Abstract
Introduction and Aims
Most women cut down or quit alcohol use during pregnancy, but return to pre-pregnancy levels of use after giving birth. Universal screening and brief intervention (SBI) for alcohol use has shown promise, but has proven challenging to implement and has rarely been evaluated with postpartum women. This trial evaluated a single 20-minute, electronic SBI (e-SBI) for alcohol use among postpartum women.
Design and Methods
In this parallel group randomised trial, 123 postpartum, low-income, primarily African-American women meeting criteria for unhealthy alcohol use were randomly assigned to either a tailored e-SBI (n=61) or a time-matched control condition (n=62), with follow-up at 3 and 6 months. Hypotheses predicted that 7-day point-prevalence abstinence and drinking days would favour the e-SBI condition.
Results
No group differences were significant. Blinded follow-up evaluation revealed 7-day point prevalence of 75% for the e-SBI condition vs. 82% for control at 3 months (odds ratio = 1.6) and 72% vs. 73%, respectively, at 6 months. Drinking days in the past 90 and mean number of drinks per week also showed no significant differences.
Discussion and Conclusions
This pilot trial failed to support a single-session e-SBI for alcohol use among postpartum women, although findings at the three month time point suggested that greater power might confirm transient effects of the e-SBI. As efficacy is likely to vary with e-SBI content and approach, future research should leverage technology’s reproducibility and modularity to isolate key components.
Keywords: pregnancy, alcohol drinking, motivation, computers, randomised clinical trial
Introduction
Alcohol use decreases during pregnancy and rapidly returns to pre-pregnancy levels following childbirth. For example, findings from the National Survey on Drug Use and Health suggest that 32.6% of non-pregnant women aged 18–44 years report binge drinking at least once in the past month; this rate drops to 1% for women in the third trimester, but increases to 15.5% for women with children five months old or younger [1]. Although postpartum alcohol use lacks the teratological potential of prenatal use, problem and disordered alcohol use is still associated with a wide range of negative prenatal and postnatal outcomes, including increased risk of child maltreatment [2–4], violence exposure, behavioural problems [5] and substance use in adolescence [6–8]. Interventions aimed at the postpartum period could potentially initiate or maintain changes in maternal alcohol use, with the potential for long-term effects on child outcomes.
Universal screening, brief intervention, and referral to treatment (SBIRT) approaches are well suited to use in the postpartum period, where a very high proportion of parenting mothers can be accessed. SBIRT has been supported in a number of controlled trials [9] and has been promoted by the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, the American College of Surgeons, and the American Congress of Obstetricians and Gynecologists, among others. Studies of brief interventions for postpartum alcohol use have been mixed. For example, Fleming and colleagues evaluated a four-session series of postpartum motivational sessions, delivered during routine medical care, and found significant reductions in several drinking-related outcomes [10]. In contrast, Rubio and colleagues examined a five-session, primarily during-pregnancy motivational intervention and found small but not significant effects on postpartum alcohol use [11]. Such findings should be considered in light of the much larger literature using general samples of adults with unhealthy alcohol use. That literature includes rigorous recent studies that have found no effect for large-scale SBIRT rollouts [12, 13], perhaps in part due to challenges with implementation [14–17].
Technology-driven screening and brief intervention (e-SBI) may offer significant advantages in terms of implementation and reproducibility. Research into technology-delivered interventions has grown rapidly in recent years, with meta-analyses showing encouraging evidence for efficacy with respect to substance abuse [18–22] as well as other health-related behaviours [23]. However, although some early studies have considered e-SBI for alcohol use during pregnancy [24,25], none have done so for postpartum drinking.
The present study was designed to evaluate a single-session e-SBI approach for alcohol use among postpartum women, provided during the postpartum hospitalisation. The e-SBI session for this study was built and delivered using a flexible e-intervention authoring platform that has been used in a number of related trials [25–29], which uses a Tablet PC to deliver screening, assessment, and intervention content as a web application (see intervention section, below, for further details). We hypothesised that women receiving the e-SBI would report higher 7-day point-prevalence abstinence from alcohol and fewer days of alcohol use.
Methods
Participants
Participants in this multi-site parallel-group randomised trial were 123 postpartum women, recruited during their inpatient hospitalisation for childbirth at one of three hospitals in the Detroit area, all of which have only private rooms for childbirth and recovery. Exclusion criteria included inability to understand spoken English, frank cognitive impairment, use of illicit drugs in the month prior to pregnancy, and recent administration of prescription pain medication. Inclusion criteria included being age 18 or older, having slept since giving birth, self-report of having had four or more standard drinks at a time at least twice per month in the 12 months prior to becoming pregnant, and scoring two or more on the T-ACE alcohol screener [30].
Procedure
Baseline data collection took place between 16 January 2008 and 15 June 2010, and follow-up data collection took place between 16 April 2008 and 4 March 2011, ending when overall recruitment goals were met. Women were approached in their private hospital rooms at one of three hospitals in Detroit, MI, and were given a brief description of the study. Those expressing potential interest were checked for preliminary eligibility (e.g. age, having slept). Women who remained eligible were then asked for verbal consent (using an information sheet) to complete an anonymous computer-delivered screen using a Tablet PC, with headphones for privacy. Participants received a small gift bag for their baby worth approximately $3 for completing the screener, regardless of the outcome of screening. All data collection procedures were approved by the institutional review boards of the Wayne State University and the Henry Ford Health System.
Those who were eligible and provided informed consent were given the computer again, which presented an approximately 30-minute assessment and then used internal algorithms to randomly assign participants in a 1:1 ratio to either the brief intervention or a time-control condition. Research assistants were thus blind to participant allocation to intervention vs. time control. Participants who completed the assessment and intervention or control process on the computer received a gift card for Target stores worth $40.
The research assistant then made arrangements for the two follow-up observations, which took place in the investigators’ offices approximately 3- and 6-months post-baseline, and were conducted by a research assistant blind to experimental condition. Participants were contacted repeatedly by mail and later by phone following published tracking guidelines [31]. They received a $75 gift certificate for completing the 3-month follow-up, assistance with transportation (either a $10 gas card or a taxi), and an additional $25 if they agreed to provide a urine sample. At the 6-month evaluation, in addition to assistance with transportation, participants received a $100 gift certificate plus an additional $25 for providing both urine and hair samples.
There is substantial evidence of under-reporting of substance use during and near pregnancy [32–34], a tendency that is likely to be at least as strong among African-American women given the extent to which they are disproportionately targeted for screening and child protection action during this period [35]. There is also evidence that anonymous and quasi-anonymous approaches result in increased disclosure of stigmatised behaviours by postpartum women [36]. We therefore chose to use a quasi-anonymous approach to protect participants in this study. In this approach, participant identifying information is collected in order to allow follow-up evaluations, but is not connected to responses via a linking table. Each participant’s experimental condition (using a code for intervention vs. control to maintain blinding) was the only participant-level data to be connected to participants’ names as well as their data, thus allowing longitudinal within-group comparisons between conditions. Although this method has the disadvantage of precluding linking of individual cases over time, this drawback may be outweighed by increased accuracy of reporting [36]. Quasi-anonymous methods may be the best available approach for research involving significant under-reporting and/or risk to participants.
Measures
Most measures for this study were completed by the participant using the software and a Tablet PC. At baseline, participants completed the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST), a well-validated brief measure developed by the World Health Organization that evaluates frequency of use as well as consequences of use, separately for all categories of substances [37]; the baseline ASSIST referred to alcohol use in the three months prior to pregnancy rather than the past three months, in order to promote disclosure and to establish a baseline more reflective of drinking when not pregnant. Participant baseline measures also included K6, a brief mental illness screener [38] that measures general symptoms of distress over the past 30 days; and items relating to current relationships, demographics, and treatment history. Participants in the e-SBI condition also completed user experience/satisfaction ratings regarding their experience of using the software. These items, many of which have been used in previous research [39], ask participants for ratings of the extent to which they found the software easy to use, respectful, helpful, likable, etc., on a 1–5 scale where 1 = not at all and 5 = very much. In this sample, these 9 items had an internal consistency (Cronbach’s Alpha) of 0.72. At each follow-up, participants again completed a computer-delivered Timeline Follow-Back interview [40] evaluating alcohol use in the past week and past 90 days. Items from the National Institute on Alcohol Abuse and Alcoholism recommended alcohol assessment battery regarding quantity-frequency and binge drinking days were also administered [41].
Intervention
The goal of the online software used in this study was to facilitate reductions in alcohol use via a single 20-minute postpartum intervention session following motivational interviewing principles [42] to the extent possible, as well as the FRAMES brief intervention model [43, 44], with significant use of synchronous interactivity, user input and empathic reflection. No keyboarding was required; all answers were provided by choosing responses from a list or by touching a visual analogue scale.
A mobile three-dimensional cartoon character capable of over 50 specific animated actions did the “talking” for the entire program. This character read each item for the participant, acted as narrator and guide throughout the process, and actively sought a non-judgmental, empathic and non-threatening demeanour using reflections and self-deprecating humour. The experience of working with the software was intended to be highly interactive, with immediate responses to most input, occasional summaries, branching based on participant characteristics, responses or preferences, and empathic reflections (e.g. “You really seem to feel two ways about this. On the one hand, you like the way that drinking helps you relax and have fun with your friends. But at the same time, you think you could really use that money on things for your baby”).
The overall intervention was broken down into components broadly focusing on: (i) eliciting the participant’s thoughts about change and their perceived advantages of doing so, if any; (ii) reviewing feedback regarding how the participant’s alcohol use compares to that of others, and of possible benefits of changing; and (iii) optional goal-setting, including a menu of change options. The intervention allowed participant input (e.g. whether or not to see more information on a certain topic), and used different branches/approaches based on participant reports of current alcohol use as well as on participants’ stated plans regarding drinking after leaving the hospital. Participants listened to the narrator via headphones to insure privacy. Notably, the intervention was not designed specifically around current active drinking. Earlier research with this platform showed very high acceptability [39].
Control Condition
Control group participants completed the same assessment measures noted above. Then, in order to control for the time spent on the intervention, maintain blinding of research assistants, and mimic the interactivity of the intervention condition, participants assigned to the control condition were asked a number of questions about their preferences in music and television, were shown brief video clips consistent with their preferences, and were asked to provide feedback regarding their opinion of the various video clips. This relatively inactive control condition was intended to minimise, as much as possible in this two-arm study, the likelihood of assessment reactivity or of an unintended brief intervention effect [45, 46].
Randomisation
Participants were randomised by the software using simple randomisation to either intervention or time control conditions in a 1:1 ratio. Randomisation by the software maintained research assistant blinding with respect to participant allocation into study conditions at the baseline data collection/intervention session.
Data Analysis
This study tested the efficacy of the e-SBI as reflected by fewer days of alcohol use, fewer days of heavy alcohol use, and higher 7-day point-prevalence abstinence from alcohol. The primary outcome of 7-day point prevalence abstinence was evaluated at 3 and 6 months using either a χ2 test or Fisher’s Exact test for variables with small numbers of events. Given non-normality of data (significant positive skew), the secondary outcome of alcohol using days in the past 90 was evaluated at 3- and 6-months using a non-parametric Wilcoxon Rank-Sum test. All analyses were conducted on an intent to treat basis considering participants in their originally assigned groups; loss to follow-up was handled by considering those lost to follow-up as positive for substance use in analyses of 7-day point prevalence, and also by simply conducting completer analysis, for comparison. Given the lack of an accepted standard for imputing missing data for continuous outcomes, as well as our use of a quasi-anonymous design (see below), the days of alcohol use outcome was analysed only for participants who had completed follow-up. The additional secondary outcomes of mean drinks per week and mean number of binge episodes per week in the past three months were analysed via independent samples t-tests.
Standardised effect sizes for point-prevalence were estimated via odds ratios (OR) and by Logit d, which was calculated using the formula where ln (OR) = the natural logarithm of the odds ratio, and π/3.5 = approximately 1.8138 [47]. Effect sizes for days of use were calculated using the probability of superiority statistic, where U is the Mann-Whitney statistic and na and nb are the sample sizes in the two groups [48].
Results
Sample Characteristics
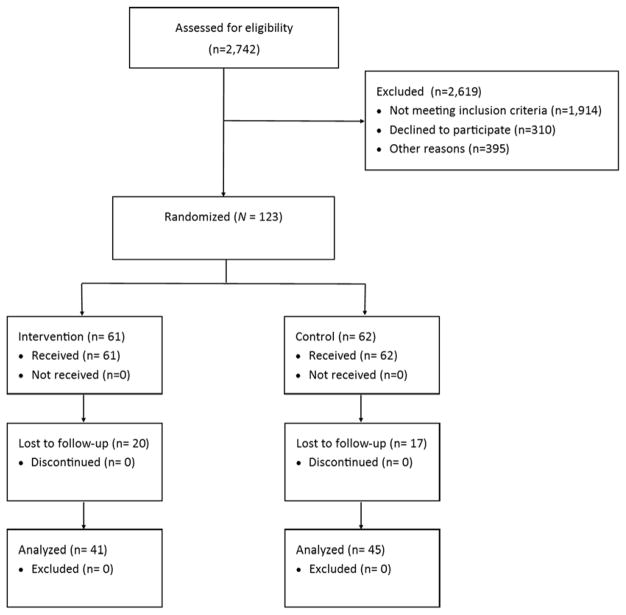
Participant flow is presented in Figure 1. A total of 2742 participants were assessed for eligibility; 2619 (95.5%) were excluded, with most of these (1914, or 73.1%) for not meeting inclusion/exclusion criteria, and 310 (11.9%) declining to participate. Within the final sample of 123 participants, 61 were allocated to the e-SBI condition and 62 to the control condition. As seen in Table 1, participants were primarily African-American women, most of whom received some form of public assistance in the past year. As also seen in Table 1, the majority of participants did not report a prior history of either mental health or substance abuse treatment. A total of 111 participants (90.2%) scored at or above the ASSIST moderate risk range on the alcohol subscale. A total of 37 participants (30.1%) did not complete either follow-up (32.5% at 3 months and 29.2% at 6 months). We did not find evidence that any participants experienced harm or unintended effects from participation in this study.
Figure 1.
Participant flow
Note. Because this trial tested a single-session intervention that was completed at the time of recruitment, all participants received the full intervention.
Table 1.
Baseline sample characteristics (N = 123)
| Categorical variables (N, %) | Total sample (N = 123) | Control (n = 62) | Intervention (n = 61) | P |
|---|---|---|---|---|
| Hispanic ethnicity | 5 (4.1) | 2 (3.3) | 3 (4.9) | 0.621 |
| Race | 0.795 | |||
| African-American | 107 (87) | 55 (88.7) | 52 (85.2) | |
| White | 5 (4.1) | 2 (3.2) | 3 (4.9) | |
| Multiracial | 5 (4.1) | 3 (4.8) | 2 (3.3) | |
| Other | 6 (4.9) | 2 (3.2) | 4 (6.6) | |
| Receipt of food assistance | 92 (74.8) | 41 (66.1) | 51 (83.6) | 0.026 |
| High school graduate or higher | 92 (74.8) | 49 (79.0) | 43 (70.5) | 0.275 |
| Currently married | 11 (8.9) | 6 (9.7) | 5 (8.2) | 0.774 |
| Worked for pay in past 6 months | 70 (56.9) | 33 (53.2) | 37 (60.7) | 0.405 |
| Prior mental health medication | 21 (17.1) | 10 (16.1) | 11 (18.0) | 0.779 |
| Prior mental health counselling | 24 (19.5) | 13 (21.0) | 11 (18.0) | 0.681 |
| Prior treatment for alcohol use | 2 (1.6) | 2 (3.2) | 0 | 0.157 |
| Pre-pregnancy binge drinking | 107 (87.0) | 54 (87.1) | 53 (86.9) | 0.972 |
| Above ASSIST alcohol BI cut-off | 111 (90.2) | 55 (88.7) | 56 (91.8) | 0.563 |
| Continuous variables (N, SD) | ||||
| Age | 27.1 (6.0) | 27.5 (6.1) | 26.7 (5.8) | 0.458 |
| K6 mental illness screener | 32.3 (5.7) | 32.4 (5.3) | 32.2 (6.0) | 0.791 |
| ASSIST alcohol score | 22.3 (8.1) | 22.1 (8.5) | 22.7 (7.9) | 0.689 |
Note. ASSIST at baseline referred to alcohol use in the three months prior to pregnancy.
ASSIST, Alcohol, Smoking, and Substance Involvement Screening Test; BI, brief intervention.
Success of Randomisation
Of the 13 comparisons in Table 1, only the baseline difference in receipt of public food assistance was significant, with participants in the intervention condition showing higher rates of assistance from this program. None of the remaining comparisons showed significant differences between intervention and control conditions.
Intervention Acceptability
The e-SBI was moderately well-received. Using a 1–5 scale reflecting level of agreement (where 1 = “not at all” and 5 = “very much”), mean scores on key satisfaction items ranged from a low of 3.4 for whether the software resulted in reconsideration of alcohol use, to highs of 3.9 for ease of use and 4.0 for respectfulness. A total of 72.4% of e-SBI participants indicated that they were more likely to change because of their interaction with the software. Of those asked, 47.6% said that they preferred using the software over talking with medical staff about their alcohol use; 14.3% said they would have preferred working with medical staff, and 38.1% were unsure. A total of 47 participants (81.0%) gave the highest possible rating for willingness to work with the computer again in a year.
Intervention Effects
As noted above, we analysed data regarding the first primary outcome (7-day point-prevalence abstinence) in two ways: by completer analysis, and presuming alcohol use among all of those lost to follow-up. As seen in Table 2, considering only participants who completed follow-up evaluation, 15 of 41 e-SBI participants (36.6%) were abstinent at 3 months, vs. 11 of 42 (26.2%) in the control condition (χ2 [1]=1.04, P = 0.307, OR = 1.6); at the 6-month follow-up, 17 of 45 e-SBI participants (41.5%) were abstinent, vs. 17 of 41 (37.8%) for the control condition (χ2 [1] =0.12, P = 0.727, OR = 1.2). Using the presumption of alcohol use among those lost to attrition did not change either the magnitude or significance of these results (ORs = 1.5 and 1.0 at 3- and 6-months, respectively).
Table 2.
Intervention effects on 7-day point-prevalence alcohol abstinence
| Observation | e-SBI (n, %) | Control (n, %) | OR (95% CI) | P | Logit d |
|---|---|---|---|---|---|
| Restricting analyses to completers | |||||
| 3 month | 15 (36.6) | 11 (26.2) | 1.6 (0.64, 4.15) | 0.307 | 0.27 |
| 6 month | 17 (41.5) | 17 (37.8) | 1.2 (0.49, 2.77) | 0.727 | 0.09 |
| Presuming those lost to follow-up are positive for alcohol | |||||
| 3 month | 15 (24.6) | 11 (17.7) | 1.5 (0.63, 3.62) | 0.352 | 0.23 |
| 6 month | 17 (27.9) | 17 (27.4) | 1.0 (0.46, 2.25) | 0.956 | 0.01 |
Note. Abstinence at each observation was determined by self-report of use in the past 7 days. N = 123 for all analyses presuming alcohol use among those lost to follow-up; for completer analysis, N = 83 at three months and 86 at six months.
CI, confidence interval; e-SBI, screening and brief intervention; OR, odds ratio.
Between-group differences in the secondary alcohol use outcomes (days of alcohol use, mean standard drinks per week, and number of binge episodes per week) are presented in Table 3. As with 7-day point prevalence, no between-group differences were significant.
Table 3.
Intervention effects on alcohol use days, mean drinks per week, and mean binges (≥ 4) per week
| 3 month follow-up | ||||
|---|---|---|---|---|
| Control (n=42) | e-SBI (n=41) | P1 | Effect size2 | |
| Alcohol use days | 19.2 (17.1) | 14.6 (20.9) | 0.071 | 0.38 |
| Mean drinks per week | 4.3 (6.2) | 3.2 (5.6) | 0.217 | 0.42 |
| Binge episodes per week | .62 (1.2) | .60 (1.1) | 0.498 | 0.46 |
| 6 month follow-up | ||||
| Control (n=46) | e-SBI (n=41) | P1 | Effect size2 | |
| Alcohol use days | 21.8 (25.8) | 15.3 (21.6) | 0.329 | 0.44 |
| Mean drinks per week | 6.4 (12.8) | 8.7 (20.0) | 0.988 | 0.50 |
| Binge episodes per week | .75 (1.6) | .56 (1.2) | 0.499 | 0.46 |
Note. Values are presented as mean (SD) for control and e-SBI groups at each time point.
Mann-Whitney U test (Wilcoxon Rank-Sum test).
, an estimate of the probability that a score randomly drawn from the control population will be greater than a score drawn randomly from the e-SBI population.
e-SBI, screening and brief intervention.
Discussion
This trial found no clear evidence for the efficacy of this single-session, computer-delivered brief motivational intervention for alcohol use among postpartum women. This study used a randomised design with blinded evaluators, strong participant protection, and—because of its basis in technology—rigorous consistency in intervention delivery as well as outcome evaluation.
The brief intervention literature contains multiple examples of positive as well as negative findings. Research in this area is currently struggling to identify the active ingredients of brief interventions [49], and the practical health impact of small reductions in alcohol use [50]. Debates regarding the ultimate utility of any brief intervention must take a number of factors into account, including the ratio of the cost of the intervention (defined broadly) and its impact. Technology-delivered interventions may be of particular interest with regard to this latter criterion: their cost per patient may prove low enough to justify their use even when effect sizes are quite small. Further, their feasibility may prove to be a crucial factor; even if other approaches show similar or superior cost-effectiveness, that advantage means little if implementation is impractical.
This potential value of even small effect sizes has clear implications for evaluating brief intervention trials, including this one. In several major meta-analyses, technology-delivered brief interventions for problem substance abuse have shown statistically significant but small effect sizes, including standardised mean difference effect sizes of 0.20 [18], 0.27 [22], and 0.24 [23]. The 3-month effects found in the present study—although not large enough to reach significance—compare favourably to these in terms of magnitude. In fact, only very highly powered trials would be able to reach significance with effect sizes in the range identified by these meta-analyses.
However, the results at 6 months suggest that these effects, if any, were transitory. The same is of course true of behavioural interventions generally, but is perhaps particularly true of brief interventions [51,52]. These approaches may be best seen in terms of regular healthcare-based check-ups rather than as a one-time treatment. Although the acceptability and efficacy of serial brief interventions has yet to be carefully studied, it is encouraging that a high proportion of participants (81.0%) expressed eagerness to work with the computer again in a year, and none were unwilling to do so.
A number of limitations must be highlighted. First, as with many trials focused on alcohol use, all outcomes were measured solely by self-report. In addition, loss to follow-up was over 30%. Further, steps taken to protect participants and enhance the accuracy of self-report also prevented linking of individual subjects over time. The relatively small sample size of this pilot trial limited our ability to detect true effects of the intervention, if any were present. Finally, the homogeneity of the study sample limits generalisability to groups other than low-socioeconomic status African-American women.
More research is needed to identify the effects of e-SBI approaches for alcohol use in pregnancy. The results of this trial are inconsistent with findings from a parallel trial conducted with postpartum women who reported drug use [26]. That trial found larger (OR = 3.3) and significant e-SBI effects on biochemically verified abstinence from drug use at a 3-month follow-up, a finding that was consistent with results from an earlier study [29]. Our weaker findings for alcohol use is inconsistent with the larger literature in this area, in which alcohol brief intervention studies show positive effects more consistently than those focusing on drug use [53]. Although the cause of this result is not clear, it is always possible that the content of the intervention was not optimal. Computer-delivered interventions can vary in as many ways as can person-delivered approaches; even subtle variations in the content and how it is presented could result in different outcomes. For example, the alcohol intervention in the present study did not include videos, which add a highly engaging element to interventions of this type and which have been well-received in prior successful interventions [28,54]. Notably, participant ratings of helpfulness, ease of use, etc., at 3.4 to 3.9 on a 5-point scale, were lower for this alcohol brief intervention than for previous tobacco and drug use brief interventions developed by our group (satisfaction ratings for which have consistently hovered near 4.5; e.g. [26]). The reasons for these lower satisfaction ratings are not clear.
A number of recent trials of brief interventions for alcohol use have demonstrated that the effects of such interventions—if any—are likely to be small, and are certain to be difficult to achieve in real-world settings (e.g. [12,50,55,56]). Such results should be carefully considered in further research using e-SBI approaches. However, technology-based approaches have significant advantages in terms of replicability and feasibility of implementation, and may be a viable alternative to person-delivered SBI in healthcare settings; their relative ease of implementation means that even smaller effects may be cost-effective. Further, e-SBI approaches are uniquely amenable to careful tailoring, dismantling, and optimisation research [57] which together may allow for cumulative increases in efficacy as research in this area progresses.
Acknowledgments
This research was supported by National Institutes of Health award DA021329 to Dr Ondersma, and by Helen Lycaki/Joseph Young, Sr. funds from the State of Michigan to Wayne State University. Dr Thacker’s efforts were supported in part by National Institutes of Health award UL1TR00058 from The National Center for Advancing Translational Sciences. Dr Ondersma is also part owner of Interva, Inc., the company that markets the intervention authoring tool used to develop the software used for this study. Neither the National Institutes of Health nor Interva, Inc. had any role in data analysis or write-up of results. This trial was registered with ClinicalTrials.gov, NCT00685074.
The authors also wish to gratefully acknowledge the invaluable assistance of the women who participated in this study, the medical staff from Hutzel Women’s Hospital, Sinai-Grace Hospital, and the Henry Ford Health System who supported recruitment and data collection, and the data collection efforts of Nerissa Germain, Veronica Gorden, Lorna Mabunda, and Veronica Connors-Burge. Finally, none of this work would have been possible without the invaluable mentorship of the late Dr Charles R. Schuster.
References
- 1.Office of Applied Studies. Substance Use among Women During Pregnancy and Following Childbirth. 2009 Available from: http://www.samhsa.gov/data/2k9/135/PregWoSubUse.htm.
- 2.Besinger BA, Garland AF, Litrownik AJ, Landsverk JA. Caregiver substance abuse among maltreated children placed in out-of- home care. Child Welfare. 1999;78:221–39. [PubMed] [Google Scholar]
- 3.Chaffin M, Kelleher K, Hollenberg J. Onset of physical abuse and neglect: psychiatric, substance abuse, and social risk factors from prospective community data. Child Abuse Negl. 1996;20:191–203. doi: 10.1016/s0145-2134(95)00144-1. [DOI] [PubMed] [Google Scholar]
- 4.Ondersma SJ. Predictors of neglect within low-SES families: the importance of substance abuse. Am J Orthopsychiatry. 2002;72:383–91. doi: 10.1037/0002-9432.72.3.383. [DOI] [PubMed] [Google Scholar]
- 5.Kendler KS, Gardner CO, Edwards A, Hickman M, Heron J, Macleod J, et al. Dimensions of parental alcohol use/problems and offspring temperament, externalizing behaviors, and alcohol use/problems. Alcohol Clin Exp Res. 2013;37:2118–27. doi: 10.1111/acer.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann JP, Su SS. Parental substance use disorder, mediating variables and adolescent drug use: a non-recursive model. Addiction. 1998;93:1351–64. doi: 10.1046/j.1360-0443.1998.93913516.x. [DOI] [PubMed] [Google Scholar]
- 7.Kilpatrick DG, Acierno R, Saunders B, Resnick HS, Best CL, Schnurr PP. Risk factors for adolescent substance abuse and dependence: data from a national sample. J Consult Clin Psychol. 2000;68:19–30. doi: 10.1037//0022-006x.68.1.19. [DOI] [PubMed] [Google Scholar]
- 8.Jacob T, Waterman B, Heath A, True W, Bucholz KK, Haber R, et al. Genetic and Environmental Effects on Offspring Alcoholism: New Insights Using an Offspring-of-Twins Design. Arch Gen Psychiatry. 2003;60:1265–72. doi: 10.1001/archpsyc.60.12.1265. [DOI] [PubMed] [Google Scholar]
- 9.Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, Brief Intervention, and Referral to Treatment (SBIRT): toward a public health approach to the management of substance abuse. Subst Abus. 2007;28:7–30. doi: 10.1300/J465v28n03_03. [DOI] [PubMed] [Google Scholar]
- 10.Fleming MF, Lund MR, Wilton G, Landry M, Scheets D. The Healthy Moms Study: the efficacy of brief alcohol intervention in postpartum women. Alcohol Clin Exp Res. 2008;32:1600–6. doi: 10.1111/j.1530-0277.2008.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubio DM, Day NL, Conigliaro J, Hanusa BH, Larkby C, McNeil M, et al. Brief motivational enhancement intervention to prevent or reduce postpartum alcohol use: a single-blinded, randomized controlled effectiveness trial. J Subst Abuse Treat. 2014;46:382–9. doi: 10.1016/j.jsat.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaner E, Bland M, Cassidy P, Coulton S, Dale V, Deluca P, et al. Effectiveness of screening and brief alcohol intervention in primary care (SIPS trial): pragmatic cluster randomised controlled trial. BMJ. 2013;346:e8501. doi: 10.1136/bmj.e8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilbink M, Voerman G, van Beurden I, Penninx B, Laurant M. A randomized controlled trial of a tailored primary care program to reverse excessive alcohol consumption. J Am Board Fam Med. 2012;25:712–22. doi: 10.3122/jabfm.2012.05.120070. [DOI] [PubMed] [Google Scholar]
- 14.Beich A, Gannik D, Malterud K. Screening and brief intervention for excessive alcohol use: qualitative interview study of the experiences of general practitioners. BMJ. 2002;325:870. doi: 10.1136/bmj.325.7369.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aalto M, Pekuri P, Seppa K. Obstacles to carrying out brief intervention for heavy drinkers in primary health care: a focus group study. Drug Alcohol Rev. 2003;22:169–73. doi: 10.1080/09595230100100606. [DOI] [PubMed] [Google Scholar]
- 16.Yarnall KS, Pollak KI, Ostbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635–41. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Beurden I, Anderson P, Akkermans RP, Grol RP, Wensing M, Laurant MG. Involvement of general practitioners in managing alcohol problems: a randomized controlled trial of a tailored improvement programme. Addiction. 2012;107:1601–11. doi: 10.1111/j.1360-0443.2012.03868.x. [DOI] [PubMed] [Google Scholar]
- 18.Rooke S, Thorsteinsson E, Karpin A, Copeland J, Allsop D. Computer-delivered interventions for alcohol and tobacco use: a meta-analysis. Addiction. 2010;105:1381–90. doi: 10.1111/j.1360-0443.2010.02975.x. [DOI] [PubMed] [Google Scholar]
- 19.Moore BA, Fazzino T, Garnet B, Cutter CJ, Barry DT. Computer-based interventions for drug use disorders: a systematic review. J Subst Abuse Treat. 2011;40:215–23. doi: 10.1016/j.jsat.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10:e1001362. doi: 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman MG, Szkodny LE, Llera SJ, Przeworski A. A review of technology-assisted self-help and minimal contact therapies for drug and alcohol abuse and smoking addiction: is human contact necessary for therapeutic efficacy? Clinical Psychol Rev. 2011;31:178–86. doi: 10.1016/j.cpr.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Riper H, Spek V, Boon B, Conijn B, Kramer J, Martin-Abello K, et al. Effectiveness of E-self-help interventions for curbing adult problem drinking: a meta-analysis. J Med Internet Res. 2011;13:e42. doi: 10.2196/jmir.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portnoy DB, Scott-Sheldon LA, Johnson BT, Carey MP. Computer-delivered interventions for health promotion and behavioral risk reduction: a meta-analysis of 75 randomized controlled trials, 1988–2007. Prev Med. 2008;47:3–16. doi: 10.1016/j.ypmed.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzilos GK, Sokol RJ, Ondersma SJ. A randomized phase I trial of a brief computer-delivered intervention for alcohol use during pregnancy. J Womens Health (Larchmt) 2011;20:1517–24. doi: 10.1089/jwh.2011.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ondersma SJ, Beatty JR, Svikis DS, Strickler RC, Tzilos GK, Chang G, et al. Computer-Delivered Screening and Brief Intervention for Alcohol Use in Pregnancy: A Pilot Randomized Trial. Alcohol Clin Exp Res. 2015;39:1219–26. doi: 10.1111/acer.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ondersma SJ, Svikis DS, Thacker LR, Beatty JR, Lockhart N. Computer-delivered screening and brief intervention (e-SBI) for postpartum drug use: a randomized trial. J Subst Abuse Treat. 2014;46:52–9. doi: 10.1016/j.jsat.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz RP, Gryczynski J, Mitchell SG, Gonzales A, Moseley A, Peterson TR, et al. Computerized versus in-person brief intervention for drug misuse: a randomized clinical trial. Addiction. 2014;109:1091–8. doi: 10.1111/add.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ondersma SJ, Svikis DS, Lam PK, Connors-Burge VS, Ledgerwood DM, Hopper JA. A randomized trial of computer-delivered brief intervention and low-intensity contingency management for smoking during pregnancy. Nicotine Tob Res. 2012;14:351–60. doi: 10.1093/ntr/ntr221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ondersma SJ, Svikis DS, Schuster CR. Computer-based brief intervention a randomized trial with postpartum women. Am J Prev Med. 2007;32:231–8. doi: 10.1016/j.amepre.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sokol RJ, Martier SS, Ager JW. The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160:863–8. doi: 10.1016/0002-9378(89)90302-5. discussion 8–70. [DOI] [PubMed] [Google Scholar]
- 31.Scott CK. A replicable model for achieving over 90% follow-up rates in longitudinal studies of substance abusers. Drug Alcohol Depend. 2004;74:21–36. doi: 10.1016/j.drugalcdep.2003.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrow-Tlucak M, Ernhart CB, Sokol RJ, Martier S, Ager J. Underreporting of alcohol use in pregnancy: relationship to alcohol problem history. Alcohol Clin Exp Res. 1989;13(3):399–401. doi: 10.1111/j.1530-0277.1989.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 33.Lowe JB, Windsor RA, Adams B, Morris J, Reese Y. Use of a bogus pipeline method to increase accuracy of self-reported alcohol consumption among pregnant women. J Stud Alcohol. 1986;47:173–5. doi: 10.15288/jsa.1986.47.173. [DOI] [PubMed] [Google Scholar]
- 34.Grekin ER, Svikis DS, Lam P, Connors V, Lebreton JM, Streiner DL, et al. Drug use during pregnancy: validating the Drug Abuse Screening Test against physiological measures. Psychol Addict Behav. 2010;24:719–23. doi: 10.1037/a0021741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chasnoff IJ, Landress HJ, Barrett ME. The prevalence of illicit-drug or alcohol use during pregnancy and discrepancies in mandatory reporting in Pinellas County, Florida [see comments] N Engl J Med. 1990;322:1202–6. doi: 10.1056/NEJM199004263221706. [DOI] [PubMed] [Google Scholar]
- 36.Chase SK, Beatty JR, Ondersma SJ. A randomized trial of the effects of anonymity and quasi anonymity on disclosure of child maltreatment-related outcomes among postpartum women. Child Maltreat. 2011;16:33–40. doi: 10.1177/1077559510387659. [DOI] [PubMed] [Google Scholar]
- 37.Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev. 2005;24:217–26. doi: 10.1080/09595230500170266. [DOI] [PubMed] [Google Scholar]
- 38.Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry. 2003;60:184–9. doi: 10.1001/archpsyc.60.2.184. [DOI] [PubMed] [Google Scholar]
- 39.Ondersma SJ, Chase SK, Svikis DS, Schuster CR. Computer-based brief motivational intervention for perinatal drug use. J Subst Abuse Treat. 2005;28:305–12. doi: 10.1016/j.jsat.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 41.National Institute on Alcohol Abuse and Alcoholism. Recommended alcohol questions 2003. Available from: http://www.niaaa.nih.gov/research/guidelines-and-resources/recommended-alcohol-questions.
- 42.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2. New York: Guilford; 2002. [Google Scholar]
- 43.Miller WR, Zweben A, DiClemente CC, Rychtarik RG. In: Motivational Enhancement Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals with Alcohol Abuse and Dependence. Mattson ME, editor. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1994. [Google Scholar]
- 44.Miller WR. Increasing motivation for change. In: Hester RK, Miller WR, editors. Handbook of alcoholism treatment approaches: Effective alternatives. 2. Needham Heights, MA, US: Allyn & Bacon; 1995. pp. 89–104. [Google Scholar]
- 45.Kypri K, Langley JD, Saunders JB, Cashell-Smith ML. Assessment may conceal therapeutic benefit: findings from a randomized controlled trial for hazardous drinking. Addiction. 2007;102:62–70. doi: 10.1111/j.1360-0443.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 46.McCambridge J, Kypri K. Can simply answering research questions change behaviour? Systematic review and meta analyses of brief alcohol intervention trials. PloS One. 2011;6:e23748. doi: 10.1371/journal.pone.0023748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasselblad V, Hedges LV. Meta-analysis of screening and diagnostic tests. Psychological Bulletin. 1995;117:167–78. doi: 10.1037/0033-2909.117.1.167. [DOI] [PubMed] [Google Scholar]
- 48.Grissom RJ, Kim JJ. Effect Sizes for Research: Univariate and Multivariate Applications. 2. New York, NY: Routledge/Taylor & Francis; 2012. [Google Scholar]
- 49.Bertholet N, Palfai T, Gaume J, Daeppen JB, Saitz R. Do brief alcohol motivational interventions work like we think they do? Alcohol Clin Exp Res. 2014;38:853–9. doi: 10.1111/acer.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heather N. Can screening and brief intervention lead to population-level reductions in alcohol-related harm? Addict Sci Clin Pract. 2012;7:15. doi: 10.1186/1940-0640-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hettema J, Steele J, Miller WR. Motivational interviewing. Annual Review of Clinical Psychology. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- 52.Vasilaki EI, Hosier SG, Cox WM. The efficacy of motivational interviewing as a brief intervention for excessive drinking: a meta-analytic review. Alcohol Alcohol. 2006;41:328–35. doi: 10.1093/alcalc/agl016. [DOI] [PubMed] [Google Scholar]
- 53.Saitz R, Palfai TP, Cheng DM, Alford DP, Bernstein JA, Lloyd-Travaglini CA, et al. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA. 2014;312:502–13. doi: 10.1001/jama.2014.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pollick SA, Beatty JR, Sokol RJ, Strickler RC, Chang G, Svikis DS, et al. Acceptability of a computerized brief intervention for alcohol among abstinent but at-risk pregnant women. Subst Abus. 2015;36:13–20. doi: 10.1080/08897077.2013.857631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saitz R. Lost in translation: The perils of implementing alcohol brief intervention when there are gaps in evidence and its interpretation. Addiction. 2014;109:1060–2. doi: 10.1111/add.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saitz R. Alcohol screening and brief intervention in primary care: Absence of evidence for efficacy in people with dependence or very heavy drinking. Drug Alcohol Rev. 2010;29:631–40. doi: 10.1111/j.1465-3362.2010.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med. 2007;32(5 Suppl):S112–8. doi: 10.1016/j.amepre.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]