Abstract
Schizophrenia presents a substantial clinical and economic burden to the health-care system. In QUAlity of LIfe with AbiliFY Maintena (QUALIFY), a randomized head-to-head study of aripiprazole once-monthly 400 mg (AOM 400) compared with paliperidone palmitate (PP; 78–234 mg/mo), AOM 400 demonstrated greater improvement in health-related quality of life and functioning in patients with stable schizophrenia. The present analysis used health economics assessment data collected during the QUALIFY study to determine the direct medical and pharmacy costs and the cost-effectiveness associated with each treatment over 6 months. Compared with those receiving PP, patients receiving AOM 400 incurred significantly lower direct total costs ($8908±186 vs $9675±190, p=0.005) and treatment costs ($7967±113 vs $8706±116, p<0.001). Effectiveness results in the subset of patients included in the cost analyses were similar to the overall population: mean (95% CI) improvement in Heinrichs-Carpenter Quality of Life Scale total score was greater with AOM 400 (5.97 [3.87; 8.08]) compared with PP (2.85 [0.56; 5.08]). Likewise, Clinical Global Impression–Severity improved more in the AOM 400 group (−0.59 [−0.71; −0.47]) compared with PP group (−0.37 [−0.46; −0.27]). Therefore, the analysis of data from stabilized patients with schizophrenia in the QUALIFY study indicated that AOM 400 is associated with lower health-care costs and greater effectiveness compared with PP and thus represents the economically dominant strategy.
Keywords: aripiprazole once-monthly, paliperidone palmitate, schizophrenia, cost-effectiveness, long-acting injectable agents, second-generation antipsychotic, health economics, patient functioning
Introduction
Schizophrenia is a chronic disease with severe symptoms, often with onset in early adulthood. If unremitted, the disease has a negative impact on social functioning and occupational status. People with schizophrenia are likely to experience depression, treatment side effects, medical comorbidities, and, for some, homelessness, all of which are associated with a poor quality of life [1]. Symptoms of poor emotional control as well as disorganized thoughts and behavior contribute to the social stigmatization and impaired cognition that limit employment. Schizophrenia is also associated with increased risk of violent and nonviolent crimes, substance use, and early mortality including suicide. Consequently, the economic and social costs of schizophrenia are high. In 2013, excess direct health-care costs accounted for 24% of the $37.7 billion US dollars (USD) overall cost of schizophrenia in the United States [2]. The rest of the overall costs consisted of direct non-health-care costs (law enforcement, research and training, and homeless shelters; 12%) and indirect costs due to unemployment, decreased work productivity, premature mortality, and caregiving (52%).
The treatment goals for patients with schizophrenia are to prevent relapse, maximize functioning and quality of life, and assist patients in achieving their life goals [3]. Relapse of schizophrenia symptoms or failure to achieve remission may result in worse mental and physical function and drive higher health-care resource utilization as well as direct medical costs [4–6].
Medication nonadherence is a persistent issue in schizophrenia and has been associated with greater risk of relapse and hospitalization, lower quality of life, and higher health-care costs [4,7]. Nonadherence has also been associated with increased use of health-care resources, poorer mental functioning, and less satisfaction with basic needs, social life, and life in general [8,9]. In a recent large retrospective claims database analysis, adherence to second-generation antipsychotics (vs nonadherence) significantly decreased total costs by almost $20,000 USD per year and reduced the risk of hospitalization by 27% [10].
Adherence to antispychotic therapy may be improved by using a long-acting injectable (LAI) formulation. Use of LAI antipsychotic agents relieves the patient from the responsibility of a daily medication schedule [11,12] and also requires regular contact between the patient and health-care professionals, allowing better monitoring of adherence. A review of mirror-image studies suggested that LAI antipsychotics were superior to oral antipsychotics in preventing hospitalization [13]. Similarly, a recent mirror-image study demonstrated that the use of LAI aripiprazole (prospective data) compared with prior oral antipsychotics (retrospective data) was associated with lower 3-month rates of inpatient psychiatric hospitalizations (2.7 vs 27.1%, respectively; p<0.0001) in a community setting [14]. In an analysis of a large Medicaid database, initiation of LAI therapy resulted in significant reductions in all-cause and mental health–related hospitalizations and significantly lower direct inpatient costs [15].
In randomized, double-blind, controlled trials, the LAI aripiprazole once-monthly 400 mg (AOM 400) was shown to improve symptoms and delay time to relapse compared with placebo [16]. It also reduced relapse rates compared with a subtherapeutic dose of AOM 50 mg [17] in patients participating in randomized, double-blind, controlled trials. The cost-effectiveness of AOM 400 compared with LAI paliperidone palmitate (PP) has previously been estimated using a 1-year decision-analytic model based on dosing and the relapse rates reported in clinical studies [18]. Compared with PP doses used in a real-world setting, AOM 400 was associated with fewer relapses, lower overall treatment cost, and greater cost-effectiveness.
More recently, in the randomized QUAlity of LIfe with AbiliFY Maintena (QUALIFY) study, AOM 400 provided significantly greater improvements in health-related quality of life and functioning compared with PP [19]. In addition, QUALIFY also assessed parameters that are important in evaluating the direct costs associated with treatment and health-care resource utilization (e.g., dosing frequency, outpatient and inpatient visits). The objective of the present analysis was to conduct a cost-effectiveness comparison of AOM 400 and PP in patients with schizophrenia from the QUALIFY study using US cost data.
Methods
Study design
A pharmacoeconomic evaluation was performed alongside a 28-week, randomized, open-label, rater-blinded, multinational, head-to-head comparative study of AOM 400 and PP in patients with schizophrenia (NCT01795547). The study procedures have been presented in detail elsewhere [19]. Briefly, eligible patients were men and women 18 to 60 years old with stable schizophrenia (diagnosed using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision [DSM-IV-TR] criteria [20]) who needed to change existing oral antipsychotic therapy because of inadequate efficacy, poor tolerability, or lack of adherence, and who, based on the investigator’s judgment, may benefit from LAI treatment. The patients were required to have ≥3 months of oral antipsychotic treatment and mild to marked illness as assessed by Clinical Global Impression–Severity (CGI-S) score of 3–5. Exclusion criteria included diagnosis of other psychiatric or Axis I disorder; intolerance or lack of efficacy with oral aripiprazole, risperidone, or paliperidone; or use of an LAI in the 6 months before screening [19]. Randomized patients underwent a conversion period in which they received either oral aripiprazole or oral paliperidone, followed by a transition to intramuscular (IM) formulations according to the prescribing information for 5 weeks. Thereafter, IM injections of AOM 400 (dose reduction to 300 mg was permitted based on individual patient tolerability) or PP (flexible dosing per label, 78–234 mg/mo) once every 4 weeks were continued for 20 weeks.
Health-related quality of life and functioning, the primary end point of the study, was assessed using Heinrichs-Carpenter Quality of Life Scale (QLS), a 21-item scale that covers the following domains: Intrapsychic Foundations, Interpersonal Relations, Instrumental Role, and Common Objects and Activities [21]. Effectiveness outcomes used in this pharmacoeconomic evaluation included mean change from baseline to week 28 in QLS total score (primary end point) and in CGI-S. Health-care resource use from the QUALIFY study was monetized in terms of the US payer perspective, including direct medical and nonmedical costs and drug acquisition costs.
Assessments and statistical analyses
A study-specific form, the health economic assessment (HEA) questionnaire, was used to collect health-care resource utilization data across the 28-week study, including oral conversion and LAI initiation periods; the questionnaire was administered at the final study visit. This standardized form captured consultation visits with primary care physicians and psychiatrists as well as contact with other health-care providers, such as psychologists and nurses. Data from other outpatient services, such as those provided by day-care centers or group therapy, and inpatient services associated with hospitalizations were also collected. Contacts with health-care providers mandated by the study protocol were not included. The data sources and unit costs used for this pharmacoeconomic analysis are presented in Table 1 [22–24]. Total cost outcomes (least squares mean per treatment group) were then estimated from an analysis of covariance model, including geographic region (Europe vs North America) and treatment as fixed effects as well as cost incurred during the 6 months before study entry and study drug exposure time as covariates.
Table 1.
Unit costs (USD) for health-care resource use.
| Resource type | Unit cost (USD) |
|---|---|
| Physician (per visit) [22] | |
| General practitioner | 62.0 |
| Psychiatrist | 50.2 |
| Cardiologist | 68.2 |
| Ear-nose-throat | 65.5 |
| Gastroenterologist | 72.2 |
| Dermatologist | 51.4 |
| Other health-care professionals (per contact) [22] | |
| Psychologist | 81.9 |
| Nurse | 49.4 |
| Physiotherapist | 69.5 |
| Occupational therapist | 82.1 |
| Social worker | 148.7 |
| Community-based day services (per contact/session) [22] | |
| Day-care center | 79.0 |
| Group therapy | 45.9 |
| Sheltered workshop | 14.6 |
| Inpatient services (per stay) | |
| Acute psychiatric ward [24] | 6160.0 |
| Psychiatric rehabilitation ward [24] | 4988.0 |
| Long-stay ward [23] | 12,815.9 |
| Emergency/crisis center (per day) [22] | 88.9 |
USD, US dollars.
Incremental cost-effectiveness ratios were used to estimate the additional cost of 1 unit of health outcome (point on the QLS or CGI-S scale) gained by a treatment compared with the alternative [25]. To evaluate the uncertainty, incremental effectiveness and incremental cost estimates were displayed on the cost-effectiveness plane [26]; 95% confidence intervals (CIs) for incremental effectiveness and cost were calculated using a bootstrapped, bias-corrected, accelerated nonparametric procedure with 10,000 iterations [27].
The prespecified pharmacoeconomic analyses were conducted using data from patients who, besides QLS, had at least one valid postbaseline assessment of the HEA questionnaire. Baseline patient characteristics were presented as frequencies and percentages for categorical variables and as means and standard deviations for continuous parameters. As was done for the primary analysis of the QUALIFY study, effectiveness outcomes (mean QLS and CGI-S score change from baseline) were analyzed using a mixed model for repeated measures with an unstructured covariance matrix including baseline score-by-visit interaction, geographic region (Europe vs North America), visit, and treatment-by-visit interactions as fixed effects. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
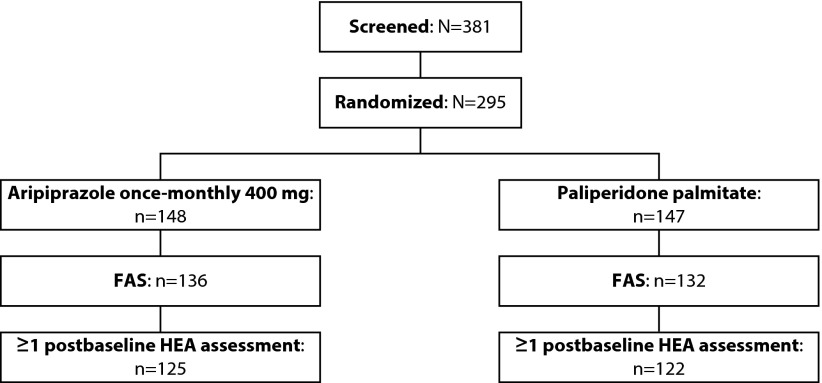
Data from HEA were available for 125 patients treated with AOM 400 and 122 patients receiving PP, representing 92% of the overall QUALIFY efficacy population (Figure 1). Baseline characteristics for this subset of the QUALIFY population are presented in Table 2.
Figure 1.
Patient disposition in the QUALIFY study.
Full-analysis set included patients who had at least one valid postbaseline assessment of the Heinrichs-Carpenter Quality of Life Scale.
FAS, full-analysis set; HEA, health economics assessment.
Table 2.
Characteristics of patients included in the cost analysis.
| Characteristic | Aripiprazole once-monthly 400 mg (n=125) | Paliperidone palmitate (n=122) |
|---|---|---|
| Mean ± SD age, y | 42.8±11.1 | 41.7±10.7 |
| Male, n (%) | 74 (59.2) | 68 (55.7) |
| Race, n (%) | ||
| White | 87 (69.6) | 83 (68.6) |
| Black/African | 37 (29.6) | 33 (27.3) |
| American | ||
| Asian | 1 (0.8) | 3 (2.5) |
| Other | 0 (0.0) | 2 (1.6) |
| Unknown | 0 (0.0) | 1 (0.8) |
| Mean ± SD baseline | 30.0±6.3 | 29.0±6.3 |
| BMI, kg/m2 | ||
| Marital status, n (%) | ||
| Single | 88 (70.4) | 83 (68.0) |
| Married/living as a couple | 18 (14.4) | 19 (15.6) |
| Separated/divorced | 18 (14.4) | 15 (12.3) |
| Widowed | 1 (0.8) | 5 (4.1) |
| Employment status, n (%) | ||
| Paid employment or self-employed | 19 (15.2) | 10 (8.2) |
| Unemployed | 56 (44.8) | 60 (49.2) |
| Student | 3 (2.4) | 4 (3.3) |
| Retired | 26 (20.8) | 20 (16.4) |
| Other | 21 (16.8) | 28 (22.9) |
| Mean ± SD baseline severity scores | ||
| QLS total score | 66.4±21.8 | 63.3±21.4 |
| CGI-S score | 4.0±0.7 | 3.9±0.6 |
BMI, body mass index; CGI-S, Clinical Global Impression–Severity; QLS, Heinrichs-Carpenter Quality of Life Scale; SD, standard deviation.
Most patients in the AOM 400 group were treated with the 400-mg dose (mean ± SE, 388.1±2.5 mg in the sample included in HEA analysis). The mean ± SE dose of PP in the HEA analysis sample was 177.8±2.9 mg, with most patients receiving 156- or 234-mg doses.
Total costs were significantly lower for AOM 400 than PP (p=0.005; Table 3). The cost of AOM 400 treatment during the QUALIFY study was approximately 9% lower (p<0.001) than the cost of PP treatment, whereas the costs associated with services delivered by health-care professionals and inpatient or outpatient providers did not differ significantly between treatment groups (Table 3).
Table 3.
Total and disaggregated costs over 6 months.*
| Cost in US dollars, LSM ± SE | Aripiprazole once-monthly (n=125) | Paliperidone palmitate (n=122) | Cost difference | p Value |
|---|---|---|---|---|
| Total | 8908±186 | 9675±190 | −767 | 0.005 |
| Treatment | 7967±113 | 8706±116 | −739 | <0.001 |
| Outpatient | 275±71 | 395±72 | −120 | 0.238 |
| Inpatient | 230±81 | 93±82 | +137 | 0.237 |
LSM, least squares mean; SE, standard error.
Total cost outcomes were estimated from an analysis of covariance model, including geographic region (Europe vs North America) and treatment as fixed effects as well as cost incurred during the 6 months before study entry and study drug exposure time as covariates. Patients in QUALIFY had stable disease at study entry.
Among patients included in the cost analysis, mean QLS total score improved by more than twice as much with AOM 400 than with PP (Table 4). The mean (95% CI) change in QLS total score for patients treated with AOM 400 was 5.97 (3.87; 8.08) compared with 2.85 (0.56; 5.08) with PP. For effectiveness based on CGI-S, results also favored AOM over PP (Table 4), with mean (95% CI) change in CGI-S scores of −0.59 (−0.71; −0.47) with AOM 400 compared with −0.37 (−0.46; −0.27) with PP.
Table 4.
Cost and effectiveness outcomes.
| Mean (bootstrapped 95% CI) | Total cost, USD | Effectiveness (change in QLS total score) | Effectiveness (change in CGI-S) |
|---|---|---|---|
| Aripiprazole once-monthly (n=125) | 8909 (8444; 9369) | 5.97 (3.87; 8.08) | −0.59 (−0.71; −0.47) |
| Paliperidone palmitate (n=122) | 9675 (9129; 10,235) | 2.85 (0.56; 5.08) | −0.37 (−0.46; −0.27) |
| Difference | −766 (−1230; −304) | 3.12 (0.12; 6.18) | −0.22 (−0.37; −0.07) |
CGI-S, Clinical Global Impression–Severity; CI, confidence interval; QLS, Heinrichs-Carpenter Quality of Life Scale; USD, US dollars.
Cost-effectiveness analysis using change in QLS and CGI-S scores from baseline as effectiveness measures indicated that AOM 400 dominated PP; AOM 400 demonstrated greater effectiveness at lower costs compared with PP over the 28-week study period.
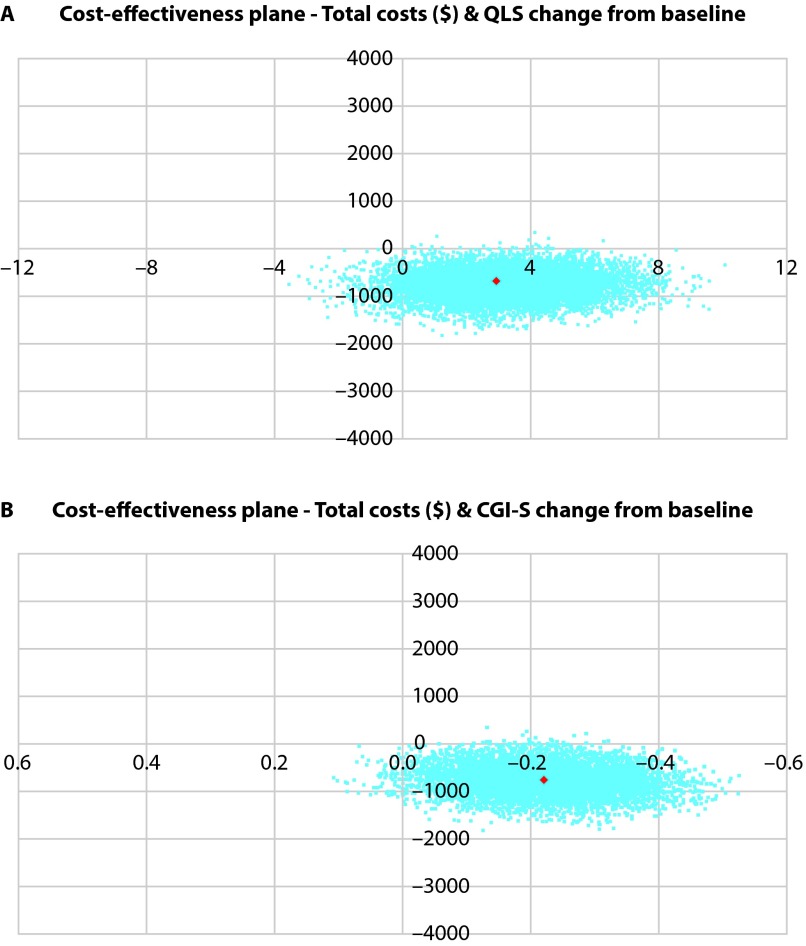
Figure 2 depicts the distribution of incremental costs and incremental effects on the cost-effectiveness plane. A majority of the points fell in the southeast quadrant of the cost-effectiveness plane, which represents negative costs and positive effects, thus indicating dominant cost-effectiveness. The cost-effectiveness acceptability curves for both QLS and CGI-S indicated that AOM 400 was the treatment of choice over PP.
Figure 2.
Cost-effectiveness planes for aripiprazole once-monthly 400 mg compared with paliperidone palmitate for (A) QLS and (B) CGI-S.
Scatter plots of bootstrapped incremental costs and effect pairs presented on the incremental cost-effectiveness plane. The southeast quadrant of the cost-effectiveness plane indicates negative costs and positive effects, thus representing the dominant strategy.
CGI-S, Clinical Global Impression–Severity;
QLS, Heinrichs-Carpenter Quality of Life Scale.
Discussion
The head-to-head design of the QUALIFY study allows the robust analysis of relative costs and effectiveness for AOM 400 compared with PP in stable patients with schizophrenia. In the present analysis of data collected from QUALIFY, the total medical costs related to AOM 400 were lower than those with PP, resulting mainly from significantly lower treatment costs. AOM 400 also demonstrated effectiveness benefit over PP, with greater improvement in QLS and CGI-S outcome measures. Cost-effectiveness analysis predicted that, whatever the willingness-to-pay threshold, AOM 400 was the economically dominant strategy compared with PP.
Second-generation LAIs, including AOM 400 and PP, have previously been shown to reduce resource utilization and associated costs, particularly inpatient hospitalization, compared with oral antipsychotics [14,15,28–30]. Increased cost of treatment with LAIs compared with oral antipsychotics partially or completely offsets savings in resource utilization. In the current study comparing two second-generation LAIs, the difference in treatment costs between AOM 400 and PP drove the difference in total direct costs. Treatment costs were estimated based on actual dosing during the study. QUALIFY was designed to reflect real-world conditions, and dosing of both LAIs could be adjusted based on tolerability and effectiveness during the study. Most patients in the AOM 400 group were treated with the 400-mg dose, and those in the PP group were treated with 156- or 234-mg doses. These dosing scenarios align closely with published dosing scenarios seen in clinical trials and real-world treatment [16,31,32].
Effectiveness per QLS and CGI-S measures in the current analysis was consistent with data reported for the full QUALIFY study patient population [19]. In the QUALIFY primary analysis, the least squares mean treatment difference in QLS total score was 4.67 (95% CI: 0.32, 9.02; p=0.036), demonstrating noninferiority per prespecified criteria and meeting the superiority criteria for AOM 400 compared with PP. Likewise, in the full sample, the least squares mean difference between treatment groups for CGI-S was −0.28 (−0.48; −0.09, p=0.004) [19].
Although not included in the current analysis, it is important to note that greater effectiveness of AOM 400 compared with PP as assessed by both QLS and CGI-S is further supported by work readiness assessment in QUALIFY using the validated Work Readiness Questionnaire (WoRQ) [33,34]. WoRQ measures a patient’s functional capacity to work based on the clinician’s assessment of seven statements related to the patient’s ability to adhere to a treatment plan; conduct daily activities; keep schedules; interact with others; and maintain acceptable appearance, behavior, and impulse control. At the conclusion of QUALIFY, the change from baseline in the total WoRQ score was significantly greater for patients receiving AOM 400 compared with PP. Among patients considered not ready to work at baseline, a greater percentage of patients treated with AOM 400 (26.4%) compared with PP (12.2%) were considered ready to work at week 28 [34]. Although readiness to work does not directly translate into compensated employment, the possibility of obtaining employment has potential economic and quality-of-life implications.
In many cost-effectiveness analyses, data for different treatment strategies have been collected from separate sources, with efforts made to reduce disparities between data sources [35]. The analysis presented here used data from a randomized, head-to-head trial that controlled for variables such as patient characteristics and the measurement of effectiveness, thus allowing for direct comparison of therapeutic interventions. Although it would be ideal to conduct economic analysis in parallel with a clinical trial, clinical trials often do not provide all of the required economic data. For example, clinical trials are conducted for relatively short periods of time, whereas cost-effectiveness decisions typically require long-term data. In such situations, computer-based modeling approaches are helpful to predict cost-effectiveness. Using a decision-analytic model, AOM 400 was demonstrated to be more cost effective than PP [18]. The present study complements the simulation method, providing a more complete picture of cost-effectiveness of AOM 400.
Limitations of this analysis include the use of data from a randomized controlled clinical trial. Because patients were enrolled based on select eligibility criteria, the study population may differ in some aspects from the general population of patients with schizophrenia. For example, patients in the QUALIFY study had stable disease and were on a stable dose of medication. In addition, patients were required by protocol to visit the investigator on a regular basis; therefore, costs may differ from the setting of usual care. Likewise, the efficacy of a drug observed in a clinical trial under supervised conditions may not reflect the effectiveness of the drug in a real-world clinical setting. It should be noted that the raters of CGI-S (but not QLS) were aware of the patient’s assigned treatment group. The sample size in this clinical trial was relatively small compared with a large database analysis. Furthermore, costs of treatment differ between countries based on the heterogeneity in treatment patterns, and US-based costs might not be representative for other countries.
Conclusion
Based on head-to-head results from QUALIFY, AOM 400 treatment provided greater improvement in functional outcomes at a lower cost compared with PP therapy. Considering the economic burden of schizophrenia for individuals and society, cost-effectiveness is a key component of the treatment decision process. AOM 400 is an economically dominant strategy over PP for the treatment of patients with stable schizophrenia in the United States.
Acknowledgments
Editorial assistance for preparation of the manuscript was provided by Vandana Sharma at C4 MedSolutions, LLC (Yardley, PA), a CHC Group company, and funded by Otsuka Pharmaceutical Development & Commercialization, Inc. and H. Lundbeck A/S.
Abbreviations:
- AOM 400
aripiprazole once-monthly 400 mg;
- BMI
body mass index;
- CGI-S
Clinical Global Impression–Severity;
- CI
confidence interval;
- FAS
full-analysis set;
- HEA
health economics assessment;
- IM
intramuscular;
- LAI
long-acting injectable;
- LSM
least squares mean;
- PP
paliperidone palmitate;
- QLS
Heinrichs-Carpenter Quality of Life Scale;
- QUALIFY
QUAlity of LIfe with AbiliFY Maintena;
- SD
standard deviation;
- SE
standard error;
- USD
US dollars;
- WoRQ
Work Readiness Questionnaire.
References
- 1.Millier A, Schmidt U, Angermeyer MC, Chauhan D, Murthy V, Toumi M, Cadi-Soussi N. Humanistic burden in schizophrenia: a literature review. J Psychiatr Res. 2014;54:85–93. doi: 10.1016/j.jpsychires.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Cloutier M, Aigbogun MS, Guerin A, Nitulescu R, Ramanakumar AV, Kamat SA, DeLucia M, Duffy R, Legacy SN, Henderson C, Francois C, Wu E. The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry. 2016;77(6):764–71. doi: 10.4088/JCP.15m10278. [DOI] [PubMed] [Google Scholar]
- 3.Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, Kreyenbuhl J, American Psychiatric Association. Steering Committee on Practice Guidelines Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 Suppl):1–56. [PubMed] [Google Scholar]
- 4.Ascher-Svanum H, Zhu B, Faries DE, Salkever D, Slade EP, Peng X, Conley RR. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry. 2010;10:2. doi: 10.1186/1471-244X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer L, Baumstarck K, Boucekine M, Blanc J, Lancon C, Auquier P. Measuring quality of life in patients with schizophrenia: an overview. Expert Rev Pharmacoecon Outcomes Res. 2013;13(3):343–9. doi: 10.1586/erp.13.15. [DOI] [PubMed] [Google Scholar]
- 6.Haynes VS, Zhu B, Stauffer VL, Kinon BJ, Stensland MD, Xu L, Ascher-Svanum H. Long-term healthcare costs and functional outcomes associated with lack of remission in schizophrenia: a post-hoc analysis of a prospective observational study. BMC Psychiatry. 2012;12:222. doi: 10.1186/1471-244X-12-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert M, Karow A, Leucht S, Schimmelmann BG, Naber D. Remission in schizophrenia: validity, frequency, predictors, and patients’ perspective 5 years later. Dialogues Clin Neurosci. 2010;12(3):393–407. doi: 10.31887/DCNS.2010.12.3/mlambert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ascher-Svanum H, Faries DE, Zhu B, Ernst FR, Swartz MS, Swanson JW. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry. 2006;67(3):453–60. doi: 10.4088/jcp.v67n0317. [DOI] [PubMed] [Google Scholar]
- 9.Ascher-Svanum H, Zhu B, Faries DE, Furiak NM, Montgomery W. Medication adherence levels and differential use of mental-health services in the treatment of schizophrenia. BMC Res Notes. 2009;2:6. doi: 10.1186/1756-0500-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Y, Ni W. Estimating the impact of adherence to and persistence with atypical antipsychotic therapy on health care costs and risk of hospitalization. Pharmacotherapy. 2015;35(9):813–22. doi: 10.1002/phar.1634. [DOI] [PubMed] [Google Scholar]
- 11.Acosta FJ, Hernandez JL, Pereira J, Herrera J, Rodriguez CJ. Medication adherence in schizophrenia. World J Psychiatry. 2012;2(5):74–82. doi: 10.5498/wjp.v2.i5.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brissos S, Veguilla MR, Taylor D, Balanza-Martinez V. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4(5):198–219. doi: 10.1177/2045125314540297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishimoto T, Nitta M, Borenstein M, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957–65. doi: 10.4088/JCP.13r08440. [DOI] [PubMed] [Google Scholar]
- 14.Kane JM, Zhao C, Johnson BR, Baker RA, Eramo A, McQuade RD, Duca AR, Sanchez R, Peters-Strickland T. Hospitalization rates in patients switched from oral anti-psychotics to aripiprazole once-monthly: final efficacy analysis. J Med Econ. 2015;18(2):145–54. doi: 10.3111/13696998.2014.979936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamat SA, Offord S, Docherty J, Lin J, Eramo A, Baker RA, Gutierrez B, Karson C. Reduction in inpatient resource utilization and costs associated with long-acting injectable antipsychotics across different age groups of Medicaid-insured schizophrenia patients. Drugs Context. 2015;4:212267. doi: 10.7573/dic.212267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kane J, Sanchez R, Perry P, Jin N, Johnson B, Forbes R, McQuade R, Carson W, Fleischhacker W. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73(5):617–24. doi: 10.4088/JCP.11m07530. [DOI] [PubMed] [Google Scholar]
- 17.Fleischhacker WW, Sanchez R, Perry PP, Jin N, Peters-Strickland T, Johnson BR, Baker RA, Eramo A, McQuade RD, Carson WH, Walling D, Kane JM. Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority study. Br J Psychiatry. 2014;205(2):135–44. doi: 10.1192/bjp.bp.113.134213. [DOI] [PubMed] [Google Scholar]
- 18.Citrome L, Kamat SA, Sapin C, Baker RA, Eramo A, Ortendahl J, Gutierrez B, Hansen K, Bentley TG. Cost-effectiveness of aripiprazole once-monthly compared with paliperidone palmitate once-monthly injectable for the treatment of schizophrenia in the United States. J Med Econ. 2014;17(8):567–76. doi: 10.3111/13696998.2014.917089. [DOI] [PubMed] [Google Scholar]
- 19.Naber D, Hansen K, Forray C, Baker RA, Sapin C, Beillat M, Peters-Strickland T, Nylander AG, Hertel P, Andersen HS, Eramo A, Loze JY, Potkin SG. Qualify: a randomized head-to-head study of aripiprazole once-monthly and paliperidone palmitate in the treatment of schizophrenia. Schizophr Res. 2015;168(1–2):498–504. doi: 10.1016/j.schres.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association . Diagnostic and statistical manual of mental disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 21.Heinrichs DW, Hanlon TE, Carpenter WT., Jr The quality of life scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388–98. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Medicare & Medicaid Services Medicare Provider Utilization and Payment Data: Physician and Other Supplier Data CY 2013. [cited 2016 May 5]. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Physician-and-Other-Supplier2013.html.
- 23.Miller LS, Martin BC. Current and future forecasts of service use and expenditures of Medicaid-eligible schizophrenia patients in the state of Georgia. Schizophr Bull. 2004;30(4):983–95. doi: 10.1093/oxfordjournals.schbul.a007147. [DOI] [PubMed] [Google Scholar]
- 24.Yu W, Wagner TH, Chen S, Barnett PG. Average cost of VA rehabilitation, mental health, and long-term hospital stays. Med Care Res Rev. 2003;60(3 suppl):40S–53S. doi: 10.1177/1077558703256724. [DOI] [PubMed] [Google Scholar]
- 25.Bang H, Zhao H. Median-based incremental cost-effectiveness ratios with censored data. J Biopharm Stat. 2016;26(3):552–64. doi: 10.1080/10543406.2015.1052482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ. 1994;3(5):309–19. doi: 10.1002/hec.4730030505. [DOI] [PubMed] [Google Scholar]
- 27.Efron B, Tibshirani RJ. An introduction to the bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 28.Baser O, Xie L, Pesa J, Durkin M. Healthcare utilization and costs of Veterans Health Administration patients with schizophrenia treated with paliperidone palmitate long-acting injection or oral atypical antipsychotics. J Med Econ. 2015;18(5):357–65. doi: 10.3111/13696998.2014.1001514. [DOI] [PubMed] [Google Scholar]
- 29.Pesa JA, Muser E, Montejano LB, Smith DM, Meyers OI. Costs and resource utilization among Medicaid patients with schizophrenia treated with paliperidone palmitate or oral atypical antipsychotics. Drugs Real World Outcomes. 2015;2(4):377–85. doi: 10.1007/s40801-015-0043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson M, Gutierrez B, Offord S, Blanchette C, Eramo A, Earnshaw S, Kamat S. Inpatient resource use and costs associated with switching from oral antipsychotics to aripiprazole once-monthly for the treatment of schizophrenia. Drugs Context. 2016;5:212273. doi: 10.7573/dic.212273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hough D, Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M. Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2010;116(2–3):107–17. doi: 10.1016/j.schres.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Kamat SA, Gutierrez B, Eramo A, Zubek D, Baker RA, Lin J, Karson C. Initial assessment of real-world usage of extended-release injectable paliperidone palmitate among Medicaid insured schizophrenia patients. Value Health. 2013;16:A61. [Google Scholar]
- 33.Potkin SG, Bugarski-Kirola D, Edgar CJ, Soliman S, Le Scouiller S, Kunovac J, Miguel Velasco E, Garibaldi GM. Psychometric evaluation of the Work Readiness Questionnaire in schizophrenia. CNS Spectr. 2014:1–8. doi: 10.1017/S1092852914000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potkin S, Loze J, Forray C, Baker R, Sapin C, Peters-Strickland T, Beillat M, Nylander A, Hertel P, Schmidt S, Eramo A, Hansen K, Naber D. Relationship between response to aripiprazole once-monthly and paliperidone palmitate on work readiness and functioning: a post-hoc analysis of QUALIFY, a head-to-head study in schizophrenia. Neuropsychopharmacology. 2015;40(S1):T187. [Google Scholar]
- 35.Saramago P, Manca A, Sutton AJ. Deriving input parameters for cost-effectiveness modeling: taxonomy of data types and approaches to their statistical synthesis. Value Health. 2012;15(5):639–49. doi: 10.1016/j.jval.2012.02.009. [DOI] [PubMed] [Google Scholar]