Epidural spinal stimulation has shown effectiveness in recovering the motor function of spinal cord transected rats by modulating neural networks in lumbosacral spinal segments [1, 2]. The state-of-the-art neuromodulation implant [3] reports a 4-channel stimulator with wireless data and power links for small animal experiments, yet weighs 6g and has a volume of 3cm3. It is preferable that the implant package has a comparable size to its bioelectronics and a high-density stimulator to support stimulation with high spatial resolution. Furthermore, the epidural electrode should be soft and flexible because a mechanical mismatch exists at the tissue-electrode interface [1]. Unlike other implant/SoCs that stimulate with pre-loaded patterns [4–5], the implant for motor function recovery should be capable of adaptively adjusting its stimulation patterns at run time in response to the subject’s varying physiological states [2]. Measuring the electrode-tissue impedance is also critical to ensure safe stimulation. Deriving the equivalent circuit model of the electrode-tissue interface determines the safe stimulation boundary (i.e. pulse width and intensity) to ensure the electrode overpotential is within the water window [6]. However, an SoC implementation of this function has not been reported.
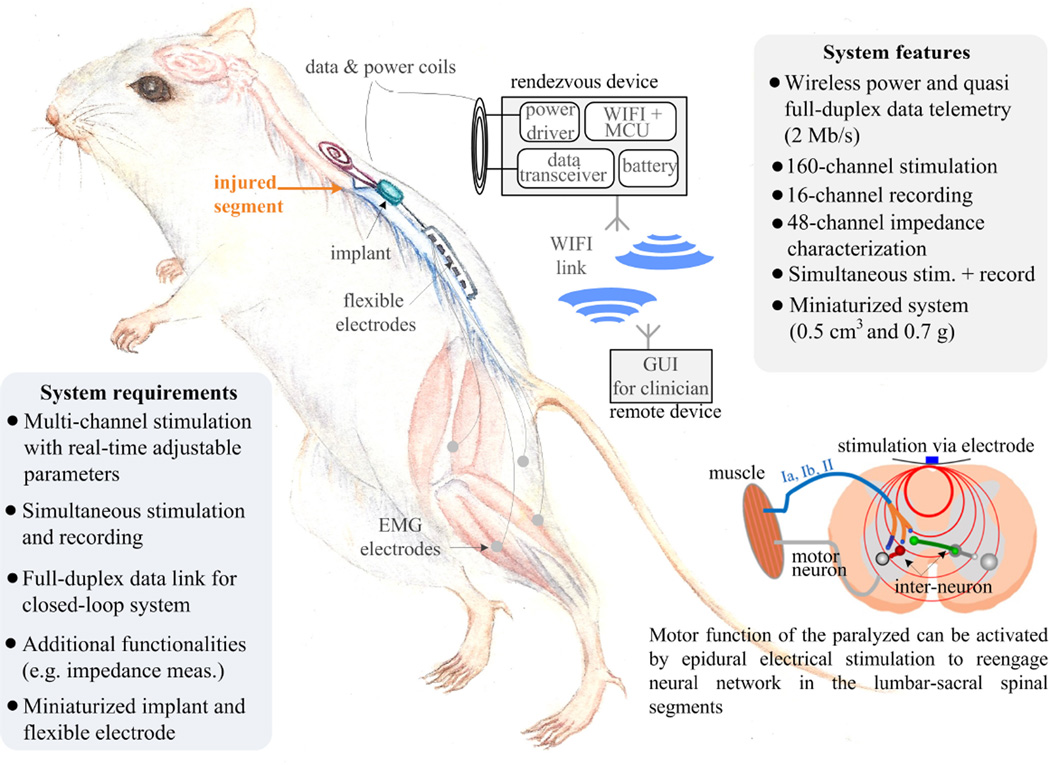
Figure 22.2.1 illustrates the implantable system performing simultaneous stimulation and full-duplex data telemetry using a rat model. The rat carries a rendezvous device that wirelessly powers the implant and links the implant and a remote device (e.g. smart phone). The implant is miniaturized to 0.5cm3 and 0.7g. A thin (8µm), flexible polyimide based platinum electrode array is placed into the epidural space, and EMG (electromyography) wire electrodes (AS632 Cooner wire, Chatsworth CA) are sutured onto leg muscles.
Figure 22.2.1.
Illustration of the implantable system and the biological mechanism for motor function recovery.
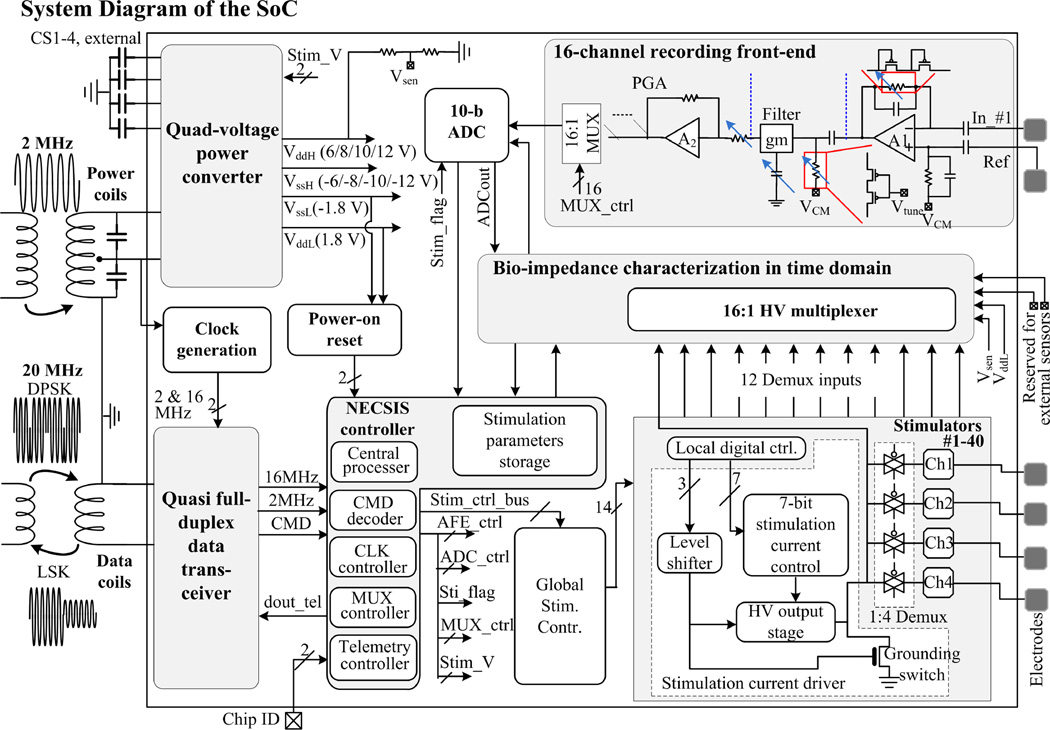
The core of the implant is a mixed-signal, multi-voltage SoC performing high-voltage (HV) 160-channel current stimulation, 16-channel recording, and 48-channel bio-impedance characterization with fully integrated power/data telemetry (Fig. 22.2.2). Improved upon [7], a power converter generates 4 different voltages to power the implant, with added capability of adjusting the supply voltages for stimulators (± 6/8/10/12 V) to accommodate various bioimpedances. A new quasi full-duplex data transceiver links the SoC and the rendezvous device at 2Mb/s. The NECSIS (Neural Command Signal Interface System) controller determines the implant operation based on the received commands (CMDs). Stimulation of 160 channels is achieved by 40 stimulation current drivers, each with a 1:4 Demux [7]. For impedance measurement, 12 out of 40 Demux inputs are selectively connected to the 16:1 MUX made of HV transistors, allowing 48 electrodes to be characterized. HV MUX is also connected to the power converter outputs and two of the MUX inputs are reserved for inertial and temperature sensors. This SoC supports chip clustering using a 2-bit ID control. By sharing the same coils, a four-SoC cluster can provide 640-channel stimulation, 64-channel recording, as well as 192-channel impedance characterization.
Figure 22.2.2.
System diagram of the SoC. Power converter, data transceiver, NECSIS controller, stimulators, and impedance characterization circuits are all integrated in the SoC. The SoC and the external device are linked inductively.
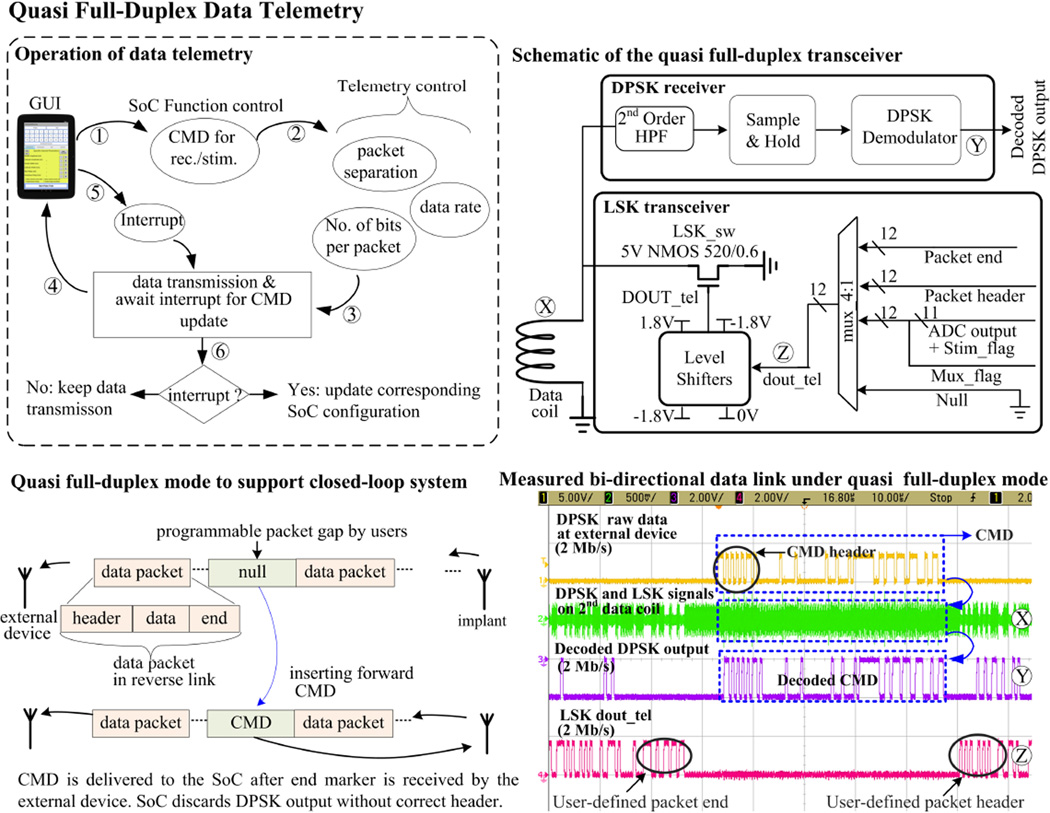
Figure 22.2.3 shows the operation of the quasi full-duplex data link and its implementation. Realizing a high data rate reverse link with a power coil is disadvantageous because high wireless power transfer efficiency and high Qfactor requirements limit the data rate. A low-Q data coil is thus used for both the forward and reverse links. In the reverse telemetry, the SoC transmits the recorded data in packets separated by programmable time gaps. Each packet contains a header, digitized data, and an end marker. Once the rendezvous device recognizes the end marker, it can send a CMD to the SoC within this time gap (Fig. 22.2.3 bottom panel). The SoC consists of a DPSK receiver for its good immunity to interference [7] and a LSK transmitter for low-power consumption (< 4µW). The test result shows both forward DPSK and reverse LSK signals can co-exist on the same coil without contention. The LSK signal may result in error bits at the DPSK-demodulated output, but they fail the CMD header check and are discarded.
Figure 22.2.3.
Operation flow and block diagram of the quasi full-duplex data link based on DPSK (forward link) and LSK (reverse link).
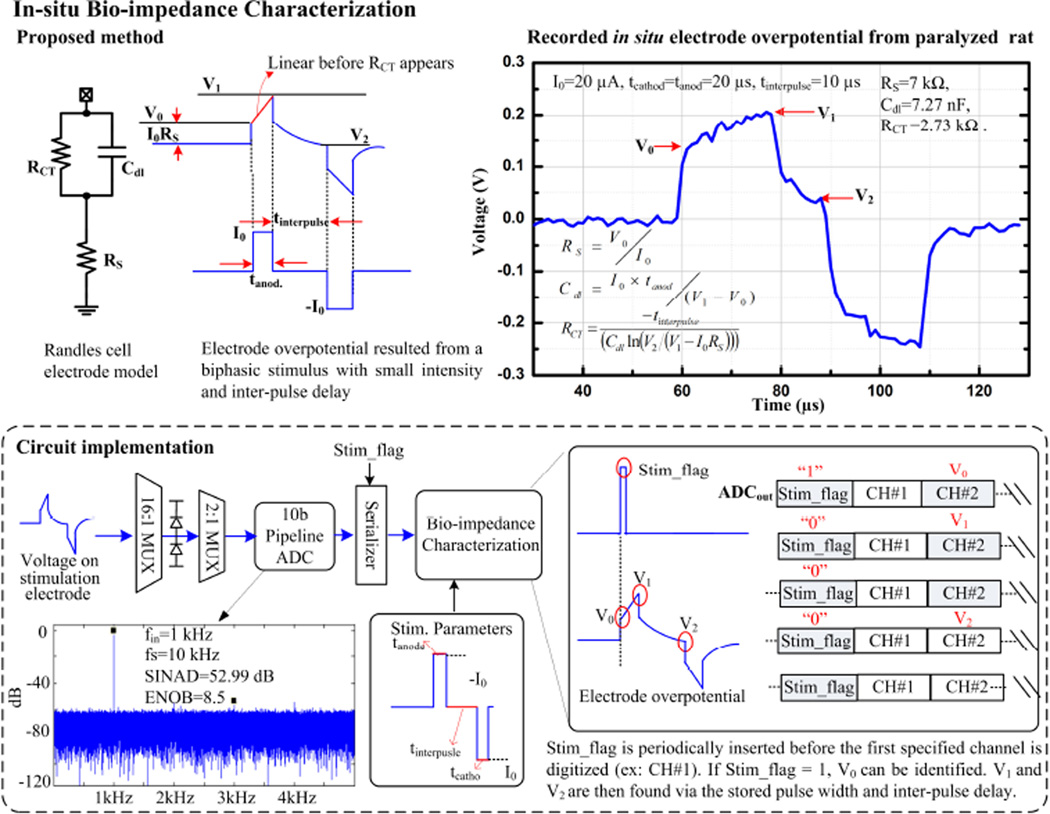
Characterizing bio-impedance across a broad spectrum requires sophisticated equipment and is time-consuming, but doing so at a fixed frequency provides limited information. We propose and implement a hardware-efficient time-domain method to characterize the Randles cell electrode model. First, a biphasic, low-intensity current stimulus with inter-pulse delay is applied to an electrode. Then, by measuring the electrode overpotentials V0, V1, and V2, tissue resistance (RS), double layer capacitance (Cdl), and charge transfer resistance (RCT) are accordingly derived. Low-intensity stimulus ensures RCT does not complicate the Cdl computation. Inter-pulse delay provides a passive discharge period for RCT acquisition (Fig. 22.2.4 top). In the circuit implementation, both recording and impedance characterization circuits share the same ADC, whose input voltage is confined by voltage-clamp diodes. A Stim_flag bit is inserted into the serialized ADC output to denote the stimulation onset. The impedance characterization module then searches for V0, V1, and V2 based on the Stim_flag bit and the given stimulation parameters (Fig. 22.2.4 bottom panel).
Figure 22.2.4.
Method and block diagram of bio-impedance characterization. Randles cell model is derived in the time domain.
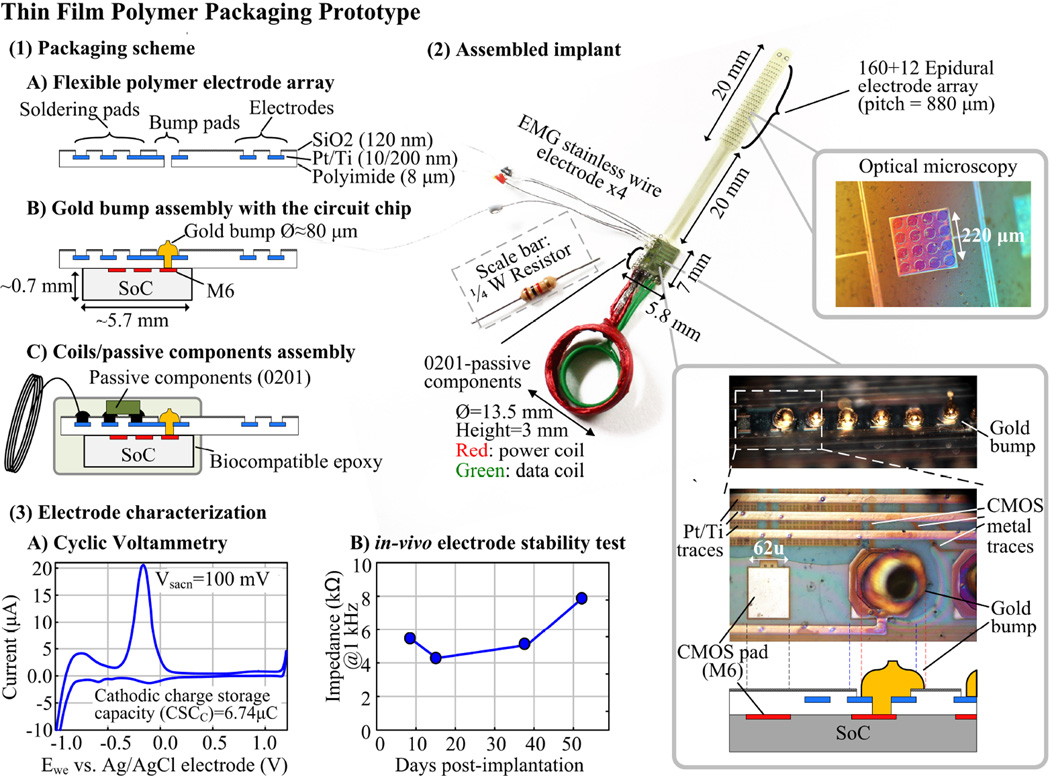
Figure 22.2.5 illustrates the prototype employing thin film polymer process with a special bump pad design. An 8µm thick polyimide substrate with an epidural electrode array serves as an interposer to connect coils, passive components, wire electrodes, and the CMOS pads via gold bumps. The prototype integrates 172 epidural electrodes, 4 EMG wire electrodes, 2 coils, 6 0201-SMD capacitors, and the SoC into a 0.7g, 0.5cm3 package. Cyclic voltammetry characterization shows the fabricated epidural electrode has a charge storage capacity of 6.74µC. The electrode in-vivo test results demonstrate < 1.5kΩ impedance standard deviations during the 52-day post-surgery period.
Figure 22.2.5.
Thin film polymer packaging prototype.
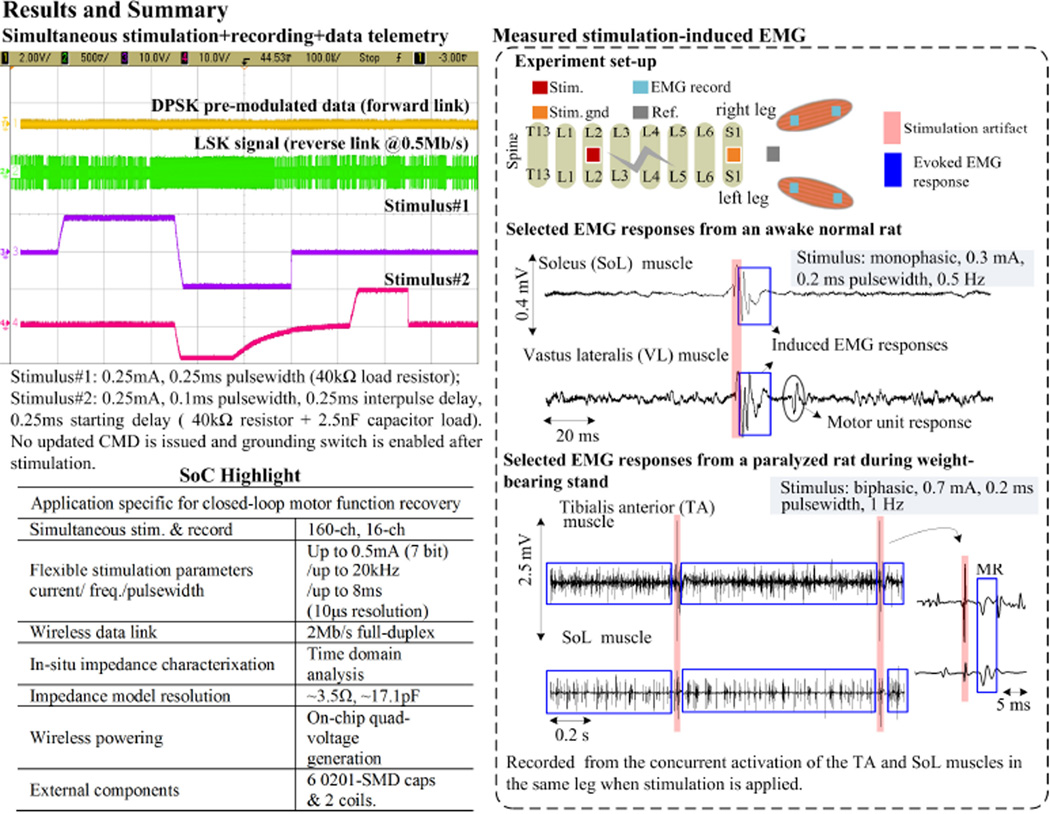
Figure 22.2.6 shows the SoC test results, its highlighted features, and the in-vivo EMG recording when stimulating the lumbosacral region of the spine in both normal and spinal cord transected rats. Stimulation-induced EMG middle responses and spontaneous onset of motor unit are observed in the leg muscles of the normal rat. Stimulating the paralyzed rat results in consistent EMG patterns required for standing. A stronger stimulation current is applied on the paralyzed rat as its brain-spinal network is injured.
Figure 22.2.6.
Experimental results and SoC performance summary.
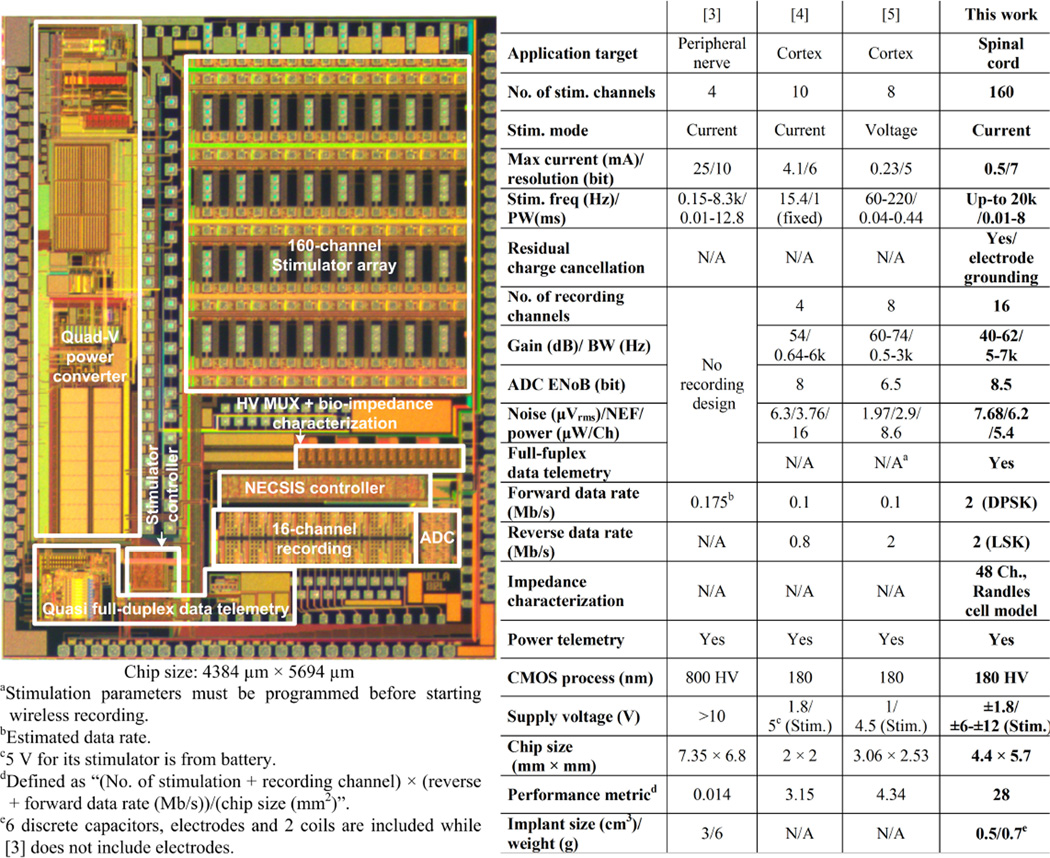
This SoC is implemented in HV 0.18µm CMOS with an area of 5.7.4.4mm2. The table of comparison with prior works is shown in Fig. 22.2.7. This SoC implant targets motor function recovery after spinal cord injury. Its versatile functionalities and highly compact form factor (0.5cm3 and 0.7g) also make it applicable in future implants for various medical applications.
Figure 22.2.7.
SoC micrograph and table of comparison.
Acknowledgments
This work is partially funded by the Endowment for the Chan Soon-Shiong Bionic Engineering Research Center at UCLA by California Capital Equity LLC, NIH BRP, NIH STTR, and UC Lab Fee Program. The authors would like to thank Dr. Hui Zhong for device implantation and Yuan Du for his technical assistance.
References
- 1.Minev IR, et al. Electronic Dura Mater for Long-term Multimodal Neural Interfaces. Science. 2015 Jan.347:159–163. doi: 10.1126/science.1260318. [DOI] [PubMed] [Google Scholar]
- 2.Wenger N, et al. Closed-loop Neuromodulation of Spinal Sensorimotor Circuits Controls Refined Locomotion after Complete Spinal Cord Injury. Science Translational Medicine. 2014 Sept.6:255ra133. doi: 10.1126/scitranslmed.3008325. [DOI] [PubMed] [Google Scholar]
- 3.Paralikar K, et al. An Implantable and Rechargeable Neuromodulation System for Animal Research; IEEE/EMBS Conf. Neural Eng; 2015. pp. 418–421. [Google Scholar]
- 4.Rhew HG, et al. A Fully Self-Contained Logarithmic Closed-Loop Deep Brain Stimulation SoC With Wireless Telemetry and Wireless Power Management. IEEE J. Solid-State Circuits. 2014 Oct.49:2213–2227. [Google Scholar]
- 5.Lin YP, et al. A Battery-Less, Implantable Neuro-Electronic Interface for Studying the Mechanisms of Deep Brain Stimulation in Rat Models. IEEE Trans. Biomed. Circuits Syst. 2015 doi: 10.1109/TBCAS.2015.2403282. [DOI] [PubMed] [Google Scholar]
- 6.Lo YK, et al. Bio-Impedance Characterization Technique with Implantable Neural Stimulator using Biphasic Current Stimulus. IEEE Int. Conf. Eng. Med. Biol. (EMBC) 2014:474–477. doi: 10.1109/EMBC.2014.6943631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo YK, et al. A Fully-integrated High-compliance Voltage SoC for Epi-retinal and Neural Prostheses. IEEE Trans. Biomed. Circuits Syst. 2013;7:761–772. doi: 10.1109/TBCAS.2013.2297695. [DOI] [PubMed] [Google Scholar]