed a microfluidic platform for the continuous synthesis of CuS NPs in aqueous media and identified their biodegradation subproducts under physiological conditions. Subcytotoxic doses of those nanoparticles have also been evaluated colorimetrically and by using flow cytometry.
Results and Discussion
Nanoparticles Synthesis
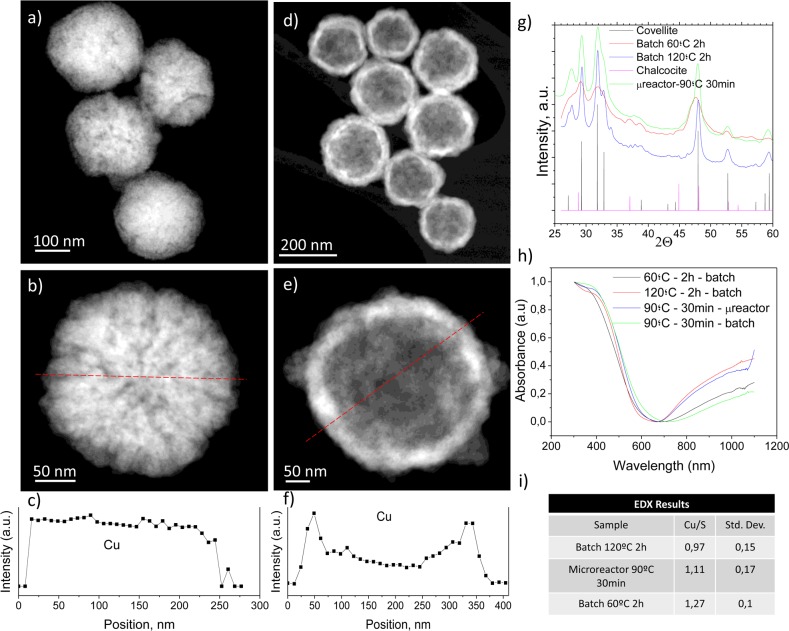
Copper sulfide can vary its optoelectronic properties depending on its stoichiometry from covellite (CuS) to djurleite (Cu1.97S), digenite (Cu1.8S), anilite (Cu1.4S), and chalcocite (Cu2S). Independent of the synthesis method, the maximum in the extinction spectrum in the NIR region at 1050 nm is reached for covellite. However, the other crystalline phases show minimal absorption at those wavelengths, and the maximum plasmon resonance wavelength decreases with increasing x in Cu2–xS.36 This maximum is attributed to an in-plane dipolar-localized surface plasmon resonant mode.37 In photothermal therapy, NIR-absorbing materials are needed to take advantage of the reduced absorption and scattering of biological chromophores, hemoglobin, and water in that region. Therefore, the morphology and absorption spectra of the resulting nanoparticles prepared in both microfluidic and batch reactors at different temperatures were analyzed in order to compare their morphological and optical properties. The nanoparticles synthesized in the batch reactor (Figure 1) show absorbance in the NIR region and crystallinity. XRD analysis showed that the materials synthesized at 60 °C show the characteristic diffraction planes of the covellite phase, but the full width at half-maximum of the diffraction peaks decreased at higher 120 °C indicative of an increased crystallinity (Figure 1g). Figure 1a,b shows the morphology of the sacrificial Cu2O nanoparticles. Cu2O nanoparticles have a spongelike morphology, but the EDS profile shows that the Cu distribution across the nanoparticle is homogeneous (Figure 1c). CuS nanoparticles produced after sulfur addition evidence the formation of hollow nanostructures with a thin shell (∼40 nm) (Figure 1d,e). The hollow structure was confirmed by the EDS analysis across a particle (Figure 1f). This EDS profile shows higher concentrations in the external area (corresponding to the particle walls), and lower concentrations in the central area, with upper and lower walls observed through the empty “core” of the particle. EDS analysis of CuS nanoparticles prepared in the batch reactor unveiled that the Cu/S atomic ratio was close to 1 when the synthesis temperature increased from 60 °C (Cu/S = 1.27 ± 0.1) to 120 °C (Cu/S = 0.97 ± 0.1) (Figure 1i). This fact also confirms that covellite was obtained during the batch synthesis production but with a higher purity and crystallinity as the synthesis temperature was increased. This was corroborated by the UV–vis absorption spectra where higher absorbances at the same concentration were obtained for the materials synthesized at 120 °C. XPS analysis revealed that the oxidation state of the CuS was +1 (see Table S1) in agreement with the previous literature.38 This hard-template-assisted technique using Cu2O nanoparticles produced hollow CuS nanoparticles based on a Kirkendall diffusion effect. Sulfur diffuses into the Cu2O template particles at the same time that the copper diffuses outward but at a slower rate. Thereby the nonreciprocal diffusion between Cu and S ultimately renders the formation of an interior cavity. Usually those processes are slow (several to tens of hours under hydrothermal or annealing conditions),39 and microfluidics can overcome those limitations. In agreement with the previous literature,40 an overall size increase was observed due to the Kirkendall effect varying the size from 198 ± 37 nm for the initial Cu2O nanoparticles to the final 208 ± 34 nm obtained for the resulting CuS nanoparticles obtained in the batch reactor at 60 °C during 2 h. The hydrodynamic size of those CuS nanoparticles (measured by DLS) was 164.1 nm.
Figure 1.
Physico-chemical characterization of the CuS NPs synthesized in the batch reactor after 2 h of synthesis. STEM-HAADF photographs of the resulting nanoparticles (60 °C, 2 h): (a) sacrificial Cu2O nanoparticles; (b) detail image of a Cu2O nanoparticle. (c) Cu EDS profile of the particle in b. Red dashed line depicts the location of the EDS profile. (d) CuS nanoparticles produced by Kirkendall diffusion. (e) Detailed image of a hollow CuS nanoparticle. (f) Cu EDS profile of the particle in e. Red dashed line in e depicts the location of the EDS profile. (g) X-ray diffractograms of the materials obtained at different temperatures (60 and 120 °C) and the characteristic covellite and chalcocite Joint Committee on Powder Diffraction Standards (JCPDS) patterns; (h) UV–vis absorption spectra of the materials synthesized in both batch and microfluidic reactors. (i) Cu/S atomic ratio of produced nanoparticles. Statistics were conducted after analyzing 20 nanoparticles.
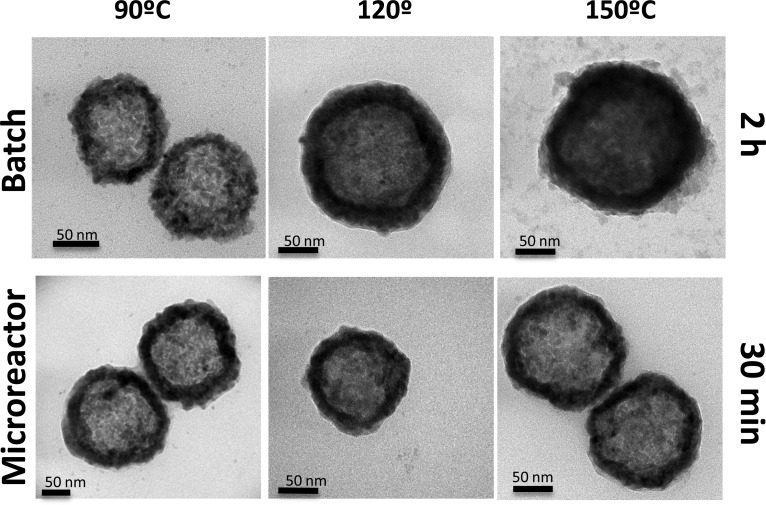
As can be seen in Figure 1h, the nanomaterials produced in the microfluidic reactor at 90 °C in only 30 min of residence time showed absorption spectrum at the same concentration similar to that of the materials synthesized in the conventional batch reactor at 120 °C during 2 h, whereas the nanoparticles synthesized during 30 min in the batch reactor at 90 °C showed a 52% decrease in their maximum absorption. Therefore, microfluidic reactors allow rapid mixing, redox reaction, and a crystallization process in reduced times compared to those of conventional batch reactors. In addition, EDS analysis for the CuS NPs produced in the microfluidic reactor at 90 °C and 30 min of synthesis rendered a Cu/S ratio of 1.1 ± 0.1 (Figure 1i). The formation of covellite is sensitive to the pH, temperature, and solvents used, and depending on those conditions, other noncrystalline CuxSy phases can be obtained.41 We demonstrated that microfluidic reactors can produce the desired phase with the required optoelectronic features. Figures 2 and S1 show how the morphology of the nanoparticles produced in both microfluidic and batch reactors is very similar but with the great advantage for the former of producing nanomaterials in a continuous manner and with a considerable reduction in the crystallization time (4-fold).
Figure 2.
TEM photographs of the nanoparticles produced in both microfluidic and batch reactors under different temperatures.
Heating efficiency was also evaluated for the resulting nanoparticles in the microfluidic reactor to validate their potential application in photothermal therapy. Figure 3 shows that the nanoparticulated colloidal suspension (1 mL) in water (0.05 mg/mL) heats up rapidly, and heating efficiency slightly decreased only ∼2° after 20 successive cycles of irradiation. Most of the nanoparticles after those 20 cycles maintained their original morphology although some fragmentation was also observed and could be responsible for that decrease in the photothermal efficiency. In agreement with Guo et al.,15 the polycrystalline CuS NPs disintegrate from the CuS shells into single CuS crystals after laser treatment.
Figure 3.
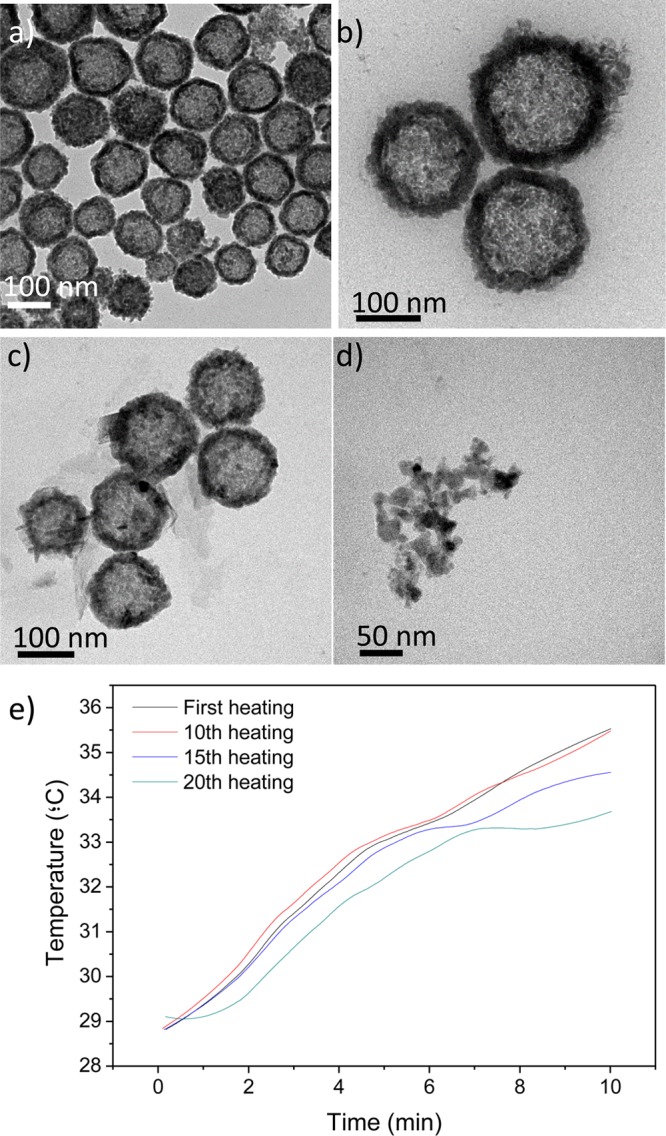
TEM images of CuS nanoparticles: (a and b) original nanoparticle suspension (1 mL, 0.05 mg/mL) obtained after 30 min of residence time in the microfluidic reactor and (c and d) after 20 successive cycles of irradiation (200 mW/cm2). (e) Photothermal heating rise after those successive laser irradiation cycles (200 mW/cm2).
For any biomedical application, a complete physiological biodegradation of the nanomaterial after use is advisable. As we mentioned before, biopersistence is a concern when using plasmonic nanoparticles, and in animal models it has been demonstrated that 90% of the injected dose of CuS NPs degraded, subsequently being mostly excreted following the hepatobiliary route 15. We characterized the degradation byproducts after immersing the nanoparticles in phosphate-buffered saline (PBS) at different temperatures (37 and 60 °C). We observed that the nanoparticles (Figure 4) lose their plasmonic absorption over time, and this degradation is kinetically accelerated at higher temperatures. The degradation of the materials in other media including RPMI and DMEM was also evaluated by following the UV–vis absorption of the materials over time, and again the plasmonic response decreased without significant differences between the media tested (see Figure S2). CuS degraded under those simulated conditions to form a mixture of water-soluble sulfates including chalcantite (CuSO4·5H2O) and brochantite (Cu4SO4(OH)6) as corroborated by XRD and by using qualitative analytical techniques such as precipitation (Figure 5). Thus, the presence of sulfate anions in the degradation byproducts of the CuS degraded nanoparticles was corroborated by precipitation with barium chloride and including sodium sulfate as control. A white precipitate was observed, indicative of the presence of sulfates. The presence of copper(II) ions was also corroborated by producing their precipitation under the presence of alkaline conditions. Under those conditions a blue-green precipitate was observed, indicative of the formation of Cu(OH)2. We can speculate that under physiological conditions CuS NPs would decompose and biodegrade to form water-soluble copper sulfates. Copper ions are essential trace elements for the body involved in many metabolic functions. An excess of copper ions is removed from the body by the liver via bile, and if that route is impaired, then some metabolites carry those ions and remove them from the body via urine.42 Sulfates are reduced in the body to elemental sulfur, which is an essential element in the protein synthesis, and an excess of sulfates is removed from the body via urine and bile.43
Figure 4.
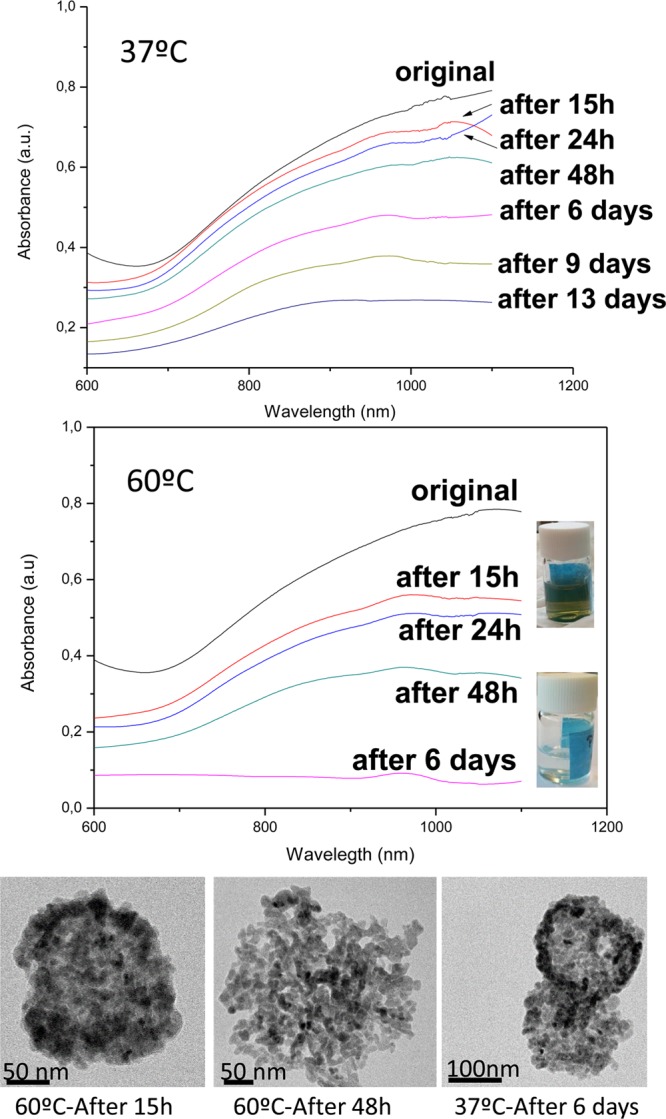
UV–vis spectra of the CuS NPs produced in the microfluidic reactor after immersion in PBS at different temperatures and times. TEM photographs showing the morphology of the nanoparticles at the different conditions. The digital images of the vials (insets) represent the initial colloidal suspension and the same sample after 6 days of aging at 60 °C.
Figure 5.
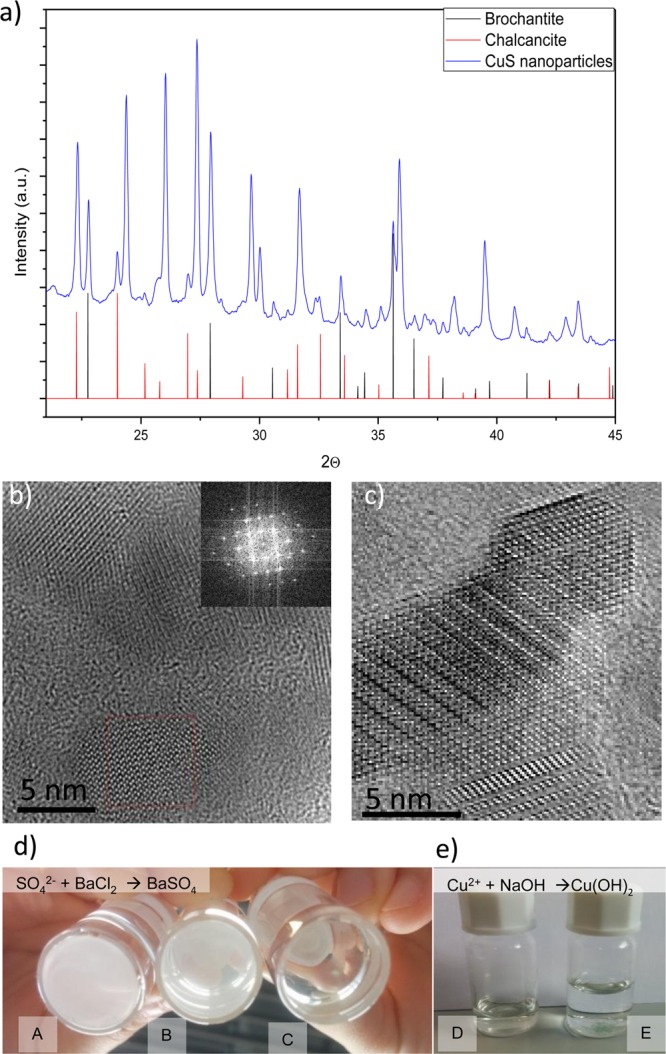
(a) XRD spectra of the materials resulting from degradation at 60 °C after 7 days in SBF (b) HRTEM image showing the polycrystalline nature of the degradation byproducts (inset is a DFT image). (c) HRTEM image with higher magnification of a degradation byproduct. (d) Precipitation of sulfates with barium sulfate to demonstrate their presence using (A) Na2(SO4) as control, (B) degraded byproducts from the CuS NPs under the presence of the barium sulfate, and (C) without barium sulfate. (e) Precipitation of copper(II) ions with sodium hydroxide to demonstrate their presence: (D) degraded byproducts from the CuS NPs without NaOH and (E) with NaOH.
Effect of CuS NPs on Cell Viability, Apoptosis, and Cell Cycle
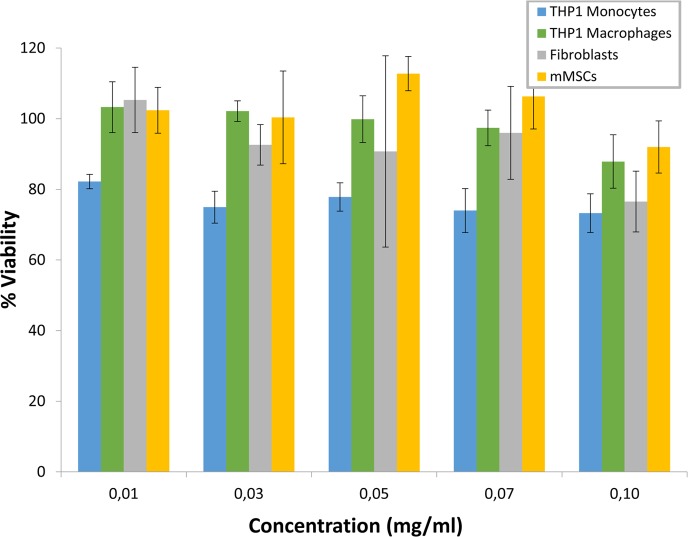
The biocompatibility of CuS NPs obtained by microfluidic reactors (90 °C, 30 min residence time) was studied at different levels in four different cell lines. The treatment of the cell types assayed with CuS NPs (0.01–0.1 mg/mL) did not significantly affect viability as was estimated by the Alamar Blue assay (Figure 6). The increase in CuS NPs concentration did not imply a significant decrease in cell viability showing percentages higher than 73% in all the cell lines and concentrations studied.
Figure 6.
Cytotoxicity of CuS NPs synthesized by microfluidics was evaluated by the Alamar Blue assay in the four cell lines assayed after 24 h. Data are presented as the mean ± SD of at least three experiments.
For further studies, 0.1 mg/mL was considered as the subcytotoxic concentration, that is, the working concentration following the recommendations of the ISO 10993-5 in which viabilities higher than 70% are not considered cytotoxic. The evaluation of cell apoptosis showed the effects on cell membrane after treatment with our CuS NPs (Table 1). The addition of CuS NPs at the considered subcytotoxic concentration (0.1 mg/mL) did not exert a significant harmful effect. In fact, necrosis showed a maximum increase of 1.60% while apoptosis displayed an increase lower than 3.5% in fibroblasts and mMSCs though for the phagocytic cell lines (monocytes and macrophages) this percentage was slightly higher (≤12.6%).
Table 1. Cell Apoptosis Evaluation by Flow Cytometry after Treatment with CuS NPsa.
| fibroblasts |
monocytes |
macrophages |
mMSCs |
|||||
|---|---|---|---|---|---|---|---|---|
| control | NPs CuS | control | NPs CuS | control | NPs CuS | control | NPs CuS | |
| necrosis | 1.5% | 1.6% | 0.3% | 0.8% | 1.4% | 3.0% | 1.4% | 0.9% |
| late apoptosis | 4.7% | 4.7% | 6.3% | 17.6% | 5.7% | 9.0% | 14.1% | 17.6% |
| early apoptosis | 5.6% | 7.1% | 9.1% | 10.4% | 9.2% | 16.4% | 11.0% | 10.9% |
| viability | 88.2% | 86.6% | 84.3% | 71.3% | 83.7% | 71.6% | 73.5% | 70.7% |
Control samples (not treated cells) were analyzed as background apoptosis level.
The changes in cell cycle after treatment with CuS NPs are shown in Figure 7. The addition of NPs to the cell cultures did not imply significant effects in cell cycle distribution except for human dermal fibroblasts. All cell lines showed a slight decrease in G1 phase being more accentuated in fibroblasts, which registered an increase of G2 phase that was almost the double that of the basal level. The percentages of G1 and G2 in fibroblasts after NPs treatment were very similar; thus, it is not considered that CuS NPs have induced the arrest of cell cycle or that DNA has been damaged.
Figure 7.
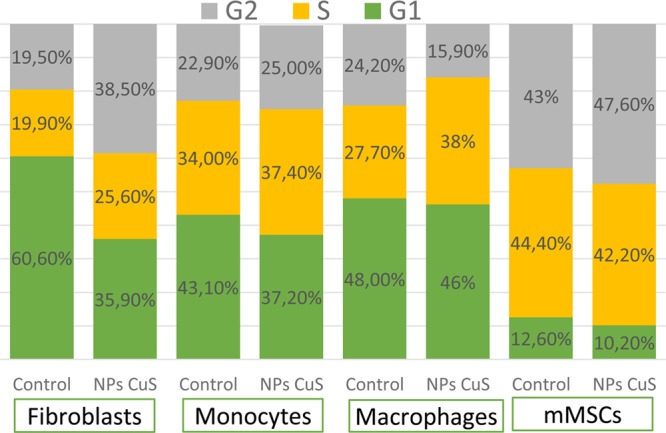
Distribution of cell cycle phases in the four cell types assayed after treatment with CuS NPs for 24 h. Control samples (not treated) were also analyzed as basal cell state.
Previous studies have shown similar effects in murine macrophages after treatment with hexagonal CuS nanoplates with an average edge length of 59.4 nm.44 Viabilities were reported close to that of the control sample for tumor cells and murine macrophages at our lower concentration decrease around 10% at our higher concentration (0.1 mg/mL), also showing a more accentuated effect in human endothelial cells at the same doses.
These results agree with our studies because human macrophages displayed viability percentages higher than 87%. In this sense, hollow CuS NPs coated with PEG tested in murine macrophage RAW264.7 cells and primary hepatocytes for 24 h did not exert cytotoxic effects displaying viability values higher than 90% at concentrations up to 0.1 mg/mL, though the addition of CuCl2 solutions to the cells implied a significant decrease in cell viability which was attributed to the fast dissociation rate of Cu ions from CuCl2 compared to that of CuS.15 Other authors have shown the biocompatibility of CuS NPs synthesized by wet chemistry in HEK293 cells after treatment for 48 h at concentrations up to 0.01 mg/mL, though at 0.1 mg/mL a significant cytotoxic effect was registered which was not displayed in our studies despite the NPs incubation times being different.5 These authors have also assayed the photothermal killing effects of these NPs in a tumor cell line (HeLa cells) after treatment for 2 h and irradiation by a near-infrared (NIR) laser showing a significant reduction in cell viability at concentrations higher than 0.02 mg/mL but only after irradiation; no toxic effects were obtained without laser treatment. In this sense, coated CuS NPs with a bovine serum albumin–folic acid (BSA-FA) complex45 or with DSPE-PEG200046 were also assayed in HeLa cells for 24 h, displaying viability values higher than 70% at concentrations up to 0.4 mg/mL, though NIR laser irradiation significantly increased cell death. However, nonaqueous copper sulfide nanocrystals obtained from a continuous-flow millifluidic chip exerted higher cytotoxic effects, decreasing viability to 50% at lower concentrations (<0.003 mg/mL) in RAW264.7 mouse macrophages which was more pronounced when cells were irradiated by a NIR laser due to the photothermal effects of CuS nanocrystals.35
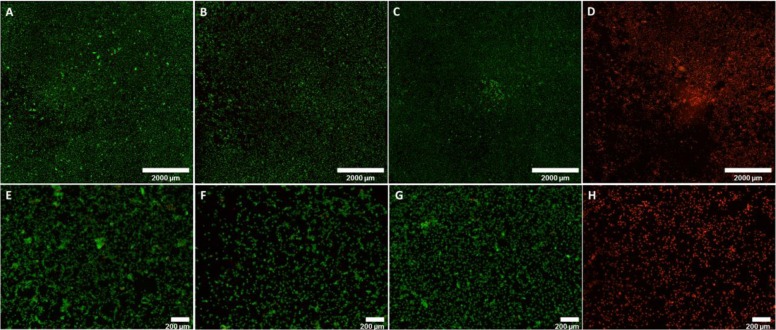
The photothermal effect in vitro was analyzed using CuS NPs obtained by microfluidics (90 °C, 30 min residence time) on murine mesenchymal stem cells (mMSCs) by culturing those cells for 24 h under the presence of subcytotoxic doses of the nanoparticles (0.1 mg/mL). NIR laser irradiation (808 nm, 200 mW/cm2, 20 min) did not reduce cell viability (Figure 8) on cells treated with the laser at that irradiance without nanoparticles. On the contrary, a reduced cell viability (measured by fluorescence microscopy through double-staining mediated by the LIVE/DEAD Viability/Cytotoxicity Kit) was observed when cells were treated with the laser in the presence of the CuS nanoparticles.
Figure 8.
Photothermal effects on mMSCs. (A–D) Composition of pictures (4× magnification) to show the whole cell culture well; (E–H) individual fields (4× magnification). (A and E) mMSCs not treated with CuS nanoparticles and not irradiated, (B and F) mMSCs treated with CuS NPs, (C and G) mMSCs laser irradiated (808 nm, 200 mW/cm2, 20 min); and (D and H) mMSCs treated with CuS NPs and laser irradiated 808 nm, 200 mW/cm2, 20 min). The cells were observed under fluorescence microscope, showing live cells in green and dead cells in red.
Finally, to decouple the photothermal effect from the photodynamic effect, we selected the specific conditions under which a chromophore (dihydrorhodamine 123, DHR123) does not degrade under heating, but its oxidation caused by reactive oxygen species (ROS) generation is easily measured by using fluorescence spectroscopy. DHR123 is a nonfluorescent probe that is easily oxidized to Rhodamine 123 (R123, a fluorescent probe) under the presence of ROS. We first evaluated the heating rate after irradiating (200 mW/cm2, 808 nm) a dispersion of our CuS nanoparticles (0.05 mg/mL) in 1 mL of ethanol. The temperature increased from the initial 37 to 68 °C in 4 min of irradiation. Figure S4 shows that the CuS NPs irradiated with the 808 nm laser produced higher oxidation of DHR123 compared to CuS NPs solution heated under the same conditions. The fluorescence intensity at 530 nm was enhanced 2-fold under laser irradiation, which was attributed to the ROS generation. DHR123 solution heated for 5 min did not show any increase in the fluorescence intensity (Figure S5), which confirmed that the enhancement in the oxidation was attributed to the photodynamic effect due to the presence of CuS NPs. CuS NPs did not yield fluorescence at the wavelength of 530 nm under the assay conditions.
All these results point to the biocompatibility of our CuS NPs obtained by microfluidics and highlight their potential in clinical applications as therapeutic carriers being a powerful material in the photothermal and photodynamic treatment of malignant cells.
Conclusions
It is possible to synthesize CuS nanoparticles with absorbance in the NIR region of the electromagnetic spectrum by using a microfluidic reactor. The physicochemical properties of the resulting nanoparticles are similar to those of the ones obtained in the conventional batch synthesis although the synthesis times are reduced 4-fold when using the microfluidic platform. Under simulated physiological conditions, CuS nanoparticles degrade into soluble copper sulfates, which highlights the great advantage of those nanomaterials compared to other conventional plasmonic nanomaterials such as gold nanostructures or carbon-based nanoparticles. Also, subcytotoxicological doses of those nanoparticles were calculated on different cell lines using the Alamar Blue assay and analyzing the cell cycle using flow cytometry. At subcytotoxic doses, those nanoparticles show an elevated photothermal effect as well as ROS generation.
Experimental Section
Chemicals and Methods
All the chemicals (polyvinylpyrrolidone, 10 000 Da Mw; polyvynilpyrrolidone K30 (PVP K30), 40 000 Da Mw; polyvinylpyrrolidone, 50 000 Da Mw; copper(II) chloride dihydrate, ACS reagent ≥99.0%; sodium sulfide nonahydrate, ACS reagent ≥98,0%; hydrazine, 35 wt % in water; sodium hydroxide, ACS reagent ≥97%, dihydrorhodamine-123 (DHR123, 95%)) were purchased from Sigma-Aldrich and used without further purification.
The synthesis of CuS NPs using a batch reactor was performed following the work of Ramadan et al.47 with some modifications. In brief, syntheses at 60 °C were carried out in an open flask by mixing 240 mg of PVP K30 dissolved in 25 mL of DDI water with 100 μL of a 0.5 M solution of CuCl2 and 25 mL of water with its pH adjusted to 9. Next, 6.4 mL of hydrazine solution was added under stirring, with the consequent formation of Cu2O seeds; finally, 200 μL of Na2S (320 mg/mL) was added to the previous dispersion while keeping it under heating at 60 °C for 2 h. All the solutions are added consecutively without dwell times. Syntheses at 120–150 °C were carried out following the same procedure but by heating the final colloidal dispersion in a PTFE-lined sealed autoclave placed in the oven. After synthesis, the resulting CuS NPs were thoroughly washed in DDI water by successive cycles of centrifugation.
Continuous microfluidic synthesis was carried out by using two consecutive Y-shaped PEEK micromixers (500 μm inner diameter). In the first micromixer, two solutions were interfaced to form the Cu2O seeds by use of syringe pumps (Harvard Apparatus) with the same flow rates (4.625 mL/h): A first solution prepared by mixing 480 mg of PVP K30 dissolved in 30 mL of DDI water with 200 μL of a 0.5 M solution of CuCl2 adjusted at a pH of 9 was mixed with a second solution composed of 12.8 μL of hydrazine in 30 mL of DDI water and 25 mL of water with its pH adjusted to 9. The resulting Cu2O dispersion was then interfaced in a second Y-shaped micromixer with a solution fed at a flow rate of 9.25 mL/h and prepared by diluting 400 μL Na2S (320 mg/mL) in 60 mL of DDI water. PTFE tubing (0.8 mm ID) was used in the first stage with the appropriate length to reach a residence time of 30 s. In the second stage, 1.5 mm ID PTFE tubing was used with different lengths to reach different residence times. A back-pressure regulator (Zaiput Flow Technologies) was used for synthesis at temperatures above 100 °C (see Figure S3). The temperature of the tubing was maintained by immersing it in a temperature-controlled oil bath.
The morphology of the nanoparticles was visualized by transmission electron microscopy (TEM; FEI Tecnai T20, operating at 200 kV). Scanning-transmission imaging with a high-angle annular dark-field detector (STEM-HAADF) and energy-dispersive X-ray spectroscopy (EDS) analysis were carried out in a transmission electron microscope (STEM; FEI Tecnai F30, operating at 300 kV). The crystallinity, purity, and structure of the materials were studied by low-angle X-ray diffraction. The patterns were recorded in a Philips X-Pert diffractometer equipped with a monochromatized Cu Kα radiation source (40 kV, 20 mA) over the range 0.6–10.0 with a step of 0.02 and an analysis time of 5 s. UV–vis absorption spectra were evaluated via a UV–vis–NIR spectrophotometer (Jasco V670, Tokyo, Japan). X-ray photoelectron spectroscopy (XPS, Axis Ultra DLD, Kratos Tech.) was used to evaluate the oxidation state of the CuS nanoparticles. Heating efficiency was measured with the aid of a 808 nm wavelength laser diode (6 × 8 mm2 spot size; Optilas model MDL-III-808-2W, Changchun New Industries Optoelectronics Technology Co., Ltd., Changchun, China) and a power controller (Model PD300-3W, Ophir Laser Measurement Group, Logan, UT, USA) with an irradiance of 200 mW/cm2. Temperature gradients were monitored using a type K thermocouple (RS Amidata, Madrid, Spain) immersed in the dispersion parallel to the path of the laser light but without being intercepted with it. The hydrodynamic diameter of aqueous dispersions of CuS nanoparticles was evaluated by dynamic light scattering (DLS) in a 90Plus Particle Size Analyzer by Brookhaven Instruments Corp., Holtsville, NY, USA.
Cell Culture
The biocompatibility of the CuS NPs synthesized was assessed at different levels regarding metabolism, cell nucleus (DNA and cell cycle), and cell membrane (induction of apoptosis). These studies were performed using mouse mesenchymal stem cells (mMSCs) kindly gifted by Dr. Pilar Martín-Duque, human dermal fibroblasts purchased from Lonza (Belgium), and THP1 human monocytes obtained from the American Type Culture Collection (USA).
Fibroblasts were grown in high-glucose DMEM (DMEM w/stable glutamine; BioWest, France) supplemented with 10% fetal bovine serum (FBS; Gibco, UK), penicillin/streptomycin (100 U/100 μg/mL; Lonza, Belgium), and amphotericin B (1.5 μg/mL; Lonza, Belgium). mMSCs were cultured in DMEM-F12 containing 1% glutamine (Gibco, UK), 10% FBS (Gibco, UK), penicillin/streptomycin (100 U/100 μg/mL; Lonza, Belgium), and amphotericin B (1.5 μg/mL; Lonza, Belgium). Monocytes were cultured in RPMI 1640 (RPMI 1640 w/stable glutamine; Biowest, France) supplemented with 10% FBS, 1% HEPES, 1% nonessential amino acids, 0.1% 2-mercaptoethanol 50 mM, 1% sodium pyruvate 100 mM, penicillin/streptomycin (100 U/100 μg/mL), and amphotericin B (1.5 μg/mL), all purchased from Gibco (UK). Macrophages were obtained by the in vitro differentiation of monocytes by adding 1 μM phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, USA) to the cell culture. All cell types were grown in humidified atmosphere at 37 °C and 5% CO2, except for mMSCs which were cultured in hypoxia (3% O2).
CuS NPs Cytotoxicity
The effects of CuS NPs treatment in cell metabolism were determined by the Alamar Blue assay (Invitrogen, US). Cells were seeded in a 96-well plate and incubated with CuS NPs (0.01–0.1 mg/mL) for 24 h. Alamar blue was then added (10%) and incubated for 4 h. The reduction of the dye to a fluorescent compound by metabolically active cells was read in a microplate reader (Multimode Synergy HT Microplate Reader; Biotek, US) at 535/590 nm ex/em. Cell viability was determined by interpolation of the emission data obtained from the treated samples and the control samples (untreated cells = 100% viability).
Evaluation of Cell Apoptosis
CuS NPs effects on cell membrane after treatment for 24 h were determined by the study of cell apoptosis by flow cytometry at the subcytotoxic concentration obtained from the alamar blue assay. After treatment with NPs, cells were harvested in PBS and double-stained with annexin V-FITC and propidium iodide. In brief, cell suspensions were stained with annexin V-FITC and treated with a solution composed of annexin V-FITC, propidium iodide, and annexin V binding buffer to be finally incubated with the binding buffer for 15 min before the analysis of the samples in the FACSARIA BD equipment and the FACSDIVA BD software (Cell Separation and Cytometry Unit, CIBA, IIS Aragon, Spain). Control samples (not treated cells) were also evaluated to determine the influence of the CuS NPs on apoptosis.
Study of Cell Cycle
The distribution of cell cycle phases after NPs treatment was assessed by flow cytometry in order to elucidate the effects of CuS NPs in cell cycle and DNA. As described above, cells were treated for 24 h at the CuS NPs subcytotoxic concentration. Then, cells were collected in PBS and fixed in 70% ice-cold ethanol. After 24 h incubation at 4 °C, DNA staining was developed by adding RNase A and propidium iodide. Samples were analyzed in a FACSARRAY BD equipment with the MODIFIT 3.0 Verity. Control samples (not treated cells) were also run to assay the normal distribution of cell cycle in the cell lines assayed.
Photothermal in Vitro Assay
mMSCs were cultured at a cell density of 60 000 cells/well in a 24-well plate for 24 h at 37 °C, 5% CO2, and hypoxia. Then, culture medium was removed, and CuS NPs (0.1 mg/mL) were added to the cells for 24 h. After incubation, samples were irradiated with the NIR diode laser (808 nm, for 20 min at 200 mW/cm2). The effects of the irradiation on cell viability was observed by fluorescence microscopy through the double-staining mediated by the LIVE/DEAD Viability/Cytotoxicity Kit (Thermo-Fisher Scientific, USA) following the manufacturer instructions. In brief, a solution containing 2 μM calcein AM and 4 μM ethidium homodimer-1 in PBS was prepared and added to the cells. Then, cells were incubated for 30 min at room temperature in the dark. The samples were evaluated in an inverted fluorescence microscope Olympus IX81. Control samples (not irradiated and/or not treated with CuS NPs) were also assayed to obtain the basal viability status of mMSCs. The experiments were run in duplicate.
Reactive Oxygen Species Generation
DHR123 was used to detect ROS generated by the presence of CuS NPs under light irradiation. DHR123 is a nonfluorescent probe that it is oxidized in the presence of ROS to form Rhodamine (R123), a highly fluorescent molecule that has an emission peak centered at 530 nm.48 CuS NPs did not yield fluorescence at the wavelength of 530 nm under the assay conditions.
A CuS nanoparticulated dispersion at 0.05 mg/mL was mixed with 250 μL of 6.6 μM DHR123 in 1 mL of ethanol. The solution was irradiated at 200 mW/cm2 (at 808 nm) to study the ROS generation upon NPs irradiation. Formation of R123 was monitored by fluorescence spectroscopy using an excitation wavelength of 485 nm, recording the emission in the 500–600 nm range. As a control, it became necessary to determine if the heating caused by the laser absorption of the CuS nanoparticles would also produce R123 without any irradiation. Therefore, control experiments were performed by heating dispersions of DHR123 and CuS NPs with DHR123. The control assays were performed using the same heating rate observed during the irradiation of the CuS nanoparticle dispersions in order to establish a comparison. The fluorescence of the samples was measured before and after 5 min of irradiation or heating.
Acknowledgments
We thank the ERC Consolidator Grant program (ERC-2013-CoG-614715, NANOHEDONISM) and EU CIG–Marie Curie under the REA grant agreement no. 321642. CIBER-BBN is an initiative funded by the VI National R&D&i Plan 2008–2011, Iniciativa Ingenio 2010, Consolider Program, CIBER Actions, and is financed by the Instituto de Salud Carlos III (Spain) with assistance from the European Regional Development Fund.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsami.6b05727.
Nanoparticles histogram, XPS data, and UV–vis characterization data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Kolny-Olesiak J. Synthesis of Copper Sulphide-based Hybrid Nanostructures and their Application in Shape Control of Colloidal Semiconductor Nanocrystals. CrystEngComm 2014, 16, 9381–9390. 10.1039/C4CE00674G. [DOI] [Google Scholar]
- Smith B. E.; Roder P. B.; Zhou X.; Pauzauskie P. J. Nanoscale Materials for Hyperthermal Theranostics. Nanoscale 2015, 7, 7115–7126. 10.1039/C4NR06164K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.; Yoon S. W.; Kim E. J.; Park J. In-situ Growth of Copper Sulfide Nanocrystals on Multiwalled Carbon Nanotubes and their Application as Novel Solar Cell and Amperometric Glucose Sensor Materials. Nano Lett. 2007, 7, 778–784. 10.1021/nl0630539. [DOI] [PubMed] [Google Scholar]
- Tian Q. W.; Tang M. H.; Sun Y. G.; Zou R. J.; Chen Z. G.; Zhu M. F.; Yang S. P.; Wang J. L.; Wang J. H.; Hu J. Q. Hydrophilic Flower-Like CuS Superstructures as an Efficient 980 nm Laser-Driven Photothermal Agent for Ablation of Cancer Cells. Adv. Mater. 2011, 23, 3542–3547. 10.1002/adma.201101295. [DOI] [PubMed] [Google Scholar]
- Li Y. B.; Lu W.; Huang Q. A.; Li C.; Chen W. Copper Sulfide Nanoparticles for Photothermal Ablation of Tumor Cells. Nanomedicine 2010, 5, 1161–1171. 10.2217/nnm.10.85. [DOI] [PubMed] [Google Scholar]
- Ku G.; Zhou M.; Song S. L.; Huang Q.; Hazle J.; Li C. Copper Sulfide Nanoparticles As a New Class of Photoacoustic Contrast Agent for Deep Tissue Imaging at 1064 nm. ACS Nano 2012, 6, 7489–7496. 10.1021/nn302782y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M.; Zhang R.; Huang M. A.; Lu W.; Song S. L.; Melancon M. P.; Tian M.; Liang D.; Li C. A Chelator-Free Multifunctional [Cu-64]CuS Nanoparticle Platform for Simultaneous Micro-PET/CT Imaging and Photothermal Ablation Therapy. J. Am. Chem. Soc. 2010, 132, 15351–15358. 10.1021/ja106855m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X. G.; Liow C. H.; Zhang M. X.; Huang R. J.; Li C. Y.; Shen H.; Liu M. Y.; Zou Y.; Gao N.; Zhang Z. J.; Li Y. G.; Wang Q. B.; Li S. Z.; Jiang J. Surface Plasmon Resonance Enhanced Light Absorption and Photothermal Therapy in the Second Near-Infrared Window. J. Am. Chem. Soc. 2014, 136, 15684–15693. 10.1021/ja508641z. [DOI] [PubMed] [Google Scholar]
- Guo L. R.; Yan D. D.; Yang D. F.; Li Y. J.; Wang X. D.; Zalewski O.; Yan B. F.; Lu W. Combinatorial Photothermal and Immuno Cancer Therapy Using Chitosan-Coated Hollow Copper Sulfide Nanoparticles. ACS Nano 2014, 8, 5670–5681. 10.1021/nn5002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J.; Liu Y. W.; Jiang X. E. Multifunctional PEG-GO/CuS Nanocomposites for Near-infrared Chemo-photothermal Therapy. Biomaterials 2014, 35, 5805–5813. 10.1016/j.biomaterials.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Libutti S. K.; Paciotti G. F.; Byrnes A. A.; Alexander H. R.; Gannon W. E.; Walker M.; Seidel G. D.; Yuldasheva N.; Tamarkin L. Phase I and Pharmacokinetic Studies of CYT-6091, a Novel PEGylated Colloidal Gold-rhTNF Nanomedicine. Clin. Cancer Res. 2010, 16, 6139–6149. 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern J. M.; Kibanov Solomonov V. V.; Sazykina E.; Schwartz J. A.; Gad S. C.; Goodrich G. P. Initial Evaluation of the Safety of Nanoshell-Directed Photothermal Therapy in the Treatment of Prostate Disease. Int. J. Toxicol. 2016, 35, 38–46. 10.1177/1091581815600170. [DOI] [PubMed] [Google Scholar]
- Tuchin V. V. Light Scattering Study of Tissues. Usp. Fiz. Nauk 1997, 167, 517–539. 10.3367/UFNr.0167.199705c.0517. [DOI] [Google Scholar]
- Hong G. S.; Diao S.; Chang J. L.; Antaris A. L.; Chen C. X.; Zhang B.; Zhao S.; Atochin D. N.; Huang P. L.; Andreasson K. I.; Kuo C. J.; Dai H. J. Through-skull Fluorescence Imaging of the Brain in a New Near-infrared Window. Nat. Photonics 2014, 8, 723–730. 10.1038/nphoton.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L. R.; Panderi I.; Yan D. D.; Szulak K.; Li Y. J.; Chen Y. T.; Ma H.; Niesen D. B.; Seeram N.; Ahmed A.; Yan B. F.; Pantazatos D.; Lu W. A Comparative Study of Hollow Copper Sulfide Nanoparticles and Hollow Gold Nanospheres on Degradability and Toxicity. ACS Nano 2013, 7, 8780–8793. 10.1021/nn403202w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y.; Yokoyama A.; Nodasaka Y.; Kohgo T.; Motomiya K.; Matsumoto H.; Nakazawa E.; Numata T.; Zhang M.; Yudasaka M.; Hara H.; Araki R.; Tsukamoto O.; Saito H.; Kamino T.; Watari F.; Tohji K. Long-term Biopersistence of Tangled Oxidized Carbon Nanotubes Inside and Outside Macrophages in Rat Subcutaneous Tissue. Sci. Rep. 2013, 3, 2516. 10.1038/srep02516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girish C. M.; Sasidharan A.; Gowd G. S.; Nair S.; Koyakutty M. Confocal Raman Imaging Study Showing Macrophage Mediated Biodegradation of Graphene In Vivo. Adv. Healthcare Mater. 2013, 2, 1489–1500. 10.1002/adhm.201200489. [DOI] [PubMed] [Google Scholar]
- Elgrabli D.; Dachraoui W.; Menard-Moyon C.; Liu X. J.; Begin D.; Begin-Colin S.; Bianco A.; Gazeau F.; Alloyeau D. Carbon Nanotube Degradation in Macrophages: Live Nanoscale Monitoring and Understanding of Biological Pathway. ACS Nano 2015, 9, 10113–10124. 10.1021/acsnano.5b03708. [DOI] [PubMed] [Google Scholar]
- Liu X. H.; Qian H. S.; Ji Y. P.; Li Z. Q.; Shao Y.; Hu Y.; Tong G. X.; Li L. C.; Guo W. D.; Guo H. C. Mesoporous Silica-coated NaYF4 Nanocrystals: Facile Synthesis, In Vitro Bioimaging and Photodynamic Therapy of Cancer Cells. RSC Adv. 2012, 2, 12263–12268. 10.1039/c2ra21688d. [DOI] [Google Scholar]
- Seo H. J.; Nam S. H.; Im H. J.; Park J. Y.; Lee J. Y.; Yoo B.; Lee Y. S.; Jeong J. M.; Hyeon T.; Who Kim J.; Lee J. S.; Jang I. J.; Cho J. Y.; Hwang D. W.; Suh Y. D.; Lee D. S. Rapid Hepatobiliary Excretion of Micelle-Encapsulated/Radiolabeled Upconverting Nanoparticles as an Integrated Form. Sci. Rep. 2015, 5, 15685. 10.1038/srep15685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman A. M.; Cao Y.; Urban C.; Neumann O.; Ayala-Orozco C.; Knight M. W.; Joshi A.; Nordlander P.; Halas N. J. The Surprising In Vivo Instability of Near-IR-absorbing Hollow Au-Ag nanoshells. ACS Nano 2014, 8, 3222–3231. 10.1021/nn405663h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan S. B.; Zou X. J.; Hossu M.; Ma L.; Yang C.; Chen W. Local Field Enhanced Au/CuS Nanocomposites as Efficient Photothermal Transducer Agents for Cancer Treatment. J. Biomed. Nanotechnol. 2012, 8, 883–890. 10.1166/jbn.2012.1486. [DOI] [PubMed] [Google Scholar]
- Wang H.; Liu K.; Chen K. J.; Lu Y. J.; Wang S. T.; Lin W. Y.; Guo F.; Kamei K. I.; Chen Y. C.; Ohashi M.; Wang M. W.; Garcia M. A.; Zhao X. Z.; Shen C. K. F.; Tseng H. R. A Rapid Pathway Toward a Superb Gene Delivery System: Programming Structural and Functional Diversity into a Supramolecular Nanoparticle Library. ACS Nano 2010, 4, 6235–6243. 10.1021/nn101908e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez L.; Sebastian V.; Irusta S.; Ibarra A.; Arruebo M.; Santamaria J. Scaled-up Production of Plasmonic Nanoparticles Using Microfluidics: from Metal Precursors to Functionalized and Sterilized nanoparticles. Lab Chip 2014, 14, 325–332. 10.1039/C3LC50999K. [DOI] [PubMed] [Google Scholar]
- Krishnadasan S.; Brown R. J. C.; deMello A. J.; deMello J. C. Intelligent Routes to the Controlled Synthesis of Nanoparticles. Lab Chip 2007, 7, 1434–1441. 10.1039/b711412e. [DOI] [PubMed] [Google Scholar]
- Lim J. M.; Swami A.; Gilson L. M.; Chopra S.; Choi S.; Wu J.; Langer R.; Karnik R.; Farokhzad O. C. Ultra-High Throughput Synthesis of Nanoparticles with Homogeneous Size Distribution Using a Coaxial Turbulent Jet Mixer. ACS Nano 2014, 8, 6056–6065. 10.1021/nn501371n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bally F.; Garg D. K.; Serra C. A.; Hoarau Y.; Anton N.; Brochon C.; Parida D.; Vandamme T.; Hadziioannou G. Improved Size-tunable Preparation of Polymeric Nanoparticles by Microfluidic Nanoprecipitation. Polymer 2012, 53, 5045–5051. 10.1016/j.polymer.2012.08.039. [DOI] [Google Scholar]
- Kastner E.; Verma V.; Lowry D.; Perrie Y. Microfluidic-controlled Manufacture of Liposomes for the Solubilisation of a Poorly Water Soluble Drug. Int. J. Pharm. 2015, 485, 122–130. 10.1016/j.ijpharm.2015.02.063. [DOI] [PubMed] [Google Scholar]
- Xia H. M.; Seah Y. P.; Liu Y. C.; Wang W.; Toh A. G. G.; Wang Z. P. Anti-solvent Precipitation of Solid Lipid Nanoparticles Using a Microfluidic Oscillator Mixer. Microfluid. Nanofluid. 2015, 19, 283–290. 10.1007/s10404-014-1517-5. [DOI] [Google Scholar]
- Schuch M.; Gross G. A.; Kohler J. M. Formation and Fluorimetric Characterization of Micelles in a Micro-flow Through System with Static Micro mixer. Sensors 2007, 7, 2499–2509. 10.3390/s7112499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C. T.; Jahn A.; Locascio L. E.; Vreeland W. N. Controlled Self-Assembly of Monodisperse Niosomes by Microfluidic Hydrodynamic Focusing. Langmuir 2010, 26, 8559–8566. 10.1021/la904616s. [DOI] [PubMed] [Google Scholar]
- Lohse S. E.; Eller J. R.; Sivapalan S. T.; Plews M. R.; Murphy C. J. A Simple Millifluidic Benchtop Reactor System for the High-Throughput Synthesis and Functionalization of Gold Nanoparticles with Different Sizes and Shapes. ACS Nano 2013, 7, 4135–4150. 10.1021/nn4005022. [DOI] [PubMed] [Google Scholar]
- Duraiswamy S.; Khan S. A. Dual-Stage Continuous-Flow Seedless Microfluidic Synthesis of Anisotropic Gold Nanocrystals. Part Part Syst. Charact. 2014, 31, 429–432. 10.1002/ppsc.201300266. [DOI] [Google Scholar]
- Gomez L.; Arruebo M.; Sebastian V.; Gutierrez L.; Santamaria J. Facile Synthesis of SiO2-Au Nanoshells in a Three-stage Microfluidic System. J. Mater. Chem. 2012, 22, 21420–21425. 10.1039/c2jm34206e. [DOI] [Google Scholar]
- Cheung T. L.; Hong L.; Rao N.; Yang C.; Wang L.; Lai W. J.; Chong P. H.; Law W. C.; Yong K. T. The Non-aqueous Synthesis of Shape Controllable Cu2-xS Plasmonic Nanostructures in a Continuous-flow Millifluidic Chip for the Generation of Photo-induced Heating. Nanoscale 2016, 8, 6609–6622. 10.1039/C5NR09144F. [DOI] [PubMed] [Google Scholar]
- Zhao Y. X.; Pan H. C.; Lou Y. B.; Qiu X. F.; Zhu J. J.; Burda C. Plasmonic Cu2-xS Nanocrystals: Optical and Structural Properties of Copper-Deficient Copper(I) Sulfides. J. Am. Chem. Soc. 2009, 131, 4253–4261. 10.1021/ja805655b. [DOI] [PubMed] [Google Scholar]
- Xie Y.; Carbone L.; Nobile C.; Grillo V.; D’Agostino S.; Della Sala F.; Giannini C.; Altamura D.; Oelsner C.; Kryschi C.; Cozzoli P. D. Metallic-like Stoichiometric Copper Sulfide Nanocrystals: Phase- and Shape-Selective Synthesis, Near-Infrared Surface Plasmon Resonance Properties, and Their Modeling. ACS Nano 2013, 7, 7352–7369. 10.1021/nn403035s. [DOI] [PubMed] [Google Scholar]
- Pattrick R. A. D.; Mosselmans J. F. W.; Charnock J. M.; England K. E. R.; Helz G. R.; Garner C. D.; Vaughan D. J. The Structure of Amorphous Copper Sulfide Precipitates: An X-ray Absorption Study. Geochim. Cosmochim. Acta 1997, 61, 2023–2036. 10.1016/S0016-7037(97)00061-6. [DOI] [Google Scholar]
- Zhu H. T.; Wang J. X.; Wu D. X. Fast Synthesis, Formation Mechanism, and Control of Shell Thickness of CuS Hollow Spheres. Inorg. Chem. 2009, 48, 7099–7104. 10.1021/ic900201p. [DOI] [PubMed] [Google Scholar]
- Bang S.; Yoon D.; Kim J.; Baik H.; Yang H.; Lee K. Formation of Double Layer Hollow Nanostars of Pd/CuIr by Utilizing a Kirkendall Effect and a Facile Cu Atom Movement Along Twinning Boundaries and their Usage as Efficient Water Splitting Catalysts. CrystEngComm 2015, 17, 4084–4088. 10.1039/C5CE00538H. [DOI] [Google Scholar]
- Luther G. W.; Theberge S. M.; Rozan T. F.; Rickard D.; Rowlands C. C.; Oldroyd A. Aqueous Copper Sulfide Clusters as Intermediates During Copper Sulfide Formation. Environ. Sci. Technol. 2002, 36, 394–402. 10.1021/es010906k. [DOI] [PubMed] [Google Scholar]
- Gray L. W.; Peng F. Y.; Molloy S. A.; Pendyala V. S.; Muchenditsi A.; Muzik O.; Lee J.; Kaplan J. H.; Lutsenko S. Urinary Copper Elevation in a Mouse Model of Wilson’s Disease Is a Regulated Process to Specifically Decrease the Hepatic Copper Load. PLoS One 2012, 7, e38327. 10.1371/journal.pone.0038327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee E. A.; Curno R.; Edmond L. M.; Cummings J. H. Contribution of Dietary Protein and Inorganic Sulfur to Urinary Sulfate: Toward a Biomarker of Inorganic Sulfur Intake. Am. J. Clin. Nutr. 2004, 80, 137–142. [DOI] [PubMed] [Google Scholar]
- Feng W.; Nie W.; Cheng Y. H.; Zhou X. J.; Chen L.; Qiu K. X.; Chen Z. G.; Zhu M. F.; He C. L. In Vitro and In Vivo Toxicity Studies of Copper Sulfide Nanoplates for Potential Photothermal Applications. Nanomedicine 2015, 11, 901–912. 10.1016/j.nano.2014.12.015. [DOI] [PubMed] [Google Scholar]
- Han L.; Zhang Y.; Chen X. W.; Shu Y.; Wang J. H. Protein-modified Hollow Copper Sulfide Nanoparticles Carrying Indocyanine Green for Photothermal and Photodynamic Therapy. J. Mater. Chem. B 2016, 4, 105–112. 10.1039/C5TB02002F. [DOI] [PubMed] [Google Scholar]
- Huang Y. Z.; Lai Y. L.; Shi S. G.; Hao S. F.; Wei J. P.; Chen X. L. Copper Sulfide Nanoparticles with Phospholipid-PEG Coating for In Vivo Near-Infrared Photothermal Cancer Therapy. Chem. - Asian J. 2015, 10, 370–376. 10.1002/asia.201403133. [DOI] [PubMed] [Google Scholar]
- Ramadan S.; Guo L. R.; Li Y. J.; Yan B. F.; Lu W. Hollow Copper Sulfide Nanoparticle-Mediated Transdermal Drug Delivery. Small 2012, 8, 3143–3150. 10.1002/smll.201200783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow J. P. Dichlorodihydrofluorescein and Dihydrorhodamine 123 are Sensitive Indicators of Peroxynitrite In Vitro: Implications for Intracellular Measurement of Reactive Nitrogen and Oxygen Species. Nitric Oxide 1997, 1, 145–157. 10.1006/niox.1996.0113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.