Abstract
Objective:
To compare magnetization transfer changes in new brain MRI lesions identified during monthly imaging in patients with multiple sclerosis (MS) randomized to treatment with 250 μg subcutaneous interferon-β-1b (IFN-β-1b) every other day or daily 20 mg glatiramer acetate (GA) in a post hoc study using data from the Betaseron Versus Copaxone for Relapsing Remitting or CIS Forms of MS Using Triple Dose Gad 3 T MRI (BECOME) trial.
Methods:
T1-weighted images acquired with and without fat saturation pulses in the BECOME study were evaluated and found to exhibit magnetization transfer ratio (MTR) effects, and were used to compute MTR images (FSMTR). Forty-three participants who had the required imaging and new lesions, from the 75 originally randomized into the BECOME study, were included in this post hoc analysis and evaluated longitudinally during treatment to determine FSMTRDrop, an experimental measure of the completeness of FSMTR recovery in new lesions. Two sets of new brain MRI lesions were defined, one based on the appearance of gadolinium contrast enhancement (Gd lesions) and the other based on FSMTR decreases (ΔFSMTR lesions).
Results:
A total of 887 Gd lesions were identified in 43 participants (19 GA, 24 IFN-β-1b) and 321 ΔFSMTR lesions in 32 participants (16 GA, 16 IFN-β-1b). Participants randomized to GA exhibited greater average postlesion FSMTR recovery than did those randomized to IFN-β-1b in both Gd (p < 0.0001) and ΔFSMTR (p < 0.0001) lesions.
Conclusions:
New brain lesions that developed during treatment with GA exhibited evidence of greater FSMTR recovery than during treatment with IFN-β-1b.
Classification of evidence:
This study provides Class III evidence that MTR recovery in patients with MS with new MRI brain lesions is greater with GA than with IFN-β-1b.
Betaseron Versus Copaxone for Relapsing Remitting or CIS Forms of MS Using Triple Dose Gad 3 T MRI (BECOME) (ClinicalTrials.gov NCT00176592)1 was a randomized head-to-head study of interferon-β-1b (IFN-β-1b) and glatiramer acetate (GA) for the treatment of relapsing-remitting multiple sclerosis (MS) or clinically isolated syndromes (CIS) suggestive of MS. In the study, monthly 3T MRI images with and without a fat saturation (FS) pulse were acquired. It has been shown previously that FS produces a magnetization transfer effect2 and this pair of scans can be used to create an FS ratio image (FSMTR), analogous to more standard magnetization transfer ratio (MTR). In MS, changes in CNS MTR have been shown to be more specific to changes in myelin than conventional imaging3; quantitative studies of fresh tissue samples show that MTR in normal-appearing white matter (NAWM) and chronic MS lesions correlates well (R = −0.84) with Luxol fast blue (LFB), a standard histologic stain for myelin.4–6 Note that the correlation between MTR and LFB is capped by the noise inherent in each measurement; in this study this was 0.81 for MTR.
We have proposed a method to quantify demyelination and remyelination using longitudinal analysis of MTR images.7,8 We applied this procedure to measure the change in FSMTR between stable postlesion and stable prelesion values (FSMTRDrop) in new brain lesions in participants in the BECOME study. This metric measures the net change of FSMTR in acute lesions, and is expected to be negative, indicating partial recovery. Previous studies have used related techniques to measure MTR recovery in gadolinium (Gd) lesions; we also examined ΔFSMTR lesions,7 which are identified based on changes in FSMTR. While Gd lesions are not always precisely co-located with MTR changes, ΔMTR lesions are, and therefore may be better at identifying areas of demyelination.7
METHODS
Participants and imaging.
Detailed inclusion criteria, demographics, and imaging information for the BECOME study have been published previously.1 Seventy-five participants with MS or a CIS suggestive of MS who provided informed consent were randomized to treatment with either GA or IFN-β-1b. Participants underwent monthly MRI on a single 3T scanner (Allegra; Siemens Medical Solutions, Malvern, PA), including proton density (PD)–weighted, T1-weighted precontrast, T2-weighted, and T1-weighted postcontrast images. Postcontrast images were obtained after administration of triple-dose Gd and delayed imaging, a protocol that had previously shown improved Gd lesion detection rates.9 Imaging was mandatory every month for the first year and at the end of the second year and some participants also had optional monthly scans during the second year. Most timepoints also included additional T1-weighted images acquired with identical parameters, except with the addition of a FS preparation pulse, which could be used to construct an MTR-like image. Only T1-weighted and FS-T1-weighted images from before contrast administration were used and only participants who had such imaging at a minimum of 3 timepoints were included in the analysis.
Fat saturation ratio validation.
To confirm that FSMTR images computed from scans with and without FS pulses are comparable to conventional MTR images, 2 sets of FSMTR and MTR images were acquired from 5 volunteers in a scan and rescan experiment. Voxel-wise correlation between MTR and FSMTR was R = 0.76. This compares with R = 0.81 for MTR with MTR and R = 0.73 for FSMTR. This experiment showed a linear relationship and high correlation between the BECOME protocol and conventional MTR, although the FSMTR images demonstrated less MT contrast, translating to lower contrast to noise ratio. Details are included in appendix e-1 and figures e-1 and e-2 at Neurology.org.
Processing.
Images from each analyzable timepoint were coregistered into a participant-specific space using a linear 9-parameter transformation calculated using minctracc, part of the MINC toolkit.10 At each timepoint, using a Bayesian classifier11 and the PD-weighted, T1-weighted, and T2-weighted images, probability maps were constructed for NAWM, normal-appearing gray matter, CSF, T2 lesions, and partial volume tissue. The T2 lesion probability maps were converted into lesion masks by thresholding. The masks from the first timepoint for each participant were reviewed and corrected by trained experts. T2 lesion masks for subsequent timepoints were then refined automatically based on the corrected first-timepoint masks.11 Gd lesions were segmented manually by an expert neuroradiologist.1 FSMTR images were constructed as the percent difference between the precontrast T1-weighted and T1-weighted + FS images.
ΔFSMTR lesions were segmented following the published procedure.7 Since the ΔFSMTR lesion segmentation algorithm depends on the variance of the NAWM, the lower contrast to noise ratio of the FS-derived FSMTR images was automatically accounted for.
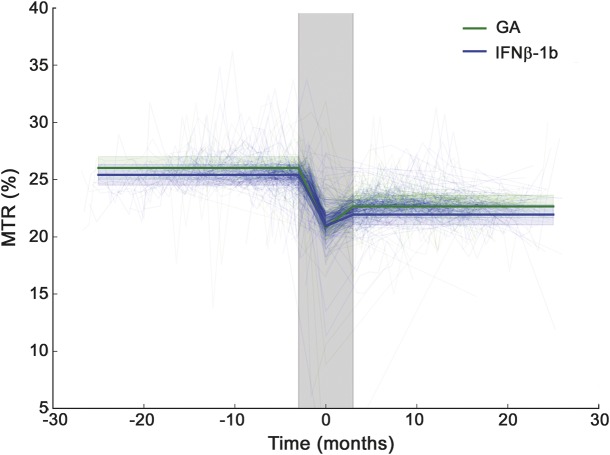
For each timepoint, the lesion mask was propagated to all timepoints, and FSMTR values within that mask were measured, producing an average FSMTR timecourse for all the new lesions at each timepoint, for each participant. In previous work we observed that FSMTR changes rapidly during lesion formation, but that average FSMTR shows long-term stability outside a 3- to 6-month window, centered on the time a new lesion is observed.7,8 FSMTR measurements outside this acute period are also less likely to be confounded by the effects of acute inflammation.7 Therefore, for analysis, samples obtained less than 3 months before or after lesion appearance were excluded. Although not included in the analysis, the sample from the timepoint when the lesion first became evident is included in the figures to better illustrate the pattern of acute FSMTR decrease and recovery.
Analysis.
Gd and ΔFSz lesions were analyzed separately, using identical procedures. FSMTR timecourses were produced, and then modeled using a random effects model,12 with each participant having a random intercept.8 The R formula was as follows:
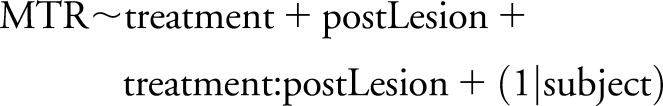 |
where postLesion is a dummy variable indicating whether the sample is from before (false) or after (true) the lesion formed, treatment was one of GA or IFN-β-1b, and postLesion:treatment is the interaction between treatment and postlesion recovery. The random effect participant accounts for correlations between measurements made in the same participant. The coefficient estimated for postLesion measures the prelesion to postlesion FSMTR difference (FSMTRDrop), which is expected to be negative, indicating incomplete recovery. The postLesion:treatment interaction measures the differential treatment effect on FSMTRDrop.
The explanatory power of the models was evaluated by testing the log-likelihoods achieved by the fully specified model and a null model with no fixed effects, using a χ2 test. If this gateway test was significant, individual effects were tested using f tests, with the denominator degrees of freedom provided by a Satterthwaite approximation using the MixMod R package.13 R2 was calculated according to the procedure suggested by Nakagawa and Schielzeth.14 The marginal R2 is the variance explained by the fixed effects alone, while the conditional R2 includes the contribution of the random effects. Shaded regions on figures show 95% confidence intervals.
Processing was done with custom software written in Python (Python Software Foundation; python.org) using the MINC tools (MINC tools; McConnell Brain Imaging Centre, Montreal, Canada) and the Scientific Python package (Scipy; www.scipy.org). Statistical analysis was performed using R15 via the Python-R bridge RPy2 (RPy2; rpy.sourceforge.net).
All investigators involved with the study were aware of which participants were assigned to each group, but were blind to which group received which treatment. Unblinding occurred only after the statistical analysis was complete.
Standard protocol approvals, registrations, and patient consents.
This work was approved by the Research Ethics Board of the Montreal Neurologic Institute and Hospital. The BECOME study and protocol was approved by the University of Medicine and Dentistry of New Jersey (now Rutgers University). Written informed consent was obtained from all patients participating in this study.
RESULTS
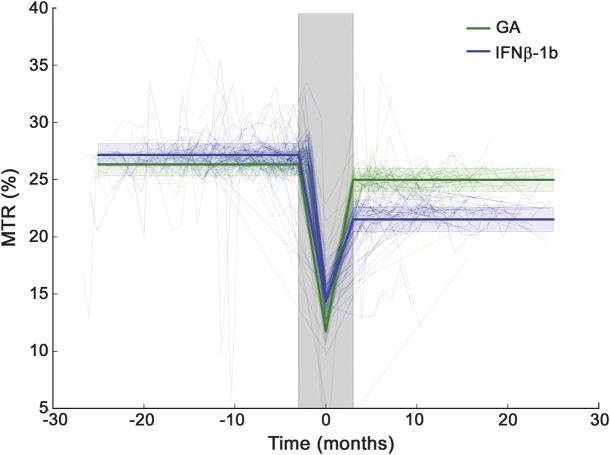
Baseline characteristics for each subgroup are shown in table 1. Forty-four participants (19 GA, 25 IFN-β-1b) had all the required imaging, as well as Gd lesions; 32 (16 GA, 16 IFN-β-1b) had ΔFSMTR lesions; 16 had Gd lesions but not ΔFSMTR lesions (5 GA, 11 IFN-β-1b); 4 had ΔFSMTR lesions but not Gd lesions (2 GA, 2 IFN-β-1b). A total of 887 Gd and 321 ΔFSMTR lesions were identified with median (interquartile range) volumes of Gd: 62 (160) and ΔFSMTR: 17 (72) mm3. FSMTR timecourses were obtained in each lesion type (figures 1 and 2). The model describing FSMTR timecourses in Gd lesions fit significantly better than the null model (p < 0.0001) with a marginal R2 = 0.24 and conditional R2 = 0.62 (table 2). Mean prelesion FSMTR was 25.4 in IFN-β-1b treated participants (intercept) and 0.603 units higher in the GA-treated group (treatment, p = 0.23). Both groups had incomplete FSMTR recovery (postLesion, p < 0.0001). New Gd lesions in the GA group recovered significantly better (treatment:postLesion, 0.110 FSMTR units, p < 0.0001). The model describing FSMTR timecourses in ΔFSMTR lesions also fit significantly (p < 0.0001), with a marginal R2 = 0.25 and conditional R2 = 0.53 (table 3). Again, no significant difference was observed in prelesion FSMTR values (IFN-β-1b 27.2, GA 0.829 units lower, p = 0.66), both groups had incomplete recovery (p < 0.0001), and the GA group showed better recovery (4.29 FSMTR units, p < 0.0001). Although not part of the planned analysis, lesional FSMTR was not significantly different between treatment groups at the time of lesion appearance (Gd: p = 0.43; ΔFSMTR: p = 0.61).
Table 1.
Baseline characteristics of analysis subgroups
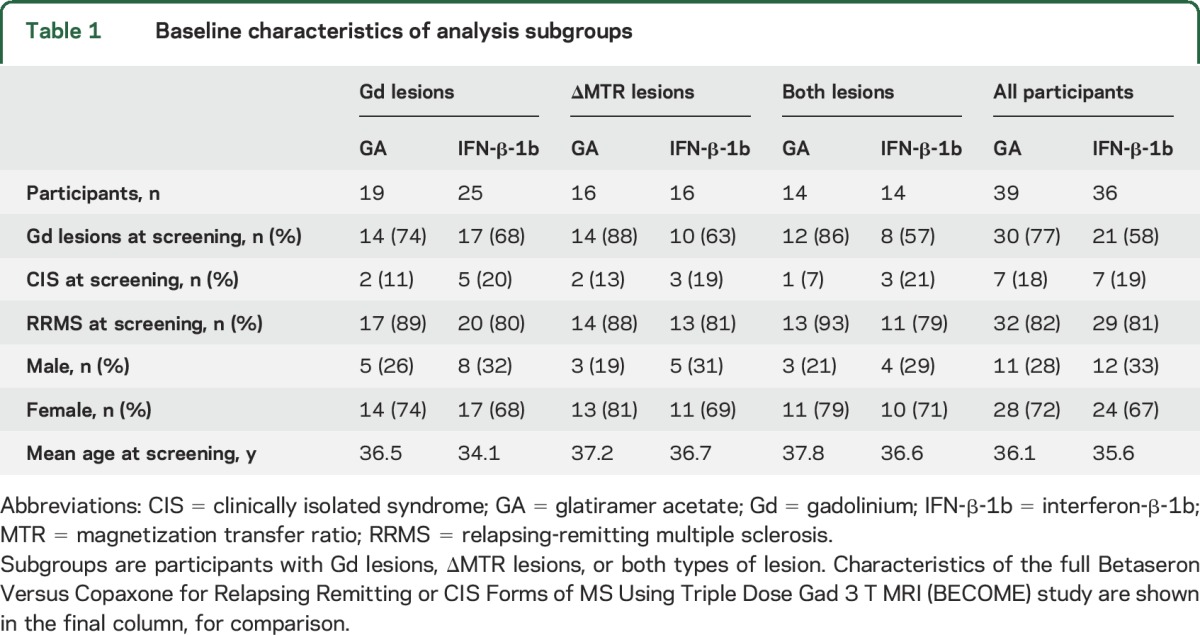
Figure 1. Magnetization transfer ratio (MTR) in new gadolinium lesions.
Light lines indicate timecourses in individual lesions while heavy lines show the model predictions. The shaded gray area indicates the acute period, 3 months before and after lesion formation; this period was not included in the model, but is shown in the figure to illustrate the full MTR timecourse. Prior to lesion formation, MTR was not significantly different in glatiramer acetate (GA) (green)– and interferon-β-1b (IFN-β-1b) (blue)–treated participants. MTR dropped to a minimum around the timepoint the lesion was identified (time 0), then recovered partially. Recovery was slightly better in GA-treated participants. Random intercepts calculated by the model have been subtracted from the individual timecourses in this figure.
Figure 2. Magnetization transfer ratio (MTR) in ΔMTR lesions.
As in figure 1, light lines indicate timecourses in individual lesions while heavy lines show the model predictions. The shaded gray area indicates the acute period, 3 months before and after lesion formation; this period was not included in the model, but is shown in the figure to illustrate the full MTR timecourse. The results in MTR lesions were consistent with gadolinium lesions: no significant difference in MTR between glatiramer acetate (GA)– and interferon-β-1b (IFN-β-1b)–treated patients before lesions formed but significantly better subsequent recovery in GA-treated patients. Random intercepts calculated by the model have been subtracted from the individual timecourses in this figure.
Table 2.
Parameter estimates of the statistical model for FSMTR in Gd lesions
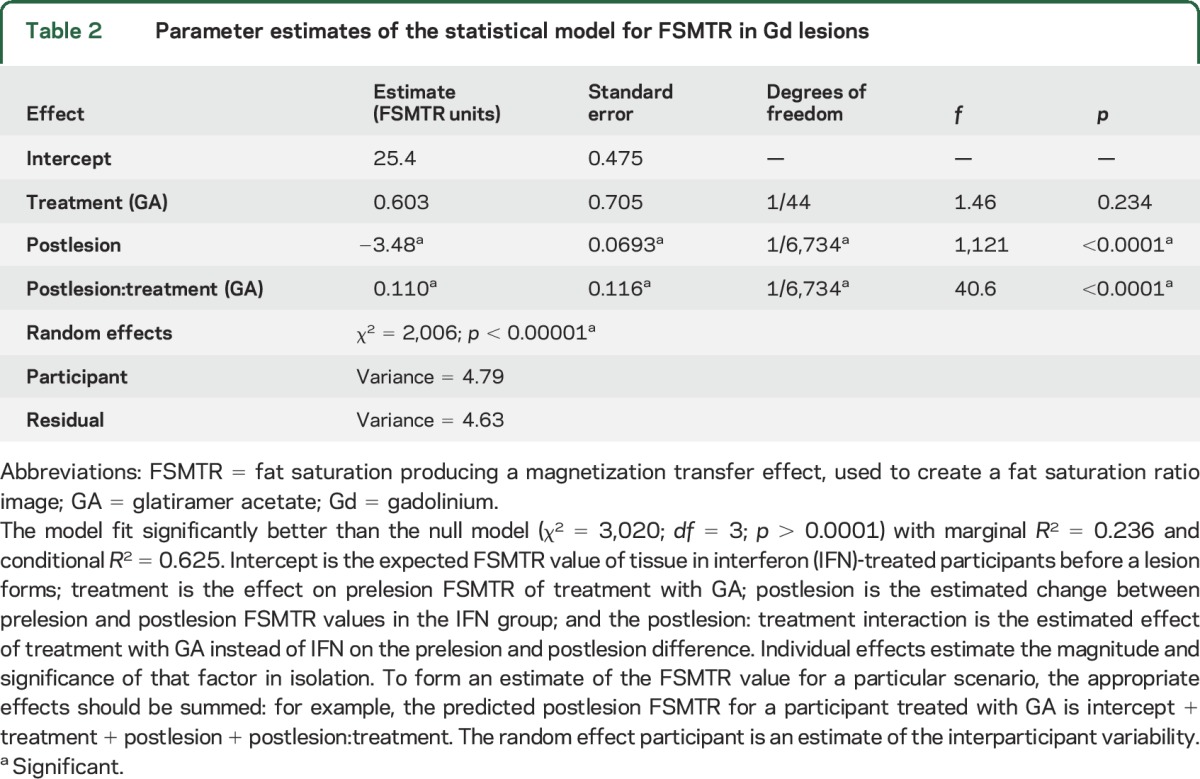
Table 3.
Parameter estimates of the statistical model for FSMTR in ΔFSMTR lesions

To examine the possibility of selection bias in the subgroups with Gd and ΔFSMTR lesions, the complete analysis was repeated in the subset of participants who had both lesion types. These results are shown in tables e-1 and e-2 and support the same conclusions as the main analyses, performed in all available participants.
DISCUSSION
Both groups exhibited FSMTR timecourses typical of lesional changes in FSMTR that we, and others, have reported previously.7,8,16 This consisted of fairly stable FSMTR before the lesion formed, a drop in FSMTR when the lesion became evident, partial recovery over the next few months, and subsequent relatively stable values. This pattern was generally similar in both Gd and ΔFSMTR lesions, and is consistent with acute demyelination and edema, resolution of edema, and partial remyelination.
In contrast to previous findings, Gd lesions were more prevalent than ΔFSMTR. This is likely due to the triple-dose contrast and delayed 3T imaging protocol used in the BECOME study,1 which provided unusually high sensitivity to Gd enhancement, as well as the high frequency of scanning. However, while the changes in FSMTR were modest and the difference between treatment groups was small in Gd lesions (0.110 units), ΔFSMTR lesions showed both greater changes in FSMTR and a much greater difference between groups (4.29 units). This may be due to previously described variable colocalization between Gd lesions and FSMTR changes.7 Gd enhancement is a dynamic phenomenon17 and the spatial extent of a Gd lesion at any point in time may not represent well the full spatial extent of the demyelinating lesion. However, the observation that ΔFSMTR lesions were also smaller on average suggests that the additional enhancing tissue detected with the high-sensitivity Gd protocol (relative to what would have been detected with a standard, single-dose, 1.5T protocol) may also have exhibited less breakdown of the blood–brain barrier,18 and less demyelination and remyelination. Overall, despite detecting fewer, smaller, lesions, the ΔFSMTR approach achieved similar power to the analysis in Gd lesions due to detection of much larger changes in FSMTR.
Less prelesion to postlesion FSMTR drop was noted in the GA group in both Gd and ΔFSMTR lesions. This suggests that participants treated with GA, compared to those on IFN-β-1b, experienced less unrepaired lesional damage. Incomplete recovery of FSMTR signal can be the result of a number of processes: (1) the myelin sheaths produced by remyelination are generally thinner and more loosely packed than de novo sheaths,19,20 (2) some axons may survive but remain chronically demyelinated,21 and (3) axons that are transected degenerate and thus are not available for remyelination. Quantitative histopathology studies have found that between 30% and 60% of axons may be lost in postacute MS lesions,4,5,22 suggesting that axonal loss may be the dominant process responsible for incomplete postlesion recovery.
Although both IFN-β-1b and GA reduce inflammation, they accomplish this via different mechanisms. IFN-β-1b impairs the ability of peripheral immune cells to cross the blood–brain barrier, reducing peripherally mediated CNS inflammation and demyelination,23 interferes with antigen presentation,24 and reduces inflammatory B and T cells.25,26 IFN-β-1b may influence remyelination by stimulating release of nerve growth factors by astrocytes27 or through a direct neuroprotective effect.28 GA appears to disproportionally promote the production of GA-specific T-helper 2 cells, which produce anti-inflammatory cytokines23 as well as neurotrophic factors, most prominently brain-derived neurotrophic factor (BDNF).29 GA-specific T-helper cells are cross-reactive to myelin antigens in the CNS, and increase production of both cytokines and BDNF.23,30 Our observations suggest that GA may have been more effective at enhancing remyelination or neuroprotection in this study. Enhanced neuroprotection with GA has been observed in vitro, and through imaging evidence in a clinical trial of CIS.31,32
In a previous study33 we found that IFN-β-1b-treated participants showed a smaller proportion of new brain Gd lesions that remained T1w hypointense (chronic black holes) at 12 months (p = 0.02). Quantitative T1 and FSMTR are correlated in MS lesions, but show different information, with FSMTR being a better measure of myelin content.5,6 T1 may underestimate the amount of myelin in chronic lesions due to increased extracellular water.34 While our present analysis quantifies the amount of FSMTR signal loss using a continuous scale, the previous work counts the number of lesions that recover to a particular threshold, as determined by visual appearance on T1-weighted imaging. The binary (yes/no) rating of lesions on T1-weighted images may correlate poorly with both quantitative T1 relaxation time and FSMTR34; T1-weighted images are a combination of different contrast mechanisms and can exhibit confounding effects, particularly from concomitant changes in T2. The difference in FSMTR between treatments in Gd lesions was modest in both this and our previous study and these methodologic differences could explain the apparent discrepancy. The previous study did not examine ΔFSMTR lesions, where we detected a much greater effect.
Images with a conventional MT pulse were not available, so FSMTR maps computed from images with a FS pulse were validated and used. Although these images were highly correlated with FSMTR, they exhibited lower contrast to noise, limiting statistical power. A further limitation is that the relationship between improved FSMTR recovery and clinical measures of disability has not yet been established.
It is important to note that FSMTR recovery quantifies the residual pathologic changes in lesions that developed on therapy. Interventions that act purely by preventing inflammatory demyelination from occurring at all will not necessarily fare well by this metric, although they are, in the broad sense, even more neuroprotective. GA may have advantages over IFN-β-1b in promoting lesion repair; however, a more complete evaluation of relative benefit requires that the ability to prevent lesions be factored in, for example, by comparing the rate of new lesion formation. This was done for the present study1 and did not identify significant differences between treatment groups. Two large clinical trials contemporary with BECOME also compared IFN and GA. In Rebif vs Glatiramer Acetate in Relapsing MS Disease (REGARD),35 no significant differences were observed in relapses, new T2 lesions, or new T1 lesions; the IFN-treated group had significantly fewer Gd-enhancing lesions. BEYOND36 saw no differences in relapses, Expanded Disability Status Scale progression, or number of T1 or Gd lesions; the IFN group had significantly less T2 lesion volume accrual. A formal meta-analysis from the Cochrane Collaboration, which included data from these 2 studies and BECOME, found no evidence for differences between IFN-β-1b and GA in relapses over 24 months, or number of new T1, T2, or Gd lesions over 24 or 36 months. Significantly lower relapse rates were reported in GA-treated participants with 36 months follow-up; T2 lesion volume increased less in the IFN group over 24 months, but not 36; and T1 lesion volume increased less with IFN over 24 months.37
We analyzed FSMTR recovery in newly formed MS brain lesions that developed during treatment with GA or IFN-β-1b in the BECOME study, and demonstrated significantly less residual FSMTR loss with GA than with IFN-β-1b. The greater stable FSMTR value after acute lesion formation and recovery is consistent with greater myelin density in the chronic lesions that persist after focal inflammatory demyelination. Further study is required to determine the relationship between improved FSMTR recovery and clinical disability.
Both overall changes in FSMTR and differences in FSMTR recovery between treatments were modest in Gd lesions and much more pronounced in ΔFSMTR lesions. Although many more Gd lesions were detected by the high-sensitivity contrast protocol used in BECOME, FSMTR analysis in ΔFSMTR lesions achieved similar power to detect treatment effects. This observation is consistent with previous findings that the ΔFSMTR lesion methodology better identifies tissue experiencing acute FSMTR changes.
Supplementary Material
GLOSSARY
- BDNF
brain-derived neurotrophic factor
- BECOME
Betaseron Versus Copaxone for Relapsing Remitting or CIS Forms of MS Using Triple Dose Gad 3 T MRI
- CIS
clinically isolated syndrome
- FS
fat saturation
- FSMTR
fat saturation producing a magnetization transfer effect, used to create a fat saturation ratio image
- GA
glatiramer acetate
- Gd
gadolinium
- IFN-β-1b
interferon-β-1b
- LFB
Luxol fast blue
- MS
multiple sclerosis
- MTR
magnetization transfer ratio
- NAWM
normal-appearing white matter
- PD
proton density
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Robert Brown performed the image processing, MTR lesion segmentation, data and statistical analyses, and prepared the manuscript. Sridar Narayanan conceptualized the validation study, performed data analysis and interpretation, and revised the manuscript for intellectual content. Nikola Stikov performed data analysis for the validation study and critical review of the manuscript. Stuart Cook was involved in the conception and funding of the study and in critical review of this manuscript. Diego Cadavid was co-principal investigator of the BECOME study and participated in conception of the study and critically reviewed the manuscript. Leo Wolansky was one of two principal investigators for the original BECOME study, designing the MRI protocol and analyzing MRI data. He provided input in design and critical manuscript review for the current study. Douglas Arnold participated in conception and design of study, critical review of manuscript, supervision, and funding of study.
STUDY FUNDING
Funding for the described work was provided by the Rutgers-New Jersey Medical School. R.A.B. received personal funding from the Multiple Sclerosis Society of Canada. The BECOME study was supported by Bayer Schering Pharma, distributors of IFN, but was investigator-initiated and remains the intellectual property of Rutgers-New Jersey Medical School, Newark, NJ.
DISCLOSURE
R. Brown has received personal compensation from NeuroRx Research for consulting services. S. Narayanan has received personal compensation from NeuroRx Research for consulting services. N. Stikov reports no disclosures relevant to the manuscript. S. Cook participated in the original BECOME study. D. Cadavid is presently a full time employee of Biogen, to which the work on the BECOME study is not related, and participated in the original BECOME study. L. Wolansky participated in the original BECOME study and received salary support from Bayer. D. Arnold is the president and CEO of NeuroRx Research. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Cadavid D, Wolansky LJ, Skurnick J, et al. . Efficacy of treatment of MS with IFN-β-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology 2009;72:1976–1983. [DOI] [PubMed] [Google Scholar]
- 2.Wolansky L, Sheynzon V, Biswal B, et al. . Magnetization transfer effect of fat suppression pulses on conspicuity of MS lesions at 3.0T: preliminary results of the “BECOME trial.” Presented at the 2006 annual meeting of the ASNR, May 1–5, 2006, San Diego, CA.
- 3.Dousset V, Grossman RI, Ramer KN, et al. . Experimental allergic encephalomyelitis and multiple sclerosis: lesion characterization with magnetization transfer imaging. Radiology 1992;182:483–491. [DOI] [PubMed] [Google Scholar]
- 4.Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol 2004;56:407–415. [DOI] [PubMed] [Google Scholar]
- 5.Schmierer K, Tozer DJ, Scaravilli F, et al. . Quantitative magnetization transfer imaging in postmortem multiple sclerosis brain. J Magn Reson Imaging 2007;26:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmierer K, Wheeler-Kingshott CA, Tozer DJ, et al. . Quantitative magnetic resonance of postmortem multiple sclerosis brain before and after fixation. Magn Reson Med 2008;59:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown RA, Narayanan S, Arnold DL. Segmentation of magnetization transfer ratio lesions for longitudinal analysis of demyelination and remyelination in multiple sclerosis. Neuroimage 2012;66:103–109. [DOI] [PubMed] [Google Scholar]
- 8.Brown RA, Narayanan S, Arnold DL. Imaging of repeated episodes of demyelination and remyelination in multiple sclerosis. Neuroimage Clin 2014;6:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolansky LJ, Bardini JA, Cook SD, Zimmer AE, Sheffet A, Lee HJ. Triple-dose versus single-dose gadoteridol in multiple sclerosis patients. J Neuroimaging 1994;4:141–145. [DOI] [PubMed] [Google Scholar]
- 10.Collins DL, Peters TM, Evans AC. Automated 3D nonlinear deformation procedure for determination of gross morphometric variability in human brain. Presented at the Proceedings of SPIE; 1994.
- 11.Francis SJ. Automatic Lesion Identification in MRI of Multiple Sclerosis Patients [dissertation]. Montreal: McGill University; 2004. [Google Scholar]
- 12.Bates D, Maechler M, Bolker B. lme4: linear mixed-effects models using S4 classes. R package. Available at: http://CRAN.R-project.org/package=lme4. Accessed April 2016.
- 13.Kuznetsova A, Brockhoff PB. MixMod: analysis of mixed models: R package. Available at: https://cran.r-project.org/web/packages/MixMod/index.html. Accessed April 2016.
- 14.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 2013;4:133–142. [Google Scholar]
- 15.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 16.Richert ND, Ostuni JL, Bash CN, Leist TP, McFarland HF, Frank JA. Interferon beta-1b and intravenous methylprednisolone promote lesion recovery in multiple sclerosis. Mult Scler 2001;7:49–58. [DOI] [PubMed] [Google Scholar]
- 17.Gaitán MI, Shea CD, Evangelou IE, et al. . Evolution of the blood–brain barrier in newly forming multiple sclerosis lesions. Ann Neurol 2011;70:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leppert IR, Narayanan S, Araújo D, et al. . Interpreting therapeutic effect in multiple sclerosis via MRI contrast enhancing lesions: now you see them, now you don't. J Neurol 2014;261:809–816. [DOI] [PubMed] [Google Scholar]
- 19.Brück W, Kuhlmann T, Stadelmann C. Remyelination in multiple sclerosis. J Neurol Sci 2003;206:181–185. [DOI] [PubMed] [Google Scholar]
- 20.Perier O, Gregoire A. Electron microscopic features of multiple sclerosis lesions. Brain 1965;88:937–952. [DOI] [PubMed] [Google Scholar]
- 21.Craner MJ, Lo AC, Black JA, Waxman SG. Abnormal sodium channel distribution in optic nerve axons in a model of inflammatory demyelination. Brain 2003;126:1552–1561. [DOI] [PubMed] [Google Scholar]
- 22.Mews I, Bergmann M, Bunkowski S, Gullotta F, Brück W. Oligodendrocyte and axon pathology in clinically silent multiple sclerosis lesions. Mult Scler 1998;4:55–62. [DOI] [PubMed] [Google Scholar]
- 23.Yong VW. Differential mechanisms of action of interferon-β and glatiramer acetate in MS. Neurology 2002;59:802–808. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H, Milo R, Swoveland P, Johnson KP, Panitch H, Dhib-Jalbut S. Interferon β-lb reduces Interferon γ-induced antigen-presenting capacity of human glial and B cells. J Neuroimmunol 1995;61:17–25. [DOI] [PubMed] [Google Scholar]
- 25.Genc K, Dona DL, Reder AT. Increased CD80 (+) B cells in active multiple sclerosis and reversal by interferon beta-1b therapy. J Clin Invest 1997;99:2664–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teleshova N, Bao W, Kivisakk P, Ozenci V, Mustafa M, Link H. Elevated CD40 ligand expressing blood T-cell levels in multiple sclerosis are reversed by interferon-beta treatment. Scand J Immunol 2000;51:312–320. [DOI] [PubMed] [Google Scholar]
- 27.Boutros T, Croze E, Yong VW. Interferon-β is a potent promoter of nerve growth factor production by astrocytes. J Neurochem 1997;69:939–946. [DOI] [PubMed] [Google Scholar]
- 28.Plioplys AV, Massimini N. Alpha/beta interferon is a neuronal growth factor. Neuroimmunomodulation 1995;2:31–35. [DOI] [PubMed] [Google Scholar]
- 29.Ziemssen T, Kümpfel T, Klinkert WE, Neuhaus O, Hohlfeld R. Glatiramer acetate-specific T-helper 1-and 2-type cell lines produce BDNF: implications for multiple sclerosis therapy. Brain 2002;125:2381–2391. [DOI] [PubMed] [Google Scholar]
- 30.Schrempf W, Ziemssen T. Glatiramer acetate: mechanisms of action in multiple sclerosis. Autoimmun Rev 2007;6:469–475. [DOI] [PubMed] [Google Scholar]
- 31.Khan O, Shen Y, Caon C, et al. . Axonal metabolic recovery and potential neuroprotective effect of glatiramer acetate in relapsing-remitting multiple sclerosis. Mult Scler 2005;11:646–651. [DOI] [PubMed] [Google Scholar]
- 32.Arnold DL, Narayanan S, Antel S. Neuroprotection with glatiramer acetate: evidence from the PreCISe trial. J Neurol 2013;260:1901–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cadavid D, Cheriyan J, Skurnick J, Lincoln JA, Wolansky LJ, Cook SD. New acute and chronic black holes in patients with multiple sclerosis randomised to interferon beta-1b or glatiramer acetate. J Neurol Neurosurg Psychiatry 2009;80:1337–1343. [DOI] [PubMed] [Google Scholar]
- 34.Levesque I, Sled JG, Narayanan S, et al. . The role of edema and demyelination in chronic T1 black holes: a quantitative magnetization transfer study. J Magn Reson Imaging 2005;21:103–110. [DOI] [PubMed] [Google Scholar]
- 35.Mikol DD, Barkhof F, Chang P, et al. . Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs glatiramer acetate in relapsing MS disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol 2008;7:903–914. [DOI] [PubMed] [Google Scholar]
- 36.O'Connor P, Filippi M, Arnason B, et al. . 250 μg or 500 μg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol 2009;8:889–897. [DOI] [PubMed] [Google Scholar]
- 37.La Mantia L, Di Pietrantonj C, Rovaris M, et al. . Interferons-beta versus glatiramer acetate for relapsing-remitting multiple sclerosis. Cochrane Database Syst Rev 2014;26:CD009333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.