Summary
The hallmark of sickle cell disease is the polymerization of sickle haemoglobin due to a point mutation in the β-globin gene (HBB). Under low oxygen saturation, sickle haemoglobin assumes the tense (T-state) deoxygenated conformation that can form polymers, leading to rigid erythrocytes with impaired blood vessel transit, compounded or initiated by adhesion of erythrocytes to endothelium, neutrophils and platelets. This process results in vessel occlusion and ischaemia, with consequent acute pain, chronic organ damage, morbidity and mortality. Pharmacological agents that stabilize the higher oxygen affinity relaxed state (R-state) and/or destabilize the lower oxygen affinity T-state of haemoglobin have the potential to delay the sickling of circulating red cells by slowing polymerization kinetics. Relevant classes of agents include aromatic aldehydes, thiol derivatives, isothiocyanates and acyl salicylates derivatives. The aromatic aldehyde, 5-hydroxymethylfurfural (5-HMF) increases oxygen affinity of sickle haemoglobin and reduces hypoxia-induced sickling in vitro and protects sickle cell mice from effects of hypoxia. It has completed pre-clinical testing and has entered clinical trials as treatment for sickle cell disease. A related molecule, GBT440, has shown R-state stabilization and increased oxygen affinity in preclinical testing. Allosteric modifiers of haemoglobin as direct anti-sickling agents target the fundamental pathophysiological mechanism of sickle cell disease.
Keywords: Sickle cell, 5-HMF, Anti-sickling, Haemoglobin allosteric effectors, GBT440, vanillin, TD-1
Oxygen Affinity of Sickle Erythrocytes
Sickle haemoglobin (Hb S) has significantly reduced oxygen affinity as compared to normal haemoglobin (Riggs & Wells, 1961; Charache et al, 1970). As indicated by the oxygen equilibrium curve (OEC), this reduction is partly due to an increase in the intracellular concentration of 2,3-diphosphogyclerate (2,3-DPG) in erythrocytes (MacDonald, 1977). 2,3-DPG is an intracellular glycolytic intermediate that controls the rate at which haemoglobin releases oxygen to the tissues. Binding of 2,3-DPG to the β-cleft of Hb S promotes polymerization by stabilizing the low-affinity tense state (T-state), which is more prone to polymerize. Decreased oxygen affinity is also reflected as an increase in the partial pressure of oxygen required to produce 50% oxygen saturation (P50) (MacDonald, 1977). P50 is a measure of oxygen affinity and its increase is described by the Bohr effect (MacDonald, 1977). P50 and 2,3-DPG levels vary widely among patients with sickle cell disease (SCD), but elevated levels appear to decrease Hb S solubility (Poillon et al, 1985, 1986; Poillon & Kim, 1990) and increase red cell sickling under hypoxia (Rogers et al, 2013; Jensen, 2009), although this has not confirmed by all investigators (Beutler et al, 1971; Swerdlow et al, 1977).
The Allosteric State of Haemoglobin and Sickle Cell Disease
Normal functional adult haemoglobin (Hb) is composed of 2 α- and 2 β-globin chains (α1β1, α2β2) arranged around a 2-fold axis of symmetry to form a central water cavity with the α-cleft and β-cleft defining entries into the cavity (Thomas & Lumb, 2012; Safo et al, 2011). It exists in dynamic equilibrium between a tense (T) state and a relaxed (R) state. Allosteric transitions (T ↔ R) occur when one state is preferentially stabilized over the other. The T → R allosteric transition is characterized by rotation of the α1β1 dimer relative to the α2β2 dimer, which significantly reshapes the central water cavity resulting in several differences between the quaternary T and R structures (Safo et al, 2011). The classical deoxygenated T-state and liganded R-state structures were used to validate the two-state Monod-Wyman-Changeux (MWC) allosteric model (Safo et al, 2011). Since then, liganded Hb has been shown to exist as an ensemble of states, including the classical R, R2, R3, RR2, RR3, etc., each with a distinct relaxed quaternary conformation (Safo et al, 2011; Jenkins et al, 2009). The term “R-state” is used in this article to represent the ensemble of relaxed Hb conformations.
Allosteric effectors of Hb preferentially bind to the surface, α-cleft, β-cleft or the middle of the central water cavity of T-state or one or multiple of the R-states to modulate Hb allosteric activity (Safo et al, 2011). Stabilization of the R-state produces a high-affinity Hb that more readily binds oxygen, shifting the OEC to the left. Stabilization of the T-state also leads to a right-shift of the OEC, resulting in a low-affinity Hb that readily releases oxygen. For example, increased endogenous H+ or 2,3-DPG concentration favours the T-conformation with concomitant increased release of oxygen (Thomas & Lumb, 2012). Loading red blood cells (RBCs) with an allosteric effector of Hb can reduce RBC sickling.
Allosteric Effectors of Haemoglobin as Potential Anti-sickling agents
Various synthetic allosteric effectors have also been identified that affect the oxygen affinity of haemoglobin. Historically, several have been studied as potential antisickling candidate drugs, based on the combination of known haemoglobin allosteric properties and the early observations of chemical modification of haemoglobin by glucose in the blood (Bookchin and Gallop 1968, Holmquist and Schroeder 1966). In-vitro proof-of-concept studies confirmed this observation (Abdella, et al 1977, Bunn, et al 1975, Haney and Bunn 1976), and helped lay the foundation for subsequent investigations on the potential of this approach to counter sickling of SS RBCs. Initial studies by Zaugg et al (1977) established that when incubated with SS RBCs, certain carbonyl compounds, including vanillin and its analogues, formed Schiff-base adducts with Hb S and increased the oxygen affinity. Several follow-up studies, in vitro, in healthy volunteers or in SCD patients, established this principle using experimental molecules (Beddell, et al, 1984, Fitzharris et al, 1985, Keidan et al, 1986 & 1989, Merrett et al, 1986, Abraham et al, 1991). Tucaresol, a newer synthetic molecule, was also extensively studied (Rolan et al, 1993, 1995; Arya et al, 1996). While ultimately none of the studies resulted in a clinically useful antisickling drug, they established a firm principle and foundation for future follow-up investigations, and provided insights to the unique challenges that hamper this approach. Subsequent, more recent studies have investigated newer compounds: 5-hydroxymethylfurfural (5-HMF), pyridyl derivatives of vanillin, GBT440 and triazol sulfide, the findings from which are summarized in this review.
Development of Allosteric Modifiers of Haemoglobin to Treat Sickle Cell Disease
5-Hydroxymethylfurfural
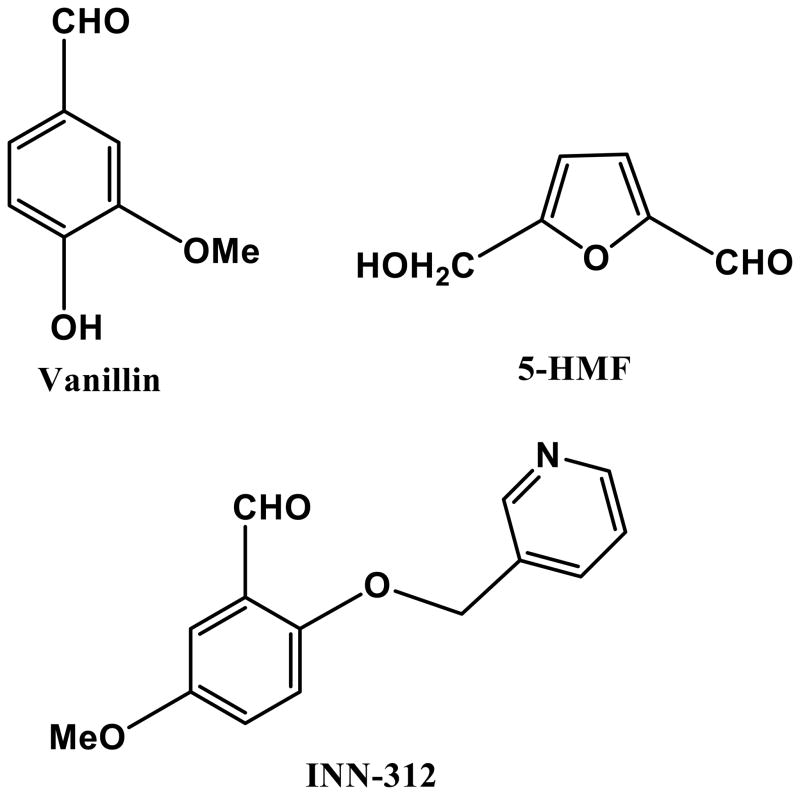
5-hydroxymethylfurfural (5-HMF; Figure 1), furfural (FUF), 5-methyl-2-furfural (5-MF), and 5-ethyl-2-furfural (5-EF) are naturally-occurring, analogous 5-membered heterocyclic aldehydes that were investigated, along with the previously known anti-sickling agent (vanillin; Figure 1) for their haemoglobin modification properties, which would translate to increased oxygen affinity, and consequently, sickling inhibition properties (Safo et al, 2004; Safo et al, 2011). The results of detailed structural studies (complexed with haemoglobin) and preliminary biochemical studies suggested that all of the compounds exhibited superior properties to vanillin, and furthermore established 5-HMF as the most potent and promising. Currently, 5-HMF (Aes-103; Bax-555) is a clinical research stage anti-sickling agent. It binds to the N-terminal valine (and possibly lysine) residues of the α-globin chains of Hb S, forming a Schiff-base adduct which stabilizes the R-state and/or destabilizes the T-state (Figure 1), shifting the OEC to the left and increasing haemoglobin oxygen affinity (Abdulmalik et al, 2005; Safo et al, 2004). 5-HMF increases oxygen affinity in sickle erythrocytes and inhibits hypoxia-induced sickling in a concentration-dependent manner (Abdulmalik et al, 2005); this effect is augmented when combined with hydroxycarbamide (Stern et al, 2012). 5-HMF had no detectable adverse effects on erythrocytes; Hb S incubated with 5-HMF did not cause haemolysis, oxidation or denaturation (Abdulmalik et al, 2005); in fact, 5-HMF inhibited haemolysis under shear stress in vitro (Mendelsohn et al, 2013). Plasma and tissue proteins do not appear to inhibit binding of 5-HMF in Hb S and 5-HMF does not appear to bind with serum albumin, myoglobin, or immunoglobulins (Abdulmalik et al, 2005). In vivo, it protects sickle mice against hypoxia-induced death (Abdulmalik et al, 2005). Single oral doses of 5-HMF given to healthy normal volunteers were well-tolerated, rapidly absorbed, and preferentially taken up into RBCs relative to plasma (Stern et al, 2012; Kato et al, 2013; Mendelsohn et al, 2013). Similarly, in a phase 1, double-blind, placebo-controlled, dose-escalation trial in adult patients with sickle cell anaemia, Aes-103 was safely tolerated without severe or recurrently observed complications over a 13-fold range of oral doses (Kato et al, 2013).
Figure 1.
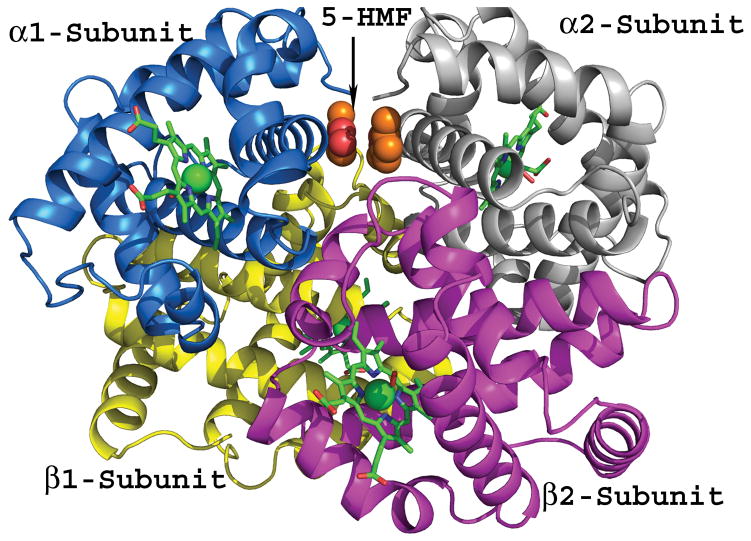
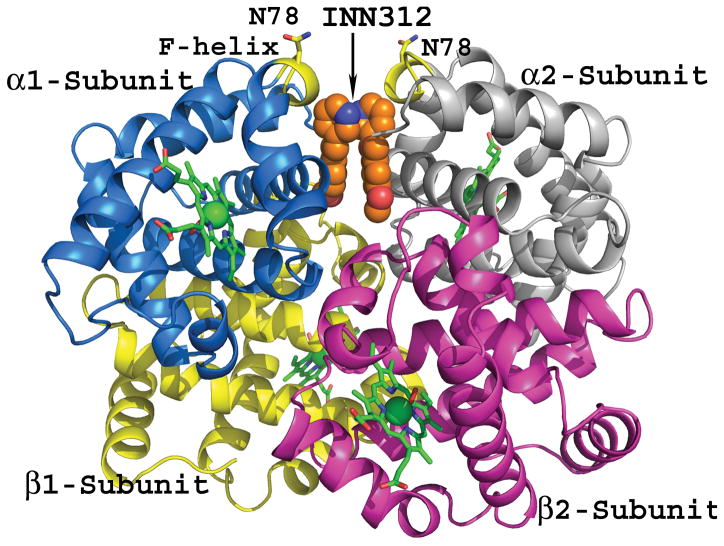
Chemical structures of aromatic aldehydes and their complexes with liganded haemoglobin. (A) Structures of aromatic aldehydes. (B) Binding of 5-hydroxymethylfurfural (5-HMF; orange) in a symmetry-related fashion at the α-cleft of liganded Hb, and through a series of inter-subunit hydrogen-bond and/or hydrophobic interactions, stabilize the R-state conformation. (C) Binding of a pyridyl derivative of vanillin, INN-312 (orange) in a symmetry-related fashion at the α-cleft of liganded Hb, which leads to stabilization of the relaxed state conformation. Additionally, the pyridine moiety of INN-312 makes hydrophobic interactions with the F-helix, perturbing the inter-strand polymer contact involving Asn78, and contributing to the anti-sickling activity of the compound.
Reports of multiple, independent investigations on 5-HMF have suggested additional benefits, either directly in SCD, or in related (or non-related) disorders. For example, 5-HMF has been shown to prevent dehydration of sickle RBCs during deoxygenation, inhibiting two of the main cation pathways that contribute to dehydration, the deoxygenation-induced cation conductance (Psickle) and the Gardos channel (Hannemann, et al 2014). Another, more recent study reported that 5-HMF dose-dependently ameliorated hypoxia-induced veno-occlusive crisis decrease in microvascular liver perfusion in the Townes mouse model of SCD (Wright et al, 2015). Additionally, Fens et al (2011) reported that 5-HMF increased the capacity of RBCs to generate nitric oxide to promote vasodilation and blood flow, as a hypothetical, added benefit to reducing the rate of Hb polymerization. Two other studies in 2011 demonstrated that 5-HMF markedly increases survival of wild-type mice under hypoxic stress by increasing blood oxygen levels (SpO2)(Li et al, 2011a); and attenuates late stage hypoxia-induced cell necrosis and apoptosis in treated ECV304 cells (Li et al, 2011b). Treatment with 5-HMF improved microvascular function during resuscitation from haemorrhagic shock in a hamster window chamber model (Villela et al, 2009), provided haemodynamics and oxygenation benefits during hypoxia: maintenance of blood pressure and heart rate; preservation of microvascular blood flow; threefold increase in perivascular pO2; and a reduction in heart and brain hypoxia areas in mice (Yalcin & Cabrales, 2012). 5-HMF has also been shown to protect from oxidative stress and provide broad antioxidant effects, as evidenced by scavenging free-radical species, reduction of reactive oxidant species and membrane protein oxidation, as well as upregulation of genes implicated in enzymatic antioxidant defence and DNA repair (Li et al, 2009; Zhao et al, 2013).
Pyridyl Derivatives of Vanillin
Based on the original investigations on the anti-sickling properties of two attractive food-based and non-toxic chemotypes, vanillin and 5-HMF (Abdulmalik et al, 2005; Abraham et al, 1991), Safo et al designed and synthesized several novel derivatives of vanillin (designated International Nonproprietary Name [INN] derivatives e.g. INN-312; Figure 1) that exhibited significantly enhanced potency (Abdulmalik et al, 2011; Nnamani et al, 2008). This generation of compounds, in addition to the primary mode of action (i.e., increased oxygen affinity of Hb), also directly destabilized polymer contacts by making hydrophobic contact with the surface located F-helix on Hb (Figure 1) (Abdulmalik et al, 2011). The αF-helix – residue Asn78 in particular – has been shown to be critical in polymer stabilization, as exemplified by the Hb variant Stanleyville (αAsn78 ↔ αLsy78), which inhibits Hb S gelation (Bunn & Forget, 1986). Further structural modifications of the INN compounds have led to the development of a third generation of anti-sickling agents (TD derivatives) that remarkably showed pharmacological properties superior to the INN compounds in vitro, particularly, a sustained duration of action and enhanced anti-polymerization properties at significantly lower doses (Abdulmalik et al, 2014). A representative of this group of compounds, TD-7 is currently undergoing preclinical investigations.
GBT440
The proof of concept piloted by 5-HMF has led to investigations by researchers in both academia and industry on other molecules that share the same general mechanism of action. One series of compounds includes GTx011 (or GBT440). Although publicly reported details in the literature are limited, GTx011 also appears to be an orally bioavailable small molecule that modifies haemoglobin oxygen affinity by binding to the N-terminal α-chain of Hb and forming a reversible Schiff base (Hutchaleelaha et al, 2015). Binding of the compound is also proposed to allosterically influence the intra-dimer interface of Hb (Hisα122 and Hisα103) and the distal valine surrounding haem pockets of both the α and β chains (Patel et al, 2014). This mechanism forms a solution phase structure that improves oxygen affinity without sterically blocking the release of oxygen (Patel et al, 2014).
GTx011 dose-dependently inhibits in vitro Hb S polymerization by maintaining a fraction of oxygenated Hb S under hypoxic conditions (Patel et al, 2014; Dufu et al, 2013, 2014). Modifying Hb S by 10–30% was sufficient to achieve an improvement in blood hyperviscosity; 300 μM concentration in whole blood was sufficient to prevent cell sickling (Patel et al, 2014). GTx011 elicits a two-fold improvement in Hb oxygen affinity even at substoichiometric concentrations (GTx011:Hb 1:3) (Patel et al, 2013). Townes’ sickle mice chronically dosed with GTx011 exhibited a prolongation of RBC half-life from 2.4 days to 3.8 days, along with a marked decrease in reticulocyte count, suggesting decreased haemolysis (Patel et al, 2014).
Renamed GBT440, this novel small molecule Hb modifier has been subjected to further investigations (Lehrer-Graiwer et al, 2015). It has been reported to be a potent and direct anti-sickling agent with high specificity for Hb; estimated Hb modification of 10–30% was safe in animal studies and effective at preventing Hb S polymerization. Pharmacokinetics studies conducted in 4 animal species (mouse, rat, dog, monkey) demonstrated that GBT440 is well absorbed following IV and oral administration, quickly partitions in to the RBC with a small fraction re-distributed into the plasma (Hutchaleelaha et al, 2015). In male rats, it distributes into Hb, blood, spleen, liver and bone marrow (Hutchaleelaha et al, 2015). Despite its high affinity binding toward Hb, it could be completely released and eliminated in faeces and urine; the major route of elimination was via both Phase I and Phase II pathways (Hutchaleelaha et al, 2015). There is good correlation between blood concentration and changes in P50, eliciting an ex vivo dose-dependent increase in Hb oxygen affinity following increasing dosage in mice (Hutchaleelaha et al, 2015). In a prospective, randomized, placebo-controlled, double blind, parallel group phase I/II study in healthy volunteers and SCD patients, GBT440 was well tolerated across a wide dose range and demonstrated dose proportional and predictable pharmacokinetics and pharmacodynamics (Lehrer-Graiwer et al, 2015). Most adverse events were mild and there were no deaths or adverse events related to tissue hypoxia. GBT440 showed a dose-dependent increase in Hb oxygen affinity without causing tissue hypoxia. In SCD patients, it rapidly reduced RBC haemolysis and reportedly improved oxygen delivery to tissues. A phase 1 study is currently underway to investigate the absorption, metabolism, and excretion of GBT440 after establishment of steady state in healthy male subjects (clinicaltrials.gov, NCT02497924).
Triazole Sulfide
Sequential high-throughput screening of small molecules based, first on their binding properties with Hb, and then on their propensity to modulate oxygen affinity, identified a novel allosteric effector of Hb. This novel thiol molecule, di(5-(2,3-dihydro-1,4-benzodioxin-2-yl)-4H-1,2,4-triazol-3-yl) disulfide (triazole disulfide; TD-1) induced a greater increase in oxygen affinity than 5-HMF, N-ethylmaleimide, or diformamidine disulfide (Nakagawa et al, 2014). Importantly, lower concentrations of TD-1 (2 mM) are required for near complete inhibition of hypoxia-induced erythrocyte sickling in vitro. Structural analysis indicated that TD-1 binds covalently to βCys93 and βCys112, as well as non-covalently to the central water cavity of the Hb tetramer, stabilizing the relaxed (R3) state), and, by sterically preventing the salt-bridge interaction between βHis146 and βAsp94, destabilizing the T-state (Nakagawa et al, 2014). Additionally, the triazole ring lends high reactivity for covalent binding to Hb, so the sum of the interactions may produce more sustained increases in oxygen affinity than other non-covalently bound allosteric effectors (Nakagawa et al, 2014). This may be of critical importance in vivo when dosing regimens are considered, as potentially less frequent dosing may elicit desirable therapeutic effects. In vitro, TD-1 dose-dependently shifts the OEC to the left, markedly reducing P50 even when the molar ratio of compound to Hb was 1:1. These effects were conserved in both intact RBCs and Hb lysates, further validating specificity for Hb that was observed during the initial high-throughput screening (Nakagawa et al, 2014). Remarkably, no adverse effects on RBCs or Hb were observed at the effective concentrations. Studies are currently ongoing to test these findings in an animal model. Based on the preliminary data and multiple modes of eliciting anti-sickling effects, positive in vivo findings would make TD-1 a very promising therapeutic candidate for SCD, warranting further detailed investigations, and possibly human clinical trials.
Challenges to Development of Anti-sickling Agents
Stoichiometry of Binding Sites
Scientific and logistical hurdles have slowed the development of anti-sickling agents. The binding target of the agents, haemoglobin, exists in far greater number in the body (250 million molecules per RBC) than the targets of other conventional drugs, such as enzymes or cell surface receptors (thousands per cell). This means that saturation of haemoglobin with any drug requires high concentrations, and this partly explains the millimolar concentrations that are optimal for activity of 5-HMF in vitro (Abdulmalik, et al., 2005). This has dampened the enthusiasm of many researchers and funding agencies. Conceptually, sickle haemoglobin polymerization is a kinetic process, and slowing the kinetics even slightly can have a big effect upon sickling (Ferrone, 2015). 5-HMF has now been a promising lead drug that has provided some proof of principle, and the newer generation 5-HMF analogues appear to have greater potency and longer half-life (Lehrer-Graiwer et al., 2015; Omar et al., 2015). In contemporary drug development approaches, such optimization from promising lead drugs is much more rapid than in years past (Hughes et al., 2011).
The Effects of Funding and Business Priorities
Historically, large, well-funded pharmaceutical companies have not focused on drug development in rare diseases, such as SCD. Academic researchers and small pharmaceutical businesses depended upon often-lean government funding, and one small company developing 5-HMF went bankrupt (Perampaladas et al., 2010), idling its commercial drug development for many years. Recent years have seen a dramatic increase in commercial interest in developing drugs for rare diseases (Gibson at al., 2015), and SCD is a beneficiary of this interest. In this new business climate, anti-sickling drugs are garnering renewed attention and robust commercial industry activity from both start-up and large pharmaceutical companies.
Conclusions
Although polymerization of Hb S is the fundamental pathology in SCD, the development of agents to counter this process has remained challenging for decades, despite significant efforts. The chief challenge largely remains the high concentration of circulating intracellular pathological Hb S present in patients, perhaps requiring high (millimolar) concentrations of allosteric modifiers to elicit direct therapeutic benefits. Consequently, efficacious doses may be hard to achieve and/or sustained. This has been further complicated by the fact that the majority of candidate molecules exhibit short plasma/blood half-life values. Emerging data from targeted drug design has led to an overall reduction in theoretical doses required to mitigate disease pathophysiology under experimental conditions (Abdulmalik et al, 2011); while the most recent reports on GBT440 suggests a significantly improved (2-fold reduction) drug:Hb stoichiometry, as well as superior partitioning of the drug into the RBC compartment (Hutchaleelaha, et al, 2015; Lehrer-Graiwer, et al, 2015), further lowering the potential efficacious doses. Another important question that continues to confound investigators developing pharmacological therapies – as well as those developing gene therapy and transplantation approaches for SCD – is the threshold of candidate therapeutics required to mitigate disease pathophysiology. We believe outcomes of the novel, ongoing diverse lines of investigations will provide valuable actionable information that may ultimately lead to a universally acceptable (patient- or sub-phenotype-specific) algorithm. Despite these challenges, recent increased government support, expanded advocacy and a shifting business climate for pharmaceutical companies have collectively accelerated the entire drug development process for SCD (Gibson et al, 2015). Additionally, improvements in drug design, synthesis and novel screening methodologies, as well as encouraging pilot studies on anti-polymerization compounds, have moved the field forward and led to greater scientific and business competitiveness. The prospects for new drugs for patients with SCD grow significantly stronger each year.
Acknowledgments
M.K. Safo gratefully acknowledges research support from the Virginia Commonwealth University Presidential Research Initiative Program Award and NIH/NIMHD grant MD009124. The structural biology resources were provided in part by the National Cancer Institute to the VCU Massey Cancer Center (CA 16059-28). During the phase-1 clinical trials of 5-HMF, G.J. Kato received research support from the National Heart, Lung and Blood Institute Division of Intramural Research (1-ZIA-HL006149) with additional project support from the National Center for Advancing Translational Sciences Therapeutics for Rare and Neglected Diseases Program (1-ZIB-TR000002-01). He acknowledges current funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health (1 R01 HL121386, 1 R01 MD009162), and the Hemostasis and Vascular Biology Research Institute. O. Abdulmalik gratefully acknowledges support from the National Institutes of Health (K01HL103186, and R01DK084188).
Footnotes
Author contributions
All authors directly contributed to the writing and editing of this manuscript.
Competing Interests
Dr M.K. Safo is a co-owner of a patent for the use of 5-HMF in sickle cell disease, and receives research funding from AesRx, LLC, a licensee for 5-HMF (Aes-103/Bax-555). Dr G.J. Kato has collaborated with and received research funding from AesRx, LLC, through a Clinical Trials Agreement between AesRx, LLC and the National Heart, Lung and Blood Institute, and has received consulting fees from Baxalta, current holder of the license for 5-HMF (Aes-103/Bax-555), and research funding from Bayer HealthCare Pharmaceuticals Inc.
References
- Abdella PM, Ritchey JM, Tam JW, Klotz IM. Glycosylation of hemoglobin S by reducing sugars and its effect on gelation. Biochim Biophys Acta. 1977;490:462–70. doi: 10.1016/0005-2795(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Abdulmalik O, Safo MK, Chen Q, Yang J, Brugnara C, Ohene-Frempong K, Abraham DJ, Asakura T. 5-hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. Br J Haematol. 2005;128:552–561. doi: 10.1111/j.1365-2141.2004.05332.x. [DOI] [PubMed] [Google Scholar]
- Abdulmalik O, Ghatge MS, Musayev FN, Parikh A, Chen Q, Yang J, Nnamani I, Danso-Danquah R, Eseonu DN, Asakura T, Abraham DJ, Venitz J, Safo MK. Crystallographic analysis of human hemoglobin elucidates the structural basis of the potent and dual antisickling activity of pyridyl derivatives of vanillin. Acta Crystallographica. Section D, Biological Crystallography. 2011;67:920–928. doi: 10.1107/S0907444911036353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulmalik O, Deshpande T, Ghatge M, Zhang Y, Venitz J, Parikh A, Safo MK. Novel structurally-modified allosteric effectors of hemoglobin exhibit superior antisickling properties. Blood. 2014;124:218. [Google Scholar]
- Abraham DJ, Mehanna AS, Wireko FC, Whitney J, Thomas RP, Orringer EP. Vanillin, a potential agent for the treatment of sickle cell anemia. Blood. 1991;77:1334–1341. [PubMed] [Google Scholar]
- Arya R, Rolan PE, Wootton R, Posner J, Bellingham AJ. Tucaresol increases oxygen affinity and reduces haemolysis in subjects with sickle cell anaemia. Br J Haematol. 1996;93:817–21. doi: 10.1046/j.1365-2141.1996.d01-1744.x. [DOI] [PubMed] [Google Scholar]
- Beddell CR, Goodford PJ, Kneen G, White RD, Wilkinson S, Wootton R. Substituted benzaldehydes designed to increase the oxygen affinity of human haemoglobin and inhibit the sickling of sickle erythrocytes. Br J Pharmacol. 1984;82:397–407. doi: 10.1111/j.1476-5381.1984.tb10775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E, Paniker NV, West C. The effect of 2,3-DPG on the sickling phenomenon. Blood. 1971;37:184–186. [PubMed] [Google Scholar]
- Bookchin RM, Gallop PM. Structure of hemoglobin AIc: nature of the N-terminal beta chain blocking group. Biochem Biophys Res Commun. 1968;32:86–93. doi: 10.1016/0006-291x(68)90430-0. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Forget GB. Hemoglobin: Molecular, Genetic and Clinical Aspects. W. B. Saunders Company; Philadelphia, PA: 1986. p. 462. [Google Scholar]
- Bunn HF, Haney DN, Gabbay KH, Gallop PM. Further identification of the nature and linkage of the carbohydrate in hemoglobin A1c. Biochem Biophys Res Commun. 1975;67:103–9. doi: 10.1016/0006-291x(75)90289-2. [DOI] [PubMed] [Google Scholar]
- Charache S, Grisolia S, Fiedler AJ, Hellegers AE. Effect of 2,3-diphosphoglycerate on oxygen affinity of blood in sickle cell anemia. J Clin Invest. 1970;49:806–812. doi: 10.1172/JCI106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufu K, Oksenberg D, Metcalf B, Sinha U. GTx011, a potent allosteric modifier of hemoglobin oxygen affinity, delays polymerization and prevents sickling. Blood. 2013;122:316. [Google Scholar]
- Dufu K, Oksenberg D, Zhou C, Hutchaleelaha A, Archer DR. GTx011, a potent allosteric modifier of hemoglobin oxygen afifinty, prevents RBC sickling in whole blood and prolongs RBC half-life in vivo in a murine model of sickle cell disease. Blood. 2014;124:217. [Google Scholar]
- Fens M, Larkin SK, Morris CR, Fitch B, Scicinski J, Oronsky B, Kuypers FA. NO or no NO, increased reduction of nitrite to nitric oxide by modified red blood cells. Blood. 2011;118:2125. [Google Scholar]
- Ferrone FA. The delay time in sickle cell disease after 40 years: A paradigm assessed. Am J Hematol. 2015;90:438–45. doi: 10.1002/ajh.23958. [DOI] [PubMed] [Google Scholar]
- Fitzharris P, McLean AE, Sparks RG, Weatherly BC, White RD, Wootton R. The effects in volunteers of BW12C, a compound designed to left-shift the blood-oxygen saturation curve. Br J Clin Pharmacol. 1985;19:471–81. doi: 10.1111/j.1365-2125.1985.tb02672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S, Raziee HR, Lemmens T. Why the Shift? Taking a Closer Look at the Growing Interest in Niche Markets and Personalized Medicine. World Med Health Policy. 2015;7:3–27. doi: 10.1002/wmh3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney DN, Bunn HF. Glycosylation of hemoglobin in vitro: affinity labeling of hemoglobin by glucose-6-phosphate. Proc Natl Acad Sci U S A. 1976;73:3534–8. doi: 10.1073/pnas.73.10.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannemann A, Cytlak UM, Rees DC, Tewari S, Gibson JS. Effects of 5-hydroxymethyl-2-furfural on the volume and membrane permeability of red blood cells from patients with sickle cell disease. J Physiol. 2014;592:4039–4049. doi: 10.1113/jphysiol.2014.277681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist WR, Schroeder WA. A new N-terminal blocking group involving a Schiff base in hemoglobin AIc. Biochemistry. 1966;5:2489–503. doi: 10.1021/bi00872a002. [DOI] [PubMed] [Google Scholar]
- Hughes JP, Rees S, Kalindjian SB, Philpott KL. Principles of early drug discovery. Br J Pharmacol. 2011;162:1239–49. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchaleelaha A, Patel M, Silva A, Oksenberg D, Metcalf B. GBT440 demonstrates high specificity for red blood cells in nonclinical species. Blood. 2015;126:2172. [Google Scholar]
- Jenkins JD, Musayev FN, Danso-Danquah R, Abraham DJ, Safo MK. Structure of relaxed-state human hemoglobin: insight into ligand uptake, transport and release. Acta Crystallographica. Section D, Structural Biology. 2009;65:41–48. doi: 10.1107/S0907444908037256. [DOI] [PubMed] [Google Scholar]
- Jensen FB. The dual roles of red blood cells in tissue oxygen delivery: oxygen carriers and regulators of local blood flow. J Exp Biol. 2009;212:3387–3393. doi: 10.1242/jeb.023697. [DOI] [PubMed] [Google Scholar]
- Kato GJ, Lawrence MP, Mendelsohn LG, Saiyed R, Wang X, Conrey AK, Starling JM, Grimes G, Taylor JG, McKew J, Minniti CP, Stern W. Phase 1 clinical trial of the candidate anti-sickling agent Aes-103 in adults with sickle cell anemia. Blood. 2013;122:1009. [Google Scholar]
- Keidan AJ, Franklin IM, White RD, Joy M, Huehns ER, Stuart J. Effect of BW12C on oxygen affinity of haemoglobin in sickle-cell disease. Lancet. 1986;1:831–4. doi: 10.1016/s0140-6736(86)90941-4. [DOI] [PubMed] [Google Scholar]
- Lehrer-Graiwer J, Howard J, Hemmaway CJ, Awogbade M, Telfer P, Layton M, Mant T, Dufu K, Hutchaleelaha A, Koller T, Oksenberg D, Patel M, Ramos E. GBT440, a potent anti-sickling hemoglobin modifier reduces hemolysis, improves anemia and nearly eliminates sickle cells in peripheral blood of patients with sickle cell disease. Blood. 2015;126:542. [Google Scholar]
- Li MM, Wu LY, Zhao T, Wu KW, Xiong L, Zhu LL, Fan M. The protective role of 5-hydroxymethyl-2-furfural (5-HMF) against acute hypobaric hypoxia. Cell Stress Chaperones. 2011a;16:529–537. doi: 10.1007/s12192-011-0264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MM, Wu LY, Zhao T, Xiong L, Huang X, Liu ZH, Fan XL, Xiao CR, Gao Y, Ma YB, Chen JJ, Zhu LL, Fan M. The protective role of 5-HMF against hypoxic injury. Cell Stress Chaperones. 2011b;16:267–273. doi: 10.1007/s12192-010-0238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YX, Li Y, Qian ZJ, Kim MM, Kim SK. In vitro antioxidant activity of 5-HMF isolated from marine red alga Laurencia undulata in free-radical-mediated oxidative systems. J Microbiol Biotechnol. 2009;19:1319–1327. doi: 10.4014/jmb.0901.00004. [DOI] [PubMed] [Google Scholar]
- MacDonald R. Red cell 2,3-diphosphoglycerate and oxygen affinity. Anaesthesia. 1977;32:544–553. doi: 10.1111/j.1365-2044.1977.tb10002.x. [DOI] [PubMed] [Google Scholar]
- Mendelsohn LG, Pedoeim L, Wang YK, Saiyed R, Brantner CA, Daniels MP, Nichols JS, Wang X, van Beers EJ, Kato GJ. The anti-sickling agent Aes-103 decreases sickle erythrocyte fragility, hypoxia-induced sickling and hemolysis in vitro. Blood. 2013;122:940. [Google Scholar]
- Merrett M, Stammers DK, White RD, Wootton R, Kneen G. Characterization of the binding of the anti-sickling compound, BW12C, to haemoglobin. Biochem J. 1986;239:387–92. doi: 10.1042/bj2390387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa A, Lui FE, Wassaf D, Yefidoff-Freedman R, Casalena D, Palmer MA, Meadows J, Mozzarelli A, Ronda L, Abdulmalik O, Bloch KD, Safo MK, Zapol WM. Identification of a small molecule that increases hemoglobin oxygen affinity and reduces SS erythrocyte sickling. ACS Chem Biol. 2014;9:2318–2325. doi: 10.1021/cb500230b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnamani IN, Joshi GS, Danso-Danquah R, Abdulmalik O, Asakura T, Abraham DJ, Safo MK. Pyridyl Derivatives of Benzaldehyde as Potential Antisickling Agents. Chem Biodiv. 2008;5:1762–1769. doi: 10.1002/cbdv.200890165. [DOI] [PubMed] [Google Scholar]
- Omar AM, Mahran MA, Ghatge MS, Chowdhury N, Bamane FH, El-Araby ME, Abdulmalik O, Safo MK. Identification of a novel class of covalent modifiers of hemoglobin as potential antisickling agents. Org Biomol Chem. 2015;13:6353–70. doi: 10.1039/c5ob00367a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Oksenberg D, Silva A, Betz A, Metcalf B, Sinha U. GTx011, a novel agent that improves rheological properties of sickle cell blood by increasing oxygen affinity for hemoglobin. Blood. 2013;122:2207. [Google Scholar]
- Patel M, Cabrales P, Dufu K, Metcalf B, Sinha U. GTx011, an anti-sickling compound, improves SS blood rheology by reduction of Hb S polymerization via allosteric modulation of O2 affinity. Blood. 2014;124:1370. [Google Scholar]
- Perampaladas K, Masum H, Kapoor A, Shah R, Daar AS, Singer PA. The road to commercialization in Africa: lessons from developing the sickle-cell drug Niprisan. BMC Int Health Hum Rights. 2010;10(Suppl 1):S11. doi: 10.1186/1472-698X-10-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poillon WN, Kim BC. 2,3-diphosphoglycerate and intracellular ph as interdependent determinants of the physiologic solubility of deoxyhemoglobin S. Blood. 1990;76:1028–1036. [PubMed] [Google Scholar]
- Poillon WN, Robinson MD, Kim BC. Deoxygenated sickle hemoglobin. Modulation of its solubility by 2,3-diphosphoglycerate and other allosteric polyanions. J Biol Chem. 1985;260:13897–13900. [PubMed] [Google Scholar]
- Poillon WN, Kim BC, Welty EV, Walder JA. The effect of 2,3-diphosphoglycerate on the solubility of deoxyhemoglobin S. Archives of Biochemistry and Biophysics. 1986;249:301–305. doi: 10.1016/0003-9861(86)90006-8. [DOI] [PubMed] [Google Scholar]
- Riggs A, Wells M. The oxygen equilibrium of sickle-cell hemoglobin. Biochim Biophys Acta. 1961;50:243–248. doi: 10.1016/0006-3002(61)90322-5. [DOI] [PubMed] [Google Scholar]
- Rogers SC, Ross JG, d’Avignon A, Gibbons LB, Gazit V, Hassan MN, McLaughlin D, Griffin S, Neumayr T, Debaun M, DeBaun MR, Doctor A. Sickle hemoglobin disturbs normal coupling among erythrocyte O2 content, glycolysis, and antioxidant capacity. Blood. 2013;121:1651–1662. doi: 10.1182/blood-2012-02-414037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolan PE, Parker JE, Gray SJ, Weatherly BC, Ingram J, Leavens W, Wootton R, Posner J. The pharmacokinetics, tolerability and pharmacodynamics of tucaresol (589C80; 4[2-formyl-3-hydroxyphenoxymethyl] benzoic acid), a potential anti-sickling agent, following oral administration to healthy subjects. Br J Clin Pharmacol. 1993;35:419–25. doi: 10.1111/j.1365-2125.1993.tb04160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolan PE, Mercer AJ, Wootton R, Posner J. Pharmacokinetics and pharmacodynamics of tucaresol, an antisickling agent, in healthy volunteers. Br J Clin Pharmacol. 1995;39:375–80. doi: 10.1111/j.1365-2125.1995.tb04465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safo MK, Abdulmalik O, Danso-Danquah R, Burnett JC, Nokuri S, Joshi GS, Musayev FN, Asakura T, Abraham DJ. Structural basis for the potent antisickling effect of a novel class of five-membered heterocyclic aldehydic compounds. J Med Chem. 2004;47:4665–4676. doi: 10.1021/jm0498001. [DOI] [PubMed] [Google Scholar]
- Safo MK, Ahmed MH, Ghatge MS, Boyiri T. Hemoglobin-ligand binding: Understanding Hb function and allostery on atomic level. Biochim Biophys Acta. 2011;1814:797–809. doi: 10.1016/j.bbapap.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Stern W, Mathews D, McKew J, Shen X, Kato GJ. A phase 1, first-in-man, dose-response study of Aes-103 (5-HMF), an anti-sickling, allosteric modifier of hemoglobin oxygen affinity in healthy normal volunteers. Blood. 2012;120:3210. [Google Scholar]
- Swerdlow PH, Bryan RA, Bertles JF, Poillon WN, Hagdoff-Fairchild B, Milner PF. Effect of 2,3-diphosphoclycerate on the solubility of deoxy-sickle hemoglobin. Hemoglobin. 1977;1:527–537. doi: 10.3109/03630267709003417. [DOI] [PubMed] [Google Scholar]
- Thomas C, Lumb AB. Physiology of haemoglobin. Continuing Education in Anaesthesia, Critical Care & Pain. 2012;12:251–256. [Google Scholar]
- Villela NR, Cabrales P, Tsai AG, Intaglietta M. Microcirculatory effects of changing blood hemoglobin oxygen affinity during hemorrhagic shock resuscitation in an experimental model. Shock. 2009;31:645–652. doi: 10.1097/SHK.0b013e31818bb98a. [DOI] [PubMed] [Google Scholar]
- Wright M, Sim D, Alonso-Galicia M, Kaalin Kauser K, Abe K. Protection of microvascular liver perfusion with 5-hydroxymethylfurfural in sickle cell disease mice following hypoxia-induced vasoocclusion. Blood. 2015;126:2282. [Google Scholar]
- Yalcin O, Cabrales P. Increased hemoglobin O2 affinity protects during acute hypoxia. American Journal of Physiology. Heart and Circulatory Physiology. 2012;303:H271–281. doi: 10.1152/ajpheart.00078.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaugg RH, Walder JA, Klotz IM. Schiff base adducts of hemoglobin. Modifications that inhibit erythrocyte sickling. J Biol Chem. 1977;252:8542–8. [PubMed] [Google Scholar]
- Zhao L, Chen J, Su J, Li L, Hu S, Li B, Zhang X, Xu Z, Chen T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. Journal of Agricultural and Food Chemistry. 2013;61:10604–10611. doi: 10.1021/jf403098y. [DOI] [PubMed] [Google Scholar]