Abstract
Objective:
To better understand cross-sectional relationships between CSF and PET measures of tau pathology, we compared regional and global measures of 18F-T807 (AV-1451) PET to CSF protein levels of total tau (t-tau), phosphorylated tau (p-tau), and β-amyloid 1–42 (Aβ42).
Methods:
T-tau, p-tau, and Aβ42 levels were assessed using INNOTEST xMAP immunoassays. Linear regression was used to compare regional and global measures of 18F-T807 standardized uptake value ratios (SUVR) to CSF protein levels using data from 31 cognitively unimpaired elderly participants in the Harvard Aging Brain study.
Results:
After controlling for sex and age, total cortical 18F-T807 binding was significantly correlated with p-tau (partial r = 0.48; p < 0.01) and at trend level with t-tau (partial r = 0.30; p = 0.12). Regional 18F-T807 measures were more strongly correlated with CSF protein levels than the global measure, with both t-tau and p-tau significantly correlated with 18F-T807 SUVR in entorhinal, parahippocampal, and inferior temporal cortical regions (partial r = 0.53–0.73). Peak correlations between CSF and PET measures of tau were similar to those between CSF and PET measures of amyloid burden. Finally, we observed significantly higher temporal T807 SUVR in individuals with high amyloid burden.
Conclusions:
These data support the link between 18F-T807 PET and CSF measures of tau pathology. In these cognitively normal individuals with 18F-T807 binding largely restricted to the temporal lobe, 18F-T807 SUVR in temporal regions appeared more reflective of CSF t-tau and p-tau than a total cortical measure.
Accumulations of β-amyloid (Aβ) and intracellular tau are defining characteristics of Alzheimer disease (AD) pathology. CSF assays of Aβ 1–42 (Aβ42), total tau (t-tau), and tau phosphorylated at residue 181 (p-tau) proteins are commonly used tools for identifying individuals harboring AD pathology. These CSF-based assays are increasingly being supplemented with imaging biomarkers reflecting molecular pathology in vivo. Following the initial description of 11C Pittsburgh compound B (PiB),1 PET-based measures of amyloid pathology have come into increasing use in research and clinical settings. Several groups have demonstrated that PET measures of amyloid burden correlate well with CSF Aβ42 measurements, and can be used in concert with other biomarkers as predictors of impending cognitive decline.2–4
More recently, several promising PET radioligands for in vivo imaging of pathologic deposits of tau have been described.5–8 As postmortem neurofibrillary tangle (NFT) burden is a predictor of global cognition9 and CSF t-tau10 and p-tau11 improve the sensitivity and specificity of CSF Aβ42 alone to identify those likely to progress to AD dementia,2 in vivo imaging of tau pathology may represent a useful biomarker in clinical and translational AD research.
18F-T807 (T807, also known as 18F AV1451) is a radioligand with high selectivity for pathologic tau aggregates (especially NFT pathology5,12) over amyloid plaques, limited nonspecific white and gray matter binding,5 and circumscribed off-target binding.12 Early experience with T807 demonstrates that ligand binding is increased in individuals with cognitive impairment.5,13 The spatial pattern of T807 binding in these early studies conforms with the distribution of NFT pathology seen in histopathologic studies in AD and in cognitively normal older individuals.13 In the present study, we examine the relationship between commonly used CSF biomarkers of AD pathology and regional measures of T807 binding in a group of well-characterized, cognitively normal elderly (CNE) participants in order to better validate T807 PET as an imaging biomarker of tau pathology.
METHODS
Participants.
Thirty-one elderly participants were recruited from the Harvard Aging Brain (HAB) study, a longitudinal examination of cognitive aging and preclinical AD based at Massachusetts General Hospital (MGH) and Brigham and Women's Hospital in Boston, Massachusetts (table 1). At enrollment in HAB, all participants underwent comprehensive medical and neurologic evaluation, and were excluded if a major medical, neurologic, psychiatric, or substance abuse–related illness was present. All participants were assessed as cognitively normal at enrollment in HAB, with global Clinical Dementia Rating14 score of 0.0, Mini-Mental State Examination (MMSE) score of 27–30 (education-adjusted), and within 1.5 SD of education-adjusted norms for the Logical Memory15 delayed paragraph recall portion of the Wechsler Memory Scale–Revised.16
Table 1.
Sample characteristics

Standard protocol approvals, registrations, and patient consents.
Study protocols and procedures were approved by the Partners Human Research Committee. All participants provided written, informed consent prior to completion of any study procedures.
PET image acquisition and processing.
All PET data were acquired using a Siemens/CTI (Knoxville, TN) ECAT HR+ scanner (3D mode, 63 image planes, 15.2 cm axial field of view, 5.6 mm transaxial resolution, and 2.4 mm slice interval) at MGH. Both 11C-PiB and 18F-T807 were produced on-site using previously published protocols.17 11C-PiB PET data were acquired following an 8.5–15 mCi bolus injection followed immediately by a 60-minute dynamic acquisition in 69 frames (12 × 15 seconds, 57 × 60 seconds). 11C-PiB PET data used in the present study are distribution volume ratios (DVR) derived from a cortical composite comprised of frontal, parietal, retrosplenial, and lateral temporal cortical regions (FLR18). T807 PET was acquired 80–100 minutes after a 10.0 ± 1.0 mCi bolus injection in 4 × 5-minute frames using previously published protocols.13,18 PET data were reconstructed and attenuation corrected, and each frame was evaluated to verify adequate count statistics and absence of head motion.
PET images were coregistered to structural images using rigid body registration as implemented in the SPM8 coreg function (Wellcome Department of Cognitive Neurology, London, UK). FreeSurfer (v5.1; http://surfer.nmr.mgh.harvard.edu)–defined cerebellar gray matter was used as a reference region to yield standardized uptake value ratios (SUVR) for T807 and DVR for PiB imaging. T807 SUVR in FreeSurfer-defined regions of interest (ROIs) averaged across both hemispheres was used to assess relationships with CSF biomarkers. Based on prior data describing regional distribution of tau NFT pathology in AD, 9 ROIs spanning early, middle, and late sites of tau deposition were chosen for analyses (table 2).19,20 In addition to these 9 ROIs, FreeSurfer-defined total cerebral cortex ROI (both hemispheres) was used as a total cortical summary measure of T807 signal. Eight of 9 chosen ROIs consisted of 1 FreeSurfer region averaged across both hemispheres; the ninth, the Broca ROI, combined 2 FreeSurfer ROIs (pars opecularis and pars triangularis) averaged across hemispheres. PET data were not partial volume–corrected.
Table 2.
Correlations between T807 standardized uptake value ratio (SUVR) and CSF measures

MRI acquisition.
Participants underwent structural T1-weighted volumetric gradient echo MRI (repetition time 6,400 ms, echo time 2.8, inversion time 900 ms, 8° flip angle, 1 × 1 × 1.2 mm voxels) on a Siemens Trio TIM 3T scanner. Images were processed as described previously, using FreeSurfer for automated segmentation.13,21
Lumbar puncture and CSF protein quantification.
Following an overnight fast, 10–15 mL of CSF was collected using Quincke or Sprotte 22G–24G spinal needles. CSF was immediately centrifuged and stored at −80°C until protein quantification. T-Tau, p-tau, and Aβ42 protein levels were determined via AlzBio3 (Fujirebio, Seguin, TX), a multiplexed immunoassay with fluorometric quantitation (xMAP), using previously described protocols.22 Due to the addition of T807 imaging well after the start of the HAB study, CSF was obtained an average of 2.03 years prior to T807 imaging (figure e-1 at Neurology.org). This delay was examined as a potential covariate in statistical models, and did not significantly contribute to models predicting regional T807 SUVR with CSF protein measures.
Vertex-wise cortical analysis.
Exploratory vertex-wise analyses of the cortical mantle were performed using standard linear regression as implemented in GLM Flex Fast (mrtools.mgh.harvard.edu). T807 data for each participant were projected to the cortical surface using Freesurfer (utilizing the FS Average template), and smoothed on the surface with the equivalent of a 10-mm full width at half maximum smoothing kernel.
Statistical approach.
Statistical analyses were performed in R (version 3.2.0, R Foundation for Statistical Computing) with nominal p values shown. Sex, age, time between CSF collection and T807 imaging, APOE ε4 status, and FreeSurfer-defined thickness or volume measures for each region were examined as covariates in linear models predicting regional T807 SUVR (table 2). To avoid model overfitting, final models included only sex, age, and CSF protein values as predictors of regional T807 SUVR. Models including more covariates showed similar correlation patterns between CSF and PET measures. In comparing T807 SUVR to previously defined pathologic cutoffs for the tau:CSF Aβ42 ratio,23 one outlying value (ratio 1.2; CSF Aβ42 144.8 pg/mL; t-tau 174.6 pg/mL; p-tau 70.2 pg/mL; inferior temporal T807 SUVR 1.29; total cortical T807 SUVR 1.12) was dropped from the analysis due to Cook d > 1 for all analyses (Cook d = 6.85 for inferior temporal and 1.35 for total cortical analyses).
RESULTS
Demographics for the study population were similar to those reported for the larger HAB study24 (tables 1 and e-1). No significant differences between the study population and the larger HAB sample were observed with respect to age, APOE ε4 status, sex, MMSE, Logical memory Immediate and Delayed Recall, or Trails A (all p > 0.05; table e-1). However, participants in the present study had significantly faster times on the Trails B task as compared with the larger HAB study population (t[304] = −2.01, p = 0.046).
Mean T807 SUVR in each FreeSurfer-defined ROI is summarized in figure 1. Nine ROIs and a total cortical ROI were selected for comparison to CSF, based on prior reports.13,19,20 Consonant with a previous study from our group,13 temporal lobe regions (both neocortical and limbic) showed the highest T807 binding in these CNE individuals (tables 1 and 2). Within the temporal neocortex, progressively lower T807 SUVR was seen when moving from the inferior to middle to superior temporal ROIs (figure 1 and table 2).
Figure 1. Mean T807 standardized uptake value ratio (SUVR) in FreeSurfer-defined regions of interest (ROIs).
FreeSurfer automated segmentation was used to identify ROIs for analysis. To illustrate the global pattern of T807 signal, the mean value for each FreeSurfer ROI is shown, with lighter colors indicating higher mean binding.
CSF t-tau and p-tau.
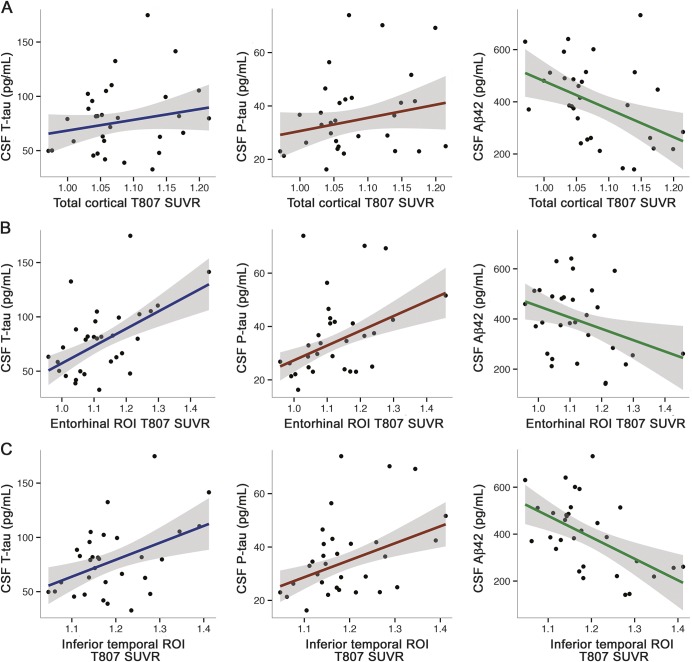
Total cortical T807 SUVR significantly correlated with p-tau but not t-tau (table 2 and figure 2A). Stronger correlations were observed between CSF and T807 SUVR in temporal lobe regions with relatively high levels of signal as compared to the total cortical ROI signal (figure 1 and table 2). Among limbic regions of the temporal lobe, entorhinal and parahippocampal T807 binding significantly correlated with CSF t-tau and p-tau (table 2 and figure 2B). T807 binding in the temporal pole correlated significantly with p-tau and at trend level with t-tau.
Figure 2. CSF biomarker correlations with T807 standardized uptake value ratio (SUVR).
T807 SUVR plotted with respect to CSF total tau (t-tau) (blue, left), phosphorylated tau (p-tau) (red, middle), and β-amyloid 1–42 (Aβ42) (green, right) in a total cortical ROI (A), the entorhinal cortex (B), and the inferior temporal cortex (C). Scatterplots shown are uncorrected for age and sex.
T807 SUVR in the inferior temporal neocortex (ITC) and middle temporal neocortex (MTC) significantly correlated with both t-tau and p-tau (figure 2C and table 2). Superior temporal cortical T807 SUVR was significantly correlated with p-tau, but not with t-tau. Except for a marginally significant relationship between p-tau and inferior parietal T807 SUVR (partial r = 0.38, p = 0.042), all selected ROIs showing significant correlations with CSF protein levels were within the temporal lobe in this sample of unimpaired participants. The peak correlation between CSF tau measures and T807 SUVR was seen in the ITC (p-tau: partial r = 0.73; p < 0.001).
To confirm that our choice of ROIs was appropriate for the topography of T807 and range of CSF protein values in this sample, we also performed an exploratory, vertex-wise, whole brain analysis (figure e-2). The majority of vertices showing strong correlations between PET and p-tau were in the medial and lateral temporal lobes, though small clusters of correlated vertices were also observed along the posterior midline. This pattern fits well with the prior observation that T807 in temporal ROIs was more highly correlated with CSF measures of t-tau and p-tau than T807 SUVR in frontal and parietal ROIs, and validates the choice of ROIs for this sample.
CSF Aβ42 and amyloid burden as measured by PET.
Lower CSF Aβ42 significantly correlated with higher T807 SUVR in temporal neocortical (ITC, MTC, superior temporal cortex) but not limbic regions (entorhinal, parahippocampal, temporal pole; table 2 and figure 2, B and C). CSF Aβ42 was a trend-level predictor of total cortical T807 SUVR (table 2). Examining interrelationships between CSF measures, we observed that Aβ42 was correlated at trend level with p-tau (partial r = −0.354, p = 0.059) and not significantly correlated with t-tau (partial r = −0.096, p = 0.621). As expected based on prior studies, a negative correlation between CSF Aβ42 and PiB FLR was observed (partial r = −0.65, p < 0.001). Used continuously, PiB FLR significantly predicted total cortical T807 SUVR (partial r = 0.380, p = 0.042).
CSF cutpoints and T807.
Prior work using data from the Alzheimer's Disease Neuroimaging Initiative (ADNI) and the same method of CSF protein quantification used in the present study (INNOTEST AlzBio3 xMAP platform) has identified optimal CSF cutpoints for separating individuals likely to progress from mild cognitive impairment (MCI) to AD dementia.23 Two separate cutpoints were derived—t-tau: CSF Aβ42 ratio >0.39 and CSF Aβ42 <192 pg/mL—and both were examined with respect to T807 SUVR in our sample (figure 3). The t-tau:CSF Aβ42 ratio was significantly correlated with total cortical (partial r = 0.40, p = 0.03) and ITC T807 SUVR (partial r = 0.62, p < 0.001). As expected in this cognitively normal sample, relatively few of the cognitively normal elderly participants in our sample were classified as having CSF in the pathologic range using these cutpoints (4 and 2 individuals for the t-tau:CSF Aβ42 ratio and CSF Aβ42 cutpoints, respectively). Both cutpoints reflected similar corresponding values for total cortical and inferior temporal T807 SUVR values in this sample (figure 3).
Figure 3. Comparison of Alzheimer's Disease Neuroimaging Initiative (ADNI)–defined CSF cutpoints to T807 standardized uptake value ratio (SUVR).
Shaw et al.23 identified a CSF total tau:CSF β-amyloid 1–42 (Aβ42) ratio cutpoint of >0.39 (A, B) and a CSF Aβ42 <192 pg/mL cutpoint (C, D) for individuals more likely to progress from mild cognitive impairment to Alzheimer disease dementia in the ADNI sample. These cutpoints are shown in relation to total cortical (A, C) and inferior temporal (B, D) T807 SUVR. Aβ = β-amyloid.
DISCUSSION
Comparison of CSF-based AD biomarkers to the pattern of binding in T807 PET is a key step in validating this novel radioligand. Utilizing data from 31 cognitively normal elderly participants across a series of ROIs that ranged from early to late sites of NFT pathology in AD, we observed that CSF measures of t-tau and p-tau significantly correlated with T807 SUVR in neocortical and limbic regions of the temporal lobe. P-tau was particularly well-correlated with T807 SUVR in temporal limbic and neocortical regions, including the entorhinal, parahippocampal, inferior, middle, and superior temporal cortex. Weaker relationships between T807 SUVR and CSF measures of tau were seen in parietal and frontal regions, as well as with a total cortical measure, consistent with the expectation that NFT pathology would be relatively limited to the temporal lobe in our unimpaired sample.
The peak correlation between p-tau and T807 SUVR (partial r = 0.73 in the ITC) is similar to the observed correlation between CSF Aβ42 and PiB FLR in this relatively small dataset (partial r = −0.65). In addition, we observed that lower CSF Aβ42 predicted higher T807 binding in temporal neocortical regions, suggesting cognitively normal individuals harboring a high amyloid burden may show increased tau pathology in these regions. In contrast, we did not observe a significant relationship between CSF Aβ42 and T807 SUVR in limbic temporal regions in this dataset. Though a great deal more work in larger samples and in impaired populations is needed to examine this possible distinction, these results are consistent with the concept that high amyloid deposition may be more strongly associated with neocortical tau deposition as compared to tau deposition in limbic temporal regions.
Established cutpoints for the INNOVA AlzBio3 xMAP platform derived from the separation of ADNI MCI progressors vs nonprogressors were applied to this dataset.23 Cutpoints using CSF Aβ42 and t-tau:CSF Aβ42 ratio yielded very similar corresponding ITC and total cortical T807 SUVR values. Focusing on the ITC, the T807 SUVR values that correspond to the CSF cutpoints reported by Shaw et al.23 (1.28 and 1.26, reflecting the ratio and CSF Aβ42 cutpoints, respectively) fit well with a recent report from our group in which separation between cognitively normal and MCI populations was seen in a similar range of T807 SUVR values.13 However, as only a small number of our sample fell within the pathologic range using these previously described cutpoints, further study in more impaired individuals will be needed to better establish T807 SUVR values that correspond to these CSF cutpoints.
Taken together, the observed correlations between CSF measures of tau pathology and T807 SUVR support the use of T807 PET as an in vivo imaging tool to assess tau pathology. Unlike PET-based measures of amyloid pathology (which are often highly collinear across frontal, temporal, and parietal cortical regions), these data highlight that PET measures of tau pathology may need to be considered regionally rather than globally. Further, the regional focus may vary based on the severity of the presumed tau pathology in a particular study population, and may well differ in more impaired vs cognitively normal individuals. Going forward, further work will be needed to identify the most informative regional patterns of T807 binding to answer specific research questions, such as prediction of cognitive performance in impaired populations, and discrimination of individuals at high and low risk of progression to AD dementia.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the participants of the Harvard Aging Brain study.
GLOSSARY
- Aβ
β-amyloid
- Aβ42
β-amyloid 1–42
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- CNE
cognitively normal elderly
- DVR
distribution volume ratio
- FLR
frontal, parietal, retrosplenial, and lateral temporal cortical regions
- HAB
Harvard Aging Brain
- ITC
inferior temporal neocortex
- MCI
mild cognitive impairment
- MGH
Massachusetts General Hospital
- MMSE
Mini-Mental State Examination
- MTC
middle temporal neocortex
- NFT
neurofibrillary tangle
- p-tau
phosphorylated tau
- PiB
Pittsburgh compound B
- ROI
region of interest
- SUVR
standardized uptake value ratio
- t-tau
total tau
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
K.A.J., J.P.C., A.P.S., R.A.S., and B.T.H. contributed to conceptualization of the study, analysis and interpretation of data, and drafting and revising the report. In addition, J.P.C., A.P.S., and K.A.J. contributed to data collection and statistical analysis. J.D., B.B., T.G.-I., C.R.S., and G.A.M. contributed to the interpretation of data and to the drafting and revision of the report. A.D.R. and M.L. contributed to drafting and revision of the report and data collection and provided technical support.
STUDY FUNDING
Data collection for this project was supported by the National Institute on Aging via P01 AG36694 (HAB; Sperling/Johnson PIs), R01 AG046396 (Johnson PI), and the Massachusetts Alzheimer's Disease Research Center (P50 AG005134). Additional support for this project was provided by the Harvard NeuroDiscovery Center Biomarker Study, supported in part by the Parkinson's Disease Biomarkers Program (NINDS U01 NS082157) and Rick and Nancy Moskovitz. The Partners Clinical Research Center provided additional support to this project through grant numbers 8UL1 TR000170-05 and 1UL1 TR001102-02, Harvard Clinical and Translational Science Center, from the National Center for Advancing Translational Science. Fellowship support for J.P.C. was provided by the BrightFocus Foundation, the American Brain Foundation/American Academy of Neurology, and the NIA (K23 AG049087). C.R.S.' work was supported in part by NIH grant U01 NS082157, the US Department of Defense, and the M.E.M.O. Hoffman Foundation.
DISCLOSURE
J. Chhatwal receives grant support from NIH (K23 AG049087) and has received grant support from the BrightFocus Foundation, the American Brain Foundation, and the American Academy of Neurology. A. Schultz, G. Marshall, B. Boot, T. Gomez-Isla, J. Durmurgier, A. Roe, M. LaPoint, and C. Scherzer report no disclosures relevant to the manuscript. B. Hyman reports grants from NIH during the conduct of the study, grants and personal fees from Siemens, personal fees from Calico, ISIS Pharma, Lilly, Neurophage, Pfizer, Biogen, Genentech, Sanofi, and AbbVie, and personal fees and other from Novartis, grants from BMS, AZTherapy, Acumen, Prothena, Fidelity Biosciences, Spark, and Intellect, outside the submitted work. R. Sperling has provided providing consulting services for Roche, Genentech, Biogen and Bracket, received support from a joint NIH-Lilly-sponsored clinical trial (A4 Study U19AG10483) and research funding from the NIH/National Institute on Aging (R01 AG046396, P01 AG036694, P50 AG00513421) and the Alzheimer's Association. B.B. is a full-time employee of Biogen. K. Johnson has provided consulting services for Lilly, Novartis, Janssen, Roche, Piramal, GE Healthcare, Siemens, ISIS Pharma, AZTherapy, and Biogen, received support from a joint NIH-Lilly-sponsored clinical trial (A4 Study U19AG10483), and received research support from NIH/National Institute on Aging (R01 AG046396, P01 AG036694, P50 AG00513421, U19AG10483, U01AG024904-S1), Fidelity Biosciences, the Michael J. Fox Foundation, and the Alzheimer's Association. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh compound-B. Ann Neurol 2004;55:306–319. [DOI] [PubMed] [Google Scholar]
- 2.Roe CM, Fagan AM, Grant EA, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology 2013;80:1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mormino EC, Betensky RA, Hedden T, et al. Synergistic effect of β-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol 2014;71:1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack CR Jr, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol 2012;71:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia CF, Arteaga J, Chen G, et al. [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer's disease. Alzheimers Dement 2013;9:666–676. [DOI] [PubMed] [Google Scholar]
- 6.Fodero-Tavoletti MT, Furumoto S, Taylor L, et al. Assessing THK523 selectivity for tau deposits in Alzheimer's disease and non-Alzheimer's disease tauopathies. Alzheimers Res Ther 2014;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamura N, Furumoto S, Harada R, et al. Novel 18F-labeled arylquinoline derivatives for noninvasive imaging of tau pathology in Alzheimer disease. J Nucl Med 2013;54:1420–1427. [DOI] [PubMed] [Google Scholar]
- 8.Harada R, Okamura N, Furumoto S, et al. Comparison of the binding characteristics of [18F]THK-523 and other amyloid imaging tracers to Alzheimer's disease pathology. Eur J Nucl Med Mol Imaging 2013;40:125–132. [DOI] [PubMed] [Google Scholar]
- 9.Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 2012;71:362–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreasen N, Minthon L, Davidsson P, et al. Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol 2001;58:373–379. [DOI] [PubMed] [Google Scholar]
- 11.Schoonenboom NSM, Pijnenburg YAL, Mulder C, et al. Amyloid beta(1-42) and phosphorylated tau in CSF as markers for early-onset Alzheimer disease. Neurology 2004;62:1580–1584. [DOI] [PubMed] [Google Scholar]
- 12.Marquié M, Normandin MD, Vanderburg CR, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol 2015;78:787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson KA, Schultz A, Betensky RA, et al. Tau PET imaging in aging and early Alzheimer's disease. Ann Neurol 2016;79:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 16.Weschler D. WMS-R: Weschler Memory Scale-Revised Manual. New York: Psychological Corporation/HBJ; 1987. [Google Scholar]
- 17.Shoup TM, Yokell DL, Rice PA, et al. A concise radiosynthesis of the tau radiopharmaceutical, [(18)F]T807. J Labelled Comp Radiopharm 2013;56:736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol 2007;62:229–234. [DOI] [PubMed] [Google Scholar]
- 19.Delacourte A, David JP, Sergeant N, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer's disease. Neurology 1999;52:1158–1165. [DOI] [PubMed] [Google Scholar]
- 20.Villemagne VL, Fodero-Tavoletti MT, Masters CL, Rowe CC. Tau imaging: early progress and future directions. Lancet Neurol 2015;14:114–124. [DOI] [PubMed] [Google Scholar]
- 21.Mormino EC, Betensky RA, Hedden T, et al. Amyloid and APOE ε4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology 2014;82:1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toledo JB, Zetterberg H, van Harten AC, et al. Alzheimer's disease cerebrospinal fluid biomarker in cognitively normal subjects. Brain 2015;138:2701–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol 2011;121:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dagley A, LaPoint M, Huijbers W, et al. Harvard Aging Brain Study: dataset and accessibility. Neuroimage Epub 2015 Apr 3. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.