Abstract
Introduction
To estimate the contribution of the prostate gland and prostatic urethral inflammation to urinary symptoms after radiation therapy for prostate cancer, we performed a secondary analysis of urinary toxicity after primary radiation to an intact prostate versus post-prostatectomy radiation to the prostatic fossa in protocols RTOG 94-08 and 96-01, respectively.
Materials and Methods
Patients randomized to the radiation alone arms (without hormone therapy) of the two trials were evaluated, including 104 men receiving primary prostate radiation to 68.4 Gy on RTOG 94-08 and 371 men receiving 64.8 Gy to the prostatic fossa on RTOG 96-01. Acute and late urinary toxicity were scored prospectively by RTOG scales. Chi-square test/logistic regression and cumulative incidence approach/Fine-Gray regression were used for analyses of acute and late toxicity, respectively.
Results
Grade≥2 acute urinary toxicity was significantly higher after primary prostatic radiation compared to post-prostatectomy radiation (30.8% versus 14.0%; p<0.001), but acute grade≥3 toxicity did not differ (3.8% versus 2.7%; p=0.54). After adjusting for age, primary radiation resulted in significantly higher grade≥2 acute urinary toxicity (OR:3.72; 95% CI:1.65–8.37; p=0.02). With median follow-up of 7.1 years, late urinary toxicity was not significantly different with primary versus post-prostatectomy radiation (5-year Grade≥2: 16.7% versus 18.3%; p=0.65; Grade≥3: 6.0% versus 3.3%; p=0.24).
Conclusions
Primary radiation to an intact prostate resulted in higher grade≥2 acute urinary toxicity than radiation to the prostatic fossa, with no difference in late urinary toxicity. Thus, a proportion of acute urinary toxicity in men with an intact prostate may be attributable to inflammation of the prostatic gland or urethra.
Keywords: Primary prostate cancer radiation therapy, post-prostatectomy radiation, urinary toxicity
Introduction
Urinary toxicity after radiation therapy in men with prostate cancer is often attributed to bladder injury. However, in males, acute urinary symptoms may also be due to radiation-induced inflammation of the prostate and prostatic urethra [1, 2]. The contribution of prostate edema and prostatic urethral inflammation to the development of symptomatic acute and late urinary toxicity has not been sufficiently quantified. The distinction between symptoms from bladder versus prostate gland and prostatic urethra radiation injury is not feasible because a clinician cannot easily distinguish the underlying cause of similar urinary symptoms, and thus, the “urinary” toxicity reported in the prostate cancer literature does not distinguish between bladder and prostate injury specifically.
A comparison of the radiation-induced urinary toxicity rates between men after prostatectomy and thus without a prostatic urethra and an internal sphincter versus those with an intact prostate using similar fields and radiation doses may provide some insight into the contribution of prostate and prostatic urethral inflammation to the symptoms of urinary toxicity.
In a series from Memorial Sloan Kettering, 42 patients treated with 3D conformal radiation to a median dose of 64.8 Gy, and a median follow-up of 2 years, experienced a 7% incidence of acute Grade 2 urinary toxicity, but this follow-up was too short for the expression of late urinary toxicity [3]. A larger multi-institutional retrospective composite series of 959 men treated with either post-prostatectomy salvage (81%) or adjuvant (19%) 3D conformal radiation with a median follow-up of 5 years also showed a very low rate of late urinary toxicity with 10% Grade 2 and 1% Grade 3 at 5 years, respectively [4]. Similarly, prospective trials of adjuvant radiation to the prostatic fossa have also reported very low (≤5%) incidences of late grade 3 urinary toxicity [5, 6].
Comparatively, the incidence of urinary toxicity reported from retrospective series of patients with primary radiation has typically been much higher. For instance, the 3D conformal primary radiation series from Memorial Sloan Kettering, Fox Chase Cancer Center and from a Dutch dose escalation trial showed a ~30–40% incidence of Grade 2 acute urinary toxicity in men with an intact prostate treated to similar doses (<70 Gy) [7–9]. The much lower rates of acute urinary toxicity seen with post-prostatectomy radiation compared to primary radiation suggests that the early urinary symptoms observed in the latter group may be in part attributable to radiation to the prostatic urethra and the prostate gland rather than the bladder.
In order to quantify the contribution of prostate and prostatic urethral symptoms to “urinary toxicity” after external beam radiation, we performed a secondary analysis of pooled data from the radiation alone arms of the RTOG 94-08 [10] and RTOG 96-01 [11] trials. Since the men in RTOG 94-08 received radiation to the intact prostate while the post-prostatectomy men in RTOG 96-01 were radiated to the prostatic fossa and a larger portion of the bladder because it was moved inferiorly to anastamose to the external sphincter, a comparison of the observed incidences of acute and late urinary toxicities between the two trials may allow an estimate of the contribution of prostate inflammation and prostatic urethral injury to early and late urinary symptoms.
Materials and Methods
Patients
This study included men randomized to the radiation alone arms of both RTOG 94-08 and 96-01. RTOG 94-08 (from 1994 to 2001) was a prospective phase III trial of 1979 stage T1b–T2b prostate cancer patients with a prostate-specific antigen (PSA) level of 20 ng/mL or less who received 68.4 Gy to the intact prostate with or without 4 months of neoadjuvant and concurrent total androgen suppression. RTOG 96-01(from 1998 to 2003) was a prospective Phase III trial of 771 post-prostatectomy patients (with high risk features pT3 or pT2 with positive margins and with an elevated post-surgery PSA) who were randomized to 24 months of bicalutamide or placebo with 64.8 Gy to the prostatic fossa.
Only patients from the radiation alone arms of these trials were included in this analysis to specifically analyze the toxicity of radiation, since the different hormonal therapy regimens on each trial could be a confounding factor, leaving 992 eligible patients from RTOG 94-08 and 383 eligible patients from RTOG 96-01 for analysis. We excluded all patients who received pelvic field radiation in RTOG 94-08 (n=888) and included only patients who received “prostate only” radiation to 68.4 Gy in RTOG 94-08, to allow for a fair comparison between the two studies since the “prostate only” radiation patients on RTOG 94-08 were treated with similar field sizes as those treated with post-prostatectomy radiation on RTOG 96-01. This left 104 patients from RTOG 94-08 and 371 patients from RTOG 96-01 available for analysis in this study.
Radiation Techniques
Patients on both trials were treated using 2D or 3D-radiation techniques, using 4-field arrangement (anterior, posterior, right and left lateral). For RTOG 94-08, the prostatic target volume measured at least 9.0 cm in craniocaudal direction and at least 8.0 cm in transverse and sagittal directions. The seminal vesicles in their entirety were not considered to be target tissues. For RTOG 96-01, the field included the prostatic bed with an expansion to account for setup variability. The typical field length was ~9.0 cm in the craniocaudal direction, and 8.0 cm in the transverse and sagittal directions.
Patients on RTOG 94-08 received a dose of 68.4 Gy in 38 fractions to the intact prostate only, while patients enrolled on RTOG 96-01 received 64.8 Gy in 36 fractions to the prostatic fossa.
Endpoints
Acute radiation toxicity
For both RTOG 94-08 and RTOG 96-01, acute radiation toxicity was scored using the RTOG Acute Radiation Morbidity Scoring Criteria (Supplemental Table 1), and defined as any radiation-related toxicity that occurred within 90 days of the treatment start date. Per protocol, all patients were evaluated weekly by their radiation oncologist during radiation, and urinary complications including urinary frequency, dysuria, hematuria, urinary tract infections, and incontinence were assessed.
Late radiation toxicity
For both RTOG 94-08 and RTOG 96-01, late radiation toxicity was scored prospectively using the Late Radiation Morbidity Scoring Scheme (Supplemental Table 1), and defined as any radiation-related toxicity that occurred >180 days from the start of treatment.
Statistical Analyses
The chi-square test and logistic regression [12] were used for univariate and multivariate analyses of acute toxicity, respectively. Potential confounders were identified and included in the regression models. Fine-Gray regression [13] was used for analyses of late toxicity. Yearly estimates were calculated using the cumulative incidence approach [14].
Results
Patient and Treatment Characteristics
The median age for the patients from RTOG 94-08 was 70 and for RTOG 96-01 was 64 (Table 1). The Gleason score (GS) distribution was significantly different between the two cohorts with higher GS tumors found in RTOG 96-01 patients (Table 1). There was a 5.5 % higher median radiation dose (by 3.6 Gy) in the RTOG 94-08 trial versus the 96-01 trial patients’ dose (64.8Gy).
Table 1.
Patient Characteristics by Trial
| RTOG 94-08 | RTOG 96-01 | ||||
|---|---|---|---|---|---|
| Characteristics | Intact Prostate (n=104) |
Post-Prostatectomy (n=371) |
|||
| Age | |||||
| Median | 70 | 64 | |||
| Range | 51–88 | 46–81 | |||
|
| |||||
| n | % | N | % | ||
|
| |||||
| KPS | |||||
| 90–100 | 96 | 92 | 367 | 99 | |
| 70–80 | 8 | 8 | 4 | 1 | |
| T-Stage* | |||||
| T1 | 47 | 45 | 0 | 0 | |
| T2 | 57 | 55 | 118 | 32 | |
| T3 | 0 | 0 | 253 | 68 | |
| PSA | |||||
| < 4 | 14 | 13 | 371 | 100 | |
| 4–20 | 90 | 87 | 0 | 0 | |
| Gleason Score** | |||||
| 2–6 | 99 | 95 | 100 | 27 | |
| 7 | 3 | 3 | 204 | 55 | |
| 8–10 | 1 | 1 | 65 | 18 | |
| Unknown | 1 | 1 | 2 | 1 | |
Clinical stage for RTOG 94-08 patients and pathologic stage for RTOG 96-01 patients
Biopsy Gleason Score for RTOG 94-08 and pathologic Gleason Score for RTOG 96-01
Incidence of Acute Urinary Toxicity
The incidences of acute urinary toxicity in each trial are shown in Table 2, and a breakdown of types of urinary toxicity is shown in Table 3. There was no acute grade 4 toxicity in any patient from either trial. Acute grade≥2 urinary toxicity was significantly higher in the RTOG 94-08 cohort with an incidence of 30.8% (n=32) versus 14.0% (n=52) in the RTOG 96-01 cohort (p<0.0001). There was no significant difference in acute grade≥3 toxicity with an incidence of 3.8% (n=4) versus 2.7% (n=10; p=0.537; Table 2).
Table 2.
Grade of Acute Urinary Toxicity by Trial
| RTOG 94-08 | RTOG 96-01 | |||||||
|---|---|---|---|---|---|---|---|---|
| Intact Prostate (n=104) Grade |
Post-Prostatectomy (n=371) Grade |
|||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Incidence of urinary toxicity (%) |
39 (37.5%) |
28 (26.9%) |
4 (3.8%) |
0 (0%) |
125 (33.7%) |
42 (11.3%) |
10 (2.7%) |
0 (0%) |
Table 3.
Incidence of Specific Acute Grade≥ 2 GU Toxicities by Trial
| RTOG 94-08 (Intact Prostate) (n=104) |
RTOG 96-01 (Post-Prostectomy) (n=371) |
P-Value | |
|---|---|---|---|
|
| |||
| Acute Grade 0–1 GU Toxicity | 72 (69.2%) | 319 (86.0%) | 0.0076 |
|
| |||
| Acute Grade ≥ 2 GU Toxicity | 32 (30.8%) | 52 (14.0%) | |
| UTI | 0 (0.0%) | 1 (0.3%) | |
| Dysuria | 4 (3.8%) | 5 (1.4%) | |
| Frequency | 10 (9.6%) | 13 (3.5%) | |
| frequency/dysuria | 0 (0.0%) | 2 (0.6%) | |
| hematuria/catheter placement | 0 (0.0%) | 1 (0.3%) | |
| hematuria/dysuria | 0 (0.0%) | 1 (0.3%) | |
| Hesitancy | 0 (0. 0%) | 1 (0.3%) | |
| Incontinence | 0 (0.0%) | 1 (0.3%) | |
| not specified | 17 (16.4%) | 26 (7.0%) | |
| obstruction–catheter needed | 1 (1.0%) | 0 (0.0%) | |
| retention w/catheter placement | 0 (0.0%) | 1 (0.3%) | |
Multivariate Analysis of Acute Urinary Toxicity
Age and GS were evaluated as potential confounders. On multivariate analysis adjusting for age, GS and radiation dose (Table 4), men with primary radiation from RTOG 94-08 had a significantly higher risk of acute grade≥2 urinary toxicity than men treated with radiation to the prostatic fossa from RTOG 96-01 (OR:3.72; 95% CI:1.65–8.37; p=0.02). Since the patient age distribution differed significantly between the two trials, we performed a sub-group analysis by age with a cut-off at the median age (66) of all patients in this study. In both age subgroups (age<66 and age≥66), there remained a statistically significant higher incidence of acute grade≥2 urinary toxicity for those men irradiated with an intact prostate (Table 5).
Table 4.
Multivariate Analysis of Acute Grade ≥ 2 Urinary Toxicity
| Covariate | Categories | HR | 95%CI | p-value* |
|---|---|---|---|---|
|
| ||||
| Radiation Dose | Continuous | 1.04 | (0.94,1.15) | 0.44 |
|
| ||||
| Study | 9601 (Post-Prostectomy) | 1.0* | – | – |
| 9408 (Intact Prostate) | 3.72 | (1.65,8.37) | 0.02† | |
|
| ||||
| Age | Continuous | 0.98 | (0.94,1.02) | 0.24 |
|
| ||||
| Gleason | 2–6 | 1.0* | – | – |
| 7 | 1.79 | (0.88,3.61) | 0.10 | |
| 8–10 | 0.86 | (0.30,2.45) | 0.78 | |
Reference group
Table 5.
Univariate* Analysis by Age Subgroup of Acute Grade ≥ 2 Toxicity by Trial
| RTOG 94-08 (n=104) |
RTOG 96-01 (n=371) |
p-value* | |
|---|---|---|---|
| Age < 66 | (n=20) | (n=201) | |
| Acute Grade ≥ 2 urinary | |||
| Toxicity | |||
| Yes | 9 (45.0%) | 33 (16.4%) | 0.0047† |
| No | 11 (55.0%) | 168 (83.6%) | |
| Age ≥ 66 | (n=84) | (n=170) | |
| Acute Grade ≥ 2 urinary | |||
| Toxicity | |||
| Yes | 23 (27.4%) | 19 (11.2%) | 0.0020† |
| No | 61 (72.6%) | 151 (88.8%) |
Based on Fisher’s exact test
Statistical Significance
Late Urinary Toxicity
The median follow-up of all patients was 7.1 years, with a median follow-up of 9.0 years in the RTOG 94-08 cohort, and 7.0 years in the RTOG 96-01 cohort. There was no significant difference at 5-years in late grade≥2 toxicity (16.7% versus 18.3%; p=0.65; Figure 1) in those men irradiated with or without an intact prostate. At least 5 years of follow-up after radiation was necessary to observe nearly full expression of late urinary toxicity in both trial cohorts (Figure 1). Late grade≥3 urinary toxicity rates were also not significantly different (6.0% versus 3.3%; p=0.24; Figure 2) in the RTOG 94-08 and RTOG 96-01 cohorts.
Figure 1.
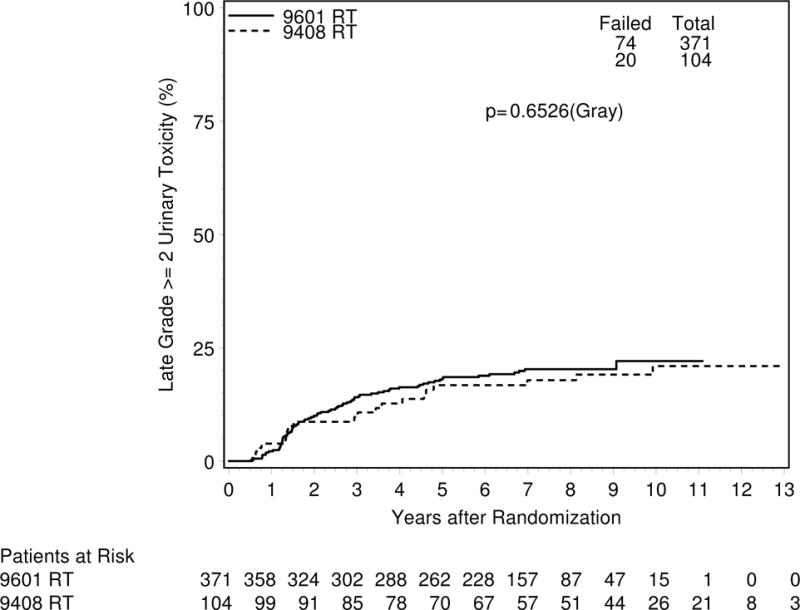
Cumulative incidence of grade ≥ 2 late urinary toxicity after radiation therapy in men with intact prostate (RTOG 9408) or post-prostatectomy (RTOG 9601)
Figure 2.
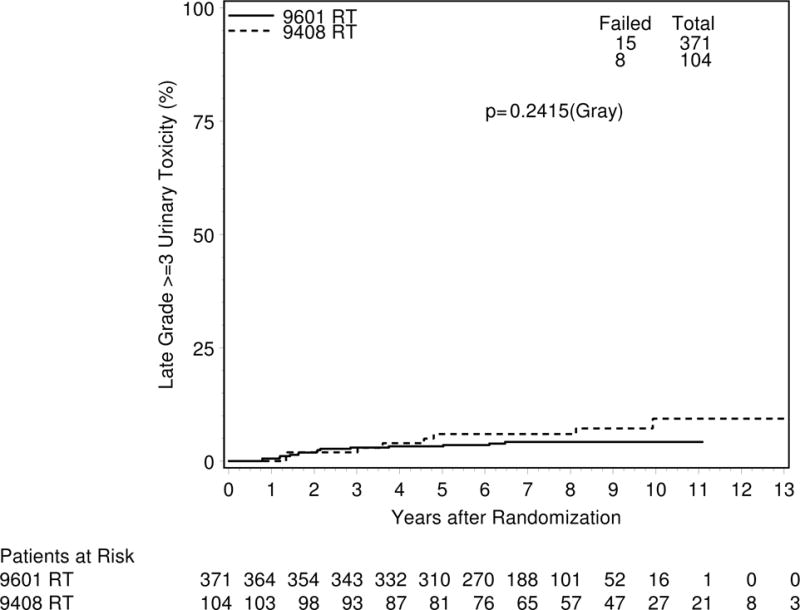
Cumulative incidence of grade ≥ 3 late urinary toxicity after radiation therapy in men with IP (RTOG 9408) or post-prostatectomy (RTOG 9601)
Discussion
In this secondary analysis of patients from two RTOG trials who received radiation alone using similar doses, field sizes and techniques, we demonstrate a higher incidence of acute grade≥2 urinary toxicity in prostate cancer patients undergoing primary radiation than post-prostatectomy radiation. The association between primary radiation and higher acute urinary toxicity was still significant after controlling for age and radiation dose as potential confounding factors on multivariate analysis, and after sub-group analysis in patients younger and older than 66. However, there was no significant difference in late grade≥2 toxicity between the patients receiving primary radiation versus post-prostatectomy radiation.
These findings suggest that acute inflammation and injury to the intact prostatic urethra or prostate may account for some of the acute urinary symptoms during radiation for prostate cancer. Of note, men who received post-prostatectomy radiation would typically have a higher volume of the bladder receiving the full radiation dose due to the post-prostatectomy urethro-vesical anastamosis shifting the caudal portion of the bladder into the radiation field. Despite this higher dose to a larger portion of the bladder, the post-prostatectomy men still had a lower incidence of acute urinary toxicity in this study. Unfortunately, since the patients on RTOG 94-08 and 96-01 were enrolled during a time period when CT-based 3D planning was not routine we do not have the percent of the bladder volume that received the full radiation dose to further study this hypothesis in greater detail.
Comparable to prior studies of patients treated to 60–70 Gy with conventional radiation techniques, the incidence of late grade≥3 urinary toxicity was low in both groups of patients and there was no significant difference. A prior pooled analysis of RTOG 7506 and 7706 showed an incidence of late grade≥3 urinary toxicity of 7.7% at 7 years and only 0.5% required surgical intervention or hospitalization [15]. Of note, half of these complications were attributed to urethral stricture. Similarly, a pooled analysis of 331 patients radiated primarily to 63–74 Gy at Massachusetts General Hospital, MD Anderson Cancer Center, and RTOG demonstrated a 5.4% incidence of stricture with a median follow-up of 6.1 years [16]. These findings in combination with the results of our study underscore the potential importance to study further the bladder dose-volume relationships in men receiving primary versus post-prostatectomy radiation.
Strengths of this study include the use of prospectively collected toxicity data and standardized radiation techniques, dose and field sizes on each trial. Additionally, we selected patients from the two trials who had comparable radiation field sizes and similar radiation dose. In contrast, prior studies comparing urinary toxicity between these two groups of patients have not identified a difference in acute urinary toxicity, but were confounded by higher radiation doses for men receiving primary versus post-prostatectomy radiation[17].
However, the findings of this study must be interpreted in the context of its design. As a retrospective secondary analysis of two prospective randomized trials, there may be potential residual confounding factors beyond age and radiation dose. In particular, differences in pre-radiation bladder function such as urinary symptom scores could not be studied in our analysis. Another limitation is this analysis did not include patient reported outcomes, which were not included in the two pooled randomized trials that were initiated two decades ago, and the reliance on physician toxicity grading systems that include medication interventaions may potentially introduce bias (e.g. earlier medical intervention for symptoms in men with intact prostate glands). Additionally, we intentionally excluded patients who received androgen-deprivation therapy from this analysis in order to focus on the effects of radiation dose, which in turn may have biased the results toward increased urinary toxicity in men with an intact prostate in this cohort given the effects of androgen-deprivation on prostate volume. Finally, although the median follow-up was 7.1 years, late urinary toxicity has been shown to have a long latency period and the risk does not appear to level out [18–21]. Thus, further follow-up is required to determine whether there will be a difference in the risk of late toxicity appearing in the second decade after radiation. Finally, the two trials compared were conducted in an era when radiation dose escalation for patients with prostate cancer was not the standard of care.
Recently reported trials of dose escalation to >70 Gy using modern IMRT techniques to treat a more conformal target volume around the intact prostate gland demonstrate lower incidences of acute grade≥2 urinary toxicity than observed in our study. For instance, in RTOG 0126 the incidence of grade≥2 urinary toxicity with dose escalation to 79.2 Gy to the prostate was only ~13% for patients treated with 3D conformal techniques and ~9% for IMRT techniques [22]. A range of incidences of grade≥2 urinary toxicity has been observed in institutional series of dose escalation with IMRT including 6.9% in a Fox Chase Cancer Center series (median dose 79.3 Gy) [23], and up to 22% with higher doses used at Memorial Sloan Kettering (prescription dose 86.4 Gy) [24].
Modern series of IMRT-based post-prostatectomy radiation report a wide range of acute grade≥2 urinary toxicity including 8% in a series from University of California San Diego (median dose 68 Gy) [25], 13.4% in a series from Memorial Sloan Kettering (94% of patients treated to ≥ 70 Gy) [26], and 26% in a series from Ghent University (median dose 74 Gy) [27], which may reflect the range of doses utilized in these studies. Further studies comparing urinary toxicity following primary versus post-prostectomy radiation in the IMRT/3D era with modern, standard high doses radiation may help quantify the proportion of acute urinary toxicity attributable to prostatic urethral injury with modern techniques.
Conclusions
In summary, we demonstrate that there is a higher incidence of acute grade≥2 urinary toxicity in patients treated to similar radiation doses with primary versus post-prostatectomy radiation. Further understanding of the mechanisms that contribute to the symptoms of “urinary toxicity” may be of particular clinical relevance in men who have pre-treatment urinary symptoms secondary to benign prostatic hyperplasia. Radical prostatectomy is often recommended for the men with significant pre-treatment urinary symptoms because external beam radiation can often exacerbate these underlying symptoms. Thus, by understanding the contribution of prostatic urethral injury and prostatic edema to “urinary toxicity” after external beam radiation, we may be able to improve patient care by identifying men who are at high risk of “urinary toxicity” and providing evidence to support the selection of alternative therapies such as radical prostatectomy.
Supplementary Material
Highlights.
Secondary analysis of RTOG protocols 94-08 and 96-01
Compared the incidence of urinary toxicity between primary radiation to an intact prostate versus post-prostatectomy radiation to the prostatic fossa
Grade≥2 acute urinary toxicity was significantly higher after primary prostatic radiation compared to post-prostatectomy radiation (30.8% versus 14.0%), but no difference in late toxicity
A proportion of acute urinary toxicity in men with an intact prostate may be attributable to inflammation of the prostatic gland or urethra.
Acknowledgments
Kathryn Winter, Asha George, and Stephanie Pugh for their assistance in statistical analysis and manuscript review.
“This project was supported by grants U10CA21661, U10CA180868, U10CA18082 from the National Cancer Institute (NCI) and AstraZeneca (RTOG9601).”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Viswanathan AN, Yorke ED, Marks LB, Eifel PJ, Shipley WU. Radiation dose-volume effects of the urinary bladder. International journal of radiation oncology, biology, physics. 2010;76:S116–22. doi: 10.1016/j.ijrobp.2009.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mak RH, Viswanathan AN, Shipley WU. Urinary Bladder ALERT• Adverse Late Effects of Cancer Treatment: Springer Berlin Heidelberg. 2014:465–94. [Google Scholar]
- 3.Zelefsky MJ, Aschkenasy E, Kelsen S, Leibel SA. Tolerance and early outcome results of postprostatectomy three-dimensional conformal radiotherapy. International journal of radiation oncology, biology, physics. 1997;39:327–33. doi: 10.1016/s0360-3016(97)00056-4. [DOI] [PubMed] [Google Scholar]
- 4.Feng M, Hanlon AL, Pisansky TM, Kuban D, Catton CN, Michalski JM, et al. Predictive factors for late genitourinary and gastrointestinal toxicity in patients with prostate cancer treated with adjuvant or salvage radiotherapy. International journal of radiation oncology, biology, physics. 2007;68:1417–23. doi: 10.1016/j.ijrobp.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 5.Wiegel T, Bottke D, Steiner U, Siegmann A, Golz R, Storkel S, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–30. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 6.Bolla M, van Poppel H, Tombal B, Vekemans K, Da Pozzo L, de Reijke TM, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911) Lancet. 2012;380:2018–27. doi: 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- 7.Zelefsky MJ, Leibel SA, Kutcher GJ, Kelson S, Ling CC, Fuks Z. The feasibility of dose escalation with three-dimensional conformal radiotherapy in patients with prostatic carcinoma. Cancer J Sci Am. 1995;1:142–50. [PubMed] [Google Scholar]
- 8.Hanks GE, Schultheiss TE, Hunt MA, Epstein B. Factors influencing incidence of acute grade 2 morbidity in conformal and standard radiation treatment of prostate cancer. International journal of radiation oncology, biology, physics. 1995;31:25–9. doi: 10.1016/0360-3016(94)00366-S. [DOI] [PubMed] [Google Scholar]
- 9.Peeters ST, Heemsbergen WD, van Putten WL, Slot A, Tabak H, Mens JW, et al. Acute and late complications after radiotherapy for prostate cancer: results of a multicenter randomized trial comparing 68 Gy to 78 Gy. International journal of radiation oncology, biology, physics. 2005;61:1019–34. doi: 10.1016/j.ijrobp.2004.07.715. [DOI] [PubMed] [Google Scholar]
- 10.Jones CU, Hunt D, McGowan DG, Amin MB, Chetner MP, Bruner DW, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–18. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 11.Shipley WU, Hunt D, Lukka H, Major P, Heney NM, Grignon D, et al. Initial Report of RTOG 9601: A Phase III Trial in Prostate Cancer: Anti-androgen Therapy (AAT) with Bicalutamide during and after Radiation Therapy (RT) Improves Freedom from Progression and Reduces the Incidence of Metastatic Disease in Patients following Radical Prostatectomy (RP) with pT2–3, N0 Disease, and Elevated PSA Levels. International journal of radiation oncology, biology, physics. 2010;78:S27. [Google Scholar]
- 12.Agresti A. A Categorical Data Analysis. New York: Wiley; 1990. [Google Scholar]
- 13.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 14.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annual Statistics. 1988;16:1141–3. [Google Scholar]
- 15.Lawton CA, Won M, Pilepich MV, Asbell SO, Shipley WU, Hanks GE, et al. Longterm treatment sequelae following external beam irradiation for adenocarcinoma of the prostate: analysis of RTOG studies 7506 and 7706. International journal of radiation oncology, biology, physics. 1991;21:935–9. doi: 10.1016/0360-3016(91)90732-j. [DOI] [PubMed] [Google Scholar]
- 16.Shipley WU, Zietman AL, Hanks GE, Coen JJ, Caplan RJ, Won M, et al. Treatment related sequelae following external beam radiation for prostate cancer: a review with an update in patients with stages T1 and T2 tumor. The Journal of urology. 1994;152:1799–805. doi: 10.1016/s0022-5347(17)32388-1. [DOI] [PubMed] [Google Scholar]
- 17.Cheng JC, Schultheiss TE, Nguyen KH, Wong JY. Acute toxicity in definitive versus postprostatectomy image-guided radiotherapy for prostate cancer. International journal of radiation oncology, biology, physics. 2008;71:351–7. doi: 10.1016/j.ijrobp.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 18.Marks LB, Carroll PR, Dugan TC, Anscher MS. The response of the urinary bladder, urethra, and ureter to radiation and chemotherapy. International journal of radiation oncology, biology, physics. 1995;31:1257–80. doi: 10.1016/0360-3016(94)00431-J. [DOI] [PubMed] [Google Scholar]
- 19.Gardner BG, Zietman AL, Shipley WU, Skowronski UE, McManus P. Late normal tissue sequelae in the second decade after high dose radiation therapy with combined photons and conformal protons for locally advanced prostate cancer. The Journal of urology. 2002;167:123–6. [PubMed] [Google Scholar]
- 20.Zelefsky MJ, Cowen D, Fuks Z, Shike M, Burman C, Jackson A, et al. Long term tolerance of high dose three-dimensional conformal radiotherapy in patients with localized prostate carcinoma. Cancer. 1999;85:2460–8. doi: 10.1002/(sici)1097-0142(19990601)85:11<2460::aid-cncr23>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 21.Eifel PJ, Levenback C, Wharton JT, Oswald MJ. Time course and incidence of late complications in patients treated with radiation therapy for FIGO stage IB carcinoma of the uterine cervix. International journal of radiation oncology, biology, physics. 1995;32:1289–300. doi: 10.1016/0360-3016(95)00118-I. [DOI] [PubMed] [Google Scholar]
- 22.Michalski JM, Yan Y, Watkins-Bruner D, Bosch WR, Winter K, Galvin JM, et al. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. International journal of radiation oncology, biology, physics. 2013;87:932–8. doi: 10.1016/j.ijrobp.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eade TN, Guo L, Forde E, Vaux K, Vass J, Hunt P, et al. Image-guided dose-escalated intensity-modulated radiation therapy for prostate cancer: treating to doses beyond 78 Gy. BJU Int. 2012;109:1655–60. doi: 10.1111/j.1464-410X.2011.10668.x. [DOI] [PubMed] [Google Scholar]
- 24.Cahlon O, Zelefsky MJ, Shippy A, Chan H, Fuks Z, Yamada Y, et al. Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: toxicity and biochemical outcomes. International journal of radiation oncology, biology, physics. 2008;71:330–7. doi: 10.1016/j.ijrobp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Nath SK, Sandhu AP, Rose BS, Simpson DR, Nobiensky PD, Wang JZ, et al. Toxicity analysis of postoperative image-guided intensity-modulated radiotherapy for prostate cancer. International journal of radiation oncology, biology, physics. 2010;78:435–41. doi: 10.1016/j.ijrobp.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Goenka A, Magsanoc JM, Pei X, Schechter M, Kollmeier M, Cox B, et al. Improved toxicity profile following high-dose postprostatectomy salvage radiation therapy with intensity-modulated radiation therapy. Eur Urol. 2011;60:1142–8. doi: 10.1016/j.eururo.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Ost P, Fonteyne V, Villeirs G, Lumen N, Oosterlinck W, De Meerleer G. Adjuvant high-dose intensity-modulated radiotherapy after radical prostatectomy for prostate cancer: clinical results in 104 patients. Eur Urol. 2009;56:669–75. doi: 10.1016/j.eururo.2009.05.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


