Summary
Unfractionated heparin (UFH) has procoagulant activity in antithrombin/heparin cofactor II (HCII)-depleted plasma. UFH prevents tissue factor pathway inhibitor alpha (TFPIα) from inhibiting the procoagulant enzyme complex, prothrombinase, providing a possible mechanism for its procoagulant activity. The procoagulant potential of UFH and various low molecular weight heparins (LMWHs) were characterized for TFPIα dependence, using thrombin generation assays performed with antithrombin/HCII-depleted plasma. UFH, the LMWHs enoxaparin and dalteparin, and the low anticoagulant LMWH 2-O, 3-O desulphated heparin (ODSH) all promoted thrombin generation, but fondaparinux did not, and this activity was blocked by a TFPIα antibody. UFH, enoxaparin, and dalteparin were anticoagulant in reactions containing 1–2% normal plasma. In prothrombinase activity assays, UFH, enoxaparin, dalteparin and ODSH blocked prothrombinase inhibition by TFPIα, while again fondaparinux did not. In both the plasma and purified assays, LMWHs displayed greater procoagulant potential than UFH, even when normalized to saccharide concentration. These biochemical data reveal that UFH and LMWHs, but not fondaparinux, block prothrombinase inhibition by TFPIα, thereby producing their paradoxical procoagulant activity observed in the absence of antithrombin/HCII. The findings may help to understand the complex pathophysiology and treatment of patients that are simultaneously bleeding and clotting, such as those with disseminated intravascular coagulation.
Keywords: Heparin, TFPI, Prothrombinase, Antithrombin
Introduction
Unfractionated heparin (UFH) is a heterogeneous mixture of sulphated polysaccharides with well-characterized anticoagulant function mediated by promoting the inhibition of the coagulation serine proteases thrombin and factor Xa (FXa) by antithrombin (Rosenberg 1989). Antithrombin binds with high affinity to a specific pentasaccharide sequence found in ~33% of UFH chains (Andersson, et al 1976, Hook, et al 1976, Lam, et al 1976). Only chains that contain the antithrombin binding sequence possess significant anticoagulant activity. UFH exhibits paradoxical procoagulant activity in human plasma, in the absence of antithrombin and heparin cofactor II (HCII) (Smith and Morrissey 2008).
The biochemical mechanism underlying this procoagulant activity of UFH is not fully understood. One possible explanation is the inhibition of tissue factor pathway inhibitor (TFPI)α anticoagulant activity, a procoagulant activity of heparin first observed more than 15 years ago (Mast and Broze 1996) and more recently biochemically characterized (Wood, et al 2013). TFPIα is a trivalent Kunitz-type protease inhibitor that dampens the initiation phase of clot formation by inhibiting tissue factor/factor VIIa (TF-FVIIa), the catalytic complex that initiates blood clotting, in a FXa-dependent manner (Baugh, et al 1998, Girard, et al 1989) and by inhibiting early forms of prothrombinase (Wood, et al 2013), the catalytic complex of FXa and factor Va (FVa) that produces thrombin (Barton, et al 1967, Nesheim, et al 1979, Rosing, et al 1980). TFPIα inhibits prothrombinase by interacting with both FXa and FVa. The second Kunitz-type inhibitor domain (K2) of TFPIα directly binds and blocks the FXa active site (Girard, et al 1989). When assembled in the prothrombinase complex, FXa is protected from inhibition by the K2 domain (Franssen, et al 1997, Mast and Broze 1996). TFPIα overcomes this protection via an exosite interaction between its C-terminus and an acidic region of the B-domain present in early forms of FVa (Wood, et al 2013), which are secreted by activated platelets (Monkovic and Tracy 1990a, Viskup, et al 1987) or produced through limited proteolysis by FXa (Monkovic and Tracy 1990b, Schuijt, et al 2013). Once thrombin is generated, it rapidly removes this acidic region from FVa (Monkovic and Tracy 1990b). Thus, TFPIα inhibits prothrombinase only during the initiation phase of blood clotting, before sufficient thrombin has been produced to remove the FVa acidic region (Mast and Broze 1996, Wood, et al 2013).
UFH blocks the interaction between the FVa B-domain acidic region and the TFPIα C-terminal basic region, in a manner that promotes thrombin generation in assays using purified proteins (Wood, et al 2013). Consistent with these findings describing the interactions between TFPIα, prothrombinase and UFH, the procoagulant activity of UFH described in antithrombin/HCII-depleted plasma was not observed if either thrombin or thrombin-activated FVa had been added to the reaction mixtures (Smith and Morrissey 2008). We hypothesized that the procoagulant activity of UFH, observed in the absence of antithrombin/HCII, is mediated by the ability of UFH to block prothrombinase inhibition by TFPIα and thereby promote the initiation phase of thrombin generation.
The dependence of the paradoxical procoagulant activity of UFH on TFPIα was characterized and compared to the procoagulant potential of the low molecular weight heparins (LMWHs) enoxaparin and dalteparin, the antithrombin-binding pentasaccharide fondaparinux, and the low anticoagulant LMWH, 2-O, 3-O desulphated heparin (ODSH).
Methods
Materials
UFH (MW ~12000–16000Da) was from Sigma Aldrich (St. Louis, MO), fondaparinux (MW = 1728Da) from Apotex (Weston, FL), enoxaparin (MW ~5000Da) from Sandoz (Princeton, NJ) and dalteparin (MW ~5000Da) from Eisai (Woodcliff Lake, NJ). ODSH (Fryer, et al 1997) was a gift from J.A. Voynow (Virginia Commonwealth University). FV810QQ, an altered form of FVa in which the B-domain residues 811–1490 are absent and the thrombin cleavage sites at Arg709 and Arg1545 have been mutated to Gln (Bos and Camire 2012), was a gift from R.M. Camire (University of Pennsylvania). Human FXa and prothrombin were from Enzyme Research Laboratories (South Bend, IN). Thrombin, the thrombin inhibitor dansylarginine N-(3-ethyl-1,5-pentanediyl)amine (DAPA) and corn trypsin inhibitor (CTI) were from Haematologic Technologies (Essex Junction, VT). Recombinant TFPIα was as described (Lockett and Mast 2002), and an inhibitory monoclonal antibody directed against the second Kunitz domain of TFPIα (anti-K2) was a gift from Novo Nordisk (Bagsvaerd, Denmark). TF (Dade Innovin) was from Siemens (Washington, DC). Thrombin and FXa chromogenic substrates (Spectrozyme TH and Spectrozyme FXa, respectively) were from Sekisui Diagnostics (Lexington, MA).
Plasma
Experiments using human plasma samples were approved by the BloodCenter of Wisconsin Institutional Review Board, and informed consent was obtained from all study participants. Blood was collected from healthy adult donors into a mixture of citrate and CTI (final concentration 50 μg/ml) and plasma was prepared by centrifugation (700×g, 15 min). Plasma was subsequently centrifuged (5000×g, 10 min) to remove any residual platelets. Antithrombin/heparin cofactor II (HCII)-depleted plasma was from Affinity Biologicals (Ancaster, ON, Canada). For some experiments, the antithrombin/HCII-depleted and normal plasma were mixed at the indicated ratios. The plasma was then diluted 1:4 with 20 mM HEPES, 150 mM NaCl, pH 7.4 (HEPES buffered saline, HBS), containing 0.1% albumin (HBSA). Dilution was necessary because the amount of non-inhibited thrombin generated in undiluted antithrombin/HCII-depleted plasma was too high to accurately quantify. Thrombin generation in normal plasma was measured without dilution.
Phospholipid vesicles
Phosphatidylcholine (PC, cat #840051), phosphatidylethanolamine (PE, cat #840026), and phosphatidylserine (PS, cat #840032), were from Avanti Polar Lipids (Alabaster, AL). Phospholipid vesicles containing PC, PE and PS at a 40:40:20 ratio (PC:PE:PS) were prepared according to the protocol of Morrissey (2001).
Calibrated automated thrombography
Plasma thrombin generation assays were performed as described (Wood, et al 2013). Briefly, 10 μl of a 6x activation mixture (either 0.6 nM FXa or 6 pM TF, and 24 μM PC:PE:PS in HBSA) was incubated with 40 μl of plasma, containing the indicated concentration of UFH, dalteparin, enoxaparin, fondaparinux or ODSH, and reactions were initiated by addition of 10 μl of a mixture of fluorogenic thrombin substrate and calcium (FluCa; Diagnostica Stago, Parsippany, NJ). Fluorescence was monitored using a Fluoroskan Ascent fluorometer (ThermoScientific, Waltham, MA.), and data were analysed using Thrombinoscope v5.0 software (Thrombinoscope, Maastricht, The Netherlands). The lag time, as calculated by the Thrombinoscope software, is the time corresponding to 6% of the peak thrombin.
Purified prothrombinase assays
Prothrombinase inhibition assays were as described (Wood, et al 2013). Briefly, FV810QQ (0.5 nM) was incubated with PC:PE:PS (20 μM), the thrombin inhibitor DAPA (3μM), and the indicated concentrations of TFPIα and heparins in 20 mM HEPES, 150 mM NaCl, pH 7.4 (HBS) containing 5 mM CaCl2 and 0.1% polyethylene glycol (PEG)-8000. Reactions were initiated by addition of prothrombin (1.4 μM) and FXa (5 nM). Aliquots were removed at timed intervals and quenched by addition to HBS containing EDTA (33.3 mM final concentration) and 0.1% PEG-8000. Thrombin concentration at each time point was measured on a SpectraMax Plus 384 microplate reader by adding 20 μl of quenched sample to 80 μl of 0.4 mM thrombin chromogenic substrate, measuring the absorbance change at 405nm, and comparison to a thrombin standard curve. TFPIα:heparin complex concentrations were calculated using a quadratic binding equation and published Kd values (Xu, et al 2002).
FXa activity assays
FXa (0.2 nM) was incubated with phospholipid vesicles (20 μM), in the presence or absence of TFPIα (0.5 nM) and UFH, enoxaparin, dalteparin, or fondaparinux (1 u/ml), and cleavage of a FXa chromogenic substrate (0.5 mM) was monitored at 405 nm.
Results
UFH, enoxaparin, and dalteparin are procoagulant in antithrombin/HCII-depleted plasma, but fondaparinux is not
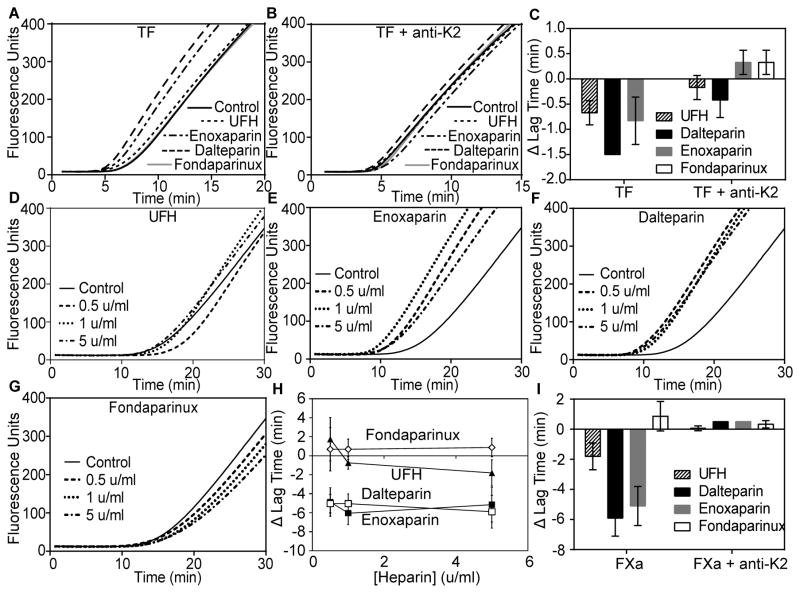
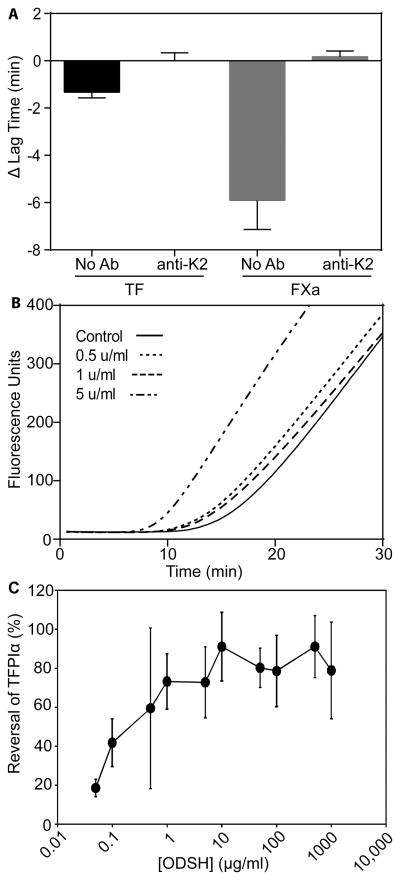
The potential procoagulant activity of the LMWHs enoxaparin and dalteparin and the pentasaccharide fondaparinux was measured using TF-initiated thrombin generation in antithrombin/HCII-depleted plasma (Fig 1A–C). UFH, dalteparin, and enoxaparin (5 u/ml of each) promoted thrombin generation, primarily by reducing the lag time for thrombin generation, while fondaparinux had no effect. Neither UFH nor enoxaparin expressed procoagulant activity when assays were performed in the presence of a monoclonal antibody against K2, while dalteparin expressed greatly reduced procoagulant activity, indicating that the procoagulant activity is dependent on TFPI anticoagulant activity. When reactions were initiated with FXa, the effect of TFPI on heparin procoagulant activity was more enticing (Fig 1D–H). In these reactions, UFH decreased the lag time for thrombin generation by 1.8±0.9 min at 5 u/ml, while the LMWHs exhibited much more pronounced procoagulant activity, with 5 u/ml enoxaparin or dalteparin decreasing the lag time by 5.1±1.3 min or 5.9±1.2 min, respectively. The procoagulant activity of UFH showed a dose response with procoagulant activity apparent at 1 u/ml, while both enoxaparin and dalteparin had full procoagulant activity at the lowest concentration tested (0.5 u/ml) (Fig 1H). By contrast, fondaparinux had minimal effect at any concentration tested (Fig 1H). As observed in the TF-initiated reactions, the procoagulant activity of the heparins in FXa-initiated reactions was dependent on the presence of plasma TFPI, with no procoagulant activity observed for any heparin when TFPI was neutralized with anti-K2 (Fig 1I).
Fig 1. UFH, enoxaparin and dalteparin, but not fondaparinux, are procoagulant in antithrombin/HCII-depleted plasma.
Thrombin generation assays initiated by addition of a mixture of phospholipid vesicles (4μM) and either TF (1pM) or factor Xa (0.1 nM) to antithrombin/HCII-depleted plasma that had been diluted 1:4 in HEPES-buffered saline containing 0.1% bovine serum albumin (A–B) Average fluorescence curves of reactions initiated with TF in the absence (A) or presence (B) of anti-K2 (50 nM) and 5 u/ml UFH, enoxaparin, dalteparin or fondaparinux (n=3). Note that in (A and B) the fondaparinux and control lines are nearly identical. (C) Average changes in lag time, relative to control, from the curves in (A–B) (mean±SD). (D–G) Average fluorescence curves of reactions initiated with FXa in the absence (black) or presence of 0.5 u/ml, 1 u/ml, or 5 u/ml UFH (D), enoxaparin (E), dalteparin (F), or fondaparinux (G) (n=3). (H) Average changes in lag time, relative to control, from the curves in (D–G) (mean±SD). (I) Average changes in lag time, relative to control, from reactions initiated with FXa in the absence or presence of anti-K2 and the different heparins (5 u/ml) (mean±SD; n=3). The lag times in the absence of heparin are reproduced from panel H for display purposes. UFH, unfractionated heparin; HCII, heparin cofactor II; TF, tissue factor; SD, standard deviation.
Heparin anticoagulant activity is restored at low antithrombin/HCII concentrations
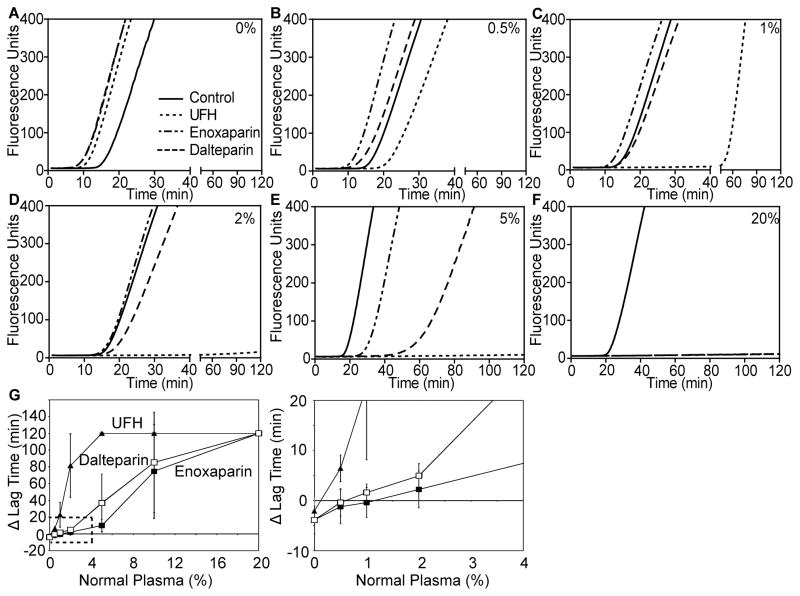
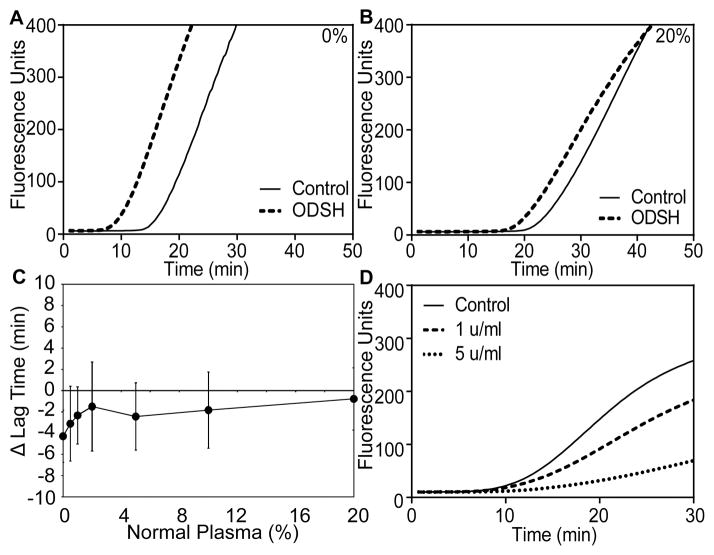
The minimum concentration of antithrombin and HCII necessary to restore anticoagulant function to the heparins was determined by mixing antithrombin/HCII-depleted plasma with normal plasma and measuring FXa-initiated thrombin generation (Fig 2). Addition of normal plasma dose-dependently prolonged the lag time for thrombin generation in the absence of heparin. Therefore, all experiments performed with heparin were compared to equivalent mixed control plasmas in the absence of heparin. In comparison to control, UFH (5 u/ml) exhibited anticoagulant function in 0.5% normal plasma and completely blocked thrombin generation in 5% normal plasma. Enoxaparin and dalteparin required greater amounts of normal plasma to display anticoagulant activity. Dalteparin was anticoagulant in 1% normal plasma and enoxaparin in 2% (Fig 2G, expanded graph). Both completely blocked thrombin generation in 20% normal plasma (Fig 2F–G).
Fig 2. The anticoagulant activity of UFH, enoxaparin and dalteparin is restored at low antithrombin/HCII concentrations.
Antithrombin/HCII-depleted plasma was mixed with various percentages of normal plasma (upper right corner of A–F) prior to dilution and factor Xa-initiated thrombin generation assays as described in Fig 1. Assays were performed in the absence (solid line) or presence of 5 u/ml UFH (dotted line), enoxaparin (dashed/dotted line), or dalteparin (dashed line). Shown are curves from one of three sets of experiments (A–F) and the average change in lag time, compared to control, as a function of normal plasma concentration, from all three experiments (G, left panel; mean ± S.D). The right panel in (G) is an expanded view of the region indicated by dashed lines included to visualize the pro- and anti-coagulant activities of enoxaparin and dalteparin in 0.5%–2% normal plasma in comparison to UFH. UFH, unfractionated heparin; HCII, heparin cofactor II.
UFH, enoxaparin and dalteparin block TFPIα-mediated prothrombinase inhibition, but fondaparinux does not
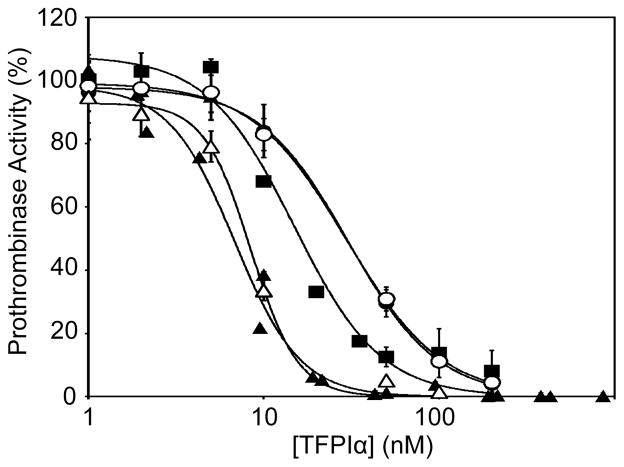
TFPIα inhibits prothrombinase only during the initiation of thrombin generation (Mast and Broze 1996, Wood, et al 2013), because once thrombin is produced, it rapidly removes the TFPIα-binding acidic region from FVa (Monkovic and Tracy 1990b). Therefore, blocking TFPIα inhibition of prothrombinase results in a decrease in the lag time of FXa-initiated thrombin generation (Wood, et al 2013), consistent with the procoagulant effect of UFH, enoxaparin and dalteparin (Fig 1). The ability of therapeutic concentrations of UFH, dalteparin, enoxaparin and fondaparinux to block TFPIα was measured in prothrombinase activity assays utilizing FV810QQ, a recombinant form of FVa that contains the acidic region that binds TFPIα and lacks the primary thrombin cleavage sites (Bos and Camire 2012). The resistance of FV810QQ to thrombin proteolysis produces an assay system, in which thrombin generated during the experiment cannot remove the FVa acidic region and thereby alter the inhibitory kinetics. In this system, TFPIα inhibited thrombin generation with 50% inhibitory concentration (IC50) of 6.8 nM in the absence of heparin (Fig 3). UFH (1 u/ml) blocked TFPIα inhibitory activity, shifting the IC50 to 14.9 nM. Consistent with their increased effect on the lag time in antithrombin/HCII-depleted plasma, enoxaparin (0.8 u/ml, IC50=30.3 nM) and dalteparin (1 u/ml, IC50=29.7 nM) were more effective at blocking prothrombinase inhibition by TFPIα than was UFH. Fondaparinux (0.8 u/ml, IC50=8.4 nM) did not significantly alter the ability of TFPIα to inhibit prothrombinase. Similar results were obtained when all heparins were used at 0.5 u/ml (data not shown).
Fig 3. UFH, enoxaparin and dalteparin block TFPIα inhibition of prothrombinase, but fondaparinux does not.
Prothrombinase activity assays were performed by incubating FV810QQ (0.5 nM) with phospholipid vesicles (20 μM), prothrombin (1.4 μM), the thrombin inhibitor dansylarginine N-(3-ethyl-1,5-pentanediyl)amine (DAPA; 3 μM) and tissue factor pathway inhibitor alpha (TFPIα), in the absence (▲) or presence of unfractionated heparin (UFH; (u/ml, ■), enoxaparin (0.8 u/ml, ●), dalteparin (1 u/ml, ○) or fondaparinux (0.8 u/ml, △), followed by the addition of FXa (5 nM) to initiate the reaction. The heparin concentrations were chosen to mimic the average recommended therapeutic concentrations of each. Shown are the initial rates of thrombin generation, expressed as the percentage of a control experiment performed in the absence of TFPIα (mean±standard error of the mean, n≥3). Note that the dalteparin and enoxaparin curves are very similar.
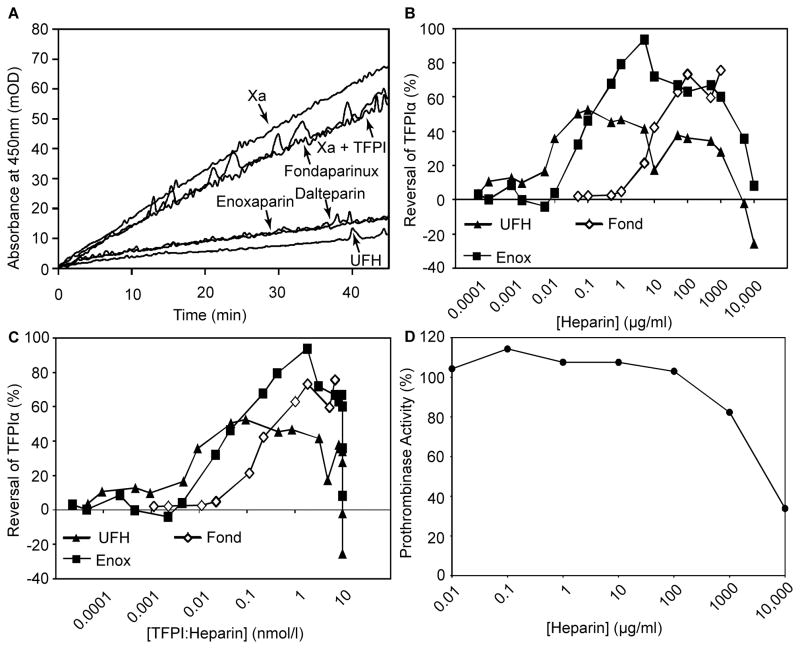
Several aetiologies for the enhanced activity of enoxaparin and dalteparin were considered. First, in addition to blocking the interaction between the TFPIα C-terminus and FVa, UFH promotes the interaction of the K2 domain of TFPIα with the FXa active site in assays performed without FVa (Huang, et al 1993, Jesty, et al 1994, Mast and Broze 1996). The ability of each of the heparins to enhance the direct inhibition of FXa was measured in a chromogenic assay (Fig 4A). Under the conditions of this assay, TFPIα slowly inhibited FXa in the absence of heparin, but rapidly inhibited in the presence of 1 u/ml UFH. FXa was also rapidly inhibited in the presence of 1 u/ml enoxaparin or dalteparin, while fondaparinux had minimal effect. Thus, the increased procoagulant activity of enoxaparin and dalteparin is not explained by decreased ability to enhance direct FXa inhibition.
Fig 4. The enhanced procoagulant activity of LMWHs is not explained by differences in FXa inhibition or saccharide concentration.
(A) Factor Xa (0.2 nM) was incubated with phospholipid vesicles (20 μM) and cleavage of a chromogenic substrate (0.5 mM) was monitored at 405 nm. Experiments were performed in the presence of tissue factor pathway inhibitor alpha (TFPI; 0.5 nM) or TFPIα plus 1 u/ml fondaparinux (Fond), enoxaparin (Enox) dalteparin or unfractionated heparin (UFH). (B–C) Prothrombinase activity experiments were performed as in Fig 3 in the presence of 10 nM TFPIα and varying concentrations of UFH, enoxaparin or fondaparinux. On average, 60% of the prothrombinase activity was inhibited by TFPIα under these conditions in the absence of heparin. The results are displayed as per cent reversal of TFPIα inhibitory activity as a function of the heparin concentration (B) or the calculated concentration of heparin:TFPIα complex (C). Heparin:TFPIα complex concentrations were calculated using the following Kd values: UFH −0.7 μM; enoxaparin −5 μM; fondaparinux −250 μM (Xu, et al 2002). (D) Experiments were performed as in panel B, in the absence of TFPIα, in the presence or absence of UFH and displayed as a percentage of prothrombinase control.
Second, given that 1 u/ml UFH has a different total saccharide concentration than 1 u/ml enoxaparin or fondaparinux, the effect of saccharide concentration on prothrombinase inhibition was examined. Prothrombinase inhibition assays were performed at constant TFPIα concentration (10 nM) and varying heparin concentrations (in μg/ml) (Fig 4B). Increasing UFH or enoxaparin concentration progressively blocked TFPIα inhibitory activity. However, the effect plateaued and then decreased at high concentrations (100–10,000 μg/ml). Fondaparinux required 100- to 1000-fold higher concentrations than UFH or enoxaparin to impede the inhibitory activity of TFPIα (Fig 4B). Published binding affinities (Xu, et al 2002) were used to normalize titration curves to the TFPIα:heparin complex concentration. The differences in the ability of the heparins to block TFPIα inhibitory activity were greatly reduced following normalization for their respective binding affinities (Fig 4C). Therefore, while higher concentrations of LMWHs than UFH are necessary to inhibit TFPIα, the LMWHs possess a greater procoagulant potential, which is not explained by differences in saccharide concentration.
Third, the potential for UFH to directly inhibit prothrombinase, independent of TFPIα, was evaluated as a potential explanation for the decreased apparent procoagulant activity of UFH compared to enoxaparin and dalteparin. However, in the absence of TFPIα, only high concentrations of UFH (≥1000 μg/ml) directly inhibited prothrombinase (Fig 4D), suggesting that TFPIα-independent prothrombinase inhibition does not impact experiments performed at lower concentrations.
The low anticoagulant LMWH ODSH has procoagulant activity at higher normal plasma concentrations than anticoagulant LMWHs
Low anticoagulant LMWHs lack the high affinity antithrombin-binding pentasaccharide, and have been proposed as treatments for a variety of conditions, including sepsis (Wildhagen, et al 2014), cancer (Pisano, et al 2005, Ritchie, et al 2011), sickle cell disease (Alshaiban, et al 2016) and heparin-induced thrombocytopenia (Rao, et al 2010). pWe hypothesized that low anticoagulant LMWHs would retain procoagulant activity at higher antithrombin/HCII concentrations than enoxaparin and dalteparin. The potential procoagulant activity of low anticoagulant LMWHs was examined using ODSH (Fryer, et al 1997). Similar to the activity of the other LMWHs, ODSH displayed procoagulant activity in antithrombin/HCII-depleted plasma initiated with either TF or FXa, but not following addition of anti-TFPI-K2 to the plasma (Fig 5A). ODSH (5 u/ml) decreased the lag time by 5.9±0.9 min in reactions initiated with FXa (Fig 5B), similar to the effect of 5 u/ml enoxaparin or dalteparin (Fig 1H). In contrast to enoxaparin and dalteparin, ODSH did not exhibit maximal procoagulant activity at 0.5 u/ml, but instead displayed a dose response with increasing concentration (Fig 5B). In prothrombinase activity assays, ODSH blocked TFPIα-mediated prothrombinase inhibition in a dose-dependent manner (Fig 5C) that was indistinguishable from enoxaparin (Fig 4B). In contrast to UFH, enoxaparin and dalteparin, ODSH retained partial procoagulant activity in 20% normal plasma (Fig 6A–C). ODSH exhibited anticoagulant activity in 100% normal plasma, as has been previously described (Fig 6D) (Fryer, et al 1997).
Fig 5. ODSH has procoagulant activity similar to enoxaparin and dalteparin in antithrombin/HCII-depleted plasma and prothrombinase activity assays.
(A) Thrombin generation assays were performed in antithrombin/heparin cofactor II (HCII)-depleted plasma in the presence or absence of 2-O, 3-O desulphated heparin (ODSH; 5 u/ml) and anti-TFPI-K2 (50 nM) and were initiated with tissue factor (1pM) or factor Xa (0.1 nM). Shown are changes in lag time, relative to control (mean±SD; n=3). (B) Thrombin generation assays were performed in antithrombin/HCII-depleted plasma as in Fig 1, in the presence of 0.5 (dotted line), 1 (dashed line), or 5 (dashed/dotted line) u/ml ODSH. Shown are the average fluorescence curves (mean±SD, n=3). (C) Prothrombinase activity assays in the presence of varying concentrations of ODSH were performed and are represented as described in Fig 4B. TFPI. TFPIα: tissue factor pathway inhibitor alpha. SD: standard deviation.
Fig 6. ODSH remains procoagulant in 20% normal plasma.
(A–B) FXa-initiated (0.1 nM) thrombin generation assays were performed in the absence or presence of 2-O, 3-O desulphated heparin (ODSH; 5 u/ml) in antithrombin/HCII-depleted plasma alone (A) or mixed with 20% normal plasma (B). Results are depicted as in Fig 2. (C) Average change in lag time (mean±standard deviation) as a function of the percentage of normal plasma. (D) FXa-initiated (0.1 nM) thrombin generation assays were performed in undiluted normal plasma in the absence or presence of 1 u/ml (dashed line) or 5 u/ml (dotted line) ODSH. Shown are the average curves from three sets of experiments.
Discussion
UFH has paradoxical procoagulant activity in thrombin generation assays performed in plasma lacking antithrombin and HCII. The experiments presented here demonstrate that LMWHs, but not the pentasaccharide fondaparinux, produce a similar procoagulant activity, as measured by a decrease in lag time for thrombin generation in TF- or FXa-initiated assays. Procoagulant activity was not observed when plasma TFPI was blocked with an anti-K2 antibody, revealing that the procoagulant activity of the heparins was mediated by their ability to obstruct the anticoagulant properties of TFPI. This finding was confirmed in prothrombinase assays using purified proteins, which demonstrated that the heparins effectively obstruct TFPIα anticoagulant activity. In addition to prothrombinase, UFH has been shown to impede TF-FVIIa inhibition by TFPIα (Jesty, et al 1994), which may have partially contributed to the procoagulant activity observed in TF-initiated assays. However, the promotion of prothrombinase activity is probably the primary procoagulant function of heparins, because the procoagulant activity is eliminated when antithrombin/HCII-depleted plasma is supplemented with either thrombin or thrombin-activated FVa (Smith and Morrissey 2008), both of which would prevent TFPIα from inhibiting prothrombinase but would not impact TF-FVIIa inhibition.
Heparins block TFPIα prothrombinase inhibitory activity by interfering with the charge-dependent interaction between the basic C-terminus of TFPIα and an acidic region in FVa (Wood, et al 2013). This effect is reliant on charge density, but does not require the specific pentasaccharide sequence necessary for antithrombin binding. Therefore, ODSH, a LMWH lacking the pentasaccharide sequence, had procoagulant activity indistinguishable from enoxaparin or dalteparin, LMWHs containing the pentasaccharide sequence. However, charge-dependent interactions do not adequately explain the differences in the procoagulant activities of UFH, LMWHs and fondaparinux. Enoxaparin and dalteparin are produced by chemical depolymerization of UFH (Hoppensteadt, et al 2003). Therefore, differences in the average glycan polymer length are likely to contribute to the differences in procoagulant activity observed between UFH, LMWHs and fondaparinux. This assertion is supported by the progressively increasing amounts (in ng/ml) of heparin polymer required, as polymer length decreased, for inhibition of TFPIα in prothrombinase activity assays. Exhibited in Fig 4B, it took 10 ng/ml UFH, 50 ng/ml enoxaparin and 10,000 ng/ml fondaparinux to reverse 40% of the TFPIα activity. This size-dependent effect could be attributed largely to the affinity of the heparins for TFPIα, as correction for differences in binding affinity for TFPIα (Xu, et al 2002) accounted for over 90% of the differences between fondaparinux and the other heparins (Fig 4C). For example, 1000 times more fondaparinux than UFH (by ng/ml) was required to achieve 40% inhibition of TFPIα, but after correcting for binding affinity, only 23 times more was necessary (by calculated TFPIα:heparin complex).
The three LMWHs promoted thrombin generation in antithrombin/HCII-depleted plasma and increased prothrombinase activity by blocking TFPIα in assays using purified proteins, to a greater extent than did UFH when examined at therapeutically relevant concentrations. For example, in prothrombinase activity assays, enoxaparin, fondaparinux and ODSH reversed 80–95% of TFPIα inhibitory activity, while UFH reversed only about 50% (Figs 4 and 5). This was unexpected for a reaction that is primarily dependent on size and charge density. Possible explanations included a difference in the ability of each heparin to promote direct FXa inhibition by TFPIα or a TFPIα-independent effect of UFH on prothrombinase activity. However, both of these explanations were disproved. First, enoxaparin and dalteparin promoted direct FXa inhibition to a similar extent as UFH. Second, while UFH did directly inhibit prothrombinase activity, it only did so at concentrations substantially higher than those used in the plasma-based assays (≥1000 μg/ml). Thus, the reason for the increased procoagulant activity of LMWHs remains uncertain but may relate to microscopic differences between full-length and low molecular weight heparins.
Similar to the heparins, polyphosphate has procoagulant activity in human plasma that is mediated, at least partially, through obstruction of prothrombinase inhibition by TFPIα (Smith, et al 2006, Wood, et al 2013). Polyphosphates display increasing ability to inhibit TFPIα with increasing phosphate chain length, suggesting that they have size-dependent procoagulant properties similar to the heparins (Smith, et al 2010). Fucoidan, a sulphated polysaccharide derived from seaweed (Liu, et al 2006) that blocks the interaction of TFPIα with FVa (Wood, et al 2013) and decreases bleeding in dogs with hemophilia A (Prasad, et al 2008), also exhibits a size-dependent capability to block TFPI anticoagulant activity in plasma (Zhang, et al 2014).
The data presented here further our understanding of the effects of heparin-based molecules on the coagulation system. Given that UFH, enoxaparin and dalteparin are mixtures of molecules, of which only ~33% contain the high affinity antithrombin binding sequence (Andersson, et al 1976, Hook, et al 1976, Lam, et al 1976), the majority of heparin chains are not anticoagulant. However, all heparin chains appear to block TFPIα from interacting with FVa and, therefore, have procoagulant activity.
Any circumstances where the procoagulant effect of heparins may be clinically relevant remain speculative. As the plasma antithrombin concentration is several orders of magnitude higher than that of TFPIα (Maclean and Tait 2007, Novotny, et al 1991), the heparins are anticoagulant under most conditions. In fact, anticoagulant activity of UFH, enoxaparin and dalteparin was restored by reconstitution of antithrombin/HCII-depleted plasma with small amounts of normal plasma (≤2%), suggesting that a severe antithrombin deficiency would be required for these drugs to have a procoagulant effect in vivo. The amount of normal plasma necessary to avoid procoagulant effects was higher with ODSH (20%), which is consistent with its weak affinity for antithrombin (Richard, et al 2009). The inhibition of TFPIα anticoagulant activity by UFH and LMWHs may become clinically relevant in severely ill patients with acquired antithrombin deficiency, such as sepsis and disseminated intravascular coagulation, or treatment with L-asparaginase; These patients can simultaneously bleed and clot and, as a result, are difficult to treat (Maclean and Tait 2007). Treatment with fondaparinux to anticoagulate these patients is an option for clinicians to consider, as it does not alter TFPIα activity at clinically effective concentrations.
Acknowledgments
We thank J.A. Voynow (Virginia Commonwealth University) for the ODSH and R.M. Camire (University of Pennsylvania) for the FV810QQ. This work was supported by National Heart, Lung, and Blood Institute grants HL068835 (to A.E.M.), HL107152 (to U.R.D.) and HL129193 (to J.P.W.), and J.P.W. was supported by training grant HL007209.
Footnotes
Author Contributions
J.P.W. performed experiments, analysed data, and wrote the manuscript. L.M.B.K. and U.R.D. analysed data and edited the manuscript. A.E.M. designed the research, analysed data and edited the manuscript.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
A.E.M. receives research grant support from Novo Nordisk.
References
- Alshaiban A, Muralidharan-Chari V, Nepo A, Mousa SA. Modulation of Sickle Red Blood Cell Adhesion and its Associated Changes in Biomarkers by Sulfated Nonanticoagulant Heparin Derivative. Clinical and Applied Thrombosis/Hemostasis. 2016;22:230–238. doi: 10.1177/1076029614565880. [DOI] [PubMed] [Google Scholar]
- Andersson LO, Barrowcliffe TW, Holmer E, Johnson EA, Sims GE. Anticoagulant properties of heparin fractionated by affinity chromatography on matrix-bound antithrombin iii and by gel filtration. Thrombosis Research. 1976;9:575–583. doi: 10.1016/0049-3848(76)90105-5. [DOI] [PubMed] [Google Scholar]
- Barton PG, Jackson CM, Hanahan DJ. Relationship between factor V and activated factor X in the generation of prothrombinase. Nature. 1967;214:923–924. doi: 10.1038/214923a0. [DOI] [PubMed] [Google Scholar]
- Baugh RJ, Broze GJ, Jr, Krishnaswamy S. Regulation of extrinsic pathway factor Xa formation by tissue factor pathway inhibitor. The Journal of Biological Chemistry. 1998;273:4378–4386. doi: 10.1074/jbc.273.8.4378. [DOI] [PubMed] [Google Scholar]
- Bos MH, Camire RM. A bipartite autoinhibitory region within the B-domain suppresses function in factor V. The Journal of Biological Chemistry. 2012;287:26342–26351. doi: 10.1074/jbc.M112.377168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen J, Salemink I, Willems GM, Wun TC, Hemker HC, Lindhout T. Prothrombinase is protected from inactivation by tissue factor pathway inhibitor: competition between prothrombin and inhibitor. Biochem J. 1997;323(Pt 1):33–37. doi: 10.1042/bj3230033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer A, Huang YC, Rao G, Jacoby D, Mancilla E, Whorton R, Piantadosi CA, Kennedy T, Hoidal J. Selective O-desulfation produces nonanticoagulant heparin that retains pharmacological activity in the lung. The Journal of Pharmacology and Experimental Therapeutics. 1997;282:208–219. [PubMed] [Google Scholar]
- Girard TJ, Warren LA, Novotny WF, Likert KM, Brown SG, Miletich JP, Broze GJ., Jr Functional significance of the Kunitz-type inhibitory domains of lipoprotein-associated coagulation inhibitor. Nature. 1989;338:518–520. doi: 10.1038/338518a0. [DOI] [PubMed] [Google Scholar]
- Hook M, Bjork I, Hopwood J, Lindahl U. Anticoagulant activity of heparin: separation of high-activity and low-activity heparin species by affinity chromatography on immobilized antithrombin. FEBS Letters. 1976;66:90–93. doi: 10.1016/0014-5793(76)80592-3. [DOI] [PubMed] [Google Scholar]
- Hoppensteadt D, Walenga JM, Fareed J, Bick RL. Heparin, low-molecular-weight heparins, and heparin pentasaccharide: basic and clinical differentiation. Hematology/Oncology Clinics of North America. 2003;17:313–341. doi: 10.1016/s0889-8588(02)00091-6. [DOI] [PubMed] [Google Scholar]
- Huang ZF, Wun TC, Broze GJ., Jr Kinetics of factor Xa inhibition by tissue factor pathway inhibitor. The Journal of Biological Chemistry. 1993;268:26950–26955. [PubMed] [Google Scholar]
- Jesty J, Wun TC, Lorenz A. Kinetics of the inhibition of factor Xa and the tissue factor-factor VIIa complex by the tissue factor pathway inhibitor in the presence and absence of heparin. Biochemistry. 1994;33:12686–12694. doi: 10.1021/bi00208a020. [DOI] [PubMed] [Google Scholar]
- Lam LH, Silbert JE, Rosenberg RD. The separation of active and inactive forms of heparin. Biochemical and Biophysical Research Communications. 1976;69:570–577. doi: 10.1016/0006-291x(76)90558-1. [DOI] [PubMed] [Google Scholar]
- Liu T, Scallan CD, Broze GJ, Jr, Patarroyo-White S, Pierce GF, Johnson KW. Improved coagulation in bleeding disorders by Non-Anticoagulant Sulfated Polysaccharides (NASP) Thrombosis and Haemostasis. 2006;95:68–76. [PubMed] [Google Scholar]
- Lockett JM, Mast AE. Contribution of regions distal to glycine-160 to the anticoagulant activity of tissue factor pathway inhibitor. Biochemistry. 2002;41:4989–4997. doi: 10.1021/bi016058n. [DOI] [PubMed] [Google Scholar]
- Maclean PS, Tait RC. Hereditary and acquired antithrombin deficiency: epidemiology, pathogenesis and treatment options. Drugs. 2007;67:1429–1440. doi: 10.2165/00003495-200767100-00005. [DOI] [PubMed] [Google Scholar]
- Mast AE, Broze GJ., Jr Physiological concentrations of tissue factor pathway inhibitor do not inhibit prothrombinase. Blood. 1996;87:1845–1850. [PubMed] [Google Scholar]
- Monkovic DD, Tracy PB. Functional characterization of human platelet-released factor V and its activation by factor Xa and thrombin. The Journal of Biological Chemistry. 1990a;265:17132–17140. [PubMed] [Google Scholar]
- Monkovic DD, Tracy PB. Activation of human factor V by factor Xa and thrombin. Biochemistry. 1990b;29:1118–1128. doi: 10.1021/bi00457a004. [DOI] [PubMed] [Google Scholar]
- Morrissey JH. Morrissey laboratory protocol for preparing phospholipid vesicles (SUV) by sonication. 2001 http://tf7.org/suv.pdf.
- Nesheim ME, Taswell JB, Mann KG. The contribution of bovine Factor V and Factor Va to the activity of prothrombinase. The Journal of Biological Chemistry. 1979;254:10952–10962. [PubMed] [Google Scholar]
- Novotny WF, Brown SG, Miletich JP, Rader DJ, Broze GJ., Jr Plasma antigen levels of the lipoprotein-associated coagulation inhibitor in patient samples. Blood. 1991;78:387–393. [PubMed] [Google Scholar]
- Pisano C, Aulicino C, Vesci L, Casu B, Naggi A, Torri G, Ribatti D, Belleri M, Rusnati M, Presta M. Undersulfated, low-molecular-weight glycol-split heparin as an antiangiogenic VEGF antagonist. Glycobiology. 2005;15:1C–6C. doi: 10.1093/glycob/cwi007. [DOI] [PubMed] [Google Scholar]
- Prasad S, Lillicrap D, Labelle A, Knappe S, Keller T, Burnett E, Powell S, Johnson KW. Efficacy and safety of a new-class hemostatic drug candidate, AV513, in dogs with hemophilia A. Blood. 2008;111:672–679. doi: 10.1182/blood-2007-07-098913. [DOI] [PubMed] [Google Scholar]
- Rao NV, Argyle B, Xu X, Reynolds PR, Walenga JM, Prechel M, Prestwich GD, MacArthur RB, Walters BB, Hoidal JR, Kennedy TP. Low anticoagulant heparin targets multiple sites of inflammation, suppresses heparin-induced thrombocytopenia, and inhibits interaction of RAGE with its ligands. American journal of physiology. Cell physiology. 2010;299:C97–110. doi: 10.1152/ajpcell.00009.2010. [DOI] [PubMed] [Google Scholar]
- Richard B, Swanson R, Olson ST. The signature 3-O-sulfo group of the anticoagulant heparin sequence is critical for heparin binding to antithrombin but is not required for allosteric activation. The Journal of Biological Chemistry. 2009;284:27054–27064. doi: 10.1074/jbc.M109.029892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JP, Ramani VC, Ren Y, Naggi A, Torri G, Casu B, Penco S, Pisano C, Carminati P, Tortoreto M, Zunino F, Vlodavsky I, Sanderson RD, Yang Y. SST0001, a chemically modified heparin, inhibits myeloma growth and angiogenesis via disruption of the heparanase/syndecan-1 axis. Clinical Cancer Research. 2011;17:1382–1393. doi: 10.1158/1078-0432.CCR-10-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg RD. Biochemistry of heparin antithrombin interactions, and the physiologic role of this natural anticoagulant mechanism. The American Journal of Medicine. 1989;87:2S–9S. doi: 10.1016/0002-9343(89)80523-6. [DOI] [PubMed] [Google Scholar]
- Rosing J, Tans G, Govers-Riemslag JW, Zwaal RF, Hemker HC. The role of phospholipids and factor Va in the prothrombinase complex. The Journal of Biological Chemistry. 1980;255:274–283. [PubMed] [Google Scholar]
- Schuijt TJ, Bakhtiari K, Daffre S, Deponte K, Wielders SJ, Marquart JA, Hovius JW, van der Poll T, Fikrig E, Bunce MW, Camire RM, Nicolaes GA, Meijers JC, van’t Veer C. Factor Xa activation of factor V is of paramount importance in initiating the coagulation system: lessons from a tick salivary protein. Circulation. 2013;128:254–266. doi: 10.1161/CIRCULATIONAHA.113.003191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Morrissey JH. Heparin is procoagulant in the absence of antithrombin. Thrombosis and Haemostasis. 2008;100:160–162. doi: 10.1160/TH08-05-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:903–908. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Choi SH, Davis-Harrison R, Huyck J, Boettcher J, Rienstra CM, Morrissey JH. Polyphosphate exerts differential effects on blood clotting, depending on polymer size. Blood. 2010;116:4353–4359. doi: 10.1182/blood-2010-01-266791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskup RW, Tracy PB, Mann KG. The isolation of human platelet factor V. Blood. 1987;69:1188–1195. [PubMed] [Google Scholar]
- Wildhagen KC, Garcia de Frutos P, Reutelingsperger CP, Schrijver R, Areste C, Ortega-Gomez A, Deckers NM, Hemker HC, Soehnlein O, Nicolaes GA. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood. 2014;123:1098–1101. doi: 10.1182/blood-2013-07-514984. [DOI] [PubMed] [Google Scholar]
- Wood JP, Bunce MW, Maroney SA, Tracy PB, Camire RM, Mast AE. Tissue factor pathway inhibitor-alpha inhibits prothrombinase during the initiation of blood coagulation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17838–17843. doi: 10.1073/pnas.1310444110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Takano R, Nagai Y, Yanagida T, Kamei K, Kato H, Kamikubo Y, Nakahara Y, Kumeda K, Hara S. Effect of heparin chain length on the interaction with tissue factor pathway inhibitor (TFPI) International Journal of Biological Macromolecules. 2002;30:151–160. doi: 10.1016/s0141-8130(02)00015-6. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Till S, Jiang C, Knappe S, Reutterer S, Scheiflinger F, Szabo CM, Dockal M. Structure-activity relationship of the pro- and anticoagulant effects of Fucus vesiculosus fucoidan. Thrombosis and Haemostasis. 2014;111:429–437. doi: 10.1160/TH13-08-0635. [DOI] [PubMed] [Google Scholar]