Introduction
Maintaining stable gait requires controlling the interaction between the center of mass (CoM) and base of support (BoS) in order to avoid instability and fall risk [1]. Stable gait can be defined as the ability to maintain functional gait without falling down, even in the presence of perturbations [2]. Healthy individuals should theoretically display optimal and stable gait, thus it is of interest to examine the interaction between their CoM and BoS. Studies have shown that center of mass motion can be approximated by trunk motion [3] while step width is an indicator of dynamic balance during gait [4, 5]. Wireless accelerometry shows promise for its ability to measure temporal and spatial parameters of trunk and foot motion outside of a laboratory and in the real world [6–8]. However, since wireless accelerometers do not allow for direct measurement of step width, which is strongly related to stability and lateral postural control [9], other accelerometer based metrics must be explored to quantify gait stability.
Previous studies have used the acceleration time series taken from these inertial measurement units to investigate changes in gait and balance using various linear and nonlinear variability analysis techniques [9, 10]. Menz et al. found that elderly individuals at risk for falls had greater step timing variability [11]. Huisinga et al. reported that persons with multiple sclerosis displayed altered variability in the form of higher Lyapunov exponents compared to age-matched controls [12]. Tochigi et al. found that accelerations at the leg during walking yielded lower sample entropy results, indicating more regularity, in older adults compared to healthy younger adults [13]. However, most studies have focused on movement patterns of one segment only, typically the movement of the trunk or foot. Stable gait requires coordination between upper and lower body segments, therefore examining these segments simultaneously during walking may provide a more comprehensive picture of stability. Kavanagh et al. found that accelerations at the head are significantly attenuated and more tightly controlled compared to accelerations at the trunk, which may indicate that the body aims to reduce motion at head during walking [14]. The current study takes a similar approach in assessing the accelerations from two separate segments simultaneously, specifically the foot and the trunk.
Thus, the purpose of this study was to examine the relationships between foot and trunk segment acceleration variability during walking in healthy adults. To examine the acceleration time series, we employed both linear and nonlinear measures of variability. Linear measures of variability such as root mean square (RMS) give information about how much variability is present in the time series [15–17]. Since walking is a cyclic and repetitive activity, it is useful to examine not only the overall magnitude of variability but also the structure of the variability throughout the time series to assess adaptive and reactive strategies during walking [18–20]. For the current study, approximate entropy (ApEn) was used to quantify the periodicity and regularity of a time series, and has previously been used to analyze acceleration time series during gait [21].
The aim of this study was to examine relationships between acceleration patterns at the trunk and at the foot during walking at self-selected pace in healthy adults. Previous studies have shown that accelerations during walking are attenuated from inferior to superior segments of body [22] and that the attenuation of these accelerations may be a result of prioritizing the stability of the trunk and head over inferior segments of the body during walking. Thus, we expected that accelerations at the trunk would be less variable (lower RMS) and more periodic (lower ApEn) than accelerations at the foot. Additionally, we also expected there to be a strong relationship between variability at the foot and at the trunk since foot motion and behavior of the center of mass are strongly linked [1].
Methods
Participants
A sample of 40 healthy adult subjects participated in this study. The participants had an average age of 43.6 + 9.8 years (range: 20 – 59 years). All participants gave informed written consent. The University of Kansas Medical Center Human Research Committee approved this study.
Protocol
Wireless inertial sensors containing accelerometers (Opal, APDM, Portland, OR, USA) were secured to the participants’ trunk and right ankle via elastic strap. The trunk accelerometer was placed over the midline of the sternum, inferior to the manubrium and superior to the xiphoid process. The right ankle accelerometer was placed over the anterior surface of the distal lower shank, superior to the ankle joint. While the sensor was not placed directly on the foot, its position on the ankle is sufficient to approximate foot motion and footfall information [23, 24]. Previous studies have shown that accelerations are attenuated inferiorly to superiorly within the trunk segment [22]; thus, we chose to place the trunk accelerometer over the sternum rather than the lumbar spine in order to measure accelerations which are maximally dampened by being in the superior portion of the trunk segment [22]. The accelerometers collected data at 128 Hz while subjects walked on a motorized treadmill (Woodway Bari-Mill, Eugene, OR, USA) at self-selected comfortable pace for 3 minutes. The participants walked at an average walking speed of 1.01 + 0.33 m/s (range: 0.48 – 1.89 m/s).
Data processing
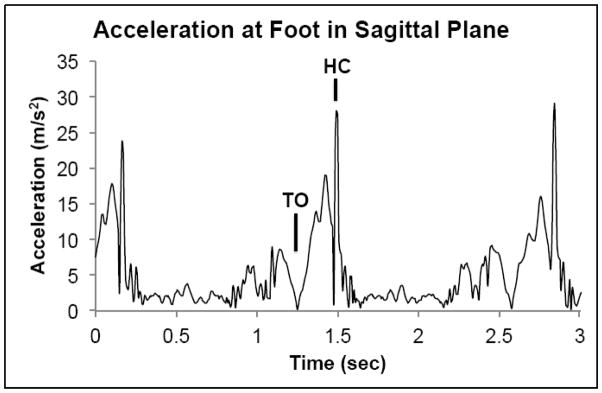
The raw acceleration time series were exported to Matlab (MATLAB version R2013b, The MathWorks, Inc., Natick, Massachusetts, USA) and translated from local Cartesian coordinates to resultant frontal and sagittal plane time series. The frontal plane time series was formed from the resultant of the X and Y acceleration time series, while the sagittal plane time series was formed from the resultant of the X and Z acceleration time series (Figure 1). All subsequent processing took place on the resultant frontal and sagittal acceleration time series. For accurate analysis of the variability and complexity within the time series, data was left unfiltered [25].
Figure 1.
Example resultant time series of accelerations at the foot in the sagittal plane. (TO – toe off, HC – heel contact)
A custom Matlab program was used to calculate RMS from both the frontal and sagittal plane acceleration time series. Root mean square was calculated as the square root of the mean of squares of the numbers in the time series, and was used to quantify the dispersion of the acceleration traces.
A custom Matlab program was used to calculate ApEn from both frontal and sagittal plane acceleration time series. A thorough explanation of the approximate entropy calculation method can be found in previous literature [26]. ApEn was calculated using customized Matlab software based upon the methodology of Pincus [26, 27], m=3 and r=0.2*(time series standard deviation). These parameters were chosen after varying r from 0.1 to 0.3 in increments of 0.02 and finding relative consistency in the results for r=0.2. The vector length m=3 was chosen after m=2 and m=4 gave very inconsistent results for all values of r. The time lag (τ) was calculated for each time series based on the average mutual information algorithm [28–31]. Time lags ranged from 5 to 30 samples, with an average time lag of 12 + 5 samples. Due to the wide range of time lags, ApEn was calculated using each time series’ specific time lag in order to appropriately capture the characteristics of each individual data set. The processing resulted in a total of four RMS and four ApEn values for each subject – trunk sagittal, trunk frontal, foot sagittal, and foot frontal.
Statistical analysis
A two-way ANOVA was performed on the ApEn and RMS values to test the effects of Location (trunk v. foot) and Plane (frontal v. sagittal) on the results. Paired t-tests were used to analyze any significant interactions. Pearson’s correlations were used to assess the relationship between sensor locations within the same plane, with correlation coefficient of 0.5–0.7 indicating a moderate correlation, and greater than 0.7 indicating a strong correlation [32]. A significance level of 0.05 was used for all analyses, and all analyses were completed in SPSS 2013 (version 22, IBM Corp., Armonk, NY).
Results
ANOVA and Paired Tests
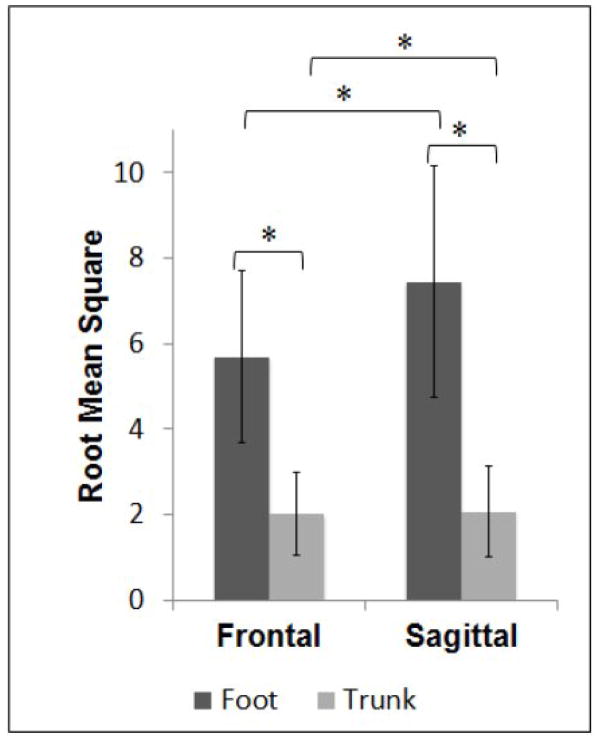
RMS of acceleration showed a main effect of Location (F=369.71, p<0.01) with RMS significantly higher at the foot compared to the trunk. RMS values showed a main effect of Plane (F=82.38, p<0.01) with RMS values significantly higher for the sagittal plane compared to the frontal plane. RMS values showed a significant interaction (F=77.66, p<0.01). Paired tests showed that RMS was significantly higher at the foot compared to the trunk in both the sagittal (t(39 =18.460, p<0.01) and frontal (t(39)=17.411, p<0.01) planes. Paired tests showed that RMS was significantly higher in the sagittal plane compared to the frontal plane at both the foot (t(39)=−9.016, p<0.01) and the trunk (t(39)=−2.355, p=0.024) (Figure 2).
Figure 2.
Means and standard deviations for root mean square of foot and trunk accelerations. Significant differences are indicated for results of paired tests where RMS was higher (larger magnitude) at the foot compared to the trunk, and higher (larger magnitude) in the sagittal plane compared to the frontal plan. * Significant difference; p < 0.05
ApEn of acceleration showed a main effect of Location (F=550.85, p<0.01); with ApEn significantly higher at the trunk compared to the foot. ApEn showed a main effect of Plane (F=117.93, p<0.01); with ApEn significantly higher for the frontal plane compared to the sagittal plane. ApEn showed a significant interaction (F=67.19, p<0.01). Paired tests showed ApEn significantly higher at the trunk compared to the foot in the sagittal (t(39)=−27.247, p<0.01) and the frontal (t(39)=−15.591, p<0.01) planes. Paired tests showed ApEn significantly higher in the frontal plane compared to the sagittal plane at both the foot (t(39)=11.058, p<0.01) and the trunk (t(39)=3.727, p<0.01) (Figure 3).
Figure 3.
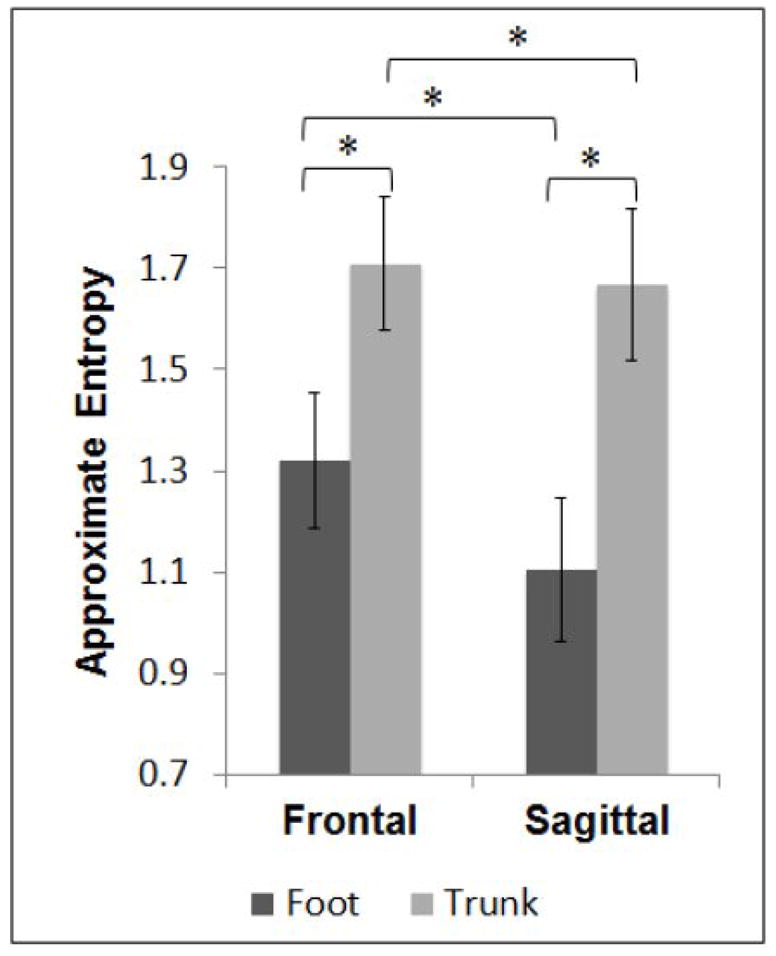
Means and standard deviations for approximate entropy of foot and trunk accelerations. Significant differences are indicated for results of paired tests where ApEn was higher (more signal irregularity) at the trunk compared to the foot, and higher (more signal irregularity) in the frontal plane compared to the sagittal plane. * Significant difference; p < 0.05
Correlations
RMS at the trunk displayed a strong, positive correlation with RMS at the foot in the sagittal plane (r=0.883, p<0.01) and in the frontal plane (r=0.811, p<0.01).
ApEn at the trunk displayed a moderate, positive correlation with ApEn at the foot in the sagittal plane (r=0.603, p<0.01), but there was not a significant correlation in the frontal plane (r=0.293, p=0.066) (Table 1).
Table 1.
Pearson’s correlations between of the foot and the trunk within the given plane for root mean square (RMS) and approximate entropy (ApEn). RMS at foot is strongly correlated with RMS at trunk in sagittal and frontal plane. ApEn at foot is moderately correlated with ApEn at trunk in sagittal plane, but a weak non-significant correlation exists in the frontal plane.
| Plane | r – value (p – value) | |
|---|---|---|
|
| ||
| Sagittal | RMS | 0.883 (<0.01)* |
| ApEn | 0.603 (<0.01)* | |
|
| ||
| Frontal | RMS | 0.811 (<0.01)* |
| ApEn | 0.293 (0.07) | |
Significant Correlation
Discussion
The goal of this study was to examine the acceleration patterns of the trunk and the foot during walking, and the relationship between these patterns. Using ApEn as a measure of variability periodicity and RMS as a measure of variability magnitude, we compared the acceleration time series from the foot and trunk in the frontal and sagittal planes. As previous studies have shown, accelerations are attenuated inferiorly to superiorly in the body during walking [22]. Therefore, we hypothesized that the trunk would display lower magnitudes of acceleration variability (lower RMS) compared to the foot and that the trunk would display more periodic acceleration patterns (lower ApEn) compared to the foot. It was also expected that there would be strong relationships between foot and trunk acceleration for both RMS and ApEn. The results show that our hypotheses were partially supported as RMS was greater at the foot compared to the trunk and there was a significant relationship between accelerations at the trunk and the foot, but ApEn was higher at the trunk compared to the foot.
Root mean square (RMS), as a linear measure of variability, gives information about the magnitude of variability present in a time series. In the current study, our RMS results displayed overall greater magnitude of acceleration variability at the foot compared to the trunk and in the sagittal plane compared to the frontal plane. These findings are in agreement with previous studies which found inferior to superior decreases in variability of acceleration time series [22]. A possible reason for decreasing variability of acceleration from inferior to superior body segments is to keep the trunk, and therefore the body’s center of mass, as stable as possible to minimize energy expenditure during walking [33, 34]. Additionally, the trunk has been shown to act as a physical low pass filter that diminishes higher frequency accelerations that can adversely affect the visual and vestibular systems during gait [35, 36]. Our results also show that there is a significantly higher magnitude of variability of accelerations in the sagittal plane compared to the frontal plane at the trunk and at the foot. As the majority of motion occurs in the sagittal plane during gait, it is reasonable that one will also find larger overall magnitudes of variability.
In both the frontal and sagittal planes, our results show that ApEn was significantly lower for accelerations at the foot compared to the trunk. Lower ApEn indicates a more periodic and therefore more predictable patterns within the time series, which in the case of walking signifies less variability from step to step. This finding suggests that the body aims to maintain predictable foot motion during each step, resulting in a consistent base of support while walking. With a consistent base of support during walking, the trunk is free to be adaptive and react to postural disturbances to maintain stability. The adaptability of the trunk relative to the foot in the present study is represented by higher ApEn of the trunk. Previous studies on stability during walking and quiet standing have illustrated that there is a level of movement variability which is healthy and demonstrates adaptability of the system [37, 38], where any change from the optimal amount of variability, either increased or decreased variability, can result in instability. In the current study, we assume the healthy individuals to have optimal variability, thus the trunk and foot accelerations here are representative of an optimal system. Our results seem to indicate that accelerations at the foot are more predictable and larger in magnitude relative to the trunk. Without a predictable base of support, the trunk may not be able to be adaptive and react to perturbations during walking, thus leading to overall decreased stability. This conclusion is supported by previous studies which found that variable foot motion is indicative of decreased stability [9, 39]. Specifically, step width variability has been shown to be an indicator of gait function [40] and our results show that accelerations at the foot and at the trunk are significantly more regular in the sagittal plane than in the frontal plane for healthy adults which may be indicative of different control mechanisms presiding over motion in the sagittal and frontal planes. This possibility is supported by studies which identify lateral foot placement variability or step width variability but not specifically step length variability as an indicator of less stable gait [4, 5]. Alternatively, this result could stem from the fact that the sagittal plane is the primary plane of motion during gait, exhibiting more regular patterns of motion in this plane compared to the frontal plane. To further understand where these differences stem from, future work should examine frontal and sagittal plane relationships in populations with known gait instability.
Our results also show a positive correlation between movement of the foot and the trunk in the sagittal plane (RMS & ApEn) and the frontal plane (RMS). Accelerations at the foot are transferred to the upper body through the physical connections of the legs, pelvis, and trunk. The physical link between these segments yields a strong relationship between magnitudes of variability at the foot and at the trunk in both the sagittal and frontal planes. As the sagittal plane is the primary plane of motion during gait (flexion/extension of the lower limb joints), a deviation from the normal pattern at the foot (i.e. a change in step length) should be reflected by a deviation from the normal pattern at the trunk, hence the significant correlation for both RMS and ApEn in the sagittal plane. The frontal plane also showed a significant correlation between foot and trunk but only for RMS. Lack of correlations in the predictability (ApEn) of foot and trunk motion may indicate that trunk motion in the frontal plane is related to the magnitude of variability of the foot acceleration but not the predictability of the foot acceleration. When taken together, these results indicate that the body may aim to primarily maintain a predictable base of support through controlling foot placement during walking, while decreasing extreme motion in superior segments of the body. Additionally, maintaining a more predictable base of support allows for greater adaptability of the center of mass, as represented by trunk motion. The ability for the trunk to control and attenuate motion during walking is critical to providing a stable base for the head which contains various balance control mechanisms [36]. Future work should examine these relationships in a population with a risk of falls to determine if these trends may be related to stability during gait.
A limitation of this study is that the data was collected on a motorized treadmill, which constrains speed. While the treadmill could have eliminated some variability in the subject’s gait, the use of this method was necessary to record a time series of sufficient length and the use of treadmill gait to assess variability of movement patterns is well established [40–43]. Similarly, subjects’ gait was only assessed at their preferred gait speed, and it is unknown how these results may change under challenging conditions such as faster or slower than preferred pace. Further, one may find different results if accelerations are recorded at different points on upper and lower body segments. Lastly, ApEn can be volatile to changing data length and other input parameters [44]. This was addressed prior to conducting our analysis, with relative consistency being established as outlined above in the Methods section. Additionally, our data sets were relatively large and should allow for the entropy calculation to be stable [44]. Future work may warrant the exploration of additional nonlinear variability measures such as sample entropy which is much less dependent on data length [44], as well as other established measures.
Employing both linear and nonlinear measures of variability in this study allowed us to more thoroughly examine the relationship between foot and trunk motion during walking. The results of this study illustrate the utility of objectively measuring motion of the body during gait, specifically with the use of small portable accelerometer available for use outside of a research laboratory. Since common gait parameters relating to stability (ie. step width) are not easily measured outside of a research laboratory, it is important to explore the application of portable tools for objective gait measurements. Future studies should aim to expand this research to populations of different ages as well as populations with neuromuscular pathologies which would allow for further examination of the coordination between foot and trunk motion during walking in altered dynamic systems.
Highlights.
Acceleration variability magnitude at feet is greater than at trunk during walking.
Structure of variability shows greater regularity at the foot than at the trunk.
Foot and trunk acceleration variability correlated in frontal and sagittal planes.
Acknowledgments
This work was supported by the National Multiple Sclerosis Society RG 4914A1/2 and the NIH National Center for Advancing Translational Science 1KL2TR00011.
Footnotes
Conflicts of Interest
None
Authorship – The conception and design of the study (JC, JH), acquisition of data (JC, AB), analysis and interpretation of data (JC, AB, JH), drafting the article (JC, JH), final approval of the version to be submitted (JC, AB, JH).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Winter DA. Human balance and posture control during standing and walking. Gait & posture. 1995;3:193–214. [Google Scholar]
- 2.Bruijn SM, Meijer OG, Beek PJ, van Dieen JH. Assessing the stability of human locomotion: a review of current measures. Journal of the Royal Society, Interface / the Royal Society. 2013;10:20120999. doi: 10.1098/rsif.2012.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moe-Nilssen R, Helbostad JL. Trunk accelerometry as a measure of balance control during quiet standing. 2002 doi: 10.1016/s0966-6362(01)00200-4. [DOI] [PubMed] [Google Scholar]
- 4.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. 1997 doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 5.O’Connor SM, Kuo AD. Direction-dependent control of balance during walking and standing. Journal of neurophysiology. 2009;102:1411–9. doi: 10.1152/jn.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zijlstra W, Hof AL. Assessment of spatio-temporal gait parameters from trunk accelerations during human walking. Gait & posture. 2003;18:1–10. doi: 10.1016/s0966-6362(02)00190-x. [DOI] [PubMed] [Google Scholar]
- 7.Kavanagh JJ, Menz HB. Accelerometry: a technique for quantifying movement patterns during walking. Gait & posture. 2008;28:1–15. doi: 10.1016/j.gaitpost.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Bruijn SM, Ten Kate WR, Faber GS, Meijer OG, Beek PJ, van Dieen JH. Estimating dynamic gait stability using data from non-aligned inertial sensors. Annals of biomedical engineering. 2010;38:2588–93. doi: 10.1007/s10439-010-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toebes MJ, Hoozemans MJ, Furrer R, Dekker J, van Dieen JH. Local dynamic stability and variability of gait are associated with fall history in elderly subjects. Gait & posture. 2012;36:527–31. doi: 10.1016/j.gaitpost.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Zhao YL, Kim H. Older Adult Inpatient Falls in Acute Care Hospitals: Intrinsic, Extrinsic, and Environmental Factors. J Gerontol Nurs. 2015;41:29–43. doi: 10.3928/00989134-20150616-05. quiz 4–5. [DOI] [PubMed] [Google Scholar]
- 11.Menz HB, Lord SR, Fitzpatrick RC. Acceleration patterns of the head and pelvis when walking are associated with risk of falling in community-dwelling older people. The journals of gerontology Series A, Biological sciences and medical sciences. 2003;58:M446–52. doi: 10.1093/gerona/58.5.m446. [DOI] [PubMed] [Google Scholar]
- 12.Huisinga JM, Mancini M, St George RJ, Horak FB. Accelerometry reveals differences in gait variability between patients with multiple sclerosis and healthy controls. Annals of biomedical engineering. 2013;41:1670–9. doi: 10.1007/s10439-012-0697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tochigi Y, Segal NA, Vaseenon T, Brown TD. Entropy analysis of tri-axial leg acceleration signal waveforms for measurement of decrease of physiological variability in human gait. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2012;30:897–904. doi: 10.1002/jor.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kavanagh JJ, Morrison S, Barrett RS. Coordination of head and trunk accelerations during walking. European journal of applied physiology. 2005;94:468–75. doi: 10.1007/s00421-005-1328-1. [DOI] [PubMed] [Google Scholar]
- 15.Sekine M, Tamura T, Yoshida M, Suda Y, Kimura Y, Miyoshi H, Kijima Y, et al. A gait abnormality measure based on root mean square of trunk acceleration. 2014 doi: 10.1186/1743-0003-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terrier P, Reynard F. Effect of age on the variability and stability of gait: a cross-sectional treadmill study in healthy individuals between 20 and 69 years of age. Gait & posture. 2015;41:170–4. doi: 10.1016/j.gaitpost.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 17.O’Connor SM, Xu HZ, Kuo AD. Energetic cost of walking with increased step variability. Gait & posture. 2012;36:102–7. doi: 10.1016/j.gaitpost.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harbourne RT, Stergiou N. Movement Variability and the Use of Nonlinear Tools: Principles to Guide Physical Therapist Practice. Physical Therapy. 2009;89:267–82. doi: 10.2522/ptj.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavanaugh JT, Mercer VS, Stergiou N. Approximate entropy detects the effect of a secondary cognitive task on postural control in healthy young adults: a methodological report. Journal of neuroengineering and rehabilitation. 2007;4:42. doi: 10.1186/1743-0003-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stergiou N, Decker LM. Human movement variability, nonlinear dynamics, and pathology: is there a connection? Human movement science. 2011;30:869–88. doi: 10.1016/j.humov.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kavanagh JJ. Lower trunk motion and speed-dependence during walking. Journal of neuroengineering and rehabilitation. 2009;6:9. doi: 10.1186/1743-0003-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kavanagh JJ, Barrett RS, Morrison S. Upper body accelerations during walking in healthy young and elderly men. Gait & posture. 2004;20:291–8. doi: 10.1016/j.gaitpost.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Bruijn SM, Van Impe A, Duysens J, Swinnen SP. Split-belt walking: adaptation differences between young and older adults. Journal of neurophysiology. 2012;108:1149–57. doi: 10.1152/jn.00018.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savin DN, Morton SM, Whitall J. Generalization of improved step length symmetry from treadmill to overground walking in persons with stroke and hemiparesis() Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2014;125:1012–20. doi: 10.1016/j.clinph.2013.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mees AI, Judd K. Dangers of geometric filtering. Physica D: Nonlinear Phenomena. 1993;68:427–36. [Google Scholar]
- 26.Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci U S A. 1991;88:2297–301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pincus SM, Gladstone IM, Ehrenkranz RA. A regularity statistic for medical data analysis. J Clin Monit. 1991;7:335–45. doi: 10.1007/BF01619355. [DOI] [PubMed] [Google Scholar]
- 28.Abarbanel H. Analysis of Observed Chaotic Data. Springer; New York: 1997. [Google Scholar]
- 29.Baker GL, Gollub JP. Chaotic Dynamics: An Introduction. Cambridge University Press; 1996. [Google Scholar]
- 30.Stergiou N. Innovative Analyses of Human Movement: Human Kinetics. 2004 [Google Scholar]
- 31.Gomez Garcia JA, Godino Llorente JI, Castellanos Dominguez G. Influence of delay time on regularity estimation for voice pathology detection. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:4217–20. doi: 10.1109/EMBC.2012.6346897. [DOI] [PubMed] [Google Scholar]
- 32.Hinkle DE, Wiersma W, Jurs SG. Applied Statistics for the Behavioral Sciences: Houghton Mifflin. 2003 [Google Scholar]
- 33.Takacs J, Kirkham AA, Perry F, Brown J, Marriott E, Monkman D, et al. Lateral trunk lean gait modification increases the energy cost of treadmill walking in those with knee osteoarthritis. Osteoarthritis and Cartilage. 2014;22:203–9. doi: 10.1016/j.joca.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 34.DeLisa JA Scientific USVHA, Section TP. Gait Analysis in the Science of Rehabilitation. Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service, Scientific and Technical Publications Section; 1998. [Google Scholar]
- 35.Menz HB, Lord SR, Fitzpatrick RC. Acceleration patterns of the head and pelvis when walking on level and irregular surfaces. Gait & posture. 2003;18:35–46. doi: 10.1016/s0966-6362(02)00159-5. [DOI] [PubMed] [Google Scholar]
- 36.Kavanagh J, Barrett R, Morrison S. The role of the neck and trunk in facilitating head stability during walking. Experimental brain research. 2006;172:454–63. doi: 10.1007/s00221-006-0353-6. [DOI] [PubMed] [Google Scholar]
- 37.Rosenblatt NJ, Hurt CP, Latash ML, Grabiner MD. An apparent contradiction: increasing variability to achieve greater precision? Experimental brain research. 2014;232:403–13. doi: 10.1007/s00221-013-3748-1. [DOI] [PubMed] [Google Scholar]
- 38.Stergiou N, Harbourne R, Cavanaugh J. Optimal movement variability: a new theoretical perspective for neurologic physical therapy. Journal of neurologic physical therapy : JNPT. 2006;30:120–9. doi: 10.1097/01.npt.0000281949.48193.d9. [DOI] [PubMed] [Google Scholar]
- 39.Hurt CP, Rosenblatt N, Crenshaw JR, Grabiner MD. Variation in trunk kinematics influences variation in step width during treadmill walking by older and younger adults. Gait & posture. 2010;31:461–4. doi: 10.1016/j.gaitpost.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Owings TM, Grabiner MD. Step width variability, but not step length variability or step time variability, discriminates gait of healthy young and older adults during treadmill locomotion. Journal of biomechanics. 2004;37:935–8. doi: 10.1016/j.jbiomech.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Dingwell JB, Robb RT, Troy KL, Grabiner MD. Effects of an attention demanding task on dynamic stability during treadmill walking. Journal of neuroengineering and rehabilitation. 2008;5:12. doi: 10.1186/1743-0003-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenblatt NJ, Grabiner MD. Measures of frontal plane stability during treadmill and overground walking. Gait & posture. 2010;31:380–4. doi: 10.1016/j.gaitpost.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Kaipust JP, Huisinga JM, Filipi M, Stergiou N. Gait variability measures reveal differences between multiple sclerosis patients and healthy controls. Motor control. 2012;16:229–44. doi: 10.1123/mcj.16.2.229. [DOI] [PubMed] [Google Scholar]
- 44.Yentes J, Hunt N, Schmid K, Kaipust J, McGrath D, Stergiou N. The Appropriate Use of Approximate Entropy and Sample Entropy with Short Data Sets. Annals of biomedical engineering. 2013;41:349–65. doi: 10.1007/s10439-012-0668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]