Abstract
Objective
The chronicity and severity of childhood onset Systemic Lupus Erythematosus (cSLE) necessitate effective transition from pediatric to adult providers. We studied transition outcomes in a cSLE cohort.
Methods
We identified patients at an adult Lupus clinic diagnosed with SLE ≤ 18 years who had been followed by a pediatric rheumatologist. Data extracted from the first 3 years in adult care (“post-transition period”) included: sociodemographics, depression, anxiety, SLE manifestations, SLE Disease Activity Index (SLEDAI) and Systemic Lupus International Collaborating Clinics/ACR Damage Index for SLE (SLICC) scores, non-adherence, and gaps in care (no appointments in the recommended time frame). Multivariable logistic regression analyses for predictors of: (1) time between pediatric and adult providers, (2) gaps in care, (3) unscheduled utilization (Emergency Department visits and admissions) (4) depression and/or anxiety were performed, as was a multivariable Poisson regression analysis for number of missed appointments.
Results
In 50 patients, SLEDAI scores were stable (mean 5.7 ± 5.0 at start vs. 4.7 ± 4.8 at year 3, p=0.2), but SLICC scores increased (0.46 ± 0.84, vs. 0.78 ± 1.25, p=0.01). Depression and anxiety increased significantly (10% vs. 26%, p=0.02). Mean time from last pediatric to first adult provider visit was almost 9 months (253 ± 392 days). Nearly 75% of patients had ≥1 gap in care. White race, low education level and non-adherence were significantly associated with missed appointments.
Conclusion
Despite moderate disease activity in this cSLE transition cohort, prolonged time between pediatric and adult providers and gaps in care in the post-transition period occurred. Anxiety and depression were frequently reported. Future work should identify methods to improve transition.
Keywords: Childhood-onset Systemic Lupus Erythematosus, Transition, Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) begins during adolescence in approximately 20% of patients and continues into adulthood nearly without exception.1 SLE disproportionately affects females, minorities, and those of low socioeconomic status,2, 3 and childhood-onset SLE (cSLE) typically occurs during adolescence, a particularly vulnerable period of development. These factors render the process of transition of cSLE patients from pediatric to adult rheumatologists potentially difficult.
The American Academy of Pediatrics, the American Academy of Family Physicians and the American College of Physicians have endorsed the need for effective transition of care4, 5 and it has been designated a public health goal per the U.S Department of Health and Human Services’ Healthy People 2020.6 Unfortunately, reports have not demonstrated significant success in achieving goals related to transition.7, 8 Minorities are at elevated risk for lack of transition preparedness,7 which has important implications in designing a transition program for patients with cSLE, given the increased incidence of cSLE in minority groups. One small study of transition-aged patients with pediatric rheumatic disease including cSLE reported increased disease activity in nearly half the cSLE patients during the year after transition,9 and a recent single center study found that more than half of cSLE patients reported difficulties during transition and that these patients were more likely to report poor symptom control.10
We studied a group of patients with cSLE during the three years following the transition from pediatric to adult care who had no formal transitioning process, and assessed patient characteristics, disease activity scores and damage scores. Our goals were to describe the post-transition period in our cohort, and to investigate potential risk factors for poor transition outcomes, including prolonged time between last pediatric and first adult provider visits, gaps in care, unscheduled utilization of healthcare via Emergency Department (ED) visits and admissions, and missed appointments.
Patients and Methods
Data Source
Within the Brigham and Women's Hospital Lupus Registry, we identified patients who met the following criteria: 1) diagnosed with SLE per American College of Rheumatology (ACR) Classification Criteria11 at ≤ 18 years of age, 2) 18–23 years old at their first visit to the Lupus Center at Brigham and Women’s Hospital, 3) followed by a pediatric rheumatologist prior to transition to adult care, 4) > 1 subsequent visit to the Lupus Center within three years of their first visit, and 5) first seen between 1992 and 2014. It was expected that a subgroup of the total cohort would transition their pediatric care from the neighboring Boston Children’s Hospital. IRB permission was obtained at both sites via a reliance agreement (Protocol 2014P001086).
Data Collection
Age at cSLE diagnosis, race, ethnicity, as well as data regarding cSLE manifestations and symptoms were extracted from the electronic medical records at Brigham and Women’s Hospital and Boston Children’s Hospital, when applicable. We also reviewed the pediatric medical records of patients who transitioned from Boston Children’s Hospital to determine time between last pediatric and first adult provider visits.
The post-transition period was defined as three years from the first adult Lupus Center visit. Information collected in detail from the first Lupus Center visit and the visit closest to the three year mark included demographic information, educational level, hydroxychloroquine (HCQ) and corticosteroid use (mg of prednisone equivalents/day), and whether non-adherence was noted in medical records. Median annual household income at first Lupus Center visit was based on residential zip code12 and 2000 US Census data. HCQ and corticosteroids were studied given their frequent use in treating lupus. Medication non-adherence was documented when patients self-reported not taking medications as prescribed, or there was physician documentation in clinic notes reporting medication nonadherence. Diagnoses of anxiety and depression were noted if the patient was referred to social work, psychology or psychiatry specifically for depression and/or anxiety, if either diagnosis was included in their problem list, or if the treating physician specifically documented these diagnoses during visits. If noted in the medical record, information regarding social work involvement was also extracted.
During the time of the study period, there was no formalized process to transition patients from Boston Children’s Hospital to Brigham and Women’s Hospital.
Suggested timing of first visit at the Lupus Center was determined on an individual basis by the pediatric provider, but within six months of last pediatric visit was typical given the need for frequent monitoring in lupus patients. Data regarding transition preparedness for patients who came from other centers were not available.
Systemic Lupus Erythematosus Disease Activity Index (SLEDAI)13 scores and Systemic Lupus International Collaborating Clinics/ACR Damage Index for Systemic Lupus Erythematosus (SLICC)14 scores were calculated for both visits. At the first visit, active disease was defined as manifestations in the six months prior to the visit, whereas past disease was defined as manifestations after the diagnosis of cSLE, but not present during the six months prior to first Lupus Center visit. Manifestations of renal disease included glomerulonephritis, nephrotic syndrome, and end-stage renal disease (ESRD). Active renal disease was defined by (1) proteinuria >0.5 grams/24 hours or, if 24 hour measurements were not available, by positive renal items on the SLEDAI,15 (2) an increase in immunosuppressive medications to treat renal disease, and/or (3) need for renal biopsy during the post-transition period. Manifestations of neuropsychiatric SLE (NPSLE) included cerebrovascular accident, transverse myelitis, seizures and psychosis.
Missed appointments in the post-transition period were defined as appointments scheduled but not kept in the Lupus Center by the patient (frequently referred to as “no shows”). In order to capture those patients who had gaps in care from lack of scheduling appointments, as opposed to those who scheduled but missed them, we also looked for periods of time ≥ 6 months per year of study wherein patients had no appointments, as visits are typically recommended at least every 6 months in the Lupus Center. Gaps in care were also recorded if the physician recommended follow-up in the Lupus Center within a specified period of time, i.e. 2 months, but the patient did not return within the recommended time period.
To assess unscheduled utilization of health care during the post-transition period, we examined the electronic medical record for visits to the ED at the two hospitals included in this study, as well as admissions per year and recorded the frequency and length of stay. Admissions for scheduled infusions were not included.
Data Analysis
Summary statistics were used to examine sociodemographic characteristics. Frequencies and percentages were determined for discrete variables. Continuous variables were summarized by mean ± SD or median ± range as appropriate. Differences in SLEDAI scores, SLICC scores, and depression and/or anxiety as a combined variable, and non-adherence between first and last Lupus Center visits were analyzed using paired t tests. Depression and/or anxiety and non-adherence were dichotomized as present versus not present.
Transition outcomes of interest included: time from last pediatric visit to first adult provider visit, gaps in care, unscheduled utilization of health care (ED visits and admissions) and missed appointments. In order to investigate risk factors for (1) having > 6 months at the transition between pediatric and adult providers, (2) ≥1 gap in care, (3) unscheduled utilization of health care (≥1 ED visit or admission), and (4) documentation of depression and/or anxiety during the post-transition period, we constructed multivariable logistic regression models including age at cSLE diagnosis (<12 years old vs. ≥12 years old), race, ethnicity, education level (incomplete high school education, completion of high school or equivalent, > 1 year of post high school education), zip code based median annual household income (<$50,000/year, $50,000–$100,000/year, ≥100,000/year), presence of renal disease and/or NPSLE, medication non-adherence, and SLEDAI score at first Lupus Center visit (≤4 vs. >4).
A multivariable Poisson regression analysis analyzing risk factors for the number of missed appointments during the post-transition period utilized the same predictors as the logistic regression. Data were analyzed with SAS version 9.2 (Cary, NC). Two-sided p values < 0.05 were considered statistically significant.
Results
Description of the Transition Cohort
We identified 83 patients with cSLE followed at the Brigham and Women’s Hospital Lupus Center. Thirteen patients had been diagnosed with cSLE, but had not been followed by a pediatric rheumatologist. Twenty patients received a second opinion in a single visit at the Lupus Center. Fifty patients fit all inclusion criteria and were seen by 16 rheumatologists in the Lupus Center; each patient had a primary rheumatologist who saw the patient for the majority of visits during the post-transition period. Twenty-eight (56%) of the patients had transitioned from the Boston Children’s Hospital next door. Forty five (90%) of the patients were female. Of the 50 patients, 42% (n=21) were White, 22% (n=11) were African American, 10% (n=5) were Asian and 22% (n=11) reported Hispanic ethnicity (Table I). Of the 13 patients with race described as “other,” eight identified Hispanic ethnicity but declined to complete race. The mean age at onset of cSLE symptoms was 14.1 years (range 6–18) and mean age at diagnosis of cSLE was 14.5 years (range 6–18). The majority of patients (n=46, 92%) were in a moderate to high annual household income. The mean age at the first Lupus Center visit was 19.5 ± 2.20 years and 60% of patients had at least one year of post high school education at that time. The mean number of days followed in the Lupus Center was 805 ± 430 days (median=1050, range 28–1652). Nearly two thirds of patients had follow up for at least two years (n=32, 64%). At the first Lupus Center visit, five patients had a pre-existing diagnosis of an anxiety or depressive disorder and were being treated for it, or had symptoms of anxiety and/or depression that warranted referral to a mental health provider, whereas over a quarter of patients (n=13) were noted to have anxiety and/or depression at the end of the post-transition period (10% vs. 26%, p=0.02, Table II). Predictors for having depression and/or anxiety were not identified in our logistic regression modeling. Over 25% of patients were evaluated by social work during the post-transition period (n=13). Reasons for social work involvement included depression, domestic violence, incarceration, financial and housing stresses, substance abuse, and non-adherence.
Table I.
Demographics of Cohort
| CHARACTERISTIC | SUBJECTS (N=50) N, (%) OR MEAN (RANGE) |
|---|---|
| Female Sex | 45 (90%) |
| Race | |
| • White | 21 (42%) |
| • African American | 11 (22%) |
| • Asian | 5 (10%) |
| • Missing/Othera | 13 (26%) |
| Hispanic | 11 (22%) |
| Age at onset of cSLEb symptoms | 14.1 years (6–18) |
| Age at diagnosis of cSLE | 14.5 years (6–18) |
| Zip Code-based Annual Household Incomec | |
| • <50,000 | 4 (8%) |
| • $50,000–100,000 | 30 (60%) |
| • $≥100,000 | 16 (32%) |
5 patients had missing data, 8 patients identified as Hispanic ethnicity in the absence of identifying Race
cSLE: Childhood-onset Systemic Lupus Erythematosus
Missing data =1
Table II.
Characteristics of 1st and last Lupus Center Visits during Post-Transition Period
| CHARACTERISTIC | 1ST LUPUS CENTER VISIT |
LAST LUPUS CENTER VISIT |
|---|---|---|
| Age in years (mean ±SD, median) | 19.5 ± 2.20, 19.7 | 21.9 ± 2.10, 21.9 |
| Educational Level, n (%) | ||
| ➢ < HSa | ➢ 10 (20%) | ➢ 3 (6%) |
| ➢ HS/GED | ➢ 10 (20%) | ➢ 7 (14%) |
| ➢ > 1 Year post HS | ➢ 30 (60%) | ➢ 32 (64%) |
| ➢ > 4 Years post HS | ➢ 3 (6%) | |
| Medications, n (% yes) | ||
| ➢ HCQ | ➢ 36 (72%) | ➢ 39 (78%) |
| ➢ Prednisone equivalents | ➢ 37 (74%) | ➢ 31 (62%) |
| • ≤10 mg/day | • 18 (36%) | • 18 (36%) |
| • > 10 mg/day | • 19 (38%) | • 13 (26%) |
| Anxiety/Depression, n (% yes) | 5 (10%) | 13 (26%)b |
| Social Work Involvement, n (%) | 13 (26%) | |
| Days of Follow Up (mean±SD, range) | 805±430, (28–1652) | |
HS: High School
Increased significantly over Post-transition Period (0.1 ± 0.3 vs. 0.26 ± 0.44, p=0.02)
Non-adherence was documented by the provider in six patients at the first and last post-transition visits, with an additional 6 patients having documentation for nonadherence at the last transition visit (12% vs. 24%, p=0.01). Of the 17 patients reported as not taking HCQ over the post-transition period, the most common causes were non-adherence or refusal due to patient’s belief it was ineffective or concern about potential side effects (n=10). Two patients had HCQ allergy, three had ocular toxicity, one had cardiomyopathy, and one reported gastrointestinal side effects.
Disease Manifestations and Disease Activity
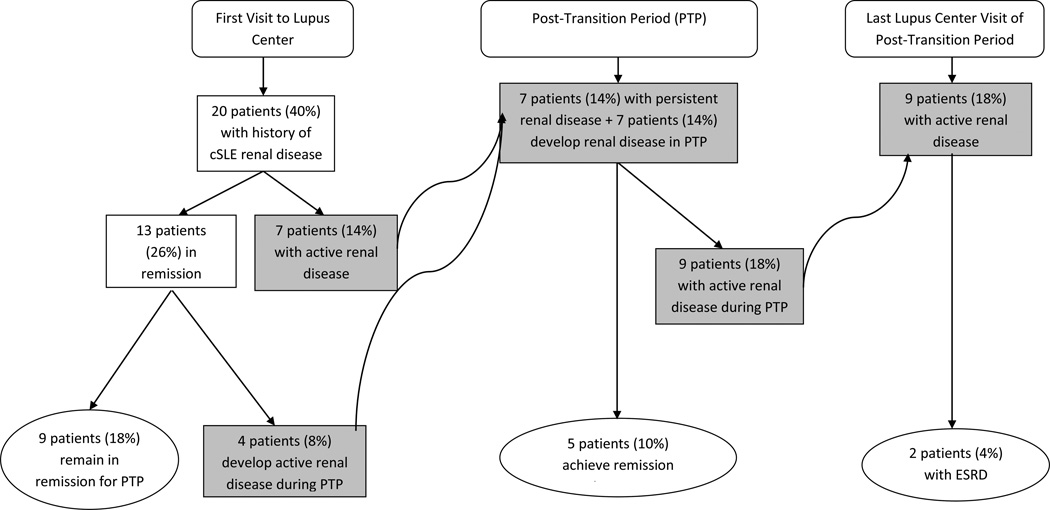
SLEDAI scores did not differ significantly from the beginning to the end of the posttransition period (mean 5.7 ± 5.0vs. 4.7 ± 4.8, range 0–18, p=0.2). Twenty of the patients (40%) had a history of lupus nephritis at the first Lupus Center visit, and 7 patients (15%) had active renal disease at their first visit (Figure I). Over a quarter of patients had active renal disease during the post-transition period (n=14, 28%). At the end of the post-transition period, nearly half of the patients (n=23, 46%) had either a history of renal disease during their pediatric care and/or developed renal disease, and two patients had ESRD and required dialysis. Fewer patients had a history of neuropsychiatric SLE (NPSLE, n=4, 8%) at the first Lupus Center visit, and none had active NPSLE at the beginning of the post-transition period. Three patients (6%) had active NPSLE during the post-transition period. No mortalities were recorded during transition.
Figure 1.
Lupus Nephritis in a Transition Cohort of patients (n = 50) with childhood-onset systemic lupus erythematosus (cSLE). Boxes in blue indicate patients with active renal disease. Active renal disease was found during the Post-Transition Period (PTP) in 28% of patients; few progressed to End Stage Renal Disease (ESRD). LC= Lupus Center.
Twenty-two patients (44%) had SLICC scores ≥1 during transition. SLICC scores were low overall, but increased significantly over the study period (0.46±0.84, range 0–3 vs. 0.78±1.25, range 0–5, p=0.01). The most common morbidities included avascular necrosis (n=5 patients) and deep venous thrombosis (n= 5 patients). Four patients had extensive skin scarring or scarring alopecia.
Transition Outcomes
We found that 28 of the 50 patients had transitioned from the local pediatric hospital. The time from last pediatric rheumatology visit to first Lupus Center visit was nearly nine months (mean of 253 ± 392 days, median 133 days, range 6–2017 days, Table III). Gaps in care were frequent, with over 70% of patients having at least one gap in care during the post-transition period (n=36, 72%).
Table III.
Transition Outcomes
| TRANSITION OUTCOME | SUBJECTS (N=50) N, (%) OR MEAN ± SD (RANGE) |
|---|---|
| Days from Last Pediatric to First Adult Provider Visit | ➢ 253 ± 392 (6–2017) |
| Patients with ≥1 gap in care during P-TPa | ➢ 36 (72%) |
| Admissions per year of P-TP (n, mean LOSb in days ± SD) | |
| ➢ Year 1 | ➢ 22, 8.79 ± 12.5 |
| ➢ Year 2 | ➢ 22, 4.07 ± 4.7 |
| ➢ Year 3 | ➢ 17, 3.82 ± 1.8 |
| Number of patients admitted during P-TP | ➢ 15 (30%) |
| ED Visits per patient during P-TP (Mean ± SD, median, range) | ➢ 1.9 ± 5.2, 0 (0–34) |
| % Missed Appointments in LC/Total LCc Appointments per Person (Mean ± SD, median, range) | ➢ 9.2%±13.5, 0 (0–55%) |
| Patients with ≥2 missed appointments during P-TP | ➢ 16 (32%) |
P-TP= Post-Transition Period
LOS= length of stay
LC= Lupus Center
Unscheduled utilization of the health care system due to ED visits was 1.9±5.2 per patient (median=0, range 0–34, Table III) during the post-transition period. Sixty one admissions were recorded in 15 patients during this time, indicating that most admissions were in a minority of patients. The majority of admissions (47/61, 77%) were due to SLE or complications of SLE therapy. The latter included serious infections or medication adverse events, such as bleeding while taking warfarin. We found that 4 patients (8%) utilized the pediatric ED following their transition to the Lupus Center. Conversely, we examined records to assess for ED utilization at the adult hospital prior to being seen in the Lupus Center and four of patients (8%) had been seen in the adult ED before officially transitioning their care to the adult hospital. Logistic regression modeling did not reveal significant predictors for time > 6 months between last pediatric and first adult provider visit, ≥1 gaps in care, or unscheduled utilization (≥1 ED visit and/or admission) during the post-transition period.
Nearly half of patients (n=23, 46%) missed at least one appointment that had been scheduled in the Lupus Center during the post-transition period (mean 2.7±5.9, range 0–32). Of those 23 patients who missed one appointment, 70% (n=16) missed more than one appointment. Missed appointments as a percentage of total scheduled appointments at the Lupus Center was 9% per patient (9.2%±13.5% per patient, median=0 and range 0–55%). Education below the high school level, medication non-adherence and white race were significantly associated with missed appointments in a Poisson regression analysis.
Discussion
The number of adolescents and young adults with chronic health conditions is growing and currently half a million of these patients are transitioning to adult care.16 The physicians involved in the care of these patients span sub-specialties across pediatrics and medicine, and although each specialty has disease activity indices or other specific markers of disease, one of the challenges in studying a transition population is defining outcomes that are applicable across subspecialty populations. Sharma et al17 have suggested that measurable outcomes of successful transition may include uninterrupted access to health care services as evidenced by lack of gaps in care and unscheduled utilization of health care services during the transition.
In this multiracial, transition aged cohort of patients with cSLE, we found that the transition from pediatric to adult providers was characterized by moderately high disease activity, prolonged time between the last pediatric and first adult provider appointments, and frequent gaps in care.
SLE disease activity as measured by SLEDAI scores was moderate and stable in our cohort, whereas SLICC scores increased significantly over the post-transition period. No mortalities occurred. Hersh et al also found stable SLEDAI scores in 16 patients with cSLE in the year after transition; one mortality in a cSLE patient was reported.9 It is of note that although a quarter of our patients had active renal disease over the post-transition period, only two patients had ESRD at the end of the post-transition period. Of the two patients with ESRD, one patient had been non-compliant with medications, had missed 10 Rheumatology and Nephrology visits, and had a prolonged gap in care (9 months) during the post-transition period. The other patient had developed cSLE at the age of 6, and although her compliance improved during the post-transition period, she had already sustained significant kidney injury and was placed on dialysis and the transplant list.
SLICC scores were low overall but increased during the post-transition period in our study, which is not unexpected given that longer disease duration is associated with increasing damage accrual.18 There is a parallel for accruing disease related damage in the transition literature in patients with Type 1 diabetes mellitus, in that increasing background incidence of retinopathy was documented in a transition aged Swedish cohort.19 To our knowledge, this is the first description of damage scores in a cSLE transition cohort, and raises consideration of intensified monitoring of patients during their transition from pediatric to adult care.
Nearly a third (n=16, 32%) of patients had >1 missed appointments during the post-transition period, which is quite similar to the number of missed appointments in the cohort described by Hersh et al.9 However, the overall percentage of missed visits (missed appointments/total number of scheduled appointments) was not high, with an average of 9% of Lupus Center visits missed per patient. This is in contrast to an urban, adult SLE cohort studied by Mosley-Williams et al, wherein patients missed ~30–40% of their appointments.20 Missed appointments were significantly associated with white race, lower educational level (< high school degree) and medication non-adherence. Interestingly, there were significantly more missed appointments in white women in Mosley-Williams’ study as well,20 although this was only marginally significant once adjusted for income. The finding of lower educational level leading to a suboptimal transition outcome may help to delineate an at-risk population that requires further support during the process of transition. It should be noted, however, that although missed appointments weren’t prominent, the majority of our patients experienced gaps in care whereby they didn’t schedule appointments within the recommended time frame per the treating physician. The lack of appropriate scheduling with its concomitant potentially serious consequences demonstrates the need to educate transition patients regarding the importance of scheduling their own appointments. To this end, scheduling of appointments by the patient is used as a transition readiness assessment item by the National Health Care Transition Center.21
Time from last pediatric visit to first adult visit was on average almost nine months, which is longer than reported in a group of transition aged patients with pediatric rheumatic disease.9 In a report of patients with T1DM, time to first adult provider visit was > 6 months in up to a third of the patients.22 We were unable to determine the cause for the delay in care given the retrospective nature of this project. Given the potential severity of SLE, decreased time to first adult provider visit should be a goal in transition programs for these patients, and provides an important target in the design and implementation of a transition program.
Unscheduled utilization in regards to hospital admission was not prominent overall in our cohort, as most patients were not admitted in the post-transition period. However, it is notable that the majority of admissions were directly related to lupus or complications of SLE therapy. Unscheduled utilization of health care via ED visits was also not frequent in our population. However, the average of approximately 2 ED visits per patient during the post-transition period is likely an underestimation, as visits to outside EDs may be not be routinely documented in the hospital electronic medical health records. Furthermore, it is likely a sign of suboptimal transition that 16% of patients continued to access the pediatric ED following their first visit to the adult Lupus Center, or obtained care through the adult ED prior to the first Lupus Center visit.
Our population of cSLE patients had a significant burden of depression and anxiety that significantly increased over the post-transition period, and more than a quarter of them required support from social work for a variety of serious circumstances including homelessness, substance abuse and incarceration. Other chronic conditions, including asthma23 and Type 1 diabetes mellitus24, have been associated with worsening of disease symptoms when co-morbidities of depression and anxiety are present. Mood disorders in SLE patients are quite complex, given the difficulty in disentangling depression and anxiety as manifestations of NP SLE vs. the psychosocial impact of a chronic disease characterized by unpredictable, potentially fatal flares of disease. In cSLE patients undergoing transition, these considerations must be further placed in the context of the challenges associated with emerging adulthood. The relationship between depression and SLE disease activity has been studied,25 but results have been conflicting.26 Findings from a recent inception cohort revealed that mood disorders in SLE are likely multi-factorial and consideration of non-lupus therapies to treat them is important.27
The substantial amount of non-adherence as documented by the providers is of interest, although is limited by the retrospective nature of this review. Given the vulnerability of transition-aged young adults, as well as the disease activity and damage scores in this group, new strategies to address non-adherence seem especially important. Ting et al studied the effect of cellular text messaging on adherence with medical visits and HCQ.28 Interestingly, text messaging was effective in increasing clinic attendance, but did not impact adherence to HCQ as measured by serum HCQ levels.
The successful transition from pediatric to adult care for all youth with chronic conditions is predicated on the ability of the individual to achieve self-efficacy and disease self-management skills, and the ability of the medical system to provide patient centered care. Given the complex interplay of chronic disease, the tasks of emerging adulthood and our complicated medical system, methods to successfully guide these patients through transition will likely need to be flexible, applicable across diseases yet responsive to differences in disease severity. These considerations prompted Huang et alto study the capability of a generic technology platform to improve disease management tasks and health related self-efficacy in a transition cohort with patients with Type 1 diabetes mellitus, inflammatory bowel disease, and cystic fibrosis.29 The results were encouraging with regard to short-term improvement in these outcomes. However, it should be noted that metrics for assessing successful transition in the long-term, such as gainful employment and low disease activity scores several years after transition, have not yet been adequately described.
A limitation in the generalizability of our study is that our cohort was of relatively high socioeconomic status. Despite this, non-adherence and gaps in care were notable. The age at symptom onset and age at diagnosis of cSLE were similar, perhaps due to a strong medical presence in an urban setting leading to more rapid diagnosis. As a retrospective review, there were limitations in data collection. Six patients did not have SLEDAI scores calculated at their last post-transition period visit due to missing laboratory data, which may have affected our finding of SLEDAI scores as stable over the post-transition period. Furthermore, documentation of depression and anxiety as well as medication non-adherence was dependent on the provider. Thus, although elevated, this is likely an underestimation of mental health issues and adherence in our population. Although our cohort is larger than other published transition cohorts of patients with pediatric rheumatic disease,9, 10 the small number of patients in our study limited the power of our analyses, likely leading to the inability to identify predictors of prolonged time to first adult provider visit, gaps in care, unscheduled utilization and depression and/or anxiety. Additionally, the lack of a formalized transition process between the pediatric and adult Rheumatology programs may have influenced outcomes, particularly the time from last pediatric visit to fist adult provider visit. Lastly, we were unable to determine insurance status during the post-transition period, as insurance status is overwritten in the electronic medical record and retrieval of prior insurance information is not possible.
In summary, the post-transition period in our cohort of cSLE patients who did not undergo a formal transition process was characterized by moderate disease activity and gaps in care. Anxiety and depression were notable, and likely underestimated in this retrospective review. This study highlights key areas to target in the design and implementation of transition programs of cSLE patients. Future areas of study include strategies to address gaps in care, non-adherence and a multi-disciplinary approach to complex patients.
Acknowledgments
Funding
This work was supported by Boston Children’s Hospital Career Development Fellowship (Dr. Son) and NIH K24 AR066109 (Dr. Costenbader).
Footnotes
Declaration of Conflicting Interests:
This manuscript was produced without any financial support or other benefits from commercial sources, nor are there any other financial interests of the authors which could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work.
References
- 1.Mina R, Brunner HI. Update on differences between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis research & therapy. 2013;15:218. doi: 10.1186/ar4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demas KL, Costenbader KH. Disparities in lupus care and outcomes. Current opinion in rheumatology. 2009;21:102–109. doi: 10.1097/BOR.0b013e328323daad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Son MB, Johnson VM, Hersh AO, Lo MS, Costenbader KH. Outcomes in hospitalized pediatric patients with systemic lupus erythematosus. Pediatrics. 2014;133:e106–e113. doi: 10.1542/peds.2013-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of P, American Academy of Family P, American College of P, Transitions Clinical Report Authoring G. Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128:182–200. doi: 10.1542/peds.2011-0969. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of P, American Academy of Family P and American College of Physicians-American Society of Internal M. A consensus statement on health care transitions for young adults with special health care needs. Pediatrics. 2002;110:1304–1306. [PubMed] [Google Scholar]
- 6.Healthy People 2020 Topics and Objectives, Disability and Health. [Accessed August 24, 2015]; [Google Scholar]
- 7.McManus MA, Pollack LR, Cooley WC, et al. Current status of transition preparation among youth with special needs in the United States. Pediatrics. 2013;131:1090–1097. doi: 10.1542/peds.2012-3050. [DOI] [PubMed] [Google Scholar]
- 8.Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Annals of internal medicine. 2004;141:533–536. doi: 10.7326/0003-4819-141-7-200410050-00009. [DOI] [PubMed] [Google Scholar]
- 9.Hersh AO, Pang S, Curran ML, Milojevic DS, von Scheven E. The challenges of transferring chronic illness patients to adult care: reflections from pediatric and adult rheumatology at a US academic center. Pediatric rheumatology online journal. 2009;7:13. doi: 10.1186/1546-0096-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felsenstein S, Reiff AO, Ramanathan A. Transition of Care and Health-Related Outcomes in Pediatric-Onset Systemic Lupus Erythematosus. Arthritis care & research. 2015;67:1521–1528. doi: 10.1002/acr.22611. [DOI] [PubMed] [Google Scholar]
- 11.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 12.Jolly M, Mikolaitis RA, Shakoor N, Fogg LF, Block JA. Education, zip codebased annualized household income, and health outcomes in patients with systemic lupus erythematosus. The Journal of rheumatology. 2010;37:1150–1157. doi: 10.3899/jrheum.090862. [DOI] [PubMed] [Google Scholar]
- 13.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis and rheumatism. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 14.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis and rheumatism. 1996;39:363–369. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 15.Miettunen PM, Pistorio A, Palmisani E, et al. Therapeutic approaches for the treatment of renal disease in juvenile systemic lupus erythematosus: an international multicentre PRINTO study. Annals of the rheumatic diseases. 2013;72:1503–1509. doi: 10.1136/annrheumdis-2012-201937. [DOI] [PubMed] [Google Scholar]
- 16.Perrin JM, Bloom SR, Gortmaker SL. The increase of childhood chronic conditions in the United States. JAMA : the journal of the American Medical Association. 2007;297:2755–2759. doi: 10.1001/jama.297.24.2755. [DOI] [PubMed] [Google Scholar]
- 17.Sharma N, O'Hare K, Antonelli RC, Sawicki GS. Transition care: future directions in education, health policy, and outcomes research. Academic pediatrics. 2014;14:120–127. doi: 10.1016/j.acap.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strand V, Gladman D, Isenberg D, Petri M, Smolen J, Tugwell P. Outcome measures to be used in clinical trials in systemic lupus erythematosus. The Journal of rheumatology. 1999;26:490–497. [PubMed] [Google Scholar]
- 19.Sparud-Lundin C, Ohrn I, Danielson E, Forsander G. Glycaemic control and diabetes care utilization in young adults with Type 1 diabetes. Diabet Med. 2008;25:968–973. doi: 10.1111/j.1464-5491.2008.02521.x. [DOI] [PubMed] [Google Scholar]
- 20.Mosley-Williams A, Lumley MA, Gillis M, Leisen J, Guice D. Barriers to treatment adherence among African American and white women with systemic lupus erythematosus. Arthritis and rheumatism. 2002;47:630–638. doi: 10.1002/art.10790. [DOI] [PubMed] [Google Scholar]
- 21.Transition CfHC. Six Core Elements of Health Care Transition. 2015 [Google Scholar]
- 22.Garvey KC, Wolpert HA, Laffel LM, Rhodes ET, Wolfsdorf JI, Finkelstein JA. Health care transition in young adults with type 1 diabetes: barriers to timely establishment of adult diabetes care. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2013;19:946–952. doi: 10.4158/EP13109.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson LP, Lozano P, Russo J, McCauley E, Bush T, Katon W. Asthma symptom burden: relationship to asthma severity and anxiety and depression symptoms. Pediatrics. 2006;118:1042–1051. doi: 10.1542/peds.2006-0249. [DOI] [PubMed] [Google Scholar]
- 24.Hood KK, Huestis S, Maher A, Butler D, Volkening L, Laffel LM. Depressive symptoms in children and adolescents with type 1 diabetes: association with diabetes-specific characteristics. Diabetes care. 2006;29:1389–1391. doi: 10.2337/dc06-0087. [DOI] [PubMed] [Google Scholar]
- 25.Kohut SA, Williams TS, Jayanthikumar J, et al. Depressive symptoms are prevalent in childhood-onset systemic lupus erythematosus (cSLE) Lupus. 2013;22:712–720. doi: 10.1177/0961203313488840. [DOI] [PubMed] [Google Scholar]
- 26.Palagini L, Mosca M, Tani C, Gemignani A, Mauri M, Bombardieri S. Depression and systemic lupus erythematosus: a systematic review. Lupus. 2013;22:409–416. doi: 10.1177/0961203313477227. [DOI] [PubMed] [Google Scholar]
- 27.Hanly JG, Su L, Urowitz MB, et al. Mood Disorders in Systemic Lupus Erythematosus: Results From an International Inception Cohort Study. Arthritis Rheumatol. 2015;67:1837–1847. doi: 10.1002/art.39111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ting TV, Kudalkar D, Nelson S, et al. Usefulness of cellular text messaging for improving adherence among adolescents and young adults with systemic lupus erythematosus. The Journal of rheumatology. 2012;39:174–179. doi: 10.3899/jrheum.110771. [DOI] [PubMed] [Google Scholar]
- 29.Huang JS, Terrones L, Tompane T, et al. Preparing adolescents with chronic disease for transition to adult care: a technology program. Pediatrics. 2014;133:e1639–e1646. doi: 10.1542/peds.2013-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]