Abstract
Regulatory T-cells (Tregs) limit autoimmunity and immunopathology using a variety of suppressive mechanisms, but their roles during pathogen-directed immune responses remain unclear. Following Herpes Simplex virus-2 (HSV-2) infection, mice lacking Tregs fail to control viral replication, pointing to a role for Tregs in facilitating productive immune responses. Using adoptive transfer of TCR transgenic CD4 T-cells into Treg-sufficient or Treg-depleted mice prior to HSV-2 infection, we found that Tregs are required for timely accumulation of HSV-2-specific CD4 T-cells within the infected tissues. Further, Tregs are critical for appropriate trafficking of dendritic cells (DCs) from the vaginal mucosa to the dLN, which results in fully effective CD4 T-cell priming, activation, and ultimately migration to the infected tissues. Using CTLA-4 conditional knockout mice, we demonstrate that Tregs impact DC migration through a CTLA-4-mediated mechanism. Together, our data highlight the critical role of Tregs in proper potentiation of adaptive immune responses to microbial infection.
Introduction
Regulatory T-cells (Tregs) are a subset of CD4 T-cells that are essential for maintaining peripheral tolerance 1, 2, yet their precise role during infections remains an active area of investigation 3–5. In the context of several infections, Tregs are required during the immune response to prevent an overly robust response that causes excessive collateral damage to self-tissue. In these cases, when Tregs are absent during the infection, the immune response is more robust and able to clear the pathogen more quickly, albeit with the risk of elevated immunopathology 3,6–10. Conversely, in other cases, the removal of Tregs prior to infection results in delayed clearance of the pathogen, suggesting that the presence of Tregs can be beneficial in facilitating an appropriately robust and protective immune response 11–14. These differing results emphasize that the role played by Tregs during infections is context-dependent.
In the setting of intravaginal (ivag) infection with HSV-2, mice acutely depleted of Tregs suffer from a higher viral burden within the vaginal tissues. The virus also infects the central nervous system more quickly in Treg-depleted mice, causing significantly earlier death 12. These observations are consistent with a diminished anti-viral immune response rather than the overly robust immune response that would be expected if the primary role of Tregs were to dampen the immune response and limit immunopathology. Consistent with a less effective immune response, Treg-depleted mice showed very early dysregulation of effector cell migration to the infected tissue 12. However, because a wild-type HSV-2 infection is rapidly lethal to Treg-depleted mice, the effect of Treg depletion on the adaptive immune response to mucosal infection remains unclear.
In most infection models that have been studied, Tregs do not appear to have a major impact on the initiation of an antigen-specific T-cell response, but rather modulate the size and intensity of the T-cell response that develops to target a potential pathogen. Early studies relying on infection with L. major, Pneumocystis carinii, HSV-1, Friend virus, Plasmodium yoelii, and others all found that when the immune response develops in the absence of Tregs, the resultant effector response is more robust and better able to target the pathogen. These studies collectively supported what has become the dominant model in the field; that Tregs are primarily important during an immune response to limit immunopathology by preventing an excessively robust effector response 6,7,10,15–18.
In contrast, the adaptive immune response was less effective in mice lacking Tregs following intranasal infection with RSV or oral infection with C. albicans. In the case of RSV, CD8 T-cell migration to the infected tissue was delayed in the absence of Tregs and in the case of C. albicans, the T-cell response was not skewed towards the expected Th17 response when Tregs were absent 11,13,14. In some models, Tregs also limit the engagement of low affinity clones of CD8 T-cells with antigen-bearing DCs so that in the absence of Tregs, a greater share of low affinity clones are activated 19. While these studies differed in their focus, and Tregs may affect both CD4 and CD8 T-cells differently, they do suggest that Tregs can also affect qualitative aspects of adaptive immunity in certain contexts.
Despite the early changes that were observed in the immune response following ivag HSV-2 infection in Treg-depleted mice, how Tregs affect the adaptive immune response is not known because ivag infection with wild-type HSV-2 is quickly lethal to mice, especially those that lack Tregs at the time of infection. The dominant model in the field that Tregs primarily modulate the size and strength of the T-cell response suggests that in the absence of Tregs, there should be more antigen-specific T-cells primed and targeting the virus in the infected tissue. Thus, to investigate the role of Tregs during the adaptive immune response against ivag HSV-2, we used a well-characterized attenuated strain of HSV-2 that mimics the wild-type virus during the initial infection of epithelial cells, but cannot productively infect neurons, allowing for the survival of infected mice 20. To assess the role of Tregs during the adaptive immune response against attenuated HSV-2, we used Foxp3DTR mice. Foxp3DTR mice have been engineered to express the human diphtheria toxin receptor (hDTR) under the control of the Foxp3 promoter, thereby allowing for the targeted depletion of Tregs following the administration of diphtheria toxin (DT) 2.
T-cell production of IFNγ at the infected tissue is the primary mechanism of viral control in the HSV-2 mouse model 21, with CD4 T-cells being the most important cell population for viral control 21,22. CD4 T-cell priming begins after antigen is carried to the draining lymph nodes (dLN) by migratory CD11b+ dendritic cells (DCs) originating in the infected tissue. Free virus does not travel to the dLN, therefore, these migratory DCs are fully responsible for CD4 T-cell priming 23. After priming, CD4 T-cells begin entering the infected tissue starting at approximately day four and are most abundant six days after infection. CD8 T-cells do not enter the tissue unless CD4 T-cells have already done so, thereby further implicating CD4 T-cells as critically essential for viral control 24. In the infected tissue, inflammatory monocytes process viral antigen and induce IFNγ production from antigen-specific T-cells. This leads to a characteristic adaptive phase wave of IFNγ in the infected vaginal tract beginning at four days post-infection 25.
Using the model of attenuated HSV-2 infection in combination with HSV-2 specific TCR transgenic T-cells, we investigated the role of Tregs in the antigen-specific CD4 T-cell response to a mucosal virus infection. Also, as the CD4 T-cell response is dependent on priming by tissue-derived migratory DCs, we examined the role of Tregs on DC migration from the infected tissues as well as antigen presentation to CD4 T-cells. Here, we demonstrate that contrary to expectations, the antigen-specific CD4 T-cell response in the tissue is severely diminished in the absence of Tregs. Furthermore, this absence seems to be the downstream effect of inefficient priming of the antigen-specific CD4 T-cell response, a phenotype that has not yet been observed in other studies that have addressed the role of Tregs during infection. Overall, our data further the understanding of the role of Tregs in the maintenance of immune homeostasis, particularly within the context of microbial invasion.
Results
HSV-2-specific CD4+ T-cells fail to accumulate in the vagina in the absence of Tregs
Our previous studies utilized wild-type HSV-2, which is lethal in mice and leads to death in Treg-depleted mice as early as day six post-infection 12. Therefore, we first wanted to validate the use of the attenuated HSV-2 186Δkpn as a viable model to study the adaptive immune response following ivag HSV-2 infection. As expected, mice depleted of Tregs prior to infection showed delayed clearance of the virus from the infected tissue (data not shown).
In wild type mice, the influx of antigen-specific CD4 T-cells into the infected tissue causes localized production of IFNγ, a step that is necessary for viral control 21,24. Earlier work has demonstrated decreased IFNγ production in the infected tissue of Treg depleted mice, despite the presence of bulk CD4 T cells, suggesting that the adaptive immune response may be diminished in Treg depleted mice 12. We also saw significantly less IFNγ production at four days post-infection within the infected tissue of Treg-depleted mice that were infected with the attenuated strain of HSV-2 (Fig. 1a). Therefore, we used this model to explore the role Tregs play in development of the antigen-specific CD4 T-cell response following ivag HSV-2 infection.
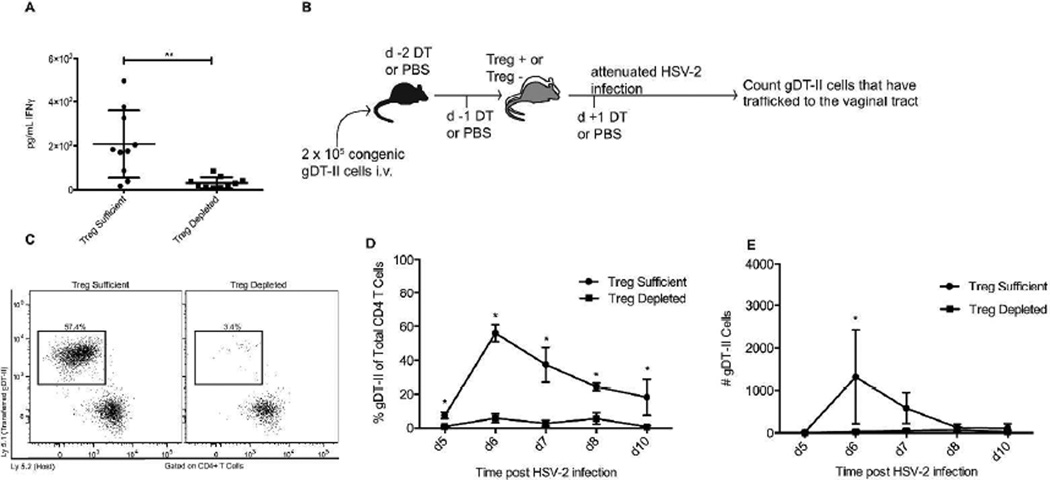
Figure 1. HSV-specific CD4 T-cells fail to accumulate in the vagina in the absence of Tregs.
Foxp3DTR mice were injected with DT on days −2, −1, and +1 relative to the day of infection. Alternatively, Treg-sufficient mice were injected with PBS instead of DT. Mice were infected intravaginally with 1 × 106 (a) or 1.88 × 105 (c-e) PFU of HSV-2 186ΔKpn, while naïve groups were left uninfected. a) Concentration of IFNγ within vaginal washes from mice infected for 4d with HSV-2. b) Experimental setup for tracking antigen-specific CD4 T-cells to the infected tissue. c) Representative flow plots showing gDT-II cells in the vaginal tract of Treg-sufficient and Treg-depleted mice 6d after intravaginal HSV-2 infection. Samples were gated on CD4 T-cells and the plots show Ly5.1 (transferred gDT-II cells) versus Ly5.2 (host cells). d) Representative data showing the fraction of total vaginal tract CD4 T-cells consisting of antigen-specific gDT-II cells at various time points after infection. e) Representative data showing the number of gDT-II cells recovered from mouse vaginal tracts. Data in A are taken from at least two independent experiments with 4–5 mice per group. Data from day 6 in D-E come from or are representative of at least 2 independent experiments with 4–5 mice per group. Data from days 5, 7, 8, and 10 come from one experiment with 4 mice per group. Each data set was first screened for outliers using the ROUT test with a Q value of 0.2%. Statistical significance in A was determined using an unpaired, two-tailed t-test. **: p ≤ 0.01. Statistical significance in D-E was determined using multiple t tests with correction for multiple comparisons using the Holm-Sidak method. *: significance determined with an alpha of 0.05.
We initially attempted to enumerate HSV-specific T-cells by stimulating cells ex vivo with inactivated HSV to produce IFNγ, but this technique resulted in many T-cells from naïve, Treg-depleted mice producing cytokines following stimulation with inactivated virus. This high level of background cytokine production in Treg-depleted mice made it impossible to distinguish cells that truly recognize viral antigen from cells that simply produced cytokine because they came out of a strongly pro-inflammatory environment. Thus, we instead relied on the transfer of T-cell receptor transgenic cells that express T-cell receptors with known specificity 26.
To assess the migration of HSV-2-specific CD4 T-cells to the infected tissue in mice lacking Tregs at the time of infection, we transferred congenically marked, TCR transgenic gDT-II cells that recognize an HSV glycoprotein D epitope 27,28 into Foxp3DTR mice, treated the mice with DT to deplete Tregs, and subsequently tracked the transferred cells’ migration into the infected vaginal tract starting five days post-infection, (Fig. 1b). As expected, Treg-sufficient mice showed an influx of CD4 T-cells into the vaginal tract following infection and this CD4 T-cell response was dominated by the congenically labeled gDT-II cells. Consistent with previous research, the greatest infiltration was seen at day six post-infection 24. Conversely, while Treg-depleted mice showed an influx of bulk CD4 T-cells into the vaginal tract following infection, the transferred gDT-II cells represented a minute fraction of the CD4 T-cell pool (Fig. 1c–d). Similarly, the total number of gDT-II cells that migrated to the infected tissue in Treg-sufficient mice was significantly higher than the number of gDT-II cells that migrated into the infected tissue of Treg-depleted mice (Fig. 1e). The number of gDT-II cells in the vaginal tracts of Treg-depleted mice remained low until at least ten days post-infection, a time when effector cells are contracting in wild type mice.
The significant decrease in the number of gDT-II cells entering the infected tissue of Treg-depleted mice supports the possibility that the non-gDT-II CD4 T cells that are present in the infected tissue are not HSV-2-specific T-cells, but rather these cells may be polyclonal CD4 T-cells activated because of the robust inflammatory conditions following Treg depletion, and then directed toward the vaginal tract due to virus-induced inflammation. This suggests that mice lacking Tregs at the time of HSV-2 infection are unable to properly combat HSV-2 in the infected tissue with an appropriate CD4 T-cell response.
Treg presence during T-cell priming is critical for subsequent accumulation of antigen-specific CD4 T-cells in the infected tissues
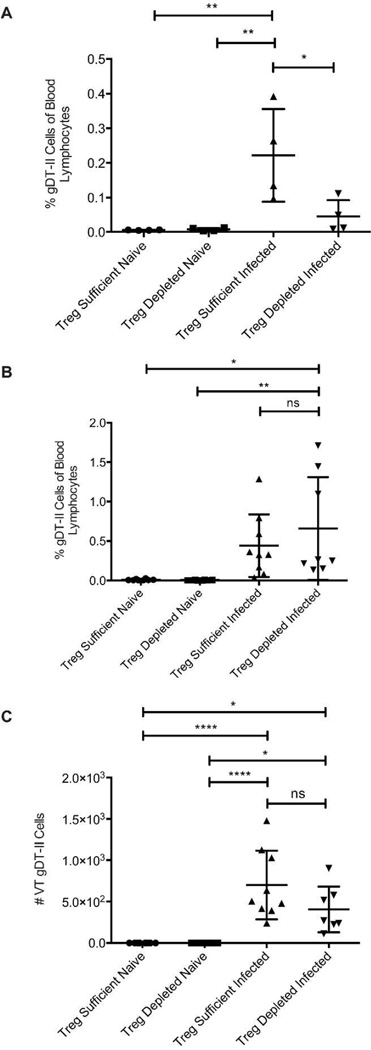
We next examined the blood of Treg-depleted mice to determine if there is an equal share of circulating gDT-II cells six days after infection as compared to Treg-replete mice. Similar to the vaginal tract, mice lacking Tregs at the time of infection had significantly fewer gDT-II cells circulating in the blood (Fig. 2a), suggesting that the decreased entry of gDT-II cells into the vaginal tract was likely caused by inefficient activation, clonal expansion, and lymph node egress of the cells as opposed to the inability to properly target the vaginal tract with activated cells from the blood. To test this idea further, we delayed administration of DT until three days after infection. At this time point, T-cell priming is underway, although very few cells have yet migrated to the infected tissue 24. When we delayed Treg depletion to a time at which T-cell priming and activation is largely completed and cells have entered the migration stage, gDT-II cells were as prevalent in the blood of Treg-depleted mice as in Treg-sufficient controls (Fig. 2b). Similarly, the number of gDT-II cells entering the infected tissue was essentially unaffected by the absence of Tregs during this phase (Fig. 2c). Together, these data suggest that the curtailed number of gDT-II cells entering the vaginal tract of mice lacking Tregs at the time of infection (Fig. 1) is not due to a migration defect of T-cells in Treg-depleted mice, but points to the potential necessity of Tregs during the initial T-cell priming phase.
Figure 2. Absence of Tregs in the post-priming phase does not diminish the antigen-specific CD4 T-cell accumulation in the vagina.
Mice were depleted of Tregs at days −2, 1, and +1 (a) or at days +3 and +4 (b and c) relative to the day of infection. Alternatively, Treg-sufficient mice were injected with PBS instead of DT. Mice were infected intravaginally with 1.88 × 105 PFU of HSV-2 186ΔKpn, while naïve groups were left uninfected. a) The fraction of gDT-II cells in the blood as a percent of total blood lymphocytes 6d after infection when Tregs were depleted prior to infection. b) The fraction of gDT-II cells in the blood as a percent of total blood lymphocytes 6d after infection when Tregs were depleted during the post-priming, migration phase of the immune response. c) The number of gDT-II cells recovered from the vaginal tracts of mice 6d after infection when Tregs were depleted during the post-priming, migration phase of the immune response. All data come from or are representative of at least 2 independent experiments with 4–5 mice in each group. Each data set was first screened for outliers using the ROUT test with a Q value of 0.2%. Statistical significance was then determined using an ordinary one-way ANOVA followed by a Tukey test to compare the mean of each group with the mean of all other groups. *: p ≤ 0.01. Error bars show standard deviation.
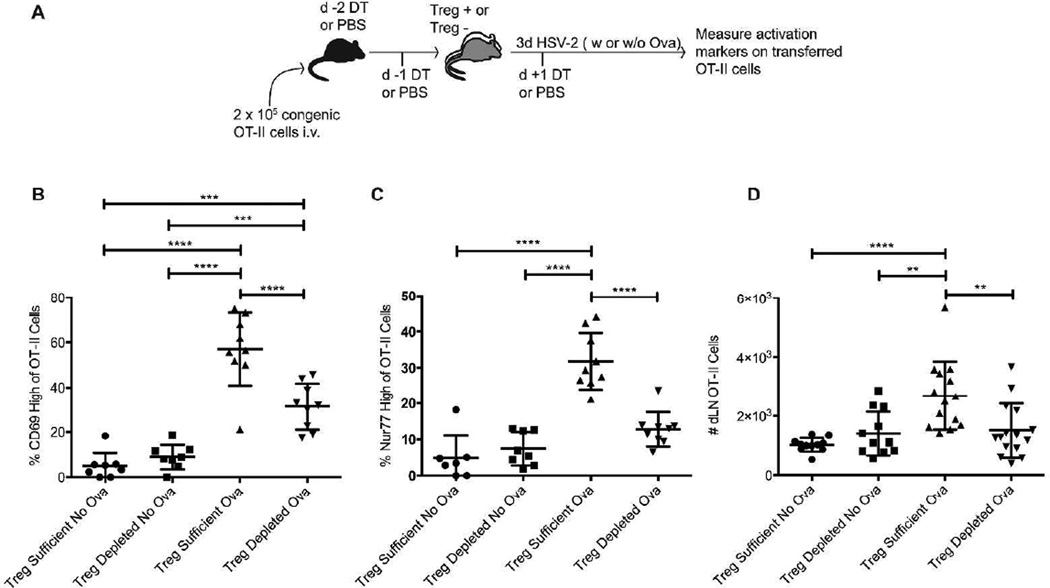
To test this hypothesis, we transferred antigen-specific CD4 T-cells into Foxp3DTR mice, depleted the mice of Tregs, infected the mice ivag with HSV-2 and then measured the activation of the transferred cells three days post-infection, a time when T-cell priming has just begun in this model 23 (Fig. 3a). In these experiments, we used TCR transgenic OT-II cells, which recognize a peptide from the chicken ovalbumin (OVA) protein, and infected the mice with a strain of attenuated HSV-2 that expresses OVA 29. As a control, attenuated HSV-2 lacking OVA expression was used for infection (“no OVA” group), enabling comparison of OT-II activation in the absence of cognate antigen but under otherwise similar in vivo conditions. OT-II cells transferred into Treg-sufficient mice infected with HSV-2-OVA showed increased expression of both CD69 and Nur77 three days post-infection, suggesting that antigen recognition had occurred, thereby triggering T-cell activation (Fig. 3b–c). OT-II cells transferred into Treg-depleted, HSV-2-OVA infected mice, however, showed very little upregulation of these markers. These data suggest that the transferred OT-II cells in the Treg-depleted mice did not “see” antigen and therefore were not activated as efficiently as the OT-II cells in Treg-sufficient mice. In addition, we found significantly more OT-II cells in the dLNs of Treg-sufficient mice as compared to Treg-depleted mice three days post- infection, likely because the OT-II cells in the Treg-sufficient mice had begun to divide more efficiently than the same cells in the Treg-depleted mice (Fig. 3d). Together, these data suggest impaired CD4 T-cell priming in Treg-depleted mice and are consistent with the diminished entry of antigen-specific cells into the infected tissue (Fig. 1) and subsequent delay in viral clearance.
Figure 3. Antigen-specific CD4 T-cell activation and priming following HSV-2 infection are compromised in the absence of Tregs.
a) Experimental setup. Foxp3DTR mice were injected with DT on days −2, −1, and +1 relative to the day of infection. Alternatively, Treg-sufficient mice were injected with PBS instead of DT. Mice were infected intravaginally with 1.88 × 105 PFU of HSV-2 186ΔKpn expressing OVA (OVA groups), while no OVA groups were infected with the same quantity of HSV-2 186ΔKpn. b) Percent of transferred OT-II cells showing increased expression of CD69 in the dLN of 3d infected mice. c) Percent of transferred OT-II cells showing increased expression of Nur77 in the dLN of 3d infected mice. d) The number of OT-II cells recovered from the dLN of mice 3d after infection. All data come from 2–3 experiments with 4–5 mice in each group. Each data set was first screened for outliers using the ROUT test with a Q value of 0.2%. Statistical significance was then determined using an ordinary one-way ANOVA followed by a Tukey test to compare the mean of each group with the mean of all other groups. **: p ≤ 0.01, ***: p ≤ 0.001, ****: p ≤ 0.0001. Error bars show standard deviation.
Tregs are critical for appropriate trafficking of dendritic cells from the vaginal mucosa to the dLN
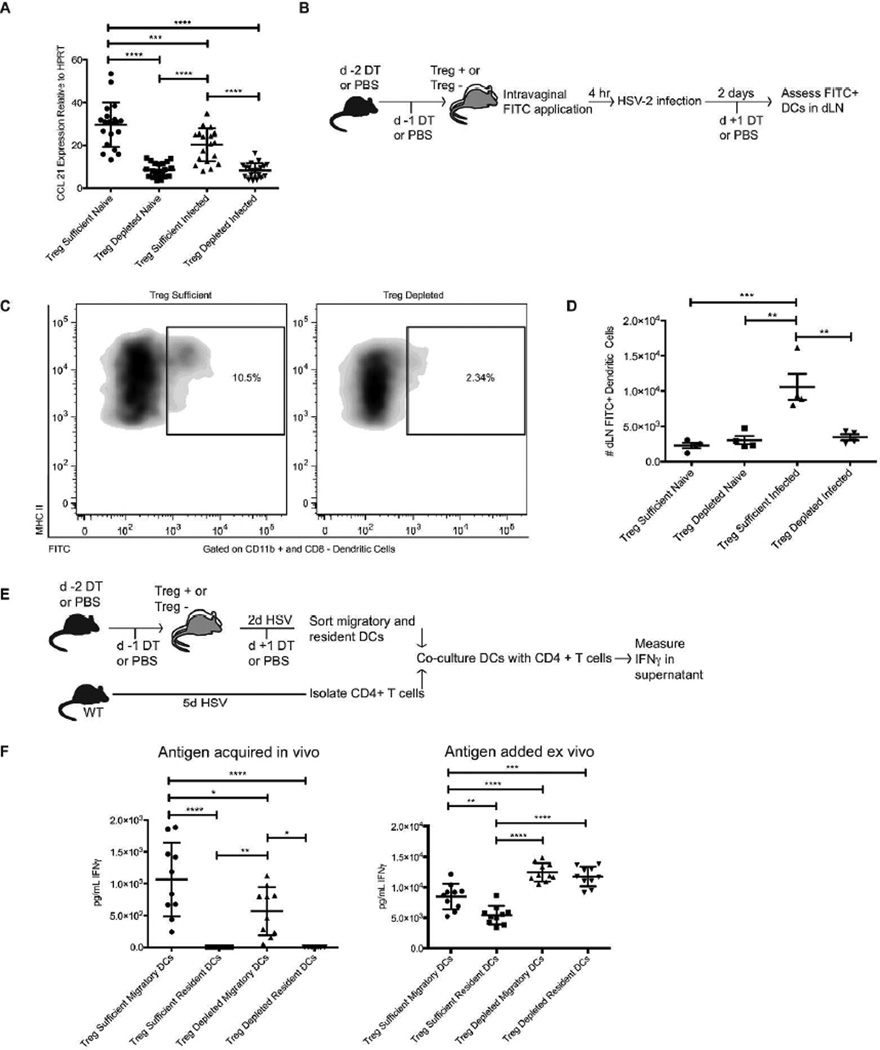
CD11b+ sub-mucosal DCs that migrate from the HSV-2-infected tissues to the dLNs present antigen to naïve CD4 T-cells and mediate effector T-cell activation 23. Since antigen-specific CD4 T-cells in Treg-depleted mice did not activate efficiently in response to infection, we hypothesized that this could be in part caused by a dysregulation in the migration of the antigen-bearing sub-mucosal DCs. This possibility was further supported by our previous work demonstrating an early drop in dLN production of CCL21 following Treg depletion 12. Migratory DCs express CCR7 to follow a gradient of CCL21 into the T-cells zone of a dLN, thereby enabling them to present cognate antigen to T-cells 30,31. As such, we reasoned that altered CCL21 production in the absence of Tregs could affect DC migration subsequent to HSV-2 infection. Thus, we confirmed that CCL21 production in the dLN was decreased in Treg-depleted mice using attenuated TK- HSV-2 12,32. Indeed, qRT-PCR analysis revealed a significant drop in CCL21 transcript production in response to Treg depletion or HSV-2 infection within two days of the infection (Fig. 4a).
Figure 4. Dendritic cell trafficking from the vaginal mucosa is impaired in the absence of Tregs.
Foxp3DTR or control mice were injected with DT and infected intravaginally with HSV-2 186ΔKpn as above. a) Expression of CCL21 in the dLN of Treg-sufficient or Treg-depleted mice 2d after infection determined by qPCR analysis (relative to the housekeeping gene HPRT). b) FITC painting experimental layout. c) Representative flow plots showing the fraction of CD11b+/CD8− DCs in the dLN that stained positive for FITC 2d after infection. Cells were first gated on MHC II/CD11c+/CD11b+ and CD8− cells. d) Representative data showing the number of FITC+ DCs that had entered the dLN from the infected vaginal tract 2d after the mice were infected. e) Ex vivo antigen presentation experimental layout. f) The amount of IFNγ produced by anti-HSV-2 CD4 T-cells after 60h of co-culture with DCs sorted from Treg-sufficient or Treg-depleted mice 2d after infection. On the left, cells were cultured without addition of exogenous antigen, whereas on the right, cells were provided HSV-2 ex vivo during culture. All data come from or are representative of at least 2 similar, independent experiments with 3–5 mice in each group that showed similar results. Each data set was first screened for outliers using the ROUT test with a Q value of 0.2%. Statistical significance was then determined using an ordinary one-way ANOVA followed by a Tukey test to compare the mean of each group with the mean of all other groups.*: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 0.001 ****: p ≤ 0.0001. Error bars show standard deviation.
To test the possibility that fewer migratory DCs trafficked from the infected tissue to the dLN, we labeled DCs within the vaginal tract by applying a solution of FITC to the vaginal canal prior to infection with HSV-2. As previously demonstrated, this procedure does not result in drainage of FITC itself to the dLN 33. The mice were infected for two days, and their dLNs were analyzed for FITC+ DCs that had originated in the vaginal tract (Fig. 4b). Significantly fewer FITC+ sub-mucosal DCs entered the dLN following infection in Treg-depleted mice as compared to Treg-sufficient mice, suggesting that the migration of antigen-bearing DCs from the infected tissues to the dLN is dysregulated in the absence of Tregs (Fig. 4c–d).
To further assess the entry of viral antigen into the dLN of Treg-depleted mice, we tested the ability of migratory DCs from the dLN of infected mice to present viral antigen to CD4 T-cells. Mice were infected for two days with HSV-2 prior to isolating MHC II-high migratory DCs from the dLNs. The sorted migratory DCs were then co-cultured ex vivo with CD4 T-cells isolated from the dLNs of WT mice infected with HSV-2 for five days and thus enriched for endogenous HSV-2-specific CD4 T-cells. Subsequent to ex vivo co-culture, levels of IFNγ in the culture supernatant were measured by ELISA as a readout for the ability of the sorted DCs to present in vivo-acquired antigen to the anti-HSV-2 CD4 T-cells (Fig. 4e). Consistent with the FITC labeling experiment, migratory DCs sorted from Treg-depleted mice induced less IFNγ production from anti-HSV-2 CD4 T-cells, despite equal numbers of these cells used in culture (Fig. 4f). As expected, only MHC II-high, migratory DCs were capable of inducing IFNγ ex vivo (Fig. 4f) 23. When the culture was supplemented with heat-inactivated HSV-2 virus ex vivo, DCs from both Treg-depleted as well as Treg-sufficient dLNs were fully capable of presenting antigen to anti-HSV-2 CD4 T-cells (Fig. 4f). Indeed, consistent with previous data showing increased expression of co-stimulatory molecules such as CD80 on DCs in the LNs of Treg-depleted mice 2, DCs sorted from Treg-depleted mice that were supplemented with HSV-2 antigen ex vivo induced more IFNγ production than the same population of cells sorted from Treg-sufficient mice (Fig. 4f). This suggests that the decreased production of IFNγ in the co-cultures where antigen was acquired in vivo was not the result of functionally-impaired DCs in the Treg-depleted group, but rather that the population of DCs sorted from Treg-depleted mice contained a smaller fraction of HSV-2-antigen-bearing cells that had entered the dLN following the infection.
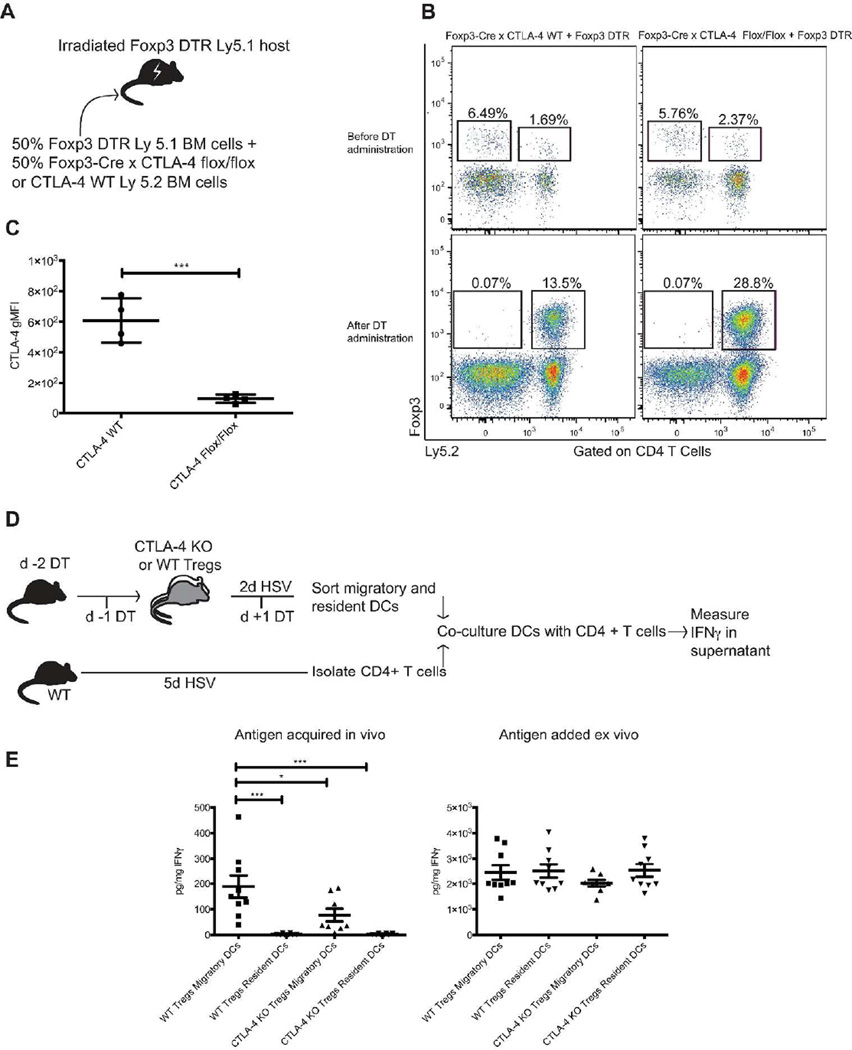
CTLA-4 expression by Tregs is critical to promote proper dendritic cell migration from the infected tissues in the context of HSV-2 infection
We next turned to the functional mechanism by which Tregs facilitate entry of antigen-bearing DCs into the dLN following vaginal HSV-2 infection. CTLA-4 is constitutively expressed by Tregs and is well known to be involved in multiple pathways of Treg-mediated suppression. Specifically, CTLA-4 can compete with CD28 for co-stimulatory molecule binding to antigen presenting cells (APCs), as well as physically remove CD80 and CD86 from the surface of APCs, thereby limiting co-stimulation available for T-cells 34. CTLA-4flox/flox x Foxp3Cre mice begin to show signs of multi-organ lymphocyte infiltration and disease at 7 weeks of age 35, demonstrating the importance of CTLA-4 expression by Tregs for the maintenance of immune homeostasis, but also presenting a challenge in using these mice in studies with further manipulations due to their early spontaneous disease. Thus, we used a mixed bone marrow chimera strategy; lethally irradiated Foxp3DTR mice were re-constituted with equal parts bone marrow from Foxp3DTRmice and either CTLA-4flox/floxx Foxp3Cre mice or CTLA-4WT/WTx Foxp3Cremice (Fig. 5a). This allowed for the development of mice that maintain a population of fully functional Tregs (from Foxp3DTRhost and donor cells) that could be depleted upon administration of DT, leaving behind only Tregs that either lack CTLA-4 expression or act as Foxp3Crecontrol cells (Fig. 5b–c). When these chimeric mice were used to isolate DCs for our ex vivo T-cell antigen presentation assay (Fig. 5d), we found that similar to Treg-depleted mice, DCs from HSV-2-infected mice with CTLA-4 deficient Tregs were less able than DCs from CTLA-4 WT mice to induce IFNγ production from T-cells without addition of exogenous HSV-2 (Fig. 5e). Thus, it appears that Tregs mediate their impact on DC migration through a CTLA-4-dependent mechanism.
Figure 5. CTLA-4 expression on Tregs is needed to promote proper migration of antigen-bearing dendritic cells from the infected tissue to the dLN.
a) Mixed bone marrow chimera setup. b) Representative flow plots from blood (before DT administration) or lymphoid tissue (after DT administration) showing two congenically distinct Treg populations in mature bone marrow chimeras. Mixed bone marrow chimera mice were injected with DT on days −2, −1, and 1 relative to the day of infection to deplete the fully functional Foxp3DTRTregs in each mouse. Following DT administration, Ly 5.1 Foxp3DTRTregs are no longer present and the populations of Foxp3Crex CTLA-4WT/WTor CTLA-4flox/floxLy 5.2 Tregs have expanded. c) Expression of CTLA-4 on Tregs measured on the day of harvest, when all fully competent, Foxp3DTRLy 5.1 cells have been eliminated. d) Experimental layout. e) Amount of IFNγ produced by anti-HSV-2 CD4 T-cells following 60 hours of co-culture with DCs sorted from mice with either wild type or CTLA-4 conditional KO Tregs 2d after infection. On the left, cells were cultured without addition of exogenous antigen, whereas on the right, cells were provided HSV-2 ex vivo during culture. All data come from or are representative of at least 2 similar, independent experiments with 4–5 mice in each group that showed similar results. Each data set was first screened for outliers using the ROUT test with a Q value of 0.2%. Statistical significance was then determined using an unpaired t test (c) or a one-way ANOVA followed by a Tukey test to compare the mean of each group with the mean of all other groups (F) *: p ≤ 0.05, ***: p ≤ 0.001. Error bars show standard deviation.
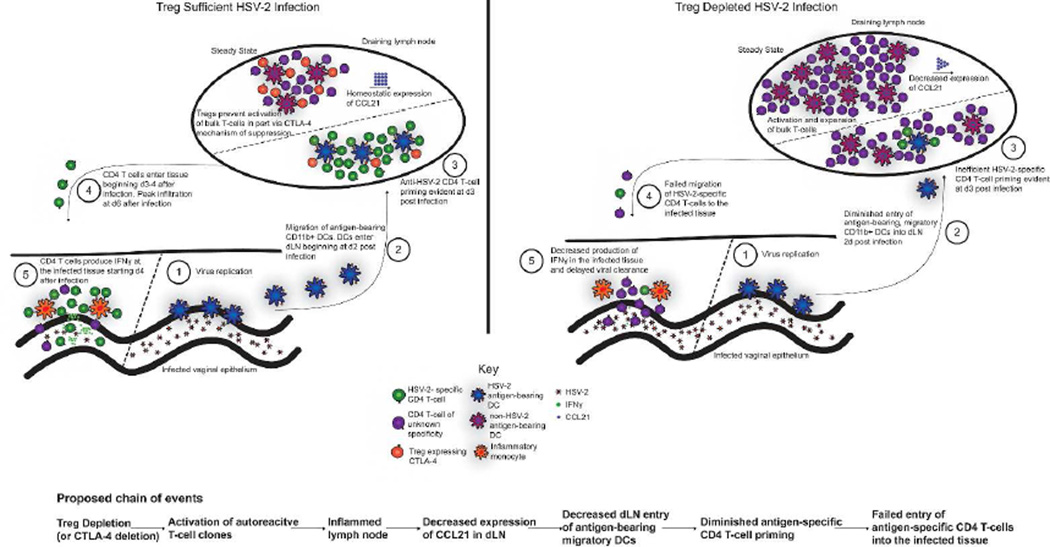
In sum, the data point to a previously unrecognized role for Tregs in maintaining the immune system in a state where it is capable of initiating an appropriate antigen-specific CD4 T-cell response upon microbial encounter (Fig. 6).
Figure 6. Visual summary of conclusions.
In the absence of Tregs, homeostasis is disrupted and, among other changes, CCL21 expression is reduced. Following intravaginal HSV-2 infection in Treg-depleted mice, the antigen-specific CD4 T-cell response fails to develop properly. Fewer antigen-bearing migratory DCs enter the dLN from the infected tissue, antigen-specific CD4 T-cell priming is inefficient, and antigen-specific CD4 T-cells ultimately fail to migrate to the infected tissue and mediate viral control.
Discussion
Current dogma places Tregs at the forefront of maintaining a well-regulated immune system. Not only do Tregs prevent autoimmunity and limit immunopathology 1,36, but we and others have demonstrated that in some cases Tregs are required for the development of an appropriate immune response 11–14. Here, our data provide further insight into why Tregs are essential in the context of a vaginal HSV-2 infection and reveal that contrary to expectations, Tregs are necessary for successful antigen-specific CD4 T-cell priming. When Tregs are depleted prior to HSV-2 infection, antigen entry into the draining lymph node is limited because of decreased trafficking of migratory DCs into the dLN where they can present cognate antigen to CD4 T-cells, thereby initiating T-cell activation and clonal expansion. This change has the downstream effect of limiting T-cell priming and migration to the tissue, steps that are necessary for timely viral control.
We propose a model whereby depletion of Tregs or elimination of CTLA-4-expressing Tregs leads to decreased entry of antigen-bearing DCs into the dLN (Fig. 6). In the steady state, Tregs bind to DCs in the lymph node via high affinity CTLA-4-B7 molecule interactions, thereby outcompeting CD28-expressing effector T-cells and preventing T-cells from forming a synapse with DCs. This maintains the LN in a homeostatic state without excessive inflammatory cytokines produced, as well as helping to prevent expansion of T-cells when there is no pathogenic threat present, including restraining activation of auto-reactive T-cells. However, in the absence of Tregs, or of CTLA-4 expression on Tregs, activation and expansion of polyclonal T-cells is unleashed, leading to an inflamed LN full of expanding T-cells producing cytokines, as well as decreased expression of CCL21.
It has been documented that following the initiation of an immune response after antigen exposure through a variety of routes, production of CCL21 in the lymphoid tissues decreases. This decrease affects CD8 T-cell priming following a subsequent infection, similar to our observation with decreased CD4 T-cell priming. In the context of LCMV infection, the drop in lymph node production of CCL21 was found to be dependent on CD4 T-cell production of IFNγ 32. Since Treg depletion causes increased production of IFNγ from CD4 T cells within the LNs 12, we initially hypothesized that if we prevented IFNγ production, we may be able to restore CCL21 to homeostatic levels, even in Treg-depleted mice, and thus restore migratory DC entry into the dLN. However, after crossing IFNγ knockout mice with Foxp3DTRmice, CCL21 production was still reduced in Treg-depleted, IFNγ knockout mice to a similar degree as in Treg-depleted IFNγ-WT mice (data not shown). However, as we previously demonstrated that Treg depletion leads to increased cytokine production by CD4 T-cells even in the absence of infection, likely due to activation of auto-reactive cells 12, it remains possible that Treg depletion results in the production of multiple inflammatory cytokines that are able to compensate for a loss of IFNγ, thereby still leading to diminished CCL21. Indeed, IL-1 and IL-12 were also implicated in leading to decreased CCL21 levels 32, so it may be that eliminating the effects of multiple cytokines would be necessary to restore homeostatic CCL21 expression in Treg-depleted mice.
Thus, following this drop in CCL21 production in the absence of Tregs, there is diminished entry of antigen-bearing, migratory DCs into the dLN following HSV-2 infection, therefore resulting in inefficient HSV-2-specific CD4 T-cell priming, and consequently, failed migration of these cells to the infected tissue. Thus, we have demonstrated for the first time that Tregs assist in focusing the CD4 T-cell response to be directed against an invading microbe upon infection.
The degree to which these findings can be extended to other infections requires further investigation. One aspect of HSV-2 infection biology that may be crucial for this observed phenotype is the relative scarcity of antigen that enters the dLN following the natural route of exposure. When other methods of exposure are used, antigen can enter the lymph node without the help of migratory dendritic cells by traveling in the lymph. In one study, HSV-1 administered into the vaginal tract tissue through needle injection moved into the draining lymph node very quickly and was presented by lymphoid organ resident DCs, while the same virus delivered to the mucosal surface of the vaginal canal resulted in antigen movement to the draining lymph node that was dependent on migratory DCs 33. Thus, during systemic infections, or infections where antigen itself drains into secondary lymphoid organs, there may be sufficient antigen presentation to prime a robust antigen-specific T-cell response in the absence of Tregs. However, following ivag HSV-2 infection in mice, antigen only moves to the dLN when carried by migratory DCs 23 and is thus subject to changes in the chemotactic gradients that facilitate migratory DC entry into the dLN. Furthermore, regardless of applicability to other infections, our study points to the crucial role that Tregs play in both restraining as well as coordinating immune responses. While it is well established that Tregs restrain T-cell responses in order to maintain peripheral tolerance, we demonstrate here that Tregs also play a pivotal role in promoting an appropriate immune response upon infection.
The data also highlight the important role that Tregs play in focusing an immune response against the invading pathogen. Although some antigen did still enter the dLN carried by migratory DCs in Treg-depleted mice, (Fig. 4), there was a near complete failure to target the antigen-specific gDT-II cells to the infected tissue (Fig. 1). Consequently, the immune response in the vaginal tract of Treg-sufficient mice was dominated by the antigen-specific CD4 T-cells that were transferred into the mice prior to infection, though these same cells were scarcely present in the vaginal tracts of Treg-depleted mice.
Tregs are known to affect both the priming of antigen-specific CD8 T-cells by preventing activation of low affinity CD8 T-cell clones 19, as well as the development of CD8 T-cell memory through the production of IL-10 37. Our data may be related to these observations; however, we focused on the priming of a specific CD4 T-cell clone and to our knowledge, the impact of Tregs on CD4 T-cell priming has not been explored in detail. Furthermore, we did not assess how Treg depletion in our model affected the development of T cell memory. In our model, the combination of decreased entry of antigen into the dLN (Fig. 4) and possible increased ability for low affinity clones to engage with antigen-bearing DCs 19,38 is a potential explanation for the decrease in priming of TCR transgenic CD4 T-cells in Treg-depleted mice.
In situations where a more robust immune response is desired, such as during chronic viral infections or cancer, it has been proposed that therapies targeted towards Treg function may be helpful. The findings presented here could have relevance when considering possible unintended consequences from such therapies. Our data suggest that very quickly after inhibition of Treg function, well before any overt signs of autoimmunity would be expected, individuals with lessened Treg function could be more susceptible to some forms of infection because of a diminished ability to mount a proper immune response.
In summary, our findings highlight an underappreciated role for Tregs in allowing the immune system to prime and target an immune response against a pathogen that is localized to a peripheral tissue, and provide further insight into the importance of Tregs in not only curtailing immunity but also in promoting appropriate immune responses against infections.
Experimental Procedures
Mice
6–8 week old female mice were used for experimental groups. All animal experiments were approved by the Fred Hutch IACUC (NIH OLAW approval #A3226-01), and this study was carried out in strict compliance with the PHS Policy on Humane Care and Use of Laboratory Animals. For more information on strains of mice used and generation of bone marrow chimeras, see supplemental experimental procedures.
Infections and Vaginal Washes
Mice were injected subcutaneously with 2 mg of Depo Provera (Greenstone, Peapack, NJ) 5 to 7 days prior to being infected with 1.88 × 105 – 1.0 × 106 PFU of HSV-2 by application of virus to the vaginal canal. HSV-2 was propagated on and titered by plaque assay on Vero cells. Vaginal washes were collected from mice by swabbing vaginal canals and washing the vaginal canals with 50 µL of titration buffer. Viral titer of vaginal washes was determined by plaque assay on Vero cells. IFNγ concentration in vaginal washes was determined using the Ready-Set-Go ELISA kit (eBioscience, San Diego CA) according to manufacturer’s instructions. For more details, see supplemental experimental procedures.
Regulatory T-cell depletion
In all experiments, Foxp3DTR mice were injected intraperitoneal with 30 µg/kg of Diphtheria toxin (DT) (EMD Millipore, Darmstadt, Germany) dissolved in PBS upon first injection and 10 µg/kg of DT for all subsequent injections. In experiments where Tregs were depleted prior to infection, DT injections were done on days −2, −1, and +1 relative to the day of infection. In experiments where Tregs were depleted after T cell priming, DT injections were done on days +3 and +4 relative to the day of infection. In some experiments, Treg-sufficient mice were a non-DTR expressing strain injected with DT, and no differences were noted upon using either Treg-sufficient control setup.
Cell sorting and Flow Cytometry
For ex vivo co-culture experiments, DCs were sorted from iliac lymph nodes based on expression of CD11c and MHC II. Cells were blocked for Fc binding then stained with anti-MHC II antibody and anti-CD11c antibody (eBioscience, San Diego, CA). Cells from dLN were sorted into two groups; an MHC II high/CD11c mid population of migratory DCs and MHC II mid/ CD11c + population of non-migratory DCs. All sorting was done on a FACSAria (BD Biosciences, San Jose, CA). CD4 T-cells that were already enriched using the CD4+ T-cell negative selection kit (Stem Cell Technologies, Vancouver, Canada) were also stained using anti-CD4 and anti-MHC II antibodies (eBioscience, San Diego, CA) and sorted into a population of CD4+ and MHC II – cells to ensure that the CD4 T-cells were not contaminated with antigen presenting cells.
For flow cytometry, cells were incubated in fixable viability dye (eBioscience, San Diego, CA), blocked for Fc binding, then stained for surface proteins. Data were analyzed using FlowJo software (Treestar, Ashland, OR). For more details, including antibodies used, see supplemental experimental procedures.
Ex vivo co-culture
DCs and CD4 T-cells were sorted as described above. 2500 – 3000 DCs were then co-cultured with 100,000 CD4 T-cells in 120 µL of volume in a 384 well culture plate. After 60 hours of co-culture at 37 degrees C, supernatant was assessed for IFNγ using the Ready-Set-Go ELISA kit (eBioscience, San Diego, CA) according to manufacturer’s instructions. Where noted, ex vivo cultures were supplemented with 1 × 105 PFU of inactivated HSV-2.
FITC Painting
Four hours prior to infection, mouse vaginal tracts were swabbed with calcium-alginate tipped swabs before having 10 µL of 1% FITC (Sigma-Aldrich, St. Louis, MO) in DMSO applied using a pipette. FITC+ DCs were enumerated in the dLN by gating on FITC+/CD11b+/CD8− DCs and multiplying the gate percentages by the number of lymphocytes in the sample. The FITC+ gate was set using cells from a mouse that had DMSO applied to the vaginal canal.
qPCR
dLN were harvested directly into RNALater (Thermo Fisher Scientific, Waltham, MA) then RNA was isolated using the RNeasy Plus mini kit (Qiagen, Hilden, Germany). Any residual genomic DNA was then eliminated using the DNase away kit (Thermo Fisher Scientific, Waltham, MA). A cDNA library was then prepared using the RT kit (Thermo Fisher Scientific, Waltham, MA). qPCR was performed on CCL21 and HPRT using TaqMan primers (Applied Biosysems) and 2x qPCR master mix (Thermo Fisher Scientific, Waltham, MA) diluted with water and 250 ng of RNA from each sample. Relative expression of CCL21 in relation to HPRT was then calculated for each sample using the delta Ct method. All kits were used according to manufacturer’s instructions.
Statistical analysis
All statistical analysis was performed using Prism software (GraphPad Software, San Diego, CA). All data sets were first screened for outliers using the ROUT test with a Q value of 0.2%. When comparing groups in experiments with more than two experimental groups, an ordinary one-way ANOVA followed by a Tukey test was conducted to determine statistical significance between groups. When comparing only two experimental groups, an unpaired, two-tailed, parametric t test was conducted to determine statistical significance. When comparing multiple rows of data, multiple t tests were performed with correction for multiple comparisons using the Holm-Sidak method. In all cases, a p value less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Tisha Graham for mouse colony maintenance and technical assistance, and Akiko Iwasaki as well as members of the Lund, Prlic, and Taylor labs for helpful discussions.
Funding was provided by the National Institute of Allergy and Infectious Diseases of the US National Institutes of Health (R01 AI087657, to JML) and the Viral Pathogenesis Training Grant (T32AI083203, to AGS).
Footnotes
Author contributions. AGS and AC performed all experiments. AGS performed data and statistical analyses. AGS and JML designed the study and conceived of the experiments wrote the first draft of the manuscript and all authors provided editorial contribution and approved the final draft.
Disclosure. The authors declare no conflicts of interest.
References
- 1.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nature reviews Immunology. 2011;11(2):119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature immunology. 2007;8(2):191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*) Annual review of immunology. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 4.Smigiel KS, Srivastava S, Stolley JM, Campbell DJ. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunological reviews. 2014;259(1):40–59. doi: 10.1111/imr.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veiga-Parga T, Sehrawat S, Rouse BT. Role of regulatory T cells during virus infection. Immunological reviews. 2013;255(1):182–196. doi: 10.1111/imr.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420(6915):502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 7.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nature immunology. 2005;6(4):353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 8.Sarangi PP, Sehrawat S, Suvas S, Rouse BT. IL-10 and natural regulatory T cells: two independent anti-inflammatory mechanisms in herpes simplex virus-induced ocular immunopathology. Journal of immunology. 2008;180(9):6297–6306. doi: 10.4049/jimmunol.180.9.6297. [DOI] [PubMed] [Google Scholar]
- 9.Sehrawat S, Suvas S, Sarangi PP, Suryawanshi A, Rouse BT. In vitro-generated antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells control the severity of herpes simplex virus-induced ocular immunoinflammatory lesions. Journal of virology. 2008;82(14):6838–6851. doi: 10.1128/JVI.00697-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. Journal of immunology. 2004;172(7):4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 11.Fulton RB, Meyerholz DK, Varga SM. Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. Journal of immunology. 2010;185(4):2382–2392. doi: 10.4049/jimmunol.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320(5880):1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruckwardt TJ, Bonaparte KL, Nason MC, Graham BS. Regulatory T cells promote early influx of CD8+ T cells in the lungs of respiratory syncytial virus-infected mice and diminish immunodominance disparities. Journal of virology. 2009;83(7):3019–3028. doi: 10.1128/JVI.00036-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernandez-Santos N, et al. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity. 2011;34(3):422–434. doi: 10.1016/j.immuni.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dittmer U, He H, Messer RJ, Schimmer S, Olbrich AR, Ohlen C, et al. Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity. 2004;20(3):293–303. doi: 10.1016/s1074-7613(04)00054-8. [DOI] [PubMed] [Google Scholar]
- 16.Hisaeda H, Maekawa Y, Iwakawa D, Okada H, Himeno K, Kishihara K, et al. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nature medicine. 2004;10(1):29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- 17.Hori S, Carvalho TL, Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. European journal of immunology. 2002;32(5):1282–1291. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. The Journal of experimental medicine. 2003;198(6):889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pace L, Tempez A, Arnold-Schrauf C, Lemaitre F, Bousso P, Fetler L, et al. Regulatory T cells increase the avidity of primary CD8+ T cell responses and promote memory. Science. 2012;338(6106):532–536. doi: 10.1126/science.1227049. [DOI] [PubMed] [Google Scholar]
- 20.Iwasaki A. The role of dendritic cells in immune responses against vaginal infection by herpes simplex virus type 2. Microbes and infection / Institut Pasteur. 2003;5(13):1221–1230. doi: 10.1016/j.micinf.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Milligan GN, Bernstein DI. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229(1):259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- 22.Milligan GN, Bernstein DI. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology. 1995;212(2):481–489. doi: 10.1006/viro.1995.1506. [DOI] [PubMed] [Google Scholar]
- 23.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. The Journal of experimental medicine. 2003;197(2):153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462(7272):510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iijima N, Mattei LM, Iwasaki A. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(1):284–289. doi: 10.1073/pnas.1005201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1(4):327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 27.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nature immunology. 2009;10(5):488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 28.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477(7363):216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 29.Dobbs ME, Strasser JE, Chu CF, Chalk C, Milligan GN. Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin- or Fas-mediated cytolytic mechanisms. Journal of virology. 2005;79(23):14546–14554. doi: 10.1128/JVI.79.23.14546-14554.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber M, Hauschild R, Schwarz J, Moussion C, de Vries I, Legler DF, et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339(6117):328–332. doi: 10.1126/science.1228456. [DOI] [PubMed] [Google Scholar]
- 31.Braun A, Worbs T, Moschovakis GL, Halle S, Hoffmann K, Bolter J, et al. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat Immunol. 12(9):879–887. doi: 10.1038/ni.2085. [DOI] [PubMed] [Google Scholar]
- 32.Mueller SN, Hosiawa-Meagher KA, Konieczny BT, Sullivan BM, Bachmann MF, Locksley RM, et al. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317(5838):670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- 33.Lee HK, Zamora M, Linehan MM, Iijima N, Gonzalez D, Haberman A, et al. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. The Journal of experimental medicine. 2009;206(2):359–370. doi: 10.1084/jem.20080601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 36.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laidlaw BJ, Cui W, Amezquita RA, Gray SM, Guan T, Lu Y, et al. Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nature immunology. 2015;16(8):871–879. doi: 10.1038/ni.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matheu MP, Othy S, Greenberg ML, Dong TX, Schuijs M, Deswarte K, et al. Imaging regulatory T cell dynamics and CTLA4-mediated suppression of T cell priming. Nature communications. 2015;6:6219. doi: 10.1038/ncomms7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.