Abstract
Lens fiber cells are highly elongated cells with complex membrane morphologies that are critical for the transparency of the ocular lens. Investigations into the molecular mechanisms underlying lens fiber cell elongation were first reported in the 1960s, however, our understanding of the process is still poor nearly 50 years later. This review summarizes what is currently hypothesized about the regulation of lens fiber cell elongation along with the available experimental evidence, and how this information relates to what is known about the regulation of cell shape/elongation in other cell types, particularly neurons.
Keywords: cytoskeleton, tubulin, actin, cell shape, differentiation
Introduction
Lens Fiber Cell Morphology
The ocular lens is a remarkable structure. It is a transparent, cellular tissue which has numerous biochemical and structural specializations that form an exquisitely tuned refractive index gradient which efficiently refracts light (Figure 1A) (Bassnett et al., 2011). The earliest histological investigations of the lens noted that it is comprised of two morphologically distinct cell types surrounded by a thickened basement membrane, the lens capsule (Thin and Ewart, 1876). Lens epithelial cells are found on the anterior surface closest to the cornea, while the profoundly elongated lens fibers form the bulk of the lens (Figure 1B) (Zampighi et al., 2000). There is considerable species and developmental variation in the length of lens fibers. Chicken primary lens fibers are 200–400μm long measured from their apical tip to their basal attachment site on the lens capsule (Shestopalov and Bassnett, 2000), while adult bovine secondary lens fibers are up to 20mm in length (Kuszak et al., 2004a).
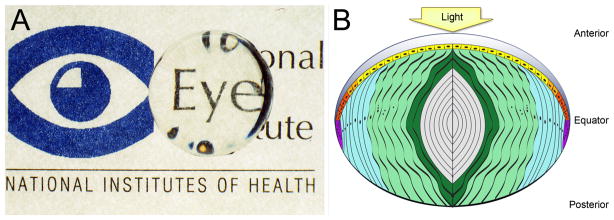
Figure 1.
A) A cow lens placed over the National Eye Institute logo showing both the clarity and refractive properties of the ocular lens. B) Parts of the lens. The lens is composed of two cell types: a monolayer of epithelial cells seen on the anterior surface, which proliferate and differentiate to fiber cells which make up the majority of the lens. Light yellow– light; dark grey– lens capsule; yellow– central epithelium; orange– germinative zone; red– transition zone; purple– meridional row region/bow region; light blue– outer cortical fiber cells; light green-inner cortical fiber cells; dark green– beginning nuclear fiber cells; light gray– nuclear fiber cells including the primary fiber cells which are found at the very center.
Lens fiber cells are organized into radial cell columns whose packing is optimized by their hexagonal cross sectional profile with two parallel sides ranging from 5 – 15μm wide and four shorter sides ranging from 1–5μm (Figure 2A) (Bassnett et al., 2011). The advent of electron microscopy revealed that lens fiber cell structure is even more complex, and can vary both between species and at different locations within the same lens (Kuszak et al., 2004a; Shestopalov and Bassnett, 2000). The straight sides of newly forming cortical fiber cells have “ball and socket” membrane specializations which are rich in gap junctions to allow for efficient cell communication in an avascular lens (Figure 3 A, B) (Bassnett et al., 2011; Kistler et al., 1986; Zhou and Lo, 2003). Each vertex at the intersection between these straight sides exhibits a very elaborate membrane structure, the membrane protrusion, which also greatly increases the surface area between cells, and may be critical for lens transparency (Figure 2B) (Kuszak et al., 1980, 2004a). In mice, fiber cells lose obvious ball and socket junctions and develop more elaborate membrane protrusions as they mature (Figure 3 C, D; Figure 4A). Overall, lens fibers at different depths within the lens have obvious differences in membrane architecture, and can develop even more complex membrane architectures, which can include the larger scale deformations of the lateral fiber cell membrane known as paddles and undulations (Figure 4) (Kuwabara, 1975). In contrast, lens fibers found in the center (the nucleus) of adult lenses tend to have only small membrane protrusions, but in many species, the straight sides develop a highly ordered membrane structure, the furrowed membrane (Figure 5), which allows lens fibers to tightly pack by reducing extracellular space, a process that may be critical to form the refractive index gradient (Al-Ghoul et al., 2001; Costello et al., 2008, 1989; Lo and Harding, 1984).
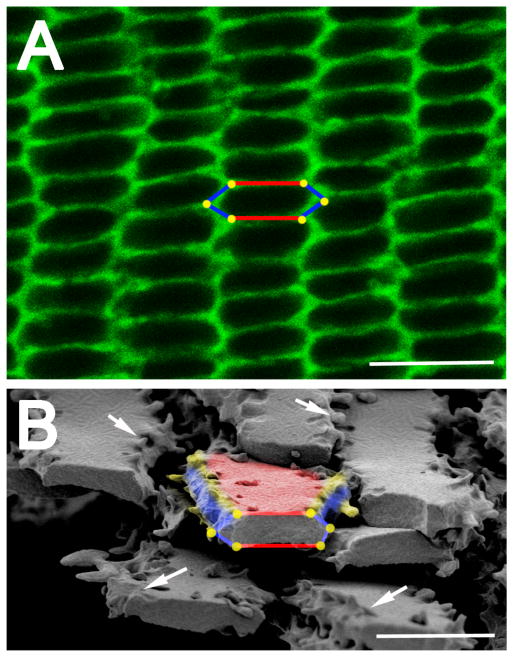
Figure 2.
Lens fiber cells have a hexagonal geometry when viewed in cross section. (A) An equatorial cross section of a mouse lens stained with fluorescent wheat germ and viewed with a confocal microscope. The hexagonal geometry of cells is highlighted in the center of the image showing the two broad sides of the cell labeled in red, the four short sides labeled in blue and the six vertices represented as yellow dots. Scale bar= 7.5μm. (B) A scanning electron micrograph showing lens fiber cells cut across their major axis allowing for viewing of their hexagonal geometry. The yellow vertices and white arrows show that there are membrane protrusions seen along these edges. Red– broad side; blue– short side; yellow– vertices/membrane protrusions; Scale bar= 5μm
Figure 3.
Scanning electron micrographs showing ball and sockets on newly differentiated fiber cells and the formation of elaborate membrane protrusions in mouse lenses. (A) An electron micrograph showing straight fiber cells dotted with ball and sockets along the broad side of the cell in the youngest lens fiber cell layers. (B) As fiber cells mature, ball and sockets are seen along the broad sides of cells, and membrane protrusions (arrowheads) are first seen along the vertices of these cells. C) Deeper in the lens cortex, morphologically obvious ball and socket junctions are not seen, but the membrane protrusions become more obvious (arrowheads) D) In the deepest layers of the lens cortex, the membrane protrusions become even more elaborate (arrowheads). arrowheads- membrane protrusions; b– ball; s– socket; Scale bar for all panels= 5μm.
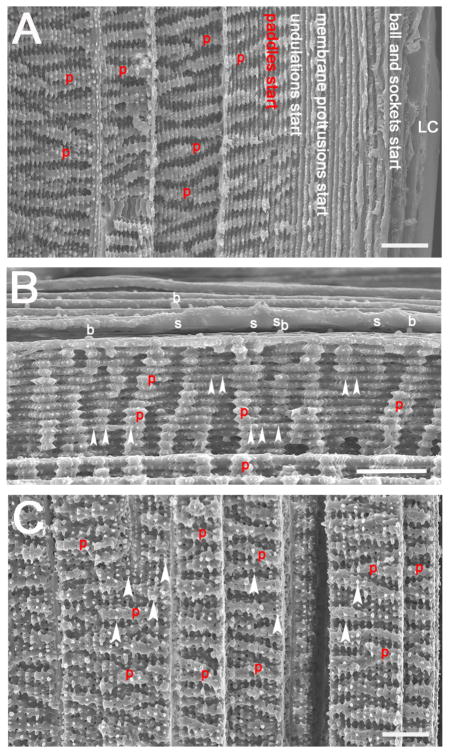
Figure 4.
Scanning electron micrographs showing longitudinal views of C57Bl/6<har> mouse lens fiber cells which highlight the changes in fiber cell morphology that occur as fiber cells mature. (A) A longitudinal view of fiber cells from the lens capsule to a depth of about 150 μm within the lens showing where different structures arise. Note though, the locations of the structures is likely to differ between mouse genetic backgrounds. (B) The interface between cells developing from young cortical fiber cells with ball and sockets to more mature fiber cells which have prominent membrane protrusions. (C) A longitudinal view of the lens at a depth of about 200 to 350 μm showing that the paddles are now more prominent and distinct membrane protrusions can also be seen along these paddles. Red p= paddle; arrowheads= membrane protrusions; b= ball; s= socket; scale bar= 10 μm
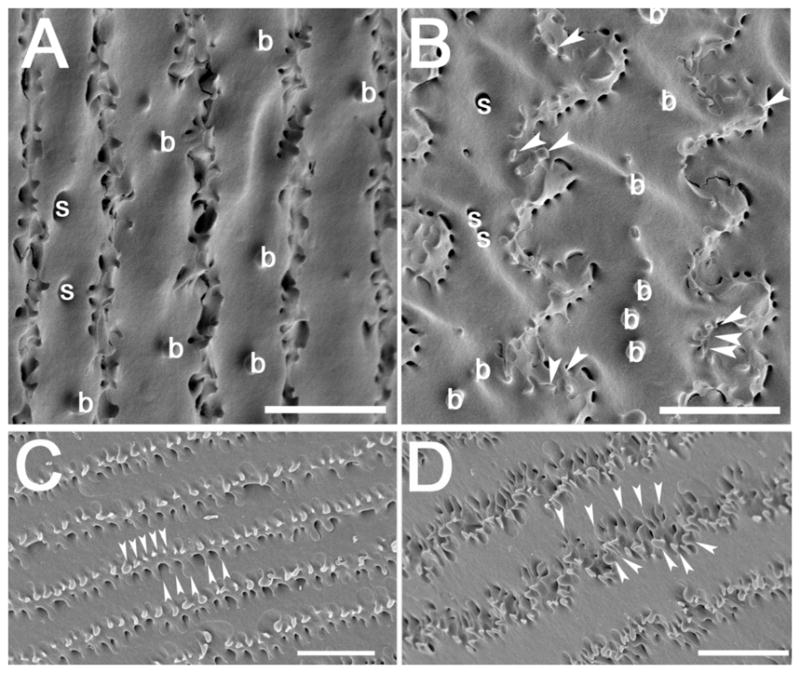
Figure 5.
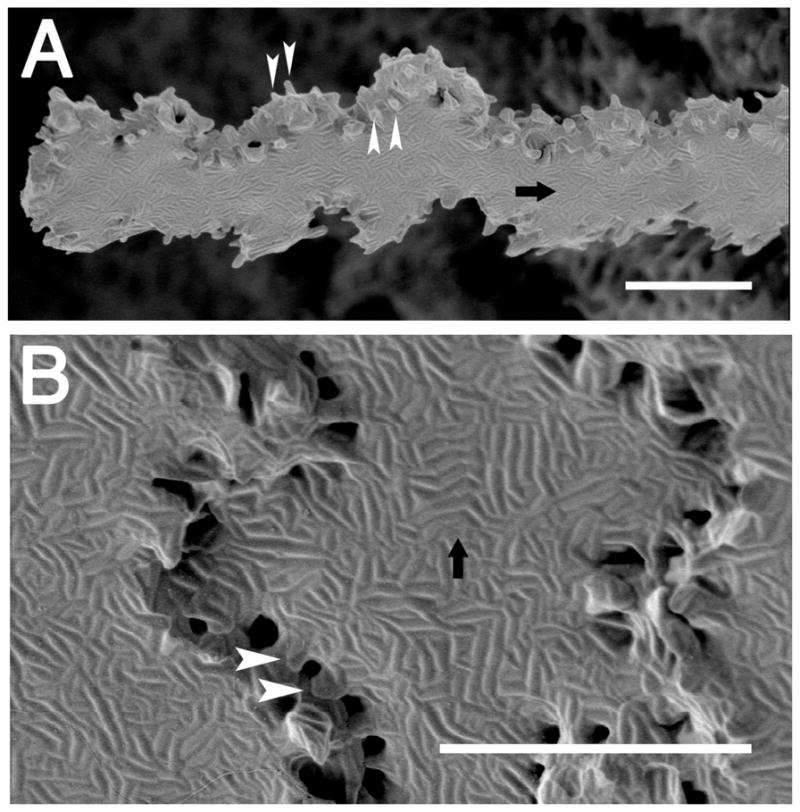
Scanning electron micrographs showing that mouse lens nuclear fiber cells have elaborate “membrane furrows” on their broad sides. (A) A single nuclear fiber cell isolated from the beginning of the lens nucleus that has a slight undulation, along with membrane furrows on its broad sides and distinct membrane protrusions at the vertices. (B) A higher magnification image of the lens nucleus showing that membrane furrows even extend out to the membrane protrusions. arrow= membrane furrows; arrowheads= membrane protrusions; scale bar= 5μm
Lens differentiation
The lens derives exclusively from the head ectoderm which receives signals produced by the optic vesicle (an outpouching of the neural tube) (Chow and Lang, 2001; Donner et al., 2006; Grainger, 1992), to form the lens placode. This structure then invaginates to form the lens pit, and closes to form the lens vesicle (Cvekl and Piatigorsky, 1996). At this point, the lens vesicle is a hollow structure comprised of a basement membrane lined with polarized epithelial cells whose apical surfaces face the vesicle interior. The cells in the anterior portion of the lens vesicle (which faces the developing cornea) are fated to become the lens epithelium which will form the proliferative cell population solely responsible for any further lens cells that form throughout life (Bhat, 2001). In contrast, the lens cells in the posterior aspect of the lens vesicle (those closest to the developing retina), terminally leave the cell cycle (Griep, 2006), downregulate the expression of a subset of genes that are active in head ectoderm/lens epithelial cells (Manthey et al., 2014; Pontoriero et al., 2009; West-Mays et al., 1999), and turn on high level expression of numerous genes that will establish the lens fiber cell proteome that is necessary for lens transparency and its refractive index gradient (Duncan et al., 2004; Hawse et al., 2005; Hoang et al., 2014; Pierscionek and Regini, 2012). Simultaneously, these cells undergo a massive cell shape change where the cuboidal/flattened epithelial cell is remodeled into the elongated lens fiber cell (Bassnett and Winzenburger, 2003; Bassnett, 2005; Shestopalov and Bassnett, 2000). These “primary” lens fibers are the shortest mature lens fibers and are found in the center of adult lenses as there is little to no lens fiber cell turnover across the lifespan (Augusteyn, 2010; Bassnett and Winzenburger, 2003; Stewart et al., 2013). Further growth of the lens occurs as lens cells formed via lens epithelial cell proliferation are crowded out of the epithelium proper towards the lens equator (Shi et al., 2015). Upon reaching the lens equator, these nascent secondary lens fiber cells come into contact with differentiation promoting FGF ligands, likely similar to those that drove primary fiber formation (Lovicu et al., 2011; de Iongh and Duncan, 2014). However, unlike primary fiber cell differentiation, secondary fiber differentiation does not appear to be as dependent on BMP (Faber et al. 2002) and Wnt/β-catenin signaling (Lovicu et al. 2011). While secondary lens fiber morphology appears more complex and tightly regulated than that of primary fibers (Bassnett et al., 2011), these cells do undergo many of the same molecular and morphological changes, leading to the formation of new fiber layers over the lens core which initially consists of only primary lens fibers (Bassnett, 2005). This process continues throughout the life span, with new lens fiber cells being added over those formerly produced to create a functional lens (Augusteyn, 2010).
Despite these significant changes in morphology during their differentiation, lens fibers initially maintain the classical apical/basal polarity characteristic of epithelial cells, maintaining a basal attachment to a basement membrane (the lens capsule) while making apical contacts with the apical side of lens epithelial cells (Zampighi et al., 2000). In primary lens fibers, this apical contact is lost as secondary lens fibers first establish apical contacts with the lens epithelium, then elongate while the apical fiber tip migrates along the apical side of the lens epithelium. When fiber cells from the same growth shell meet another fiber cell from the opposite side of the lens, they lose their apical-apical attachment with the lens epithelium and create an apical-apical interaction with another lens fiber, forming the anterior lens suture. Similarly, the basal surface of a newly formed lens fiber maintains its attachment to the lens capsule. As the fiber cell elongates, its basal tip simultaneously migrates towards the opposite side of the lens. This results in the first secondary lens fibers displacing the basal tips of primary lens fibers from the capsule. Eventually, the basal end of these secondary fibers will meet their partner from the opposite lens hemisphere, detach from the capsule, and generate the basal-basal cell contact of the posterior lens suture (Augusteyn, 2010; Joy et al., 2010; Kuszak et al., 2004a). It is notable that lens suture morphology varies between species, ranging from the umbilical sutures of birds and reptiles. the simple (line) sutures of frogs and rabbits, the Y-sutures of most mammals, and the star sutures of humans (Kuszak et al., 2004a; Kuszak et al., 2004b). The differences may arise from the constraints of lens growth (Kuszak et al., 2004a), the need to precisely position the refractive discontinuities generated by sutures in respect to the retina (Banh et al., 2006), the ability of the suture to facilitate the alterations in lens shape necessary for accommodation (Kuszak et al., 2006), and physiological considerations such as the need for fluid flow within the lens (Vaghefi et al., 2012).
Notably, while it is apparent that the hallmark of lens fiber cell differentiation is dramatic reorganization of cell shape from a cuboidal lens epithelial cell to the structurally complex fiber cell, our understanding of the molecular mechanisms underlying this lag far behind that for other critical aspects of fiber cell differentiation including the identity of the key transcription and growth factors critical for the process (Kawauchi et al., 1999; Lovicu et al., 2011; Nishiguchi et al., 1998; Wigle et al., 1999), the terminal exit of lens fibers from the cell cycle (Griep, 2006), the onset of crystallin expression (Cvekl et al., 2015), and the establishment of the lens circulation (Gao et al., 2011; Mathias et al., 2010). This review seeks to summarize our current understanding of the molecular mechanisms regulating lens fiber cell elongation.
Our current understanding of lens fiber elongation
Multiple hypotheses have been put forth to explain the profound changes in cell shape that occur during lens fiber differentiation. Here, each hypothesis and the associated evidence will be presented.
Do microtubules play a role in lens fiber cell elongation?
In 1964, Byers and Porter demonstrated that microtubules assemble at high densities during both lens placode thickening and primary lens fiber cell elongation. In primary lens fibers, these microtubules underlay the longitudinal membranes and are oriented along the axis of cell elongation. Polymerized microtubules are highly concentrated at the apical tip of the actively elongating fiber cell, while microtubules are less numerous at the posterior end of the cell and “seem to end freely”. These microtubules largely disappear as primary lens fiber cell elongation ends, but abundant microtubules are still seen in secondary lens fibers that are actively elongating. (Byers and Porter, 1964). These findings have been replicated in many other species, so the presence of organized microtubules underlying the membrane of elongating lens fiber cells is well accepted (Farnsworth et al., 1980; Lo et al., 2003; Piatigorsky, 1975). In 2003, Lo et al. reported that most lens microtubules are organized with their minus end towards the apical tip of lens fibers, while the plus ends face the basal end of the fibers. This finding is consistent with more recent studies that demonstrate the presence of microtubule organizing centers/centrosomes (MTOCs) at the apical tips of lens fibers, structures which are known to anchor the minus end of microtubules (Dahm et al., 2007; Dawes et al., 2013; Lodish, 2000; Manning et al., 2008; Sugiyama et al., 2016).
This correlation between microtubule assembly and fiber cell elongation led to the hypothesis that microtubules play important roles in lens fiber cell elongation. This proposal was first supported experimentally when embryonic day 6 (E6) chicken lens epithelial explants induced to differentiate by treatment with vitreous humor failed to elongate when treated with the microtubule disassociating agents colchicine and vinblastine (Piatigorsky, 1975; Piatigorsky et al., 1973, 1972). However, David Beebe’s group suggested that microtubules were not required for the elongation of E6 chicken lens epithelium explants in response to vitreous humor as disruption of the microtubule network with a, then new, microtubule inhibitor, Nocodazole did not block the elongation of cultured chicken lens epithelial explants treated with vitreous humor (Beebe et al., 1979). Further, they showed that colchicine inhibited lens cell elongation in this model at doses too small to affect microtubule stability, suggesting that microtubules do not play a role in fiber cell elongation. Since then, little new experimental evidence has been published either supporting or refuting the idea that microtubules are critical for lens fiber cell elongation, however, this is an area needing new investigation.
When the current understanding of lens fiber elongation is considered, it is unclear whether the day six chick epithelial explant model, in which the initial investigations of microtubule function were performed, is the most appropriate one to study the mechanisms controlling lens fiber cell elongation. Under the best circumstances, cultured lens epithelial cells only double in length from 10 μm to 20 μm after 5 hours of vitreous humor treatment, while growth slows so that cells only reach 30 μm in length during the first day in culture (Beebe and Feagans, 1981; Beebe et al., 1979). This is in stark contrast to chicken fibers in vivo whose secondary lens fibers elongate approximately 150 micrometers per day (Bassnett and Winzenburger, 2003). Further, the chick lens explant system never recapitulates the full elongation/structure of a mature secondary lens fiber cell, which has a complex lateral membrane structure and can reach 20 mm or more in length (Kuszak et al., 2004a).
Notably, lens fiber cells have been compared to neurons as they share some morphological features, including the need to greatly elongate during differentiation (Frederikse et al., 2012). This observation is supported by the discovery that lens fibers express several common neuronal markers (Bitel et al., 2010; Frederikse et al., 2015). Since the prior studies on microtubules in lens fiber elongation during the 1970s, it has been demonstrated that elongating bundled microtubules at the tip of an axonal growth cone can provide the motile force to move cell plasma membranes forward to drive neuronal elongation while also regulating axonal pathfinding during neural development (Etienne-Manneville, 2013; Laan et al., 2008; Suter and Miller, 2011), even in the absence of motile forces provided by actin and myosin (Bradke and Dotti, 1999; Das et al., 2015; Dehmelt et al., 2006; Etienne-Manneville, 2013; Laan et al., 2008; Tanaka et al., 1995). Future studies might evaluate whether the interplay of microtubules and actin at the apical tip of an elongating fiber cell is structurally analogous to the neuronal growth cone. While such an analog would likely be molecularly distinct from the neuronal growth cone since the orientation of the microtubules in the lens is opposite that of neurons, it could involve the interplay between the apically localized microtubule organizing centers of elongating lens fibers (Dahm et al., 2007) and the actin cytoskeleton via the recruitment of binding proteins that link actin and microtubule networks as is seen in other epithelia (Bazellières et al., 2012).
However, microtubules may have other important roles in lens fiber cell differentiation outside of just providing a motile force for membrane extension. Lens fiber cell elongation requires a large increase in membrane area (Bassnett, 2005; Borchman and Yappert, 2010; Piatigorsky, 1981), and the production/membrane insertion of fiber preferred membrane proteins such as aquaporin 0 (Bassnett et al., 2009; Chepelinsky, 2003; Sindhu Kumari et al., 2015). In neurons and other cell types, such membrane growth occurs via release of membranous vesicles from the endoplasmic reticulum/Golgi network, and trafficking of these vesicles to their site of membrane insertion. In neurons, it is established that such trafficking is, in part, driven by microtubules in partnership with motor proteins of the kinesin/dynein families which are able to move vesicular cargos to cellular locations distant from their synthesis (Hausott and Klimaschewski, 2015). While this mechanism has not been functionally investigated in the lens, the microtubules of elongating lens fiber cells are closely associated with the Golgi network in the central region of these cells, and contact numerous vesicles throughout the lens fiber, suggesting that microtubules can transport vesicular cargos necessary to increase fiber cell membrane area during cellular elongation (Lo et al., 2003). Notably, mitochondria, a microtubule associated cargo in other cell types (Suter and Miller, 2011), including elongating neurons, have been reported to move up to 18.5 μm/minute in lens fiber cells (Bantseev and Sivak, 2005). Alternatively, microtubules have been recently shown to provide key directional information needed for planar cell polarity (Matis et al., 2014), a process critical to regulate the migration of the apical lens fiber cell tips necessary to form the anterior lens sutures during fiber cell elongation (Dawes et al., 2014; Sugiyama and McAvoy, 2012; Sugiyama et al., 2011).
Microtubules may also play other important roles in lens fiber cell function. Treatment of intact lenses with microtubule inhibitors results in lens opacities (Mikuni et al., 1981), while some human cataracts have been reported to lose microtubules (Kuwabara, 1968). Mutations in FYOC1, an autophagy associated protein which can bind to microtubule associated kinesin motors, results in human cataracts that may be caused by defects in vesicle transit and/or disposal of autophagosomes during lens fiber cell differentiation (Chen et al., 2011). Thus, microtubules may play important roles in lens fiber cells independent of fiber cell elongation per se. Overall, the complex functions that microtubules are likely to play in the lens are an important topic for future investigation.
Fiber cell elongation driven by increases in cell volume?
After demonstrating that the ability of colchicine to block lens fiber cell elongation in the chicken epithelial cell explant model was unlikely to be due to its effect on microtubules (Beebe et al., 1982, 1979), David Beebe’s group pursued the hypothesis that the initial elongation of lens fiber cells was driven by a net increase in cell volume in physically constrained cells (Beebe et al., 1979). They found that although sodium (Na+) and most metabolite concentrations did not differ significantly between lens epithelial and lens fiber cells, lens fibers had a significantly lower efflux of potassium (K+) which could increase fluid influx into these cells, resulting in cell swelling in cultured explants (Parmelee and Beebe, 1988). This was supported by their observation that culturing LECs in K+-free media blocked LEC elongation in response to treatment with vitreous humor, while culture of lens epithelial explants with an inhibitor of K+ efflux led to LEC elongation similar to the levels observed in LECs stimulated to differentiate in response to vitreous humor. This hypothesis was supported when they demonstrated that the three fold increase in chicken lens cell length observed in this culture model occurred in proportion to the increase in volume of these cells (Beebe et al., 1982). Notably, the Beebe group later demonstrated the feasibility of this mechanism as the elongation of head ectoderm cells into the thickened lens placode was largely driven by cell proliferation and the resulting crowding of the basal tips of these cells when they physically constrained to a restricted area of basement membrane (Huang et al., 2011). However, this model did not explain the additional factors or conditions that must be met to facilitate the 100 fold increase or more in cell length occurring during lens fiber cell differentiation (Bassnett and Winzenburger, 2003), nor does it explain the highly ordered membrane elaborations that lens fibers exhibit in vivo. Further, the initial stages of mouse secondary lens fiber differentiation in vivo occurred without an increase in lens cell volume (Bassnett, 2005), suggesting that either primary and secondary fiber cell elongation occur by different mechanisms or the chicken lens explant model does not recapitulate lens fiber cell elongation mechanisms in vivo. This could be either an intrinsic difference between in vitro and in vivo mechanisms or a difference between the initial stages of epithelial-fiber cell differentiation and those occurring later. It could also be a difference between mammals and birds, particularly since chicken lenses exhibit an exceptionally high fluid content in their developing lens as compared to other species, as well as a relatively depolarized membrane potential that could alter solute retention and osmosis in chicken lens fibers (Bassnett et al., 1992). Overall, the role of volume regulation in driving lens fiber cell elongation is an open question, although it is unlikely to fully explain the differentiation of structurally elaborate lens fiber cells.
Does actin cytoskeletal dynamics drive fiber cell elongation?
In addition to microtubules, the lens fiber cell cytoskeleton consists of both F-actin containing microfilament networks and intermediate filaments (Rao and Maddala, 2006). Current data suggests that intermediate filaments do not play a role in lens fiber cell elongation as lens fiber cells elongate normally in animals lacking the lens preferred CP49/filensin beaded filament network (Alizadeh et al., 2002; Sandilands et al., 2004; Simirskii et al., 2006) despite the formation of cataracts in humans and animals with mutations in these genes (Song et al., 2009). Further, vimentin expression is largely restricted to the lens epithelium although lens fiber cell defects do arise in response to vimentin mutations (Matsuyama et al., 2013; Müller et al., 2009). Thus, only the potential roles for the actin cytoskeleton in lens fiber cell elongation will be discussed here.
The lens has a complex actin cytoskeletal network. In lens epithelial cells, F-actin is found in stress fibers along the basal cell membrane and in cortical rings along the lateral membranes (Weber and Menko, 2006). In some mouse strains, sequestered actin bundles are also seen at the apical tips of epithelial cells (Rafferty and Scholz, 1985). In lens fiber cells, cortical actin is found along the membranes while bundles of actin at the vertices are associated with fingerlike extensions (the “membrane protrusions”) (Kibbelaar et al., 1980; Lo et al., 1997; Lo, 1988). The basal tips of lens fibers exhibit actin networks that apparently are involved in stabilizing fiber cell/capsule interactions (Bassnett et al., 1999) which reorganize when the basal tips release from the lens capsule to form the posterior suture (Lu et al., 2008). Finally, the tips of lens fibers participating in lens sutures exhibit focal areas of actin, “the terminal web” which has been proposed to be important for stabilizing suture structure (Al-Ghoul et al., 2010). Thus, the actin cytoskeletal network of the lens reorganizes as lens fibers differentiate from lens epithelial cells, and again as fibers differentiate into their final form.
The disruption of actin interactions with N-cadherin, a transmembrane protein whose cytoplasmic tail can serve as a scaffold for actin assembly, disrupts the formation of F-actin and reduces, but does not abolish, fiber cell elongation in a chick lens explant model (Leonard et al., 2011). Removal of β1–integrin, another transmembrane protein able to serve as a scaffold for actin assembly, from either the entire lens or lens fibers exclusively, attenuates F-actin levels, and results in profound defects in lens fiber cell structure, although fiber cell elongation/differentiation per se proceeds normally (Scheiblin et al., 2014; Simirskii et al., 2007). Similarly, removal and/or inhibition of numerous regulators of actin cytoskeletal dynamics/organization such as Rho GTPases (Maddala et al., 2004), Rac1 GTPase (Maddala et al., 2011), tropomodulin (Nowak et al., 2009), and dystrophin (Fort et al., 2014), lead to disruptions in lens fiber cell structure without a profound effect on fiber cell elongation per se. Thus the role of actin in the regulation of lens fiber cell elongation is still an open question.
Treatment of cultured rat lens epithelial cells with the F-actin inhibitor, cytochalasin D blocked both their elongation and the onset of expression of the fiber cell differentiation marker, γ-crystallin (Mousa and Trevithick, 1977). In cultured chick lenses explants, Beebe’s group found that cytochalasin D treatment blocked fiber cell elongation. although they attributed the effect to a role in cytoskeleton in regulating potassium efflux and cell volume (see above) (Beebe and Cerrelli, 1989). More recently, Menko’s laboratory found that transient disassembly of the actin stress fibers of the lens epithelium by cytochalasin D drove lens fiber cell differentiation as measured by the expression of the fiber cell preferred intermediate filament components, CP49 and filensin, although prolonged treatment resulted in apoptosis (Weber and Menko, 2006). All of these findings suggest an important role for actin cytoskeletal dynamics in lens fiber cell elongation/differentiation, although the direct relationship is not well understood.
If the actin cytoskeleton does play a role in lens fiber cell elongation, it could be via different, but not necessarily mutually exclusive, routes. In neurons, the tip of an elongating axon, the growth cone, develops structures that are analogous to filopodia and lammellipodia, and exhibit highly dynamic actin assembly which can drive the membrane forward, and disassembly which is critical to allow microtubules into the growth cone, suggesting that actin and tubulin networks could cooperate in fiber cell elongation (Ledesma and Dotti, 2003; Suter and Miller, 2011). Further, like microtubules, the actin cytoskeleton interacts with molecular motors, predominately of the myosin family, to move vesicular cargos within cells (Lu et al., 2014), Notably, actin-myosin motors often collaborate with tubulin-kinesin motors for final positioning of cargos such as organelles (Hammer and Sellers, 2012), again suggesting that microtubule and actin cytoskeletal networks may collaborate in lens fiber cell elongation.
Future Directions for Study
Our understanding of the molecular mechanisms driving lens fiber cell elongation lags behind that of many other aspects of lens biology, likely because we lack culture models that fully recapitulate the process of elongation that occurs in vivo. However, both chicken and rat lens explants do start the process when treated with factors known to drive lens fiber cell differentiation such as vitreous humor or bFGF (Beebe and Feagans, 1981; Iyengar et al., 2007; Menko and Boettiger, 1988; Musil, 2012; Piatigorsky et al., 1972; Zelenka et al., 2009), and could potentially be refined into quantitative models using modern imaging techniques. Alternatively, in vivo manipulation of early chick (Reza et al., 2007; Shestopalov and Bassnett, 2000) or frog embryos (Nakayama et al., 2015) could provide important insight into the mechanisms driving lens fiber cell elongation.
We have also underexploited mutant mice exhibiting defective lens fiber cell elongation as these animals have the potential to reveal fundamental regulators of this process. For instance, mutations in three different transcription factors, Prox1 (Wigle et al., 1999), cMaf (Kawauchi et al., 1999; Ring et al., 2000) and Sox1 (Nishiguchi et al., 1998), lead to a failure of lens fiber cell elongation, although why these transcription factors are important is unknown. Non-biased assessment of the transcriptome of these mutant lenses using microarrays or RNAseq could reveal novel regulators of lens fiber cell elongation. We recently used this technique to demonstrate that Prox1 regulates a large subset of the lens-preferred transcriptome, which includes many genes encoding functionally uncharacterized cytoskeletal regulators (Audette et al., 2016). Similar techniques could be applied to study the abnormally shaped lenses of Sfrp2 transgenic mice (Chen et al., 2008) and the malformed sutures of Epha2 null mouse lenses (Shi et al., 2012), as these might elucidate the mechanisms involved in the organization and packing of lens fibers.
Similarly, removal of the fibroblast growth factor (FGF) receptors FGFR1, FGFR2 and FGFR3, from the mouse lens also leads to a failure of lens fiber cell elongation (Zhao et al., 2008). This could also result from changes in the expression of important cytoskeletal regulators as FGF signaling can alter transcription factor expression and function (Xie et al., 2016; Zhao et al., 2008, Audette et al., 2016). Alternatively, FGF signaling through MAPK, AKT and PI3-kinase pathways could change the function of actin regulators which can drive cell shape changes directly (Eswarakumar et al., 2005; Harding and Nechiporuk, 2012). Thus, investigation of such regulation in the lens could be a fruitful avenue of study.
Overall, new studies on the mechanisms regulating lens fiber cell elongation are needed to answer this long standing question. The answers will reveal important basic knowledge of how a very complex, but tightly regulated, cell shape is regulated during development to yield a functional tissue. Further, this information may further our understanding of how lenses can regenerate after cataract surgery to yield transparent lenses, an approach that has been long proposed as a solution to the problem of posterior capsular opacification (Gwon and Gruber, 2010; Gwon, 2005). Notably, a recent report demonstrated that lens regeneration can be induced following surgery for congenital cataract in human infants, yielding lenses capable of supporting good visual acuity (Lin et al., 2016).
Highlights.
We review and provide new data regarding lens fiber anatomy in the adult and developing lens
Multiple hypothesis for the molecular mechanisms underlying lens fiber development are presented and contrasted
We review the historical advances made in studying lens fiber elongation and suggest future strategies for study
Acknowledgments
This work was supported by National Eye Institute Grants EY012221 and EY015279. It includes text and figures that were included in the dissertations submitted in partial fulfillment of the Ph.D. degrees conferred by the University of Delaware to DSA and DAS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Ghoul KJ, Lindquist TP, Kirk SS, Donohue ST. A novel terminal web-like structure in cortical lens fibers: architecture and functional assessment. Anat Rec (Hoboken) 2010;293:1805–15. doi: 10.1002/ar.21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ghoul KJ, Nordgren RK, Kuszak AJ, Freel CD, Costello MJ, Kuszak JR. Structural Evidence of Human Nuclear Fiber Compaction as a Function of Ageing and Cataractogenesis. Exp Eye Res. 2001;72:199–214. doi: 10.1006/exer.2000.0937. [DOI] [PubMed] [Google Scholar]
- Alizadeh A, Clark JI, Seeberger T, Hess J, Blankenship T, Spicer A, FitzGerald PG. Targeted genomic deletion of the lens-specific intermediate filament protein CP49. Invest Ophthalmol Vis Sci. 2002;43:3722–7. [PubMed] [Google Scholar]
- Audette DS, Anand D, So T, Rubenstein TB, Lachke SA, Lovicu FJ, Duncan MK. Prox1 and fibroblast growth factor receptors form a novel regulatory loop controlling lens fiber differentiation and gene expression. Development. 2016;143:318–28. doi: 10.1242/dev.127860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusteyn RC. On the growth and internal structure of the human lens. Exp Eye Res. 2010;90:643–54. doi: 10.1016/j.exer.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banh A, Bantseev V, Choh V, Moran KL, Sivak JG. The lens of the eye as a focusing device and its response to stress. Prog Retin Eye Res. 2006;25:189–206. doi: 10.1016/j.preteyeres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Bantseev V, Sivak JG. Confocal laser scanning microscopy imaging of dynamic TMRE movement in the mitochondria of epithelial and superficial cortical fiber cells of bovine lenses. Mol Vis. 2005;11:518–23. [PubMed] [Google Scholar]
- Bassnett S. Three-dimensional reconstruction of cells in the living lens: The relationship between cell length and volume. Exp Eye Res. 2005;81:716–723. doi: 10.1016/j.exer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Becker TM, Beebe DC. Ion concentrations, fluxes and electrical properties of the embryonic chicken lens. Exp Eye Res. 1992;55:215–224. doi: 10.1016/0014-4835(92)90185-U. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Missey H, Vucemilo I. Molecular architecture of the lens fiber cell basal membrane complex. J Cell Sci. 1999;112(Pt 1):2155–65. doi: 10.1242/jcs.112.13.2155. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Shi Y, Vrensen GFJM. Biological glass: structural determinants of eye lens transparency. Philos Trans R Soc Lond B Biol Sci. 2011;366:1250–1264. doi: 10.1098/rstb.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S, Wilmarth PA, David LL. The membrane proteome of the mouse lens fiber cell. Mol Vis. 2009;15:2448–63. [PMC free article] [PubMed] [Google Scholar]
- Bassnett S, Winzenburger Pa. Morphometric analysis of fibre cell growth in the developing chicken lens. Exp Eye Res. 2003;76:291–302. doi: 10.1016/S0014-4835(02)00315-9. [DOI] [PubMed] [Google Scholar]
- Bazellières E, Massey-Harroche D, Barthélémy-Requin M, Richard F, Arsanto JP, Le Bivic A. Apico-basal elongation requires a drebrin-E-EB3 complex in columnar human epithelial cells. J Cell Sci. 2012;125:919–31. doi: 10.1242/jcs.092676. [DOI] [PubMed] [Google Scholar]
- Beebe D, Cerrelli S. Cytochalasin Prevents Cell Elongation and Increases Potassium Efflux from Embryonic Lens Epithelial Cells: Implications for the Mechanism of Lens Fiber Cell Elongation. Lens Eye Toxic Res. 1989;6:589–601. [PubMed] [Google Scholar]
- Beebe DC, Compart PJ, Johnson MC, Feagans DE, Feinberg RN. The mechanism of cell elongation during lens fiber cell differentiation. Dev Biol. 1982;92:54–59. doi: 10.1016/0012-1606(82)90149-X. [DOI] [PubMed] [Google Scholar]
- Beebe DC, Feagans DE. A tissue culture system for studying lens cell differentiation. Vision Res. 1981;21:113–8. doi: 10.1016/0042-6989(81)90143-7. [DOI] [PubMed] [Google Scholar]
- Beebe DC, Feagans DE, Blanchette-Mackie EJ, Nau ME. Lens epithelial cell elongation in the absence of microtubules: evidence for a new effect of colchicine. Science. 1979;206:836–838. doi: 10.1126/science.493982. [DOI] [PubMed] [Google Scholar]
- Bhat SP. The ocular lens epithelium. Biosci Rep. 2001;21:537–563. doi: 10.1023/A:1017952128502. [DOI] [PubMed] [Google Scholar]
- Bitel CL, Perrone-Bizzozero NI, Frederikse PH. HuB/C/D, nPTB, REST4, and miR-124 regulators of neuronal cell identity are also utilized in the lens. Mol Vis. 2010;16:2301–16. [PMC free article] [PubMed] [Google Scholar]
- Borchman D, Yappert MC. Lipids and the ocular lens. J Lipid Res. 2010;51:2473–2488. doi: 10.1194/jlr.R004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–1934. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- Byers B, Porter KR. Oriented microtubules in elongating cells of the developing lens rudiment after induction. Proc Natl Acad Sci U S A. 1964;52:1091–9. doi: 10.1073/pnas.52.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ma Z, Jiao X, Fariss R, Kantorow WL, Kantorow M, Pras E, Frydman M, Pras E, Riazuddin S, Riazuddin SA, Hejtmancik JF. Mutations in FYCO1 cause autosomal-recessive congenital cataracts. Am J Hum Genet. 2011;88:827–38. doi: 10.1016/j.ajhg.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Stump RJW, Lovicu FJ, Shimono A, McAvoy JW. Wnt signaling is required for organization of the lens fiber cell cytoskeleton and development of lens three-dimensional architecture. Dev Biol. 2008;324:161–76. doi: 10.1016/j.ydbio.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepelinsky AB. The ocular lens fiber membrane specific protein MIP/Aquaporin 0. J Exp Zool. 2003;300A:41–46. doi: 10.1002/jez.a.10307. [DOI] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–96. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Costello MJ, Johnsen S, Metlapally S, Gilliland KO, Ramamurthy B, Krishna PV, Balasubramanian D. Ultrastructural analysis of damage to nuclear fiber cell membranes in advanced age-related cataracts from India. Exp Eye Res. 2008;87:147–58. doi: 10.1016/j.exer.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello MJ, McIntosh TJ, Robertson JD. Distribution of gap junctions and square array junctions in the mammalian lens. Invest Ophthalmol Vis Sci. 1989;30:975–89. [PubMed] [Google Scholar]
- Cvekl A, McGreal R, Liu W. Lens Development and Crystallin Gene Expression. Prog Mol Biol Transl Sci, Progress in Molecular Biology and Translational Science. 2015;134:129–67. doi: 10.1016/bs.pmbts.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Piatigorsky J. Lens development and crystallin gene expression: many roles for Pax-6. Bioessays. 1996;18:621–30. doi: 10.1002/bies.950180805. [DOI] [PubMed] [Google Scholar]
- Dahm R, Procter JE, Ireland ME, Lo WK, Mogensen MM, Quinlan RA, Prescott AR. Reorganization of centrosomal marker proteins coincides with epithelial cell differentiation in the vertebrate lens. Exp Eye Res. 2007;85:696–713. doi: 10.1016/j.exer.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Das S, Sillitoe I, Lee D, Lees JG, Dawson NL, Ward J, Orengo CA. CATH FunFHMMer web server: protein functional annotations using functional family assignments. Nucleic Acids Res. 2015;43:W148–53. doi: 10.1093/nar/gkv488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes LJ, Sugiyama Y, Lovicu FJ, Harris CG, Shelley EJ, McAvoy JW. Interactions between lens epithelial and fiber cells reveal an intrinsic self-assembly mechanism. Dev Biol. 2014;385:291–303. doi: 10.1016/j.ydbio.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes LJ, Sugiyama Y, Tanedo AS, Lovicu FJ, McAvoy JW. Wnt-Frizzled signaling is part of an FGF-induced cascade that promotes lens fiber differentiation. Invest Ophthalmol Vis Sci. 2013;54:1582–90. doi: 10.1167/iovs.12-11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Iongh RU, Duncan MK. Lens Epithelium and Posterior Capsular Opacification. In: Saika S, Werner L, Lovicu FJ, editors. Lens Epithelium and Posterior Capsular Opacification. Springer; Japan, Tokyo: 2014. pp. 81–104. [DOI] [Google Scholar]
- Dehmelt L, Nalbant P, Steffen W, Halpain S. A microtubule-based, dynein-dependent force induces local cell protrusions: Implications for neurite initiation. Brain Cell Biol. 2006;35:39–56. doi: 10.1007/s11068-006-9001-0. [DOI] [PubMed] [Google Scholar]
- Donner AL, Lachke SA, Maas RL. Lens induction in vertebrates: variations on a conserved theme of signaling events. Semin Cell Dev Biol. 2006;17:676–85. doi: 10.1016/j.semcdb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Duncan M, Cvekl A, Kantorow M, Piatigorsky J. Lens Crystallins, in: Development of the Ocular Lens. Cambridge University Press; 2004. pp. 119–150. [Google Scholar]
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–49. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. Microtubules in Cell Migration. Annu Rev Cell Dev Biol. 2013;29:471–499. doi: 10.1146/annurev-cellbio-101011-155711. [DOI] [PubMed] [Google Scholar]
- Faber SC, Robinson ML, Makarenkova HP, Lang RA. Bmp signaling is required for development of primary lens fiber cells. Development. 2002;129:3727–37. doi: 10.1242/dev.129.15.3727. [DOI] [PubMed] [Google Scholar]
- Farnsworth PN, Shyne SE, Caputo SJ, Fasano AV, Spector A. Microtubules: a major cytoskeletal component of the human lens. Exp Eye Res. 1980;30:611–5. doi: 10.1016/0014-4835(80)90044-5. [DOI] [PubMed] [Google Scholar]
- Fort PE, Darche M, Sahel JA, Rendon A, Tadayoni R. Lack of dystrophin protein Dp71 results in progressive cataract formation due to loss of fiber cell organization. Mol Vis. 2014;20:1480–90. [PMC free article] [PubMed] [Google Scholar]
- Frederikse PH, Kasinathan C, Kleiman NJ. Parallels between neuron and lens fiber cell structure and molecular regulatory networks. Dev Biol. 2012;368:255–60. doi: 10.1016/j.ydbio.2012.05.022. [DOI] [PubMed] [Google Scholar]
- Frederikse PH, Nandanoor A, Kasinathan C. Fragile X Syndrome FMRP Co-localizes with Regulatory Targets PSD-95, GABA Receptors, CaMKIIα, and mGluR5 at Fiber Cell Membranes in the Eye Lens. Neurochem Res. 2015 doi: 10.1007/s11064-015-1702-2. [DOI] [PubMed] [Google Scholar]
- Gao J, Sun X, Moore LC, White TW, Brink PR, Mathias RT. Lens intracellular hydrostatic pressure is generated by the circulation of sodium and modulated by gap junction coupling. J Gen Physiol. 2011;137:507–20. doi: 10.1085/jgp.201010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger RM. Embryonic lens induction: shedding light on vertebrate tissue determination. Trends Genet. 1992;8:349–55. doi: 10.1016/0168-9525(92)90280-h. [DOI] [PubMed] [Google Scholar]
- Griep AE. Cell cycle regulation in the developing lens. Semin Cell Dev Biol. 2006;17:686–97. doi: 10.1016/j.semcdb.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwon A. Lens regeneration in mammals: a review. Surv Ophthalmol. 2005;51:51–62. doi: 10.1016/j.survophthal.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Gwon A, Gruber L. Engineering the crystalline lens with a biodegradable or non-degradable scaffold. Exp Eye Res. 2010;91:220–8. doi: 10.1016/j.exer.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Hammer JA, Sellers JR. Walking to work: roles for class V myosins as cargo transporters. Nat Rev Mol Cell Biol. 2012;13:13–26. doi: 10.1038/nrm3248. [DOI] [PubMed] [Google Scholar]
- Harding MJ, Nechiporuk AV. Fgfr-Ras-MAPK signaling is required for apical constriction via apical positioning of Rho-associated kinase during mechanosensory organ formation. Development. 2012;139:3130–3135. doi: 10.1242/dev.082271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausott B, Klimaschewski L. Membrane turnover and receptor trafficking in regenerating axons. Eur J Neurosci. 2015 doi: 10.1111/ejn.13025. [DOI] [PubMed] [Google Scholar]
- Hawse JR, DeAmicis-Tress C, Cowell TL, Kantorow M. Identification of global gene expression differences between human lens epithelial and cortical fiber cells reveals specific genes and their associated pathways important for specialized lens cell functions. Mol Vis. 2005;11:274–83. [PMC free article] [PubMed] [Google Scholar]
- Hoang TV, Kumar PKR, Sutharzan S, Tsonis PA, Liang C, Robinson ML. Comparative transcriptome analysis of epithelial and fiber cells in newborn mouse lenses with RNA sequencing. Mol Vis. 2014;20:1491–517. [PMC free article] [PubMed] [Google Scholar]
- Huang J, Rajagopal R, Liu Y, Dattilo LK, Shaham O, Ashery-Padan R, Beebe DC. The mechanism of lens placode formation: a case of matrix-mediated morphogenesis. Dev Biol. 2011;355:32–42. doi: 10.1016/j.ydbio.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar L, Wang Q, Rasko JEJ, McAvoy JW, Lovicu FJ. Duration of ERK1/2 phosphorylation induced by FGF or ocular media determines lens cell fate. Differentiation. 2007;75:662–8. doi: 10.1111/j.1432-0436.2007.00167.x. [DOI] [PubMed] [Google Scholar]
- Joy A, Mohammed TA, Al-Ghoul KJ. Abnormal fiber end migration in Royal College of Surgeons rats during posterior subcapsular cataract formation. Mol Vis. 2010;16:1453–66. [PMC free article] [PubMed] [Google Scholar]
- Kawauchi S, Takahashi S, Nakajima O, Ogino H, Morita M, Nishizawa M, Yasuda K, Yamamoto M. Regulation of lens fiber cell differentiation by transcription factor c-Maf. J Biol Chem. 1999;274:19254–60. doi: 10.1074/jbc.274.27.19254. [DOI] [PubMed] [Google Scholar]
- Kibbelaar MA, Ramaekers FCS, Ringens PJ, Selten-Versteegen AME, Poels LG, Jap PHK, van Rossum AL, Feltkap TEW, Bloemendal H. Is actin in eye lens a possible factor in visual accomodation? Nature. 1980;285:506–508. doi: 10.1038/285506a0. [DOI] [PubMed] [Google Scholar]
- Kistler J, Gilbert K, Brooks HV, Jolly RD, Hopcroft DH, Bullivant S. Membrane interlocking domains in the lens. Invest Ophthalmol Vis Sci. 1986;27:1527–34. [PubMed] [Google Scholar]
- Kuszak J, Alcala J, Maisel H. The surface morphology of embryonic and adult chick lens-fiber cells. Am J Anat. 1980;159:395–410. doi: 10.1002/aja.1001590406. [DOI] [PubMed] [Google Scholar]
- Kuszak JR, Zoltoski RK, Sivertson C. Fibre cell organization in crystalline lenses. Exp Eye Res. 2004a;78:673–687. doi: 10.1016/j.exer.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Kuszak JR, Zoltoski RK, Tiedemann CE. Development of lens sutures. Int J Dev Biol. 2004b;48:889–902. doi: 10.1387/ijdb.041880jk. [DOI] [PubMed] [Google Scholar]
- Kuszak JR, Mazurkiewicz M, Jison L, Madurski A, Ngando A, Zoltoski RK. Quantitative analysis of animal model lens anatomy: accommodative range is related to fiber structure and organization. Vet Ophthalmol. 2006;9:266–80. doi: 10.1111/j.1463-5224.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- Kuwabara T. The maturation of the lens cell: a morphologic study. Exp Eye Res. 1975;20:427–43. doi: 10.1016/0014-4835(75)90085-8. [DOI] [PubMed] [Google Scholar]
- Kuwabara T. Microtubules in the lens. Arch Ophthalmol (Chicago, Ill 1960) 1968;79:189–95. doi: 10.1001/archopht.1968.03850040191017. [DOI] [PubMed] [Google Scholar]
- Laan L, Husson J, Munteanu EL, Kerssemakers JWJ, Dogterom M. Force-generation and dynamic instability of microtubule bundles. Proc Natl Acad Sci U S A. 2008;105:8920–5. doi: 10.1073/pnas.0710311105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledesma MD, Dotti CG. Membrane and cytoskeleton dynamics during axonal elongation and stabilization. Int Rev Cytol. 2003;227:183–219. doi: 10.1016/s0074-7696(03)01010-6. [DOI] [PubMed] [Google Scholar]
- Leonard M, Zhang L, Zhai N, Cader A, Chan Y, Nowak RB, Fowler VM, Menko AS. Modulation of N-cadherin junctions and their role as epicenters of differentiation-specific actin regulation in the developing lens. Dev Biol. 2011;349:363–77. doi: 10.1016/j.ydbio.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Ouyang H, Zhu J, Huang S, Liu Z, Chen S, Cao G, Li G, Signer RAJ, Xu Y, Chung C, Zhang Y, Lin D, Patel S, Wu F, Cai H, Hou J, Wen C, Jafari M, Liu X, Luo L, Zhu J, Qiu A, Hou R, Chen B, Chen J, Granet D, Heichel C, Shang F, Li X, Krawczyk M, Skowronska-Krawczyk D, Wang Y, Shi W, Chen D, Zhong Z, Zhong S, Zhang L, Chen S, Morrison SJ, Maas RL, Zhang K, Liu Y. Lens regeneration using endogenous stem cells with gain of visual function. Nature. 2016 doi: 10.1038/nature17181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WK, Shaw AP, Wen XJ. Actin Filament Bundles in Cortical Fiber Cells of the Rat Lens. Exp Eye Res. 1997;65:691–701. doi: 10.1006/exer.1997.0375. [DOI] [PubMed] [Google Scholar]
- Lo WK, Wen XJ, Zhou CJ. Microtubule configuration and membranous vesicle transport in elongating fiber cells of the rat lens. Exp Eye Res. 2003;77:615–26. doi: 10.1016/s0014-4835(03)00176-3. [DOI] [PubMed] [Google Scholar]
- Lo WK. Adherens junctions in the ocular lens of various species: ultrastructural analysis with an improved fixation. Cell Tissue Res. 1988;254:31–40. doi: 10.1007/BF00220014. [DOI] [PubMed] [Google Scholar]
- Lo WK, Harding CV. Square arrays and their role in ridge formation in human lens fibers. J Ultrastruct Res. 1984;86:228–45. doi: 10.1016/s0022-5320(84)90103-5. [DOI] [PubMed] [Google Scholar]
- Lodish H. Molecular cell biology. W.H. Freeman; New York: 2000. [Google Scholar]
- Lovicu FJ, McAvoy JW, de Iongh RU. Understanding the role of growth factors in embryonic development: insights from the lens. Philos Trans R Soc Lond B Biol Sci. 2011;366:1204–18. doi: 10.1098/rstb.2010.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JY, Mohammed TA, Donohue ST, Al-Ghoul KJ. Distribution of basal membrane complex components in elongating lens fibers. Mol Vis. 2008;14:1187–203. [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Li J, Zhang M. Cargo recognition and cargo-mediated regulation of unconventional myosins. Acc Chem Res. 2014;47:3061–70. doi: 10.1021/ar500216z. [DOI] [PubMed] [Google Scholar]
- Maddala R, Chauhan BK, Walker C, Zheng Y, Robinson ML, Lang RA, Rao PV. Rac1 GTPase-deficient mouse lens exhibits defects in shape, suture formation, fiber cell migration and survival. Dev Biol. 2011;360:30–43. doi: 10.1016/j.ydbio.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddala R, Deng PF, Costello JM, Wawrousek EF, Zigler JS, Rao VP. Impaired cytoskeletal organization and membrane integrity in lens fibers of a Rho GTPase functional knockout transgenic mouse. Lab Invest. 2004;84:679–92. doi: 10.1038/labinvest.3700105. [DOI] [PubMed] [Google Scholar]
- Manning JA, Colussi PA, Koblar SA, Kumar S. Nedd1 expression as a marker of dynamic centrosomal localization during mouse embryonic development. Histochem Cell Biol. 2008;129:751–64. doi: 10.1007/s00418-008-0392-0. [DOI] [PubMed] [Google Scholar]
- Manthey AL, Lachke SA, FitzGerald PG, Mason RW, Scheiblin DA, McDonald JH, Duncan MK. Loss of Sip1 leads to migration defects and retention of ectodermal markers during lens development. Mech Dev. 2014;131:86–110. doi: 10.1016/j.mod.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias RT, White TW, Gong X. Lens gap junctions in growth, differentiation, and homeostasis. Physiol Rev. 2010;90:179–206. doi: 10.1152/physrev.00034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matis M, Russler-Germain DA, Hu Q, Tomlin CJ, Axelrod JD. Microtubules provide directional information for core PCP function. Elife. 2014;3:e02893. doi: 10.7554/eLife.02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama M, Tanaka H, Inoko A, Goto H, Yonemura S, Kobori K, Hayashi Y, Kondo E, Itohara S, Izawa I, Inagaki M. Defect of mitotic vimentin phosphorylation causes microophthalmia and cataract via aneuploidy and senescence in lens epithelial cells. J Biol Chem. 2013;288:35626–35. doi: 10.1074/jbc.M113.514737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menko AS, Boettiger D. Inhibition of chicken embryo lens differentiation and lens junction formation in culture by pp60v-src. Mol Cell Biol. 1988;8:1414–20. doi: 10.1128/mcb.8.4.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuni I, Fujiwara T, Obazawa H. Microtubules in experimental cataracts: disappearance of microtubules of epithelial cells and lens fibers in colchicine-induced cataracts. Tokai J Exp Clin Med. 1981;6:297–303. [PubMed] [Google Scholar]
- Mousa GY, Trevithick JR. Differentiation of rat lens epithelial cells in tissue culture. II Effects of cytochalasins B and D on actin organization and differentiation. Dev Biol. 1977;60:14–25. doi: 10.1016/0012-1606(77)90107-5. [DOI] [PubMed] [Google Scholar]
- Müller M, Bhattacharya SS, Moore T, Prescott Q, Wedig T, Herrmann H, Magin TM. Dominant cataract formation in association with a vimentin assembly disrupting mutation. Hum Mol Genet. 2009;18:1052–7. doi: 10.1093/hmg/ddn440. [DOI] [PubMed] [Google Scholar]
- Musil LS. Primary cultures of embryonic chick lens cells as a model system to study lens gap junctions and fiber cell differentiation. J Membr Biol. 2012;245:357–68. doi: 10.1007/s00232-012-9458-y. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Fisher M, Nakajima K, Odeleye AO, Zimmerman KB, Fish MB, Yaoita Y, Chojnowski JL, Lauderdale JD, Netland PA, Grainger RM. Xenopus pax6 mutants affect eye development and other organ systems, and have phenotypic similarities to human aniridia patients. Dev Biol. 2015 doi: 10.1016/j.ydbio.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi S, Wood H, Kondoh H, Lovell-Badge R, Episkopou V. Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes Dev. 1998;12:776–81. doi: 10.1101/gad.12.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak RB, Fischer RS, Zoltoski RK, Kuszak JR, Fowler VM. Tropomodulin1 is required for membrane skeleton organization and hexagonal geometry of fiber cells in the mouse lens. J Cell Biol. 2009;186:915–28. doi: 10.1083/jcb.200905065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmelee JT, Beebe DC. Decreased membrane permeability to potassium is responsible for the cell volume increase that drives lens fiber cell elongation. J Cell Physiol. 1988;134:491–496. doi: 10.1002/jcp.1041340323. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens cell elongation in vitro and microtubules. Ann N Y Acad Sci. 1975;253:333–47. doi: 10.1111/j.1749-6632.1975.tb19211.x. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J, Rothschild SS, Wollberg M. Stimulation by insulin of cell elongation and microtubule assembly in embryonic chick-lens epithelia. Proc Natl Acad Sci U S A. 1973;70:1195–8. doi: 10.1073/pnas.70.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatigorsky J, Webster HF, Wollberg M. Cell elongation in the cultured embryonic chick lens epithelium with and without protein synthesis. Involvement of microtubules. J Cell Biol. 1972;55:82–92. doi: 10.1083/jcb.55.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierscionek BK, Regini JW. The gradient index lens of the eye: An opto-biological synchrony. Prog Retin Eye Res. 2012;31:332–349. doi: 10.1016/j.preteyeres.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Pontoriero GF, Smith AN, Miller LAD, Radice GL, West-Mays JA, Lang RA. Cooperative roles for E-cadherin and N-cadherin during lens vesicle separation and lens epithelial cell survival. Dev Biol. 2009;326:403–17. doi: 10.1016/j.ydbio.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafferty NS, Scholz DL. Actin in polygonal arrays of microfilaments and sequestered actin bundles (SABs) in lens epithelial cells of rabbits and mice. Curr Eye Res. 1985;4:713–8. doi: 10.3109/02713688509017667. [DOI] [PubMed] [Google Scholar]
- Rao PV, Maddala R. The role of the lens actin cytoskeleton in fiber cell elongation and differentiation. Semin Cell Dev Biol. 2006;17:698–711. doi: 10.1016/j.semcdb.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reza HM, Nishi H, Kataoka K, Takahashi Y, Yasuda K. L-Maf regulates p27kip1 expression during chick lens fiber differentiation. Differentiation. 2007;75:737–44. doi: 10.1111/j.1432-0436.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- Ring BZ, Cordes SP, Overbeek PA, Barsh GS. Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development. 2000;127:307–17. doi: 10.1242/dev.127.2.307. [DOI] [PubMed] [Google Scholar]
- Sandilands A, Wang X, Hutcheson AM, James J, Prescott AR, Wegener A, Pekny M, Gong X, Quinlan RA. Bfsp2 mutation found in mouse 129 strains causes the loss of CP49 and induces vimentin-dependent changes in the lens fibre cell cytoskeleton. Exp Eye Res. 2004;78:109–23. doi: 10.1016/j.exer.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Scheiblin DA, Gao J, Caplan JL, Simirskii VN, Czymmek KJ, Mathias RT, Duncan MK. Beta-1 integrin is important for the structural maintenance and homeostasis of differentiating fiber cells. Int J Biochem Cell Biol. 2014;50:132–45. doi: 10.1016/j.biocel.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestopalov VI, Bassnett S. Three-dimensional organization of primary lens fiber cells. Investig Ophthalmol Vis Sci. 2000;41:859–863. [PubMed] [Google Scholar]
- Shi Y, De Maria A, Bennett T, Shiels A, Bassnett S. A role for epha2 in cell migration and refractive organization of the ocular lens. Invest Ophthalmol Vis Sci. 2012;53:551–9. doi: 10.1167/iovs.11-8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, De Maria A, Lubura S, Šikić H, Bassnett S. The penny pusher: a cellular model of lens growth. Invest Ophthalmol Vis Sci. 2015;56:799–809. doi: 10.1167/iovs.14-16028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simirskii VN, Lee RS, Wawrousek EF, Duncan MK. Inbred FVB/N mice are mutant at the cp49/Bfsp2 locus and lack beaded filament proteins in the lens. Invest Ophthalmol Vis Sci. 2006;47:4931–4. doi: 10.1167/iovs.06-0423. [DOI] [PubMed] [Google Scholar]
- Simirskii VN, Wang Y, Duncan MK. Conditional deletion of beta1-integrin from the developing lens leads to loss of the lens epithelial phenotype. Dev Biol. 2007;306:658–68. doi: 10.1016/j.ydbio.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhu Kumari S, Gupta N, Shiels A, FitzGerald PG, Menon AG, Mathias RT, Varadaraj K. Role of Aquaporin 0 in lens biomechanics. Biochem Biophys Res Commun. 2015;462:339–45. doi: 10.1016/j.bbrc.2015.04.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Landsbury A, Dahm R, Liu Y, Zhang Q, Quinlan RA. Functions of the intermediate filament cytoskeleton in the eye lens. J Clin Invest. 2009;119:1837–48. doi: 10.1172/JCI38277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DN, Lango J, Nambiar KP, Falso MJS, FitzGerald PG, Rocke DM, Hammock BD, Buchholz BA. Carbon turnover in the water-soluble protein of the adult human lens. Mol Vis. 2013;19:463–75. [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Lovicu FJ, McAvoy JW. Planar cell polarity in the mammalian eye lens. Organogenesis. 2011;7:191–201. doi: 10.4161/org.7.3.18421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, McAvoy JW. Analysis of PCP defects in mammalian eye lens. Methods Mol Biol. 2012;839:147–56. doi: 10.1007/978-1-61779-510-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Shelley EJ, Yoder BK, Kozmik Z, May-Simera HL, Beales PL, Lovicu FJ, McAvoy JW. Non-essential role for cilia in coordinating precise alignment of lens fibres. Mech Dev. 2016;139:10–7. doi: 10.1016/j.mod.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter DM, Miller KE. The emerging role of forces in axonal elongation. Prog Neurobiol. 2011;94:91–101. doi: 10.1016/j.pneurobio.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, Ho T, Kirschner MW. The role of microtubule dynamics in growth cone motility and axonal growth. J Cell Biol. 1995;128:139–55. doi: 10.1083/jcb.128.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thin G, Ewart JC. A Contribution to the Anatomy of the Lens. J Anat Physiol. 1876;10:i3–230. [PMC free article] [PubMed] [Google Scholar]
- Vaghefi E, Walker K, Pontre BP, Jacobs MD, Donaldson PJ. Magnetic resonance and confocal imaging of solute penetration into the lens reveals a zone of restricted extracellular space diffusion. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1250–9. doi: 10.1152/ajpregu.00611.2011. [DOI] [PubMed] [Google Scholar]
- Weber GF, Menko AS. Actin filament organization regulates the induction of lens cell differentiation and survival. Dev Biol. 2006;295:714–729. doi: 10.1016/j.ydbio.2006.03.056. [DOI] [PubMed] [Google Scholar]
- West-Mays JA, Zhang J, Nottoli T, Hagopian-Donaldson S, Libby D, Strissel KJ, Williams T. AP-2alpha transcription factor is required for early morphogenesis of the lens vesicle. Dev Biol. 1999;206:46–62. doi: 10.1006/dbio.1998.9132. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21:318–22. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- Xie Q, McGreal R, Harris R, Gao CY, Liu W, Reneker LW, Musil LS, Cvekl A. Regulation of c-Maf and αA-Crystallin in Ocular Lens by Fibroblast Growth Factor Signaling. J Biol Chem. 2016;291:3947–58. doi: 10.1074/jbc.M115.705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi Ga, Eskandari S, Kreman M. Epithelial organization of the mammalian lens. Exp Eye Res. 2000;71:415–435. doi: 10.1006/exer.2000.0895. [DOI] [PubMed] [Google Scholar]
- Zelenka PS, Gao CY, Saravanamuthu SS. Preparation and culture of rat lens epithelial explants for studying terminal differentiation. J Vis Exp. 2009 doi: 10.3791/1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yang T, Madakashira BP, Thiels CA, Bechtle CA, Garcia CM, Zhang H, Yu K, Ornitz DM, Beebe DC, Robinson ML. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008;318:276–88. doi: 10.1016/j.ydbio.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CJ, Lo WK. Association of clathrin, AP-2 adaptor and actin cytoskeleton with developing interlocking membrane domains of lens fibre cells. Exp Eye Res. 2003;77:423–32. doi: 10.1016/s0014-4835(03)00171-4. [DOI] [PubMed] [Google Scholar]