Abstract
Purpose
In the general population, psychological symptoms frequently co-occur; however, profiles of symptom comorbidities have not been examined among adolescent survivors of childhood cancer.
Patients and Methods
Parents of 3,893 5-year survivors of childhood cancer who were treated between 1970 and 1999 and who were assessed in adolescence (age 12 to 17 years) completed the Behavior Problems Index. Age- and sex-standardized z scores were calculated for symptom domains by using the Childhood Cancer Survivor Study sibling cohort. Latent profile analysis identified profiles of comorbid symptoms, and multivariable multinomial logistic regression modeling examined associations between cancer treatment exposures and physical late effects and identified symptom profiles. Odds ratios (ORs) and 95% CIs for latent class membership were estimated and analyses were stratified by cranial radiation therapy (CRT; CRT or no CRT).
Results
Four symptoms profiles were identified: no significant symptoms (CRT, 63%; no CRT, 70%); elevated anxiety and/or depression, social withdrawal, and attention problems (internalizing; CRT, 31%; no CRT, 16%); elevated headstrong behavior and attention problems (externalizing; CRT, no observed; no CRT, 9%); and elevated internalizing and externalizing symptoms (global symptoms; CRT, 6%; no CRT, 5%). Treatment with ≥ 30 Gy CRT conferred greater risk of internalizing (OR, 1.7; 95% CI, 1.0 to 2.8) and global symptoms (OR, 3.2; 95% CI, 1.2 to 8.4). Among the no CRT group, corticosteroid treatment was associated with externalizing symptoms (OR, 1.9; 95% CI, 1.2 to 2.8) and ≥ 4.3 g/m2 intravenous methotrexate exposure was associated with global symptoms (OR, 1.5; 95% CI, 0.9 to 2.4). Treatment late effects, including obesity, cancer-related pain, and sensory impairments, were significantly associated with increased risk of comorbid symptoms.
Conclusion
Behavioral, emotional, and social symptoms frequently co-occur in adolescent survivors of childhood cancer and are associated with treatment exposures and physical late effects. Assessment and consideration of symptom profiles are essential for directing appropriate mental health treatment for adolescent survivors.
INTRODUCTION
Survivors of childhood cancer are at risk for disease and treatment-related physical late effects, which may increase vulnerability to emotional and behavioral problems, particularly during adolescence, a developmental period marked by transition and increasing expectations of independence. Past studies have reported risk of attention, learning, and social difficulties as well as anxiety and depression in adolescent survivors of childhood cancer.1-6 A recent systematic review indicated that 13% to 29% of adolescent survivors experience problems with psychological distress and emotional functioning.6 CNS-directed therapies and physical scarring or disfigurement have been associated with increased risk of these symptoms.2,5,7-9
To the best of our knowledge, no study has examined profiles or predictors of behavioral, social, and emotional symptom comorbidities in adolescent survivors of childhood cancer. In noncancer populations, mental health symptoms are highly comorbid, particularly during adolescence.10 Identifying symptom profiles, especially for survivors at risk for comorbidities, has implications for screening guidelines as well as treatment and future complications. For example, even though treatment with stimulant medication is recommended for adolescents with primary attention problems, if attention problems co-occur with anxiety, alternate treatment approaches should be considered.11 Furthermore, adolescents with combined attention problems and antisocial behaviors are at risk for substance abuse as adults,12 and they may benefit from programs to prevent substance abuse.
In this context, the aims of this study were to identify profiles of comorbid behavioral, social and emotional symptoms; examine childhood cancer treatment exposures associated with profiles of comorbidities; and examine treatment-related physical late effects associated with symptom profiles.
PATIENTS AND METHODS
Childhood Cancer Survivor Study
The Childhood Cancer Survivor Study (CCSS) is a multi-institutional study of > 5-year survivors of childhood cancer diagnosed before 21 years of age.13,14 Survivors were treated at one of 31 institutions between 1970 and 1999. Study participants completed a baseline questionnaire beginning in 1992 for survivors diagnosed between 1970 and 1986 and in 2008 for survivors diagnosed between 1987 and 1999. Information regarding primary cancer diagnosis and treatment was abstracted from medical records at each treating institution. Local institutional review boards approved study procedures, and parental informed consent was obtained for all participants younger than age 18 years. Because a larger proportion of the eligible population of childhood cancer survivors were acute leukemia survivors, for survivors diagnosed between 1987 and 1999, acute leukemia survivors were under-recruited to establish a more uniform distribution of all childhood cancer diagnoses for the entire cohort. Survivors in our sample were between 12 and 17 years of age at study baseline, and questionnaires were completed by a parent or guardian. Survivors with neurodevelopmental disorders that preceded cancer diagnosis were excluded (ie, Down syndrome, Klinefelter syndrome, Turner syndrome, fragile X syndrome, or spina bifida with neural tube defect).
Primary Outcome
The primary outcome was behavioral, social, and emotional symptoms as measured by the Behavior Problems Index (BPI).15 The BPI is a subset of 28 questions derived from the Child Behavior Checklist16 and provides scores for five symptom domains: depression/anxiety, headstrong behavior, attention deficit, peer conflict/social withdrawal, and antisocial behavior. Adequate construct validity and internal consistency (α = .80 to .87) of BPI subscales have been documented.5
Treatment Exposures and Covariates
Treatment exposures included corticosteroids (none, dexamethasone, nondexamethasone), intravenous (IV) methotrexate (none, < 4.3 g/m2, ≥ 4.3 g/m2), intrathecal (IT) methotrexate (none, < 230 mg/m2, ≥ 230 mg/m2), cytarabine (yes/no), anthracyclines (none, < 300 mg/m2, ≥ 300 mg/m2), or cranial radiation therapy (CRT; highest maximum dose to one of four brain regions [posterior fossa, temporal lobe, frontal lobe, parieto-occipital lobe]: none, < 30 Gy, ≥ 30 Gy).17,18 Physical late effects of childhood cancer included parent report of overweight or obesity defined as a body mass index > 25 kg/m2; growth hormone deficiency defined as a report of growth hormone deficiency or use of injections of growth hormone; scarring or disfigurement of the head, neck, or face; scarring or disfigurement of an extremity or amputation of an arm, leg, or foot; sensory impairment defined as hearing impairment, speech deficits, or vision problems; cancer-related pain defined as moderate or severe pain; and the presence of migraines or severe headaches. Demographic covariates included age at time of survey completion, sex, race/ethnicity (white, black, Hispanic, other), and household income (< $60,000 v ≥ $60,000 per year).
Data Analysis
Descriptive statistics were calculated for all exposures, covariates, and outcomes. For BPI symptom domains, if fewer than 50% of items contributing to a domain were missing, values were imputed by using the mean of items on the same symptom scale. The z scores were calculated for each domain by using the CCSS sibling cohort as a normative population with z scores standardized to the sex and age (12 to 14 years and 15 to 17 years) of siblings. For each domain, the expected mean is zero, and higher scores indicate greater symptom presence or worse functioning. Because of expected differences in symptom frequency by treatment with CRT, all analyses were stratified by CRT exposure (ie, CRT or no CRT). Weights were incorporated into all analyses to account for probability of sampling because of undersampling of the survivors of acute lymphoblastic leukemia. Latent profile analysis (LPA) was used to identify unobserved classes of symptoms. Specifically, LPA uses measured variables (ie, symptom scores derived from the BPI) to identify groups of survivors that differ from one another, but the number and nature of the groups is unknown. Several statistical indices were calculated to assess model fit, including Bayesian information criterion (BIC) and the sample size–adjusted BIC (ABIC), Vuong-Lo-Mendell-Rubin likelihood ratio (VLMR) test and sample size–adjusted VLMR P values, entropy, and minimum class membership size. Models were fit with two to five classes. The optimal model was chosen on the basis of both model fit statistics and substantive meaning. Treatment exposures19-26 associated with class membership were examined by using multivariable multinomial logistic regression. Odds ratios (ORs) and 95% CIs were calculated for each exposure and covariate in the final models. The same multivariable modeling approach was used to examine the associations between late effects9,27-31 and class membership. For all multivariable models, exposures and outcomes were selected a priori on the basis of review of the literature and established associations between childhood cancer therapies and adverse neurobehavioral outcomes. Because our analyses involved multiple comparisons, we used the false discovery rate approach to account for false positives at P < .05 by using a false discovery rate threshold of 10%.32
RESULTS
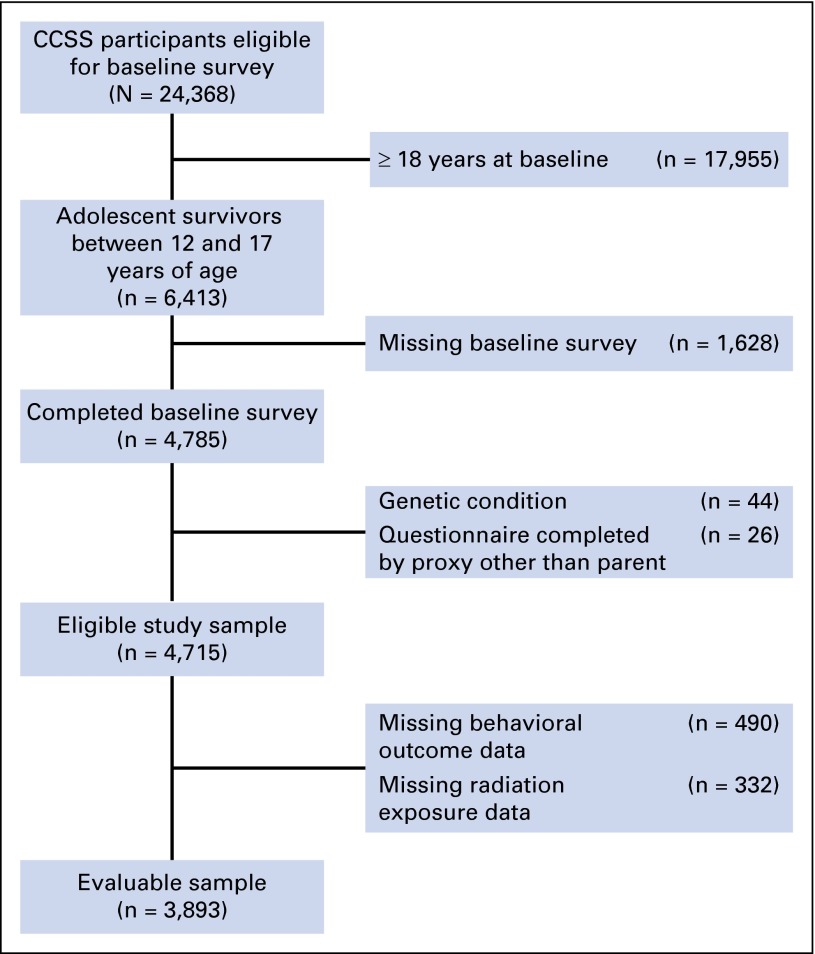
Among 24,369 participants in CCSS, 6,413 were adolescents at the time of the baseline survey (Fig 1), 4,715 were eligible for our analysis, and 3,893 (83%) contributed data to our analysis. Characteristics of survivors with parent-reported behavioral data are listed in Table 1.
Fig 1.
CONSORT diagram of adolescent survivor study participation. CCSS, Childhood Cancer Survivor Study.
Table 1.
Survivor Characteristics Stratified by CRT Exposure
| Characteristic | No CRT (n = 2,770) | CRT (n = 1,123) | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | % | Median | Range | No. | % | Median | Range | |
| Age, years | ||||||||
| Current | 15.0 | 12.0-17.0 | 15.0 | 12.0-17.0 | ||||
| At diagnosis | 2.6 | 0.0-9.9 | 3.1 | 0.1-9.4 | ||||
| Time since diagnosis, years | 12.4 | 7.9-17.9 | 12.0 | 7.9-17.5 | ||||
| Sex | ||||||||
| Male | 1,438 | 51.9 | 619 | 55.1 | ||||
| Female | 1,332 | 48.1 | 504 | 44.9 | ||||
| Race/ethnicity | ||||||||
| White | 2,286 | 84.9 | 932 | 84.3 | ||||
| Black | 149 | 5.5 | 58 | 5.2 | ||||
| Hispanic | 159 | 5.9 | 59 | 5.3 | ||||
| Other | 99 | 3.7 | 57 | 5.2 | ||||
| CRT, Gy | ||||||||
| None | 2,770 | 100.0 | 0 | 0.0 | ||||
| < 30 | 0 | 0.0 | 711 | 65.9 | ||||
| ≥ 30 | 0 | 0.0 | 368 | 34.1 | ||||
| IT methotrexate, mg/m2 | ||||||||
| None | 1,856 | 68.6 | 438 | 41.6 | ||||
| < 230 | 330 | 12.2 | 381 | 36.2 | ||||
| ≥ 230 | 521 | 19.2 | 234 | 22.2 | ||||
| IV methotrexate | ||||||||
| No | 2,209 | 80.8 | 827 | 75.7 | ||||
| Yes | 525 | 19.2 | 266 | 24.3 | ||||
| High-dose IV methotrexate (≥ 4.3 g/m2) | ||||||||
| No | 2,390 | 87.4 | 1,024 | 93.7 | ||||
| Yes | 344 | 12.6 | 69 | 6.3 | ||||
| Cytarabine | ||||||||
| No | 2,029 | 73.5 | 547 | 49.3 | ||||
| Yes | 732 | 26.5 | 563 | 50.7 | ||||
| Corticosteroids | ||||||||
| None | 1,685 | 61.0 | 393 | 35.4 | ||||
| Nondexamethasone | 738 | 26.7 | 498 | 44.9 | ||||
| Dexamethasone | 338 | 12.2 | 219 | 19.7 | ||||
| Anthracyclines, mg/m2 | ||||||||
| None | 1,529 | 56.6 | 563 | 51.9 | ||||
| < 300 | 904 | 33.5 | 359 | 33.1 | ||||
| ≥ 300 | 268 | 9.9 | 162 | 14.9 | ||||
| Diagnosis | ||||||||
| CNS tumor | 293 | 10.6 | 314 | 28.0 | ||||
| Leukemia | 890 | 32.1 | 658 | 58.6 | ||||
| Wilms tumor | 598 | 21.6 | 0 | 0.0 | ||||
| Neuroblastoma | 591 | 21.3 | 54 | 4.8 | ||||
| Other* | 398 | 14.4 | 97 | 8.6 | ||||
Abbreviations: CRT, cranial radiation therapy; IT, intrathecal; IV, intravenous.
Other diagnoses include Hodgkin lymphoma, non-Hodgkin lymphoma, soft tissue sarcomas, and bone tumors.
Symptom Profiles
Model fit indices for two to five class solutions derived from latent profile analysis are provided in Appendix Tables A1 and A2 (online only). The proportion of survivors composing each latent class did not differ significantly by treatment era (Appendix Tables A3 and A4, online only).
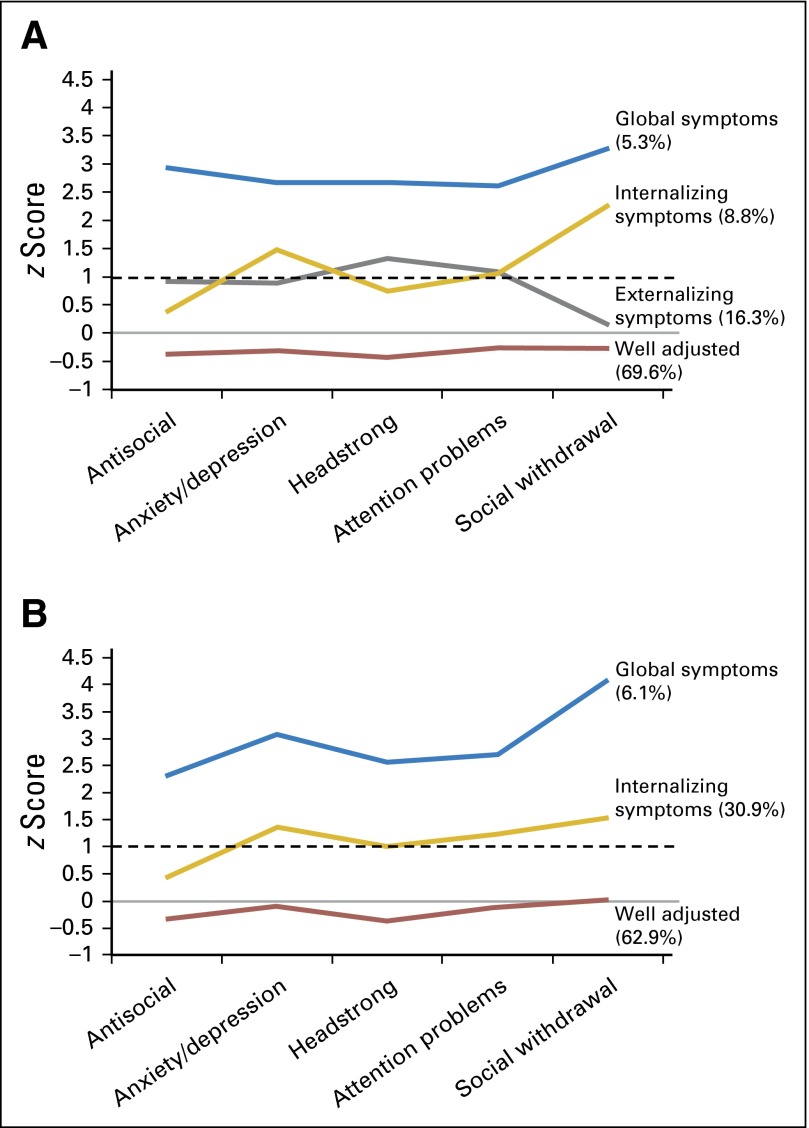
Four profiles of symptoms were identified for the no CRT group: (1) survivors without any increased symptoms (well adjusted; 69.6%); (2) survivors with greater symptoms of headstrong behavior and attention deficit (externalizing; 16.3%); (3) survivors with greater symptoms of anxiety or depression, social withdrawal or peer conflict, and attention deficit (internalizing; 8.8%); and (4) survivors with increased symptoms across all domains (externalizing and internalizing symptoms [global symptoms]; 5.3%; Fig 2A).
Fig 2.
Symptom profiles of behavioral, social, and emotional symptoms. Solid line represents expected mean z score of zero. Dashed line represents the upper limit of the average range (z score of 1). Symptom profiles of survivors treated (A) without CRT and (B) with CRT.
Among survivors treated with CRT, three profiles of symptoms were identified: (1) survivors without any increased symptom clusters (well adjusted; 62.9%); (2) survivors with increased symptoms of anxiety or depression, attention deficit, and social withdrawal or peer conflict (internalizing; 30.9%); and (3) survivors with increased symptoms across all domains (externalizing and internalizing symptoms [global symptoms]; 6.1%; Fig 2B). An externalizing class was not observed for survivors treated with CRT.
Treatment Exposures
In multivariable models among those who had no CRT, a higher dose of IV methotrexate compared with a lower dose and nondexamethasone corticosteroid treatment compared with no corticosteroid treatment were associated with lower odds of internalizing symptoms compared with being well adjusted (high-dose IV methotrexate: OR, 0.53; 95% CI, 0.35 to 0.82; nondexamethasone: OR, 0.51; 95% CI, 0.29 to 0.93; Table 2). Similarly, higher doses of IV methotrexate were associated with reduced odds of externalizing symptoms (OR, 0.5; 95% CI, 0.32 to 0.79). Nondexamethasone treatment compared with no corticosteroid treatment was associated with greater odds of externalizing symptoms (OR, 1.9; 95% CI, 1.2 to 2.8), whereas a higher dose of IV methotrexate compared with a lower dose was associated with greater odds of global symptoms than of being well adjusted (OR, 1.5; 95% CI, 0.9 to 2.4). Treatment with a lower dose of IT methotrexate was associated with reduced odds of externalizing symptoms (OR, 0.5; 95% CI, 0.28 to 0.78) and increased odds of internalizing symptoms (OR, 2.0; 95% CI, 1.0 to 3.9). In models that included terms for cancer diagnosis but not treatment exposures, survivors of neuroblastoma had greater odds of externalizing symptoms (OR, 1.4; 95% CI, 0.9 to 2.0), whereas survivors of CNS tumors had greater odds of internalizing symptoms (OR, 1.6; 95% CI, 1.0 to 2.7; Appendix Table A5, online only).
Table 2.
Treatment Exposures Associated With Latent Class Membership: No CRT
| Variable | Externalizing Versus Well Adjusted | Internalizing Versus Well Adjusted | Global Symptoms Versus Well Adjusted | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age, years | ||||||
| At diagnosis | 1.0 | 1.0 to 1.1 | 1.0 | 0.9 to 1.0 | 1.1 | 1.0 to 1.2 |
| At survey | ||||||
| 12-14 | 1.2 | 1.0 to 1.5 | 1.4 | 1.1 to 1.8 | 1.8 | 1.3 to 2.5 |
| 15-17 | 1.0 | 1.0 | 1.0 | |||
| Sex | ||||||
| Female | 1.0 | 1.0 | 1.0 | |||
| Male | 1.0 | 0.8 to 1.2 | 0.8 | 0.6 to 1.0 | 1.2 | 0.9 to 1.7 |
| Race | ||||||
| White | 1.0 | 1.0 | 1.0 | |||
| Black | 1.3 | 0.9 to 2.0 | 0.5 | 0.3 to 1.1 | 2.0 | 1.1 to 3.5 |
| Other | 1.4 | 1.0 to 2.0 | 1.1 | 0.7 to 1.7 | 1.1 | 0.6 to 2.0 |
| IT methotrexate, mg/m2 | ||||||
| None | 1.0 | 1.0 | 1.0 | |||
| < 230 | 0.5 | 0.3 to 0.8 | 2.0 | 1.0 to 3.9 | 1.6 | 0.7 to 3.9 |
| ≥ 230 | 0.5 | 0.3 to 0.8 | 1.1 | 0.6 to 2.0 | 0.7 | 0.3 to 1.7 |
| High-dose IV methotrexate, g | ||||||
| < 4.3 | 1.0 | 1.0 | 1.0 | |||
| ≥ 4.3 | 1.1 | 0.8 to 1.5 | 0.5 | 0.3 to 0.8 | 1.5 | 0.9 to 2.4 |
| Cytarabine | ||||||
| None | 1.0 | 1.0 | 1.0 | |||
| Yes | 1.1 | 0.8 to 1.7 | 1.6 | 0.9 to 2.7 | 1.0 | 0.5 to 1.8 |
| Corticosteroids | ||||||
| None | 1.0 | 1.0 | 1.0 | |||
| Nondexamethasone | 1.9 | 1.2 to 2.8 | 0.5 | 0.3 to 0.9 | 1.0 | 0.5 to 2.1 |
| Dexamethasone | 1.1 | 0.7 to 1.7 | 0.8 | 0.5 to 1.5 | 0.6 | 0.3 to 1.4 |
| Anthracyclines, mg/m2 | ||||||
| None | 1.0 | 1.0 | 1.0 | |||
| < 300 | 1.1 | 0.8 to 1.4 | 0.8 | 0.6 to 1.1 | 0.8 | 0.6 to 1.3 |
| ≥ 300 | 1.0 | 0.7 to 1.5 | 0.8 | 0.5 to 1.3 | 0.7 | 0.4 to 1.3 |
NOTE. In all, 2,584 participants were included in this analysis. Bold font indicates estimates with a false discovery rate ≤ 10%.
Abbreviations: CRT, cranial radiation therapy; IT, intrathecal; IV, intravenous; OR, odds ratio.
In multivariable models among those treated with CRT, treatment with ≥ 30 Gy CRT compared with treatment with < 30 Gy was associated with greater odds of global symptoms (OR, 3.2; 95% CI, 1.2 to 8.4) and internalizing symptoms (OR, 1.7; 95% CI, 1.0 to 2.8) compared with being well adjusted (Table 3). In addition, survivors treated with ≥ 300 mg/m2 anthracyclines compared with survivors who were not treated with anthracyclines were more likely to have internalizing symptoms (OR, 1.9; 95% CI, 1.2 to 3.0). In models that included terms for cancer diagnosis but not treatment exposures, diagnosis of a CNS tumor was associated with greater odds of global symptoms (OR, 2.6; 95% CI, 1.0 to 6.6) and internalizing symptoms (OR, 1.9; 95% CI, 1.2 to 3.0; Appendix Table A6, online only).
Table 3.
Treatment Exposures Associated With Latent Class Membership: CRT
| Variable | Internalizing Versus Well Adjusted | Global Symptoms Versus Well Adjusted | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age, years | ||||
| At diagnosis | 0.9 | 0.9 to 1.0 | 1.0 | 0.9 to 1.2 |
| At survey | ||||
| 12-14 | 0.9 | 0.7 to 1.2 | 1.8 | 1.0 to 3.2 |
| 15-17 | 1.0 | 1.0 | ||
| Sex | ||||
| Female | 1.0 | 1.0 | ||
| Male | 0.9 | 0.6 to 1.1 | 1.1 | 0.6 to 1.8 |
| Race/ethnicity | ||||
| White | 1.0 | 1.0 | ||
| Black | 1.2 | 0.6 to 2.2 | 2.3 | 0.9 to 5.8 |
| Other | 1.3 | 0.8 to 2.0 | 1.2 | 0.5 to 3.0 |
| CRT, Gy | ||||
| < 30 | 1.0 | 1.0 | ||
| ≥ 30 | 1.7 | 1.0 to 2.8 | 3.2 | 1.2 to 8.4 |
| IT methotrexate, mg/m2 | ||||
| None | 1.0 | 1.0 | ||
| < 230 | 0.9 | 0.5 to 1.6 | 2.2 | 0.7 to 6.9 |
| ≥ 230 | 1.3 | 0.7 to 2.3 | 3.2 | 0.9 to 11.9 |
| IV methotrexate | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.2 | 0.8 to 1.8 | 1.7 | 0.8 to 3.8 |
| Cytarabine | ||||
| No | 1.0 | 1.0 | ||
| Yes | 0.8 | 0.5 to 1.3 | 0.4 | 0.2 to 1.2 |
| Corticosteroids | ||||
| None | 1.0 | 1.0 | ||
| Nondexamethasone | 1.2 | 0.7 to 2.0 | 1.0 | 0.4 to 2.8 |
| Dexamethasone | 1.0 | 0.6 to 1.7 | 1.3 | 0.5 to 3.3 |
| Anthracyclines, mg/m2 | ||||
| None | 1.0 | 1.0 | ||
| < 300 | 1.0 | 0.7 to 1.6 | 0.9 | 0.4 to 2.3 |
| ≥ 300 | 1.9 | 1.2 to 3.0 | 1.8 | 0.7 to 4.3 |
NOTE. In all, 979 participants were included in this analysis.
Abbreviations: CRT, cranial radiation therapy; IT, intrathecal; IV, intravenous; OR, odds ratio.
Physical Late Effects
Survivors treated without CRT who were overweight or obese compared with survivors of normal weight had greater odds of internalizing (OR, 2.0; 95% CI, 1.5 to 2.9) and global symptoms (OR, 1.8; 95% CI, 1.1 to 2.8) compared with being well adjusted. Survivors who had a sensory impairment compared with those who did not were 2.5 times more likely to have internalizing symptoms than to be well adjusted (OR, 2.5; 95% CI, 1.7 to 3.6). Scarring or disfigurement to the chest or abdomen compared with no scarring or disfigurement in this region was associated with increased odds of externalizing symptoms (OR, 1.3; 95% CI, 1.0 to 1.6). Both cancer-related pain and migraines or severe headaches compared with no pain were associated with increased internalizing and externalizing symptoms (Table 4).
Table 4.
Late Effects Associated With Latent Class Membership: No CRT
| Variable | Externalizing Versus Well Adjusted | Internalizing Versus Well Adjusted | Global Symptoms Versus Well Adjusted | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age at survey, years | ||||||
| 12-14 | 1.1 | 0.9 to 1.4 | 1.7 | 1.3 to 2.3 | 1.7 | 1.1 to 2.5 |
| 15-17 | 1.0 | 1.0 | 1.0 | |||
| Sex | ||||||
| Female | 1.0 | 1.0 | 1.0 | |||
| Male | 1.0 | 0.8 to 1.3 | 0.8 | 0.6 to 1.0 | 1.3 | 0.9 to 1.9 |
| Race/ethnicity | ||||||
| White | 1.0 | 1.0 | 1.0 | |||
| Black | 1.3 | 0.8 to 2.0 | 0.6 | 0.3 to 1.4 | 1.7 | 0.8 to 3.2 |
| Other | 1.5 | 1.1 to 2.2 | 1.6 | 1.0 to 2.5 | 0.7 | 0.3 to 1.5 |
| Household income, $ | ||||||
| < 60,000 | 1.0 | 1.0 | 1.0 | |||
| ≥ 60,000 | 1.9 | 1.5 to 2.4 | 1.2 | 0.9 to 1.6 | 1.9 | 1.3 to 2.8 |
| Body mass index | ||||||
| Normal | 1.0 | 1.0 | 1.0 | |||
| Underweight | 1.2 | 0.9 to 1.6 | 1.3 | 0.9 to 1.9 | 0.9 | 0.5 to 1.5 |
| Overweight/obese | 1.1 | 0.8 to 1.5 | 2.0 | 1.5 to 2.9 | 1.8 | 1.1 to 2.8 |
| Scarring or disfigurement of head, neck, or face | ||||||
| No | 1.0 | 1.0 | 1.0 | |||
| Yes | 1.1 | 0.8 to 1.5 | 1.2 | 0.8 to 1.7 | 1.1 | 0.6 to 1.7 |
| Scarring or disfigurement of extremity | ||||||
| No | 1.0 | 1.0 | 1.0 | |||
| Yes | 0.9 | 0.6 to 1.3 | 1.0 | 0.6 to 1.5 | 1.2 | 0.7 to 2.0 |
| Scarring or disfigurement of chest or abdomen | ||||||
| No | 1.0 | 1.0 | 1.0 | |||
| Yes | 1.3 | 1.0 to 1.6 | 1.2 | 0.9 to 1.6 | 1.1 | 0.7 to 1.6 |
| Sensory impairment | ||||||
| No | 1.0 | 1.0 | 1.0 | |||
| Yes | 1.3 | 0.9 to 1.8 | 2.5 | 1.7 to 3.6 | 1.2 | 0.7 to 2.0 |
| Cancer-related pain | ||||||
| No | 1.0 | 1.0 | 1.0 | |||
| Yes | 1.5 | 1.1 to 2.1 | 1.4 | 0.9 to 2.1 | 2.7 | 1.7 to 4.3 |
| Migraines or severe headaches | ||||||
| No | 1.0 | 1.0 | 1.0 | |||
| Yes | 1.6 | 1.3 to 2.1 | 1.4 | 1.0 to 2.0 | 1.9 | 1.2 to 2.9 |
NOTE. In all, 2,135 participants were included in this analysis. Bold font indicates estimates with a false discovery rate ≤ 10%.
Abbreviations: CRT, cranial radiation therapy; OR, odds ratio.
Similar to survivors in the no CRT group, survivors treated with CRT who were overweight or obese compared with those of normal weight had greater odds of internalizing (OR, 1.7; 95% CI, 1.2 to 2.3) and global symptoms (OR, 2.0; 95% CI, 0.9 to 4.1; Table 5). Survivors with a sensory impairment (OR, 2.2; 95% CI, 1.0 to 4.6) or pain compared with survivors without such conditions were twice as likely to have global symptoms (cancer-related pain: OR, 2.7; 95% CI, 1.3 to 5.8; migraines or severe headaches: OR, 2.3; 95% CI, 1.2 to 4.6). Cancer-related pain compared with no pain was also associated with increased odds of internalizing symptoms compared with being well adjusted (OR, 1.9; 95% CI, 1.2 to 2.9).
Table 5.
Late Effects Associated With Latent Class Membership: CRT
| Variable | Internalizing Versus Well Adjusted | Global Symptoms Versus Well Adjusted | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age at survey, years | ||||
| 12-14 | 0.9 | 0.7 to 1.3 | 1.6 | 0.8 to 3.0 |
| 15-17 | 1.0 | 1.0 | ||
| Sex | ||||
| Female | 1.0 | 1.0 | ||
| Male | 0.9 | 0.7 to 1.2 | 0.9 | 0.5 to 1.7 |
| Race/ethnicity | ||||
| White | 1.0 | 1.0 | ||
| Black | 1.2 | 0.6 to 2.4 | 0.8 | 0.2 to 3.8 |
| Other | 1.2 | 0.7 to 2.0 | 1.1 | 0.4 to 3.3 |
| Household income, $ | ||||
| < 60,000 | 1.0 | 1.0 | ||
| ≥ 60,000 | 1.4 | 1.0 to 1.9 | 1.2 | 0.6 to 2.3 |
| Body mass index | ||||
| Normal | 1.0 | 1.0 | ||
| Underweight | 1.1 | 0.7 to 1.7 | 1.8 | 0.8 to 4.4 |
| Overweight/obese | 1.7 | 1.2 to 2.3 | 2.0 | 0.9 to 4.1 |
| Growth hormone deficiency | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.1 | 0.8 to 1.5 | 0.5 | 0.2 to 1.0 |
| Scarring or disfigurement of head, neck, or face | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.2 | 0.8 to 1.6 | 1.0 | 0.5 to 2.0 |
| Scarring or disfigurement of extremity | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.4 | 0.9 to 2.2 | 1.2 | 0.5 to 2.9 |
| Scarring or disfigurement of chest or abdomen | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.3 | 0.9 to 1.8 | 1.1 | 0.5 to 2.2 |
| Sensory impairment | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.1 | 0.8 to 1.6 | 2.2 | 1.0 to 4.6 |
| Cancer-related pain | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.9 | 1.2 to 2.9 | 2.7 | 1.3 to 5.8 |
| Migraines or severe headaches | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.3 | 0.9 to 1.8 | 2.3 | 1.2 to 4.6 |
NOTE. In all, 834 participants were included in this analysis. Bold font indicates estimates with a false discovery rate ≤ 10%.
DISCUSSION
Adolescence is a critical period for emotional, social, and behavioral development and is associated with an increase in psychological symptoms in the general population33; however, few studies have examined profiles of mental health symptoms among adolescent survivors of childhood cancer.6 In our sample of adolescent survivors, we identified distinct profiles and a high prevalence of comorbid psychological symptoms. These findings have implications for routine screening of mental health morbidities as well as management of treatment of symptoms in adolescent survivors of childhood cancer.
Consistent with past reports, our results suggest that the majority of survivors do not have increased psychological symptoms.5 This suggests largely positive emotional development in adolescent survivors of childhood cancer, at least from the perspective of their parents. Among the CRT group, 31% of survivors had a profile characterized by increased symptoms of anxiety or depression, attention problems, and social withdrawal or peer conflict (ie, internalizing symptoms); 16% of survivors who were not treated with CRT had these symptoms. However, among those in the no CRT group, approximately 9% had increased comorbid symptoms of headstrong behavior and attention problems (ie, externalizing symptoms), a profile that was not observed among the CRT group. The absence of an externalizing profile among survivors treated with CRT is consistent with past reports. Although executive dysfunction is commonly observed in this population, many of the behavioral symptoms include inattention, slowed cognitive processing, and lack of initiation rather than hyperactivity or impulsivity.34-38
Importantly, attention problems, which co-occurred with both internalizing and externalizing symptoms, are relatively nonspecific (eg, present in a number of psychological disorders) and may reflect underlying emotional problems such as anxiety or depression (eg, difficulty concentrating) or behavioral dysregulation (eg, restlessness).39 Clinically, treatment approaches vary on the basis of the presence of internalizing or externalizing symptoms. Efficacious treatment of internalizing symptoms include psychotherapy (eg, cognitive behavior therapy, behavioral activation) and/or pharmacotherapy with antidepressants.40-42 Conversely, psychotherapies for externalizing problems in adolescence often include parent training, family therapy, and problem-solving training.43 Although pharmacologic approaches for the management of oppositional behaviors are typically less efficacious, psychostimulants are effective for treating symptoms of inattention and hyperactivity.44
We did not observe a symptom profile that was restricted to a single symptom. This is consistent with data from the general population that comorbidity is the rule rather than the exception regarding adolescent psychopathology.45 Although comorbid symptoms were observed in > 30% of survivors, only a small proportion of survivors had increased internalizing and externalizing symptoms (ie, global symptoms). This is also consistent with the general population in which comorbidity tends to occur within rather than across internalizing and externalizing symptom domains. Unfortunately, children and adolescents with comorbid symptoms are less likely to achieve an acute treatment response or symptom remission or to maintain treatment gains.45,46 In addition, adolescents with comorbid symptoms often require more intensive multimodal approaches to treatment (eg, combined psychotherapy and pharmacotherapy).47
Among survivors treated with CRT, treatment with ≥ 30 Gy CRT was associated with increased odds of internalizing and global symptoms. Models restricted to diagnosis indicated that risk for internalizing and global symptoms among those treated with CRT was largely driven by CNS tumor diagnosis. Among the no CRT group, survivors treated with nondexamethasone corticosteroids were twice as likely to have externalizing symptoms and 50% less likely to have internalizing symptoms as those who did not receive corticosteroids. Similar risk was not observed among survivors treated with dexamethasone. However, because cumulative dose is not captured for these oral medications, direct comparisons are difficult to interpret. Higher dose of dexamethasone has been associated with greater behavioral problems on therapy,48 although recent studies suggest similar effects with prednisone.49 Treatment with IT methotrexate was associated with reduced likelihood of externalizing problems, and treatment with high-dose IV methotrexate was associated with reduced risk of internalizing symptoms. It is unlikely that methotrexate is protective; rather, we speculate confounding on the basis of primary cancer diagnosis such that survivors who did not receive methotrexate had increased likelihood of such symptoms. Specifically, survivors of neuroblastoma, for whom methotrexate is not a front-line therapy, were 40% more likely to have externalizing symptoms, and survivors of CNS tumors who did not receive methotrexate were 1.6 times more likely to have internalizing symptoms.
Late effects of childhood cancer therapies were associated with observed symptom profiles and often conferred a greater risk than therapeutic exposures. Survivors who were overweight or obese were nearly twice as likely to have comorbid internalizing and global symptoms. This is consistent with data from noncancer populations, indicating that attention deficits, conduct disorder, and depressive symptoms are more common in obese than nonobese children.30 The potential psychosocial consequences of obesity are particularly concerning in our population, given the high prevalence of obesity among certain childhood cancer survivors.31 Survivors with pain were more likely to have comorbid internalizing and externalizing symptoms. The contribution of pain to cognitive and behavioral dysregulation is well established as are the adverse side effects of medications used to manage pain symptoms. Because survivors are more likely to report pain and use of prescription analgesics compared with siblings,29,50 interventions targeting pain symptoms may be beneficial. Finally, sensory impairment was associated with increased odds of internalizing and global symptoms. Notably, children and adolescents with hearing loss and speech deficits are at risk for social isolation.28 Survivors treated with platinum-based therapies and/or CRT are at risk for serious hearing loss, which has been associated with educational and social attainment difficulties in long-term survivors.27 Our results suggest that such late effects may have serious behavioral and emotional consequences for survivors as well.
Our results should be considered in the context of several limitations. Behavioral outcomes were based on parent report. Past studies indicate that adolescent self-report of symptoms is often discrepant from parent report, particularly for internalizing symptoms.51 We found evidence of developmental differences in symptoms between survivors in early versus later adolescence. Specifically, younger adolescents were more likely to have increased internalizing and global symptoms compared with older adolescents. It is unclear whether the observed difference reflects heightened risk of symptoms in younger adolescents or parental sensitivity to typical developmental changes as adolescents first begin to assert their independence in early adolescence. Our cross-sectional design precludes assessment of temporal associations between exposures and outcomes; however, treatment exposures temporally preceded the report of symptom profiles. We do not have information regarding length of treatment, which may be associated with the development of behavioral symptoms. Use of a sibling comparison group as a normative sample is a potential limitation because symptoms of siblings may not be normal as a result of exposure to a family member with childhood cancer. Finally, although the CCSS expansion cohort includes survivors diagnosed through 1999, our results may not be generalizable to survivors treated more recently.
Although most adolescent survivors of childhood cancer do not have clinically significant behavioral, social, and emotional symptoms, we identified subgroups at risk of comorbid symptoms. Recent attention has been paid to screening for psychological distress symptoms among childhood cancer survivors,52 and the Children’s Oncology Group Long-Term Follow-Up Guidelines recommend yearly evaluations for depression, anxiety, post-traumatic stress, and suicide ideation (ie, internalizing symptoms).53 However, screening for these symptoms alone may be insufficient, because many survivors have comorbid externalizing symptoms and social difficulties. Robust screening efforts during adolescence could help identify survivors for whom interventions may remediate psychological symptoms and potentially offset the persistence or worsening of symptoms into adulthood. Our results suggest that survivors of CNS disease and/or survivors treated with CNS-directed therapies as well as those who develop physical late effects, including obesity, pain, and sensory impairments, may benefit from targeted screening during this developmental period. Screening efforts should incorporate parent and survivor report of symptoms. Future research should examine the etiology and developmental course of comorbid symptoms during therapy, stability of profiles over time, and functional outcomes associated with symptom profiles.
Appendix
Table A1.
Model Fit Indices for 2 Through 5 Class Solutions for BPI Scores: No CRT
| Model | BIC | ABIC | VLMR P | Adjusted VLMR P* | Entropy | Minimum Class (%) |
|---|---|---|---|---|---|---|
| Class | ||||||
| 2 | 36,834 | 36,783 | .0 | .0 | 0.94 | 18.7 |
| 3 | 34,975 | 34,905 | .03 | .03 | 0.92 | 6.3 |
| 4 | 34,228 | 34,139 | .54 | .55 | 0.92 | 5.3 |
| 5 | 33,460 | 33,352 | .39 | .40 | 0.93 | 2.5 |
Abbreviations: ABIC, sample size–adjusted BIC; BIC, Bayesian information criterion; BPI, Behavior Problems Index; CRT, cranial radiation therapy; VLMR, Vuong-Lo-Mendell likelihood ratio test.
For sample size–adjusted VLMR.
Table A2.
Model Fit Indices for 2 Through 5 Class Solutions for BPI Scores: CRT
| Model | BIC | ABIC | VLMR P | Adjusted VLMR P* | Entropy | Minimum Class (%) |
|---|---|---|---|---|---|---|
| Class | ||||||
| 2 | 15,653 | 15,602 | .04 | .039 | 0.91 | 20.6 |
| 3 | 14,905 | 14,836 | .0 | .0 | 0.90 | 6.1 |
| 4 | 14,720 | 14,632 | .24 | .24 | 0.85 | 4.2 |
| 5 | 1,488 | 14,380 | .35 | .36 | 0.88 | 3.5 |
Abbreviations: ABIC, sample size–adjusted BIC; BIC, Bayesian information criterion; BPI, Behavior Problems Index; CRT, cranial radiation therapy; VLMR, Vuong-Lo-Mendell likelihood ratio test.
For sample size–adjusted VLMR.
Table A3.
Proportion of Survivors in Each Latent Class by Treatment Era: No CRT
| Latent Class | 1970-1979 | 1980-1989 | 1990-1999 | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Well adjusted | 81 | 73.6 | 1,077 | 66.9 | 747 | 71.2 |
| Externalizing symptoms | 18 | 16.4 | 299 | 18.6 | 152 | 14.5 |
| Internalizing symptoms | 7 | 6.4 | 142 | 8.8 | 102 | 9.7 |
| Global symptoms | 4 | 3.6 | 93 | 5.8 | 48 | 4.6 |
Abbreviation: CRT, cranial radiation therapy.
Table A4.
Proportion of Survivors in Each Latent Class by Treatment Era: CRT
| Latent Class | 1970-1979 | 1980-1989 | 1990-1999 | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Well adjusted | 21 | 47.7 | 539 | 63.3 | 153 | 67.4 |
| Internalizing symptoms | 20 | 45.5 | 260 | 30.5 | 59 | 26.0 |
| Global symptoms | 3 | 6.8 | 53 | 6.2 | 15 | 6.6 |
Abbreviation: CRT, cranial radiation therapy.
Table A5.
Latent Class Membership by Primary Childhood Cancer Diagnosis: No CRT
| Variable | Externalizing Versus Well Adjusted | Internalizing Versus Well Adjusted | Global Symptoms Versus Well Adjusted | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age, years | ||||||
| At diagnosis (per 1 year) | 1.1 | 1.0 to 1.1 | 0.93 | 0.86 to 1.0 | 1.1 | 1.0 to 1.2 |
| At survey | ||||||
| 12-14 | 1.2 | 1.0 to 1.5 | 1.3 | 1.0 to 1.7 | 1.7 | 1.2 to 2.4 |
| 15-17 | 1.0 | 1.0 | 1.0 | |||
| Sex | ||||||
| Male | 1.0 | 0.8 to 1.2 | 0.7 | 0.6 to 0.9 | 1.2 | 0.8 to 1.6 |
| Female | 1.0 | 1.0 | 1.0 | |||
| Race/ethnicity | ||||||
| White | 1.0 | 1.0 | 1.0 | |||
| Black | 1.4 | 0.9 to 2.1 | 0.6 | 0.3 to 1.2 | 2.0 | 1.2 to 3.6 |
| Other | 1.5 | 1.1 to 2.0 | 1.3 | 0.9 to 1.9 | 1.1 | 0.6 to 1.9 |
| Diagnosis | ||||||
| Wilms tumor | 1.0 | 0.7 to 1.4 | 0.8 | 0.5 to 1.3 | 0.8 | 0.4 to 1.5 |
| Neuroblastoma | 1.4 | 0.9 to 2.0 | 0.8 | 0.5 to 1.4 | 1.3 | 0.7 to 2.4 |
| Leukemia | 0.9 | 0.7 to 1.3 | 1.0 | 0.6 to 1.5 | 0.9 | 0.5 to 1.5 |
| CNS tumor | 0.9 | 0.6 to 1.5 | 1.6 | 1.0 to 2.7 | 1.5 | 0.8 to 2.8 |
| Other | 1.0 | 1.0 | 1.0 | |||
Abbreviations: CRT, cranial radiation therapy; OR, odds ratio.
Table A6.
Latent Class Membership by Primary Childhood Cancer Diagnosis: CRT
| Variable | Internalizing Versus Well Adjusted | Global Symptoms Versus Well Adjusted | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age, years | ||||
| At diagnosis (per 1 year) | 1.0 | 0.9 to 1.0 | 1.0 | 0.9 to 1.2 |
| At survey | ||||
| 12-14 | 1.0 | 0.7 to 1.3 | 1.8 | 1.0 to 3.0 |
| 15-17 | 1.0 | 1.0 | ||
| Sex | ||||
| Male | 0.9 | 0.7 to 1.1 | 0.9 | 0.5 to 1.5 |
| Female | 1.0 | 1.0 | ||
| Race | ||||
| White | 1.0 | 1.0 | ||
| Black | 1.3 | 0.7 to 2.3 | 1.1 | 0.7 to 1.7 |
| Other | 1.1 | 0.7 to 1.7 | 1.5 | 0.7 to 3.3 |
| Diagnosis | ||||
| Leukemia | 1.4 | 0.9 to 2.1 | 1.6 | 0.7 to 3.9 |
| CNS | 1.9 | 1.2 to 3.0 | 2.5 | 1.0 to 6.6 |
| Other | 1.0 | 1.0 | ||
Abbreviations: CRT, cranial radiation therapy; OR, odds ratio.
Footnotes
Supported by Grant No. CA55727 from the National Cancer Institute, by Cancer Center Support CORE Grant No. CA21765, and by the American Lebanese Syrian Associated Charities.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Tara M. Brinkman, Chenghong Li, Kathryn Vannatta, Deokumar Srivastava, Leslie L. Robison, Gregory T. Armstrong Kevin R. Krull
Financial support: Leslie L. Robison, Gregory T. Armstrong
Provision of study materials or patients: Leslie L. Robison, Gregory T. Armstrong
Collection and assembly of data: Leslie L. Robison, Gregory T. Armstrong
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Behavioral, Social, and Emotional Symptom Comorbidities and Profiles in Adolescent Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Tara M. Brinkman
No relationship to disclose
Chenghong Li
No relationship to disclose
Kathryn Vannatta
No relationship to disclose
Jordan G. Marchak
No relationship to disclose
Jin-Shei Lai
No relationship to disclose
Pinki K. Prasad
No relationship to disclose
Cara Kimberg
No relationship to disclose
Stefanie Vuotto
No relationship to disclose
Chongzhi Di
No relationship to disclose
Deokumar Srivastava
No relationship to disclose
Leslie L. Robison
No relationship to disclose
Gregory T. Armstrong
No relationship to disclose
Kevin R. Krull
No relationship to disclose
REFERENCES
- 1.Moore IM, Challinor J, Pasvogel A, et al. Online exclusive: Behavioral adjustment of children and adolescents with cancer—Teacher, parent, and self-report. Oncol Nurs Forum. 2003;30:E84–E91. doi: 10.1188/03.ONF.E84-E91. [DOI] [PubMed] [Google Scholar]
- 2.Brinkman TM, Palmer SL, Chen S, et al. Parent-reported social outcomes after treatment for pediatric embryonal tumors: A prospective longitudinal study. J Clin Oncol. 2012;30:4134–4140. doi: 10.1200/JCO.2011.40.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wengenroth L, Rueegg CS, Michel G, et al. Concentration, working speed and memory: Cognitive problems in young childhood cancer survivors and their siblings. Pediatr Blood Cancer. 2015;62:875–882. doi: 10.1002/pbc.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gianinazzi ME, Rueegg CS, Wengenroth L, et al. Adolescent survivors of childhood cancer: Are they vulnerable for psychological distress? Psychooncology. 2013;22:2051–2058. doi: 10.1002/pon.3249. [DOI] [PubMed] [Google Scholar]
- 5.Schultz KA, Ness KK, Whitton J, et al. Behavioral and social outcomes in adolescent survivors of childhood cancer: A report from the childhood cancer survivor study. J Clin Oncol. 2007;25:3649–3656. doi: 10.1200/JCO.2006.09.2486. [DOI] [PubMed] [Google Scholar]
- 6. Mertens AC, Gilleland Marchak J: Mental health status of adolescent cancer survivors. Clinical Oncology in Adolescents and Young Adults 2015:87-95, 2015.
- 7.Mabbott DJ, Spiegler BJ, Greenberg ML, et al. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23:2256–2263. doi: 10.1200/JCO.2005.01.158. [DOI] [PubMed] [Google Scholar]
- 8.Vannatta K, Gartstein MA, Short A, et al. A controlled study of peer relationships of children surviving brain tumors: Teacher, peer, and self ratings. J Pediatr Psychol. 1998;23:279–287. doi: 10.1093/jpepsy/23.5.279. [DOI] [PubMed] [Google Scholar]
- 9.Kinahan KE, Sharp LK, Seidel K, et al. Scarring, disfigurement, and quality of life in long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor study. J Clin Oncol. 2012;30:2466–2474. doi: 10.1200/JCO.2011.39.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krull KR: Attention deficit hyperactivity disorder in children and adolescents: Overview of treatment and prognosis. UpToDate. http://www.uptodate.com/contents/attention-deficit-hyperactivity-disorder-in-children-and-adolescents-overview-of-treatment-and-prognosis?source=search_result&search=Attention+deficit+hyperactivity+disorder+in+children+and+adolescents%3A+Overview+of+treatment+and+prognosis&selectedTitle=1∼150. [Google Scholar]
- 12.Lee SS, Humphreys KL, Flory K, et al. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clin Psychol Rev. 2011;31:328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 14.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson JL, Zill N. Marital disruption, parent-child relationships, and behavior problems in children. J Marriage Fam. 1986;48:295–307. [Google Scholar]
- 16.Achenbach TM, Edelbrock CS. Behavioral problems and competencies reported by parents of normal and disturbed children aged four through sixteen. Monogr Soc Res Child Dev. 1981;46:1–82. [PubMed] [Google Scholar]
- 17.Armstrong GT, Jain N, Liu W, et al. Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neuro Oncol. 2010;12:1173–1186. doi: 10.1093/neuonc/noq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: Use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 19.Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23:5511–5519. doi: 10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- 20.Waber DP, McCabe M, Sebree M, et al. Neuropsychological outcomes of a randomized trial of prednisone versus dexamethasone in acute lymphoblastic leukemia: Findings from Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. Pediatr Blood Cancer. 2013;60:1785–1791. doi: 10.1002/pbc.24666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waber DP, Turek J, Catania L, et al. Neuropsychological outcomes from a randomized trial of triple intrathecal chemotherapy compared with 18 Gy cranial radiation as CNS treatment in acute lymphoblastic leukemia: Findings from Dana-Farber Cancer Institute ALL Consortium Protocol 95-01. J Clin Oncol. 2007;25:4914–4921. doi: 10.1200/JCO.2007.10.8464. [DOI] [PubMed] [Google Scholar]
- 22.Bhojwani D, Sabin ND, Pei D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32:949–959. doi: 10.1200/JCO.2013.53.0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krull KR, Sabin ND, Reddick WE, et al. Neurocognitive function and CNS integrity in adult survivors of childhood Hodgkin lymphoma. J Clin Oncol. 2012;30:3618–3624. doi: 10.1200/JCO.2012.42.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kesler SR, Blayney DW. Neurotoxic effects of anthracycline- vs nonanthracycline-based chemotherapy on cognition in breast cancer survivors. JAMA Oncol. 2016;2:185–192. doi: 10.1001/jamaoncol.2015.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krull KR, Brinkman TM, Li C, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: A report from the St Jude lifetime cohort study. J Clin Oncol. 2013;31:4407–4415. doi: 10.1200/JCO.2012.48.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinkman TM, Krasin MJ, Liu W, et al. Long-term neurocognitive functioning and social attainment in adult survivors of pediatric CNS tumors: Results from the St Jude Lifetime Cohort Study. J Clin Oncol. 2016;34:1358–1367. doi: 10.1200/JCO.2015.62.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brinkman TM, Bass JK, Li Z, et al. Treatment-induced hearing loss and adult social outcomes in survivors of childhood CNS and non-CNS solid tumors: Results from the St. Jude Lifetime Cohort Study. Cancer. 2015;121:4053–4061. doi: 10.1002/cncr.29604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Most T, Ingber S, Heled-Ariam E. Social competence, sense of loneliness, and speech intelligibility of young children with hearing loss in individual inclusion and group inclusion. J Deaf Stud Deaf Educ. 2012;17:259–272. doi: 10.1093/deafed/enr049. [DOI] [PubMed] [Google Scholar]
- 29.Lu Q, Krull KR, Leisenring W, et al. Pain in long-term adult survivors of childhood cancers and their siblings: A report from the Childhood Cancer Survivor Study. Pain. 2011;152:2616–2624. doi: 10.1016/j.pain.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turer CB, Lin H, Flores G. Health status, emotional/behavioral problems, health care use, and expenditures in overweight/obese US children/adolescents. Acad Pediatr. 2013;13:251–258. doi: 10.1016/j.acap.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Smith WA, Li C, Nottage KA, et al. Lifestyle and metabolic syndrome in adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort Study. Cancer. 2014;120:2742–2750. doi: 10.1002/cncr.28670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 33.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 34.Carpentieri SC, Meyer EA, Delaney BL, et al. Psychosocial and behavioral functioning among pediatric brain tumor survivors. J Neurooncol. 2003;63:279–287. doi: 10.1023/a:1024203323830. [DOI] [PubMed] [Google Scholar]
- 35.Patel SK, Lai-Yates JJ, Anderson JW, et al. Attention dysfunction and parent reporting in children with brain tumors. Pediatr Blood Cancer. 2007;49:970–974. doi: 10.1002/pbc.21151. [DOI] [PubMed] [Google Scholar]
- 36.Winter AL, Conklin HM, Tyc VL, et al. Executive function late effects in survivors of pediatric brain tumors and acute lymphoblastic leukemia. J Clin Exp Neuropsychol. 2014;36:818–830. doi: 10.1080/13803395.2014.943695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahalley LS, Conklin HM, Tyc VL, et al. ADHD and secondary ADHD criteria fail to identify many at-risk survivors of pediatric ALL and brain tumor. Pediatr Blood Cancer. 2011;57:110–118. doi: 10.1002/pbc.22998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willard VW, Hardy KK, Allen TM, et al. Sluggish cognitive tempo in survivors of pediatric brain tumors. J Neurooncol. 2013;114:71–78. doi: 10.1007/s11060-013-1149-8. [DOI] [PubMed] [Google Scholar]
- 39.Racer KH, Dishion TJ. Disordered attention: Implications for understanding and treating internalizing and externalizing disorders in childhood. Cognit Behav Pract. 2012;19:31–40. doi: 10.1016/j.cbpra.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Craske MG, Roy-Byrne PP, Stein MB, et al. Treatment for anxiety disorders: Efficacy to effectiveness to implementation. Behav Res Ther. 2009;47:931–937. doi: 10.1016/j.brat.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cuijpers P, van Straten A, Andersson G, et al. Psychotherapy for depression in adults: A meta-analysis of comparative outcome studies. J Consult Clin Psychol. 2008;76:909–922. doi: 10.1037/a0013075. [DOI] [PubMed] [Google Scholar]
- 42.Cuijpers P, Sijbrandij M, Koole SL, et al. The efficacy of psychotherapy and pharmacotherapy in treating depressive and anxiety disorders: A meta-analysis of direct comparisons. World Psychiatry. 2013;12:137–148. doi: 10.1002/wps.20038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weisz JR, Hawley KM, Doss AJ: Empirically tested psychotherapies for youth internalizing and externalizing problems and disorders. Child Adolesc Psychiatr Clin N Am 13:729-815, 2004 . [DOI] [PubMed]
- 44. doi: 10.1001/archpsyc.56.12.1073. The MTA Cooperative Group: Multimodal treatment study of children with ADHD: A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 56:1073-1086, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40:57–87. [PubMed] [Google Scholar]
- 46.Rapee RM, Lyneham HJ, Hudson JL, et al. Effect of comorbidity on treatment of anxious children and adolescents: Results from a large, combined sample. J Am Acad Child Adolesc Psychiatry. 2013;52:47–56. doi: 10.1016/j.jaac.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Halldorsdottir T, Ollendick TH, Ginsburg G, et al. Treatment outcomes in anxious youth with and without comorbid ADHD in the CAMS. J Clin Child Adolesc Psychol. 2015;44:985–991. doi: 10.1080/15374416.2014.952008. [DOI] [PubMed] [Google Scholar]
- 48.Hinds PS, Hockenberry MJ, Gattuso JS, et al. Dexamethasone alters sleep and fatigue in pediatric patients with acute lymphoblastic leukemia. Cancer. 2007;110:2321–2330. doi: 10.1002/cncr.23039. [DOI] [PubMed] [Google Scholar]
- 49.Mrakotsky CM, Silverman LB, Dahlberg SE, et al. Neurobehavioral side effects of corticosteroids during active treatment for acute lymphoblastic leukemia in children are age-dependent: Report from Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. Pediatr Blood Cancer. 2011;57:492–498. doi: 10.1002/pbc.23060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brinkman TM, Ullrich NJ, Zhang N, et al. Prevalence and predictors of prescription psychoactive medication use in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Cancer Surviv. 2013;7:104–114. doi: 10.1007/s11764-012-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moretti MM, Fine S, Haley G, et al. Childhood and adolescent depression: Child-report versus parent-report information. J Am Acad Child Psychiatry. 1985;24:298–302. doi: 10.1016/s0002-7138(09)61090-6. [DOI] [PubMed] [Google Scholar]
- 52.Recklitis C, O’Leary T, Diller L. Utility of routine psychological screening in the childhood cancer survivor clinic. J Clin Oncol. 2003;21:787–792. doi: 10.1200/JCO.2003.05.158. [DOI] [PubMed] [Google Scholar]
- 53. Children’s Oncology Group: Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancer, version 4.0. http://www.survivorshipguidelines.org/pdf/LTFUGuidelines_40.pdf. [Google Scholar]