Abstract
Background
TUBB8 is a primate-specific β-tubulin isotype whose expression is confined to oocytes and the early embryo. We previously found that mutations in TUBB8 caused oocyte maturation arrest. The objective was to describe newly discovered mutations in TUBB8 and to characterize the accompanying spectrum of phenotypes and modes of inheritance.
Methods and Results
Patients with oocyte maturation arrest were sequenced with respect to TUBB8. We investigated the effects of identified mutations in vitro, in cultured cells, and in mouse oocytes. Seven heterozygous missense and two homozygous mutations were identified. These mutations cause a range of folding defects in vitro, different degrees of microtubule disruption upon expression in cultured cells, and interfere to varying extents in the proper assembly of the meiotic spindle in mouse oocytes. Several of the newly discovered TUBB8 mutations result in phenotypic variability. For example, oocytes harboring any of three missense mutations (I210V, T238M and N348S) could extrude the first polar body. Moreover, they could be fertilized, although the ensuing embryos became developmentally arrested. Surprisingly, oocytes from patients harboring homozygous TUBB8 mutations that in either case preclude the expression of a functional TUBB8 polypeptide nonetheless contained identifiable spindles.
Conclusions
Our data substantially expand the range of dysfunctional oocyte phenotypes incurred by mutation in TUBB8, underscore the independent nature of human oocyte meiosis and differentiation, extend the class of genetic diseases known as the tubulinopathies, and provide new criteria for the qualitative evaluation of MII oocytes for IVF.
Keywords: TUBB8, mutation, oocyte maturation arrest, female infertility
INTRODUCTION
An essential feature of successful mammalian reproduction is the fusion of a sperm with a metaphase II oocyte.1–2 Oocytes are first arrested at prophase I and resume meiosis in response to luteinizing hormone (LH).3–4 Following spindle assembly and extrusion of the first polar body, oocytes are again arrested at metaphase II until fertilization.5–6 This complex process is regulated by several signaling pathways.7–14 A number of mouse genes have been identified with mutations that cause an oocyte maturation arrest phenotype.15–19 However, in humans, only a few cases of primary infertility caused by oocyte maturation arrest have been reported,20–25 and the genetic etiology of human oocyte maturation arrest is still largely unknown.
Although the mammalian oocyte meiotic spindle differs from the mitotic spindle in somatic cells in several respects,26–27 microtubules are the principal structural components in each case. These are dynamic filamentous polarized polymers assembled from α/β tubulin heterodimers; the latter consist of one α-tubulin and one β-tubulin polypeptide in tight association with one another. The productive folding of α- and β-tubulins and their integration into heterodimers cannot occur spontaneously. Rather, these processes require the interaction of newly synthesized polypeptides with several molecular chaperones. These include Prefoldin, which binds to and stabilizes nascent α- and β-tubulin polypeptides;28–29 the cytosolic chaperonin, CCT,30 which provides a sequestered environment in which non-native tubulins can partition to a quasi-native conformation in the absence of off-pathway interactions that would otherwise lead to aggregation;31 and five tubulin-specific chaperones termed TBCA-E that function in concert as a GTP-dependent heterodimer assembly nanomachine.32
The α-and β-tubulins are each encoded by a multigene family containing seven (α) or nine (β) members. Each encoded protein is termed an isotype; these are highly homologous in amino acid sequence, and differ from other family members by a relatively small number of conservative amino acid substitutions throughout the polypeptide as well as a more distinctively different acidic C-terminal tail.33 The amino acid sequence of each isotype is rigidly conserved across species boundaries, and the expression patterns of different α- and β-tubulin isotypes vary both as a function of development and in a tissue-specific manner. Not surprisingly, therefore, there is mounting evidence that the isotype composition of microtubules can contribute to their functional behavior.33–36 The essential biological contribution of various α-and β-tubulin isotypes is reflected in the recent discovery of a large number of naturally occurring mutations in the genes that encode them; these mutations cause a range of mostly devastating developmental disorders collectively termed tubulinopathies.37–39
Among the isotypes encoding β-tubulin, TUBB8 is unusual in that it exists only in primate species, where its biological function was unknown. Recently, we identified seven missense mutations in TUBB8 in patients with oocyte maturation arrest.40 We found that TUBB8 is uniquely expressed in oocytes and the early embryo, where it is the preponderant β-tubulin isotype. Based on an assessment of their effects upon expression in HeLa cells, yeast cells, mouse and human oocytes, we concluded that these mutations exert their effects via dominant negative effects on microtubule behavior. These findings uncovered an essential role for TUBB8 in human oocyte maturation and female fertility.
Here we report the identification of nine novel TUBB8 mutations in patients with oocyte maturation arrest. Among seven missense mutations, three (S176L, V255M and R262W) are de novo (of which one – S176L – is recurrent), one (T238M) is inherited, and three (I210V, T285P and N348S) are of an unknown inheritance pattern. We also identify two homozygous patients with either an internal deletion of seven amino acids (p.(E27_A33del)) or a deletion leading to a frame shift (T143Dfs*12) in TUBB8, in either case precluding the expression of a functional TUBB8 polypeptide. Surprisingly, oocytes from patients with homozygous mutations each have a visible spindle, implicating spindle microtubule assembly from pre-existing non-TUBB8 isotypes expressed at an earlier developmental stage. Equally surprisingly, oocytes from patients with certain TUBB8 missense mutations (I210V, T238M and N348S) are capable of extruding the first polar body in the absence of a properly assembled spindle; these oocytes can be fertilized and undergo cleavage to generate embryos that fail to develop beyond the 4- or 8-cell stage. These key findings dramatically extend the dysfunctional phenotypes of oocytes caused by mutation in TUBB8 and demonstrate that in humans, oocyte differentiation occurs independently of meiosis.
MATERIALS AND METHODS
Human subjects and permission for animal experimentation
Nine families with oocyte maturation arrest were referred from the reproductive medicine center at Ninth Hospital affiliated with Shanghai Jiao Tong University and Shanghai Ji Ai Genetics. All studies on human subjects were approved by the Fudan University Institutional Medical Review Board. A 32-year old patient (BMI index 23.2) had a single attempt at ICSI, because of problems relating to her husband’s sperm quality. Four immature oocytes and 7 MII oocytes were retrieved. Control MII oocytes for immunostaining were from 4 immature oocytes undergoing maturation in vitro, while the 7 MII oocytes were used either for morphologic evaluation by light or polarization microscopy, or for fertilization and embryonic development. MI oocytes were retrieved from patients diagnosed with oocyte MI arrest and were cultivated for maturation in vitro for about 20–24 h, followed by fixation for immunostaining. All oocytes from normal controls and patients were obtained with written informed consent signed by donor couples. The study was also approved by the Reproductive Study Ethics Committee of the Ninth Hospital affiliated with Shanghai Jiao Tong University. All protocols for the experimental use and euthanasia of mice were reviewed and approved by the Medical College of Fudan University and were in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care Guidelines.
Sequencing analysis of TUBB8
Patient genomic DNA samples and those from their family members and controls were extracted from peripheral blood using standard methods. All exons and splicing sites of TUBB8 were amplified. Amplified fragments were directly sequenced using an ABI 3100 DNA analyzer (Applied Biosystems, Foster city, CA, USA).
Evaluation of phenotypes of oocytes
Oocytes obtained from donors undergoing routine clinical ICSI were viewed by phase contrast and polarization microscopy with an OLYMPUS IX71 inverted microscope system. A method of oocyte immunostaining was adapted from that previously described.40 Briefly, oocytes were first fixed in 1.5% paraformaldehyde containing 0.1% BSA. Meiotic oocyte spindles were stained with an anti-β-tubulin antibody (1:400, F2043, Sigma-Aldrich) or an anti-α-tubulin antibody (1:500, T9026, Sigma-Aldrich). In microinjection experiments, mouse oocytes were tested using the above anti-β-tubulin antibody and an anti-FLAG antibody (1:500, A9594, Sigma-Aldrich). Hoechst 33342 (1:600, BD) was used to label DNA. Oocytes were mounted on glass slides and examined using a Leica TCS SP8 confocal laser-scanning microscope platform.
Molecular modeling
A high-resolution cryo-EM structure of the microtubule41 (PDB ID: 3JAS) was used to map the TUBB8 mutations described in this study onto the atomic model of the α/β-tubulin heterodimer.
Expression of wild type and mutant forms of TUBB8 in cultured Cells
Constructs engineered for the expression of C-terminally FLAG-tagged TUBB8 (wild type and mutant) were expressed by transfection into HeLa cells and their microtubule phenotypes examined by immunofluorescence as described previously.40
Folding kinetics of wild type and mutant forms of TUBB8
Constructs encoding full length wild type or mutant forms of TUBB8 in plasmid vectors containing a T7 promoter were used to drive expression in a rabbit reticulocyte lysate cell free system (TNT, Promega) supplemented with 35S-methionine. The kinetic analysis was done and reaction products analyzed either by SDS-PAGE or on non-denaturing polyacrylamide gels as described previously.40 Reactions designed to “chase” folding intermediates into heterodimers by providing a source of unlabeled α-tubulin were done by adding unlabeled native tubulin heterodimers to coupled TNT reactions after 90 mins, and the continuing the incubation for a further 60 min as described previously. 32
Mouse oocyte collection, meiotic maturation and culture
GV-stage oocytes were isolated from the ovaries of 4–6 week-old female ICR mice cultured in M16 medium and collected for immunostaining as described previously.40
Expression constructs, generation of cRNAs and microinjection into mouse oocytes
Site-directed mutagenesis, transcription of full-length TUBB8 cDNAs contained in a vector that included a CMV promoter and a C-terminal FLAG tag, and microinjection of wild type and mutant cRNAs into mouse GV oocytes was done as described previously.40
RESULTS
Mutations in TUBB8 result in a variety of oocyte phenotypes
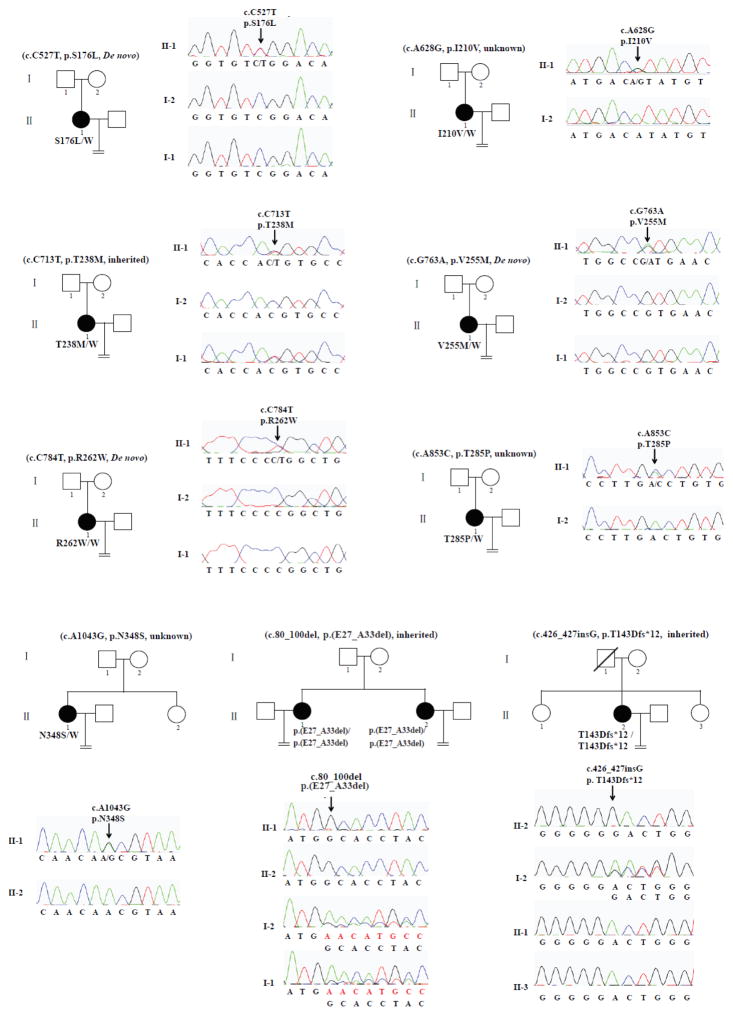
We recruited ten patients from nine independent and hitherto uncharacterized families that included one or more female members with persistent infertility. This resulted in the identification of nine mutations in TUBB8, of which eight are novel (Fig 1). S176L is a recurrent de novo mutation whose occurrence we reported previously.40 p.T238M is an inherited heterozygous missense mutation, while p.V255M and p.R262W are de novo mutations. Patients with homozygous mutations have either an internal deletion of seven amino acids (p.(E27_A33del)) or a deletion (p.T143Dfs*12) resulting in a truncated polypeptide of 154 amino acids. Because of a lack of parental information regarding their fathers, p.I210V, p.T285P and p.N348S are mutations with an unknown inheritance pattern. The location of all these mutations as well as those previously identified within TUBB8 40 is shown in S1A Fig; all residues implicated are strictly evolutionarily conserved among primate species (S1B Fig).
Figure 1. Pedigree and TUBB8 mutations leading to oocyte meiotic arrest.
Pedigrees of nine families with mutations in TUBB8. The S176L, V255M and R262W mutations are de novo. The T238M mutation is inherited from her father, while the E27_A33del mutation and the T143Dfs*12 mutation are both homozygous, in each case inherited from heterozygous parents. The I210V, T285P and N348S mutations have unknown inheritance patterns. Sanger sequencing chromatograms are shown defining the nature and location of the mutations in each family. Squares denote male family members and circles female family members. Black circles represent affected individuals. Slash indicates deceased individual. An equal sign denotes infertility.
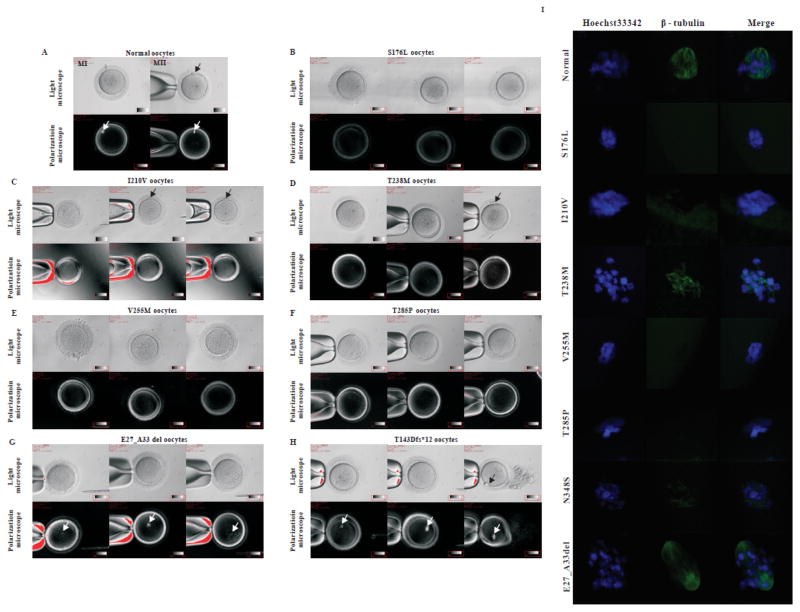
All patients had primary infertility over the course of several years; their spouses had normal sperm counts, sperm morphology and motility. Each patient underwent 2–7 failed IVF/ICSI attempts (Table 1). Specific information on the stage of oocytes retrieved is summarized in Table S1. We found several significant phenotypic differences among the patients’ oocytes. Patients p.S176L, p.V255M, p.R262W and p.T285P had no morphologically identifiable MII oocytes (Figs 2B, E, and F; Table S1). However, in patients p.I210V, p.T238M, p.E27_A33del and p.T143Dfs*12, there were some morphologically identifiable MII oocytes with an extruding first polar body (Figs 2C, D, G, and H). The relative proportion of morphological MII oocytes differed among these patients. The p.I210V patient had the highest proportion of MII oocytes (4/15), and in her latest attempt at ICSI, four out of five oocytes retrieved were morphologically MII with an extruding first polar body (Fig 2C). In the latest ICSI attempt with the p.T238M patient, two out of nine oocytes retrieved were morphologically MII, with an extruding first polar body and no visible spindle (Fig 2D). Upon immunostaining, oocytes of patients with heterozygous mutations had no detectable spindle (p.S176L, p.I210V, p.V255M and p.T285P), a conspicuously disorganized spindle (p.T238M), or a spindle that gave only a barely detectable immunofluorescence signal with an anti-β-tubulin antibody (p.N348S) (Fig 2I).
Table 1.
Clinical characteristics of oocyte maturation arrest patients from the nine families.
| Case | Age (years) | BMI (kg/m2) | Duration of infertility (years) | Previous IVF/ICSI cycles | Total No. of oocyte retrieved |
|---|---|---|---|---|---|
| S176L | 29 | 19.57 | 3 | 3 | 32 |
| I210V | 36 | 20.31 | 3 | 2 | 14 |
| T238M | 29 | 20.83 | 5 | 6 | 30 |
| V255M | 33 | 20.58 | 7 | 2 | 29 |
| R262W | 34 | 20.90 | 3 | 2 | 36 |
| T285P | 32 | 22.06 | 10 | 4 | 11 |
| N348S | 34 | 23.62 | 7 | 4 | 55 |
| E27_A33del | 23 | 23.88 | 3 | 3 | 33 |
| T143Dfs*12 | 42 | 23.83 | 18 | 7 | 19 |
Figure 2. Oocyte phenotypes from patients with maturation arrest.
A normal oocyte (A) and oocytes from patients in the families indicated (B–H) were separated from granulosa cells and examined by light and polarization microscopy. Note that a normal MII oocyte has a first polar body (black arrow in A) and that normal MI and MII oocytes have visible spindles (white arrows in A). Patient oocytes S176L (B), V255M (E) and T285P (F) are at MI and none have a first polar body or a visible spindle. Some oocytes in the patient I210V (C) and T238M (D) have a first polar body (black arrow in C, D), but none of the oocytes from this patient have a visible spindle. All oocytes from patients E27_A33 del and T143Dfs*12 have a visible spindle (G, H), and an oocyte from the patient T143Dfs*12 has a first polar body (H). (I) Oocytes from control and various patients (n=1 for S176L; n=1 for T210V; n=2 for T238M; n=1 for V255M; n=2 for T285P; n=3 for N348S; n=2 for E27_A33del) were immunolabeled with antibodies against β-tubulin (to visualize the spindle, shown in green) and counterstained with Hoechst 33342 (shown in blue) to visualize DNA. No oocytes from the T143DFs*12 patient were available for this analysis and the spindle in the oocyte from the N348S patient is only very weakly visible compared to the wild-type control.
We previously established that TUBB8 is by a large margin the most abundant β-tubulin isotype expressed in human oocytes.40 In spite of the fact that the two homozygous mutations (p.T143Dfs*12 and p.(E27_A33del)) are incapable of generating a functional β-tubulin polypeptide (see below), oocytes harboring these two mutations had a visible spindle by polarization microscopy, although most of them lacked a first polar body (Fig 2G, H). To see whether the absence of any functional TUBB8 in p.T143Dfs*12 oocytes might be compensated by an increase in expression of non-TUBB8 β-tubulin isotypes, we measured the levels of non-TUBB8 mRNAs in a single p.T143Dfs*12 oocyte, but found no such increase (data not shown). In addition, compared with a normal oocyte, immunofluorescence analysis of a p.(E27_A33del) oocyte showed the spindle to be less compact, with fewer polar microtubules (and possibly a lack of kinetochore microtubules) compared with a normal oocyte (Fig 2I). We conclude that different TUBB8 mutations contribute to an extensive variety of oocyte phenotypes with respect to extrusion of the first polar body and spindle morphology, and that TUBB8 expression is essential for proper primate spindle morphology and function.
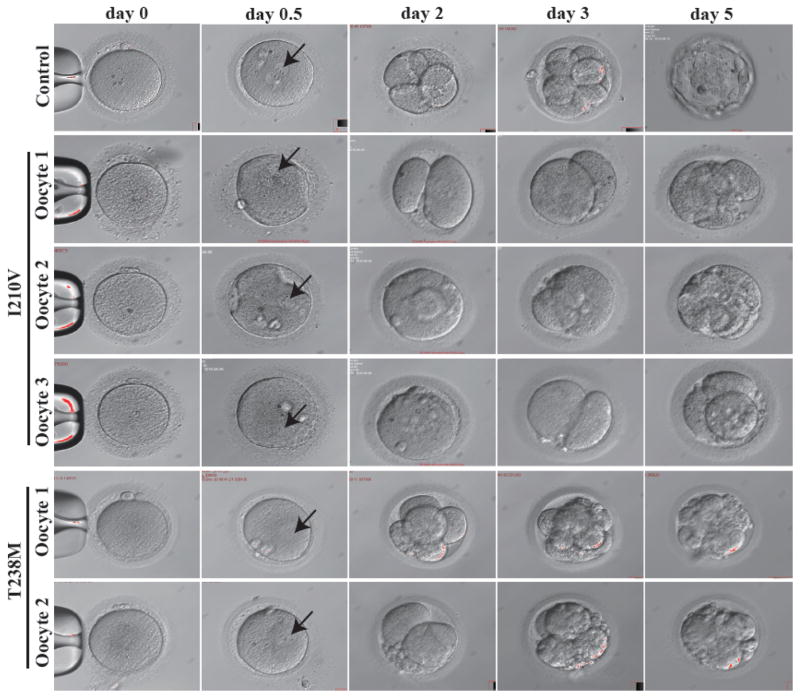
While many patient MII oocytes had no visible spindle by polarization microscopy, we were surprised to discover that in her latest IVF attempt, three out of four MII oocytes from the I210V patient could nonetheless be fertilized and underwent cleavage. However, all embryos became arrested at the 2-cell stage on day 3, and by day 5 there were no viable embryos (Fig 3). In the case of MII oocytes from the T238M patient, although two were abnormally fertilized in the sense that they had a 1PN pronucleus, each nonetheless underwent cleavage and formed either a class III 8-cell embryo or a class III 4-cell embryo on day 3. Thereafter, in both cases, the embryos became arrested on day 5 (Fig 3). The patient with the N348S mutation had a similar phenotype: eight oocytes became fertilized, but all embryos were nonviable by day 3 (data not shown). These observations further extend the range of dysfunctional oocyte phenotypes incurred by mutation in TUBB8 (summarized in Table 2).
Figure 3. Morphology of wild type, I120V and T238M MII oocytes and early embryos.
The morphologies of control MII oocytes (n=1), MII oocytes (n=3 for I210V; n=2 for T238M) from patients harboring the indicated mutations in TUBB8, fertilized oocytes (on day 0.5), and embryos on days 2, 3 and 5 were examined by phase contrast light microscopy. Black arrows indicate the pronucleus.
Table 2.
Spindle status, polar body extrusion, fertilization and ensuing embryonic development in wild-type and patient oocytes harboring mutations in TUBB8. See text and Figures 2 and 3 for details.
| W.T. | S176L | I210V | T238M | V255M | R262W | T285P | N348S | 27_33del | T143 Dfs*12 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Spindle | Present | Absent | Absent | Present | Absent | / | Absent | Present | Present | Present |
| Spindle Morphology | Normal | / | / | Disorganized | / | / | / | Disorganized and Low Fluorescent Intensity | Aberrant | / |
| Polar Body Extrusion | Yes (100% oocytes) | No | Yes (4/15,26.7% oocytes) | Yes (6/30,20% oocytes) | No | No | No | Yes (8/55,14.5% oocytes) | Yes (1/33, 3% oocytes) | Yes (1/19, 5.2% oocytes) |
| Fertilization and embryo development | Yes | / | Normal fertilization; Early embryonic arrest | Abnormal fertilization; Early embryonic arrest | / | / | / | Normal fertilization; Early embryonic arrest | Abnormal fertilization; No cleavage | Abnormal fertilization; No cleavage |
/ : Not Applicable or No data were collected
Structural implications of TUBB8 mutations
Previous analysis based on the known atomic structure of the α/β tubulin heterodimer (PDB: 3JAS)41 implied that the S176L mutation may result in the disruption of longitudinal interactions between heterodimers in the microtubule lattice and thereby affect microtubule stability.40 The p.T143Dfs*12 mutation will result in a truncated protein (154 N-terminal amino acids rather than the full length 444 amino acid polypeptide) that cannot be incorporated into heterodimers. We mapped the remaining affected TUBB8 residues onto the same tubulin atomic structure. As shown in S2 Fig, T285 is located within the M-loop, which is essential for lateral interactions within the microtubule. I210, N348, V255 and T238 are buried within the β-tubulin structure. Their mutation could affect folding and stability. Residues 27–33 may also contribute to β-tubulin stability. Amino acid R262 may form a salt bridge with D417, which is involved in kinesin binding;42 the R262W mutation may therefore weaken this interaction. In summary, the newly discovered TUBB8 mutations described here have the potential to interfere with microtubule function via tubulin deficit, to cause defects in microtubule behavior by affecting β-tubulin folding, heterodimer assembly, or tubulin dynamics (via interfering with lateral contacts within the microtubule lattice), or to affect interaction with kinesin and perhaps other microtubule associated proteins (MAPs).
α/β heterodimer assembly in vitro and effect of mutant TUBB8 expression on microtubule architecture in cultured cells
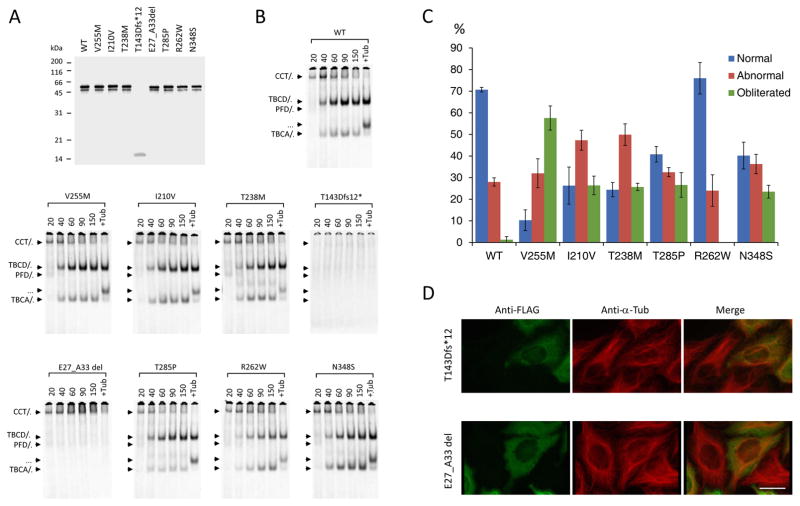
To investigate whether the newly discovered mutations in TUBB8 indeed cause defects in β-tubulin folding and α/β heterodimer formation, we followed coupled transcription/translation reactions kinetically. This allowed us to monitor the transit of newly synthesized polypeptides through the various β-tubulin/chaperone complexes that contribute to the chaperone-dependent assembly pathway.43 None of the mutations had a substantial influence on translational efficiency (Fig 4A); the minor band migrating at about 50 kDa (just below the major full-length product at 55 kDa and present in all the TUBB8 sequences analyzed with the exception of the T143D*fs mutation, which produces a prematurely terminated translation product migrating at about 16 kDa) is probably a product of internal translational initiation. On the other hand, most of the TUBB8 missense mutants displayed a spectrum of differences compared to the wild-type control, including in many cases a reduction in the yield of de novo assembled heterodimers that could result in tubulin haploinsufficiency. This is particularly evident in the case of I120V, T238M and R262W (Fig 4B). For the p.T143Dfs*12 and p.(E27_A33del) mutations, there is either a complete absence of folding intermediates (in the case of p.T143Dfs*12), or the generation of only a binary complex with the cytoplasmic chaperonin, CCT 32 (in the case of p.(E27_A33del)). As expected, in neither case are de novo synthesized polypeptides containing either of these mutations capable of assembly into native α/β heterodimers. We conclude that both these mutations can be considered as functionally null.
Figure 4. Kinetics of heterodimer assembly in vitro and microtubule phenotypes resulting from expression of wild type and mutant forms of TUBB8 in cultured cells.
(A) Analysis by 12% SDS-PAGE of the products of 35S-methionine-labeled transcription-translation reactions driven by plasmids encoding wild type and mutant forms of TUBB8. (B) Analysis on 4.5% native polyacrylamide gels of the products of transcription-translation reactions done in an identical manner to those shown in (A) for the times shown (in mins) above each panel. Arrows (top to bottom) show the migration positions of the binary complex formed between newly translated polypeptides and the cytosolic chaperonin, CCT (CCT/β), the TBCD/β-tubulin complex, the Prefoldin/β-tubulin complex, the native α/β tubulin heterodimer and the TBCA/β-tubulin complex, respectively 32. (C) Quantitative analysis of microtubule phenotypes in HeLa cells expressing high levels of FLAG-tagged constructs encoding the mutations shown. The phenotypes (characterized as normal, abnormal or obliterated: examples shown in S3 Fig.) were analyzed and scored as described previously 40. About 200 transfected cells were assessed for each category in each of three independent experiments. The figure shows mean percentages (+/− SD) of cells expressing wild-type or mutant TUBB8 and assigned to each category. (D) Expression of the T143Dfs*12 and E27_A33del TUBB8 mutations. HeLa cells transfected with constructs engineered for the expression of the truncation and 7 amino acid internally deleted mutant forms of TUBB8, each bearing an in-frame C-terminal FLAG tag, were examined by immunofluorescence microscopy using an anti-FLAG antibody (to detect the transgene, shown in green) and an anti-α-tubulin antibody (to detect the endogenous microtubule network, shown in red). Note the appearance of the transgene as a diffuse mottled stain throughout the cytoplasm in each case, with no evidence of co-assembly into microtubules. Bar = 10 microns.
To investigate the effect of the various TUBB8 mutants on microtubule behavior, we transfected FLAG-tagged constructs into cultured HeLa cells. Consistent with our previously described analysis of missense mutations in TUBB8,40 most of the newly discovered missense mutations had a deleterious effect on the organization of cytoplasmic microtubules (S3 Fig.). A quantitative analysis of the microtubule phenotypes observed upon expression of wild type and missense TUBB8 mutations showed that, with the exception of R262W, all had a greater propensity than the wild type protein to cause microtubule obliteration (Fig. 4C). In contrast, cells transfected with either of the two functionally null mutations expressed the transgene in a diffuse mottled pattern distributed throughout the cytoplasm, with little or no effect on the organization of the endogenous microtubule network (Fig 4D). Given that the deletions in question each result in the excision of a critical portion of the β-tubulin polypeptide, we conclude that expression of these mutant proteins results in their deposition as insoluble cytoplasmic aggregates. The R262Q mutation was previously reported to cause a substantially diminished yield of properly assembled α/β-tubulin heterodimers in vitro, but had a relatively modest effect on the microtubule network upon expression in cultured cells.40 Notably, a different mutation at the same locus, R262W, also resulted in a reduced yield of assembled heterodimers (Fig 4B). However, similar to cells transfected with wildtype, cells transfected with R262W had microtubules with a generally normal appearance (Fig 4C). Thus, as would be expected, different amino acid substitutions at the same locus can cause different effects and result in variable phenotypes.
TUBB8 mutations and spindle assembly
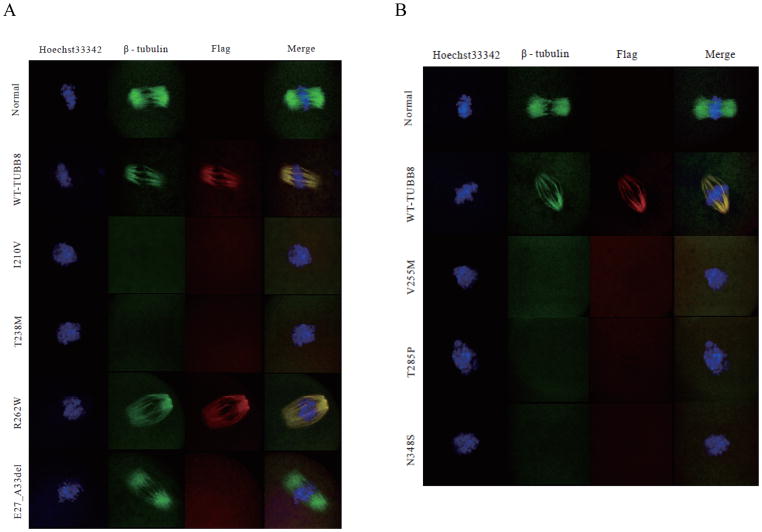
To establish a causal relationship between mutations and phenotypes, we microinjected wild-type and mutant TUBB8-encoding RNAs into mouse oocytes Although mice (in common with all other non-primate species) do not harbor a gene encoding TUBB8, our previous work showed that expression of wild type TUBB8 induced in mouse oocytes as a result of microinjection of recombinant RNA does result in co-assembly of TUBB8-containing heterodimers into meiotic spindle microtubules, thus setting a precedent for the usefulness of this approach 40 Compared with the appearance of uninjected control oocytes, oocytes microinjected with wild-type TUBB8 RNA had a spindle with a less dense appearance (Fig 5), reinforcing our inference that β-tubulin isotype composition itself influences spindle morphology. In addition, expression of microinjected RNA encoding I210V, T238M, V255M, T285P and N348S TUBB8 in mouse oocytes resulted in completely or severely impaired spindle assembly (Fig 5A, B), precisely recapitulating the spindle status in the corresponding patient oocytes. The absence of an anti-FLAG signal in the spindles of oocytes microinjected with RNA encoding p.(E27_A33del) confirmed that the effect of this mutation is equivalent to a null. Microinjection of R262W RNA resulted in a normal spindle (Fig 5), consistent with the observation that expression of this mutant TUBB8 in HeLa cells does not disrupt microtubule architecture. In this respect, R262W is unique among known TUBB8 missense mutations.
Figure 5. TUBB8 RNA microinjection, spindle morphology and assembly.
(A,B) Immunostaining of mouse oocytes 12h after GVBD. GV oocytes were injected with a high (1000 ng/μl) concentration of either wild type (n=143) or mutant (I210V (n=67), T238M (n=53), R262W (n=66) and E27_A33del (n=81), panel A; V255M (n=58), T285P (n=50) or N348S (n=57), panel B) RNA encoding C-terminally FLAG-tagged TUBB8. Normal mouse GV oocytes (n=131) without injection were cultivated in vitro for maturation. In each case the oocytes were stained to visualize chromosomes (Hoechst 33342; blue), spindles (β-tubulin; green) and TUBB8 (FLAG; red) and examined by confocal microscopy.
DISCUSSION
In this study, we identified eight novel heterozygous/homozygous mutations and one recurrent de novo mutation in TUBB8 from nine independent families with infertile females (Fig 1 and S1 Fig). Seven are heterozgyous missense, while two are homozygous mutations resulting in the expression of either a polypeptide with an internal deletion of seven amino acids or a truncated protein caused by a nucleotide insertion mutation and containing only 154 β-tubulin N-terminally-derived amino acids. Taken together with our previous analysis, 40 we have now identified 16 patients carrying a mutation in TUBB8 out of a total of 43 infertile women (i.e. 36.3%) with MI oocyte arrest. It follows that other as yet unknown genetic defects can also contribute to infertility caused by oocyte maturation arrest. The discovery of the patients described here extends the spectrum of phenotypes in oocytes with meiotic arrest as a result of mutation in TUBB8 in two important respects. First, some morphologically MII oocytes can be fertilized, but the ensuing embryos become arrested at an early developmental stage, and second, some MI oocytes have a visible spindle (Figs 2 and 3). Among missense mutations, most are predicted to affect microtubule behavior by impacting either β-tubulin stability or lateral contacts between protofilaments (S2 Fig). Indeed, many of these were found to affect α/β heterodimer assembly in vitro, particularly with respect to the yield of material migrating as native heterodimer (Fig 4B). Moreover, in several cases they disrupted microtubule organization upon expression in cultured cells (Fig 4D) and interfered with proper spindle assembly upon expression in mouse oocytes (Fig 5).
We highlighted the location of all mutations identified in this and our previous study 40 in the sequence of TUBB8 (Fig. S1A). Most are located in exon 4. Our previous analyses showed that various TUBB8 missense mutations resulted in impaired microtubule behavior – including proper spindle assembly - by dominant negative effects.40 This mechanism also applies to some of the newly discovered heterozygous missense mutations reported here. However in at least one case (R262W), the mutation had no discernable effect on microtubule architecture in cultured cells or on spindle assembly upon expression in mouse oocytes. The R262W mutation therefore seems more likely to exert its effect either via haploinsufficiency (reflected in the reduced yield of native tubulin heterodimers generated in in vitro folding assays, Fig 4B) or as a result of one or more predicted alterations in the binding of kinesin (S2 Fig) rather than a dominant negative effect on microtubule stability or dynamics (Fig 5B). On the other hand, oocyte maturation arrest can also be caused by homozygous mutations in TUBB8 (p.T143Dfs*12 and p.(E27_A33del), each with a recessive inheritance pattern. We were surprised to find spindle structures in oocytes carrying these mutations, since they both render the expressed protein functionally null (Figs 4A–C). At least in the latter case, a defective spindle was observed (Fig 2I). It therefore seems probable that spindle microtubules in these homozygous mutant-bearing oocytes are polymerized from pre-existing non-TUBB8-containing tubulin heterodimers expressed at an earlier developmental stage, without compensatory up-regulated expression of other β-tubulin isotypes. Because patient oocytes were not readily obtainable, the latter conclusion was based on mRNA-based analysis from a single oocyte. Nonetheless, we interpret the spindle defects resulting from this mutation as reflecting an essential contribution of heterodimers containing TUBB8 to proper spindle structure in primates. Consistent with this inference, the spindles of mouse oocytes microinjected with wild-type TUBB8 RNA were morphologically distinct from uninjected controls (Fig 5). We conclude that TUBB8 has a key role in determining the specific morphology and normal function of the primate meiotic spindle, and that the class of genetically determined diseases known as the tubulinopathies37–38 must now be extended to include female infertility caused by mutations in TUBB8
Because of the scarcity of human oocytes available for experimental purposes, we were constrained in the number of these cells we could use for analysis by immunostaining. Nonetheless, our data are sufficiently compelling to justify the phenotypic classification of oocytes harboring the TUBB8 mutations we describe into three distinct classes: MI oocytes with an impaired spindle (heterozygous mutations: S176L, T238M, V255M, T285P, N348S); MI oocytes that are dysfunctional but that have a visible but morphologically defective spindle (homozygous mutations p.T143Dfs*12 and p.(E27_A33del)); and morphological MII oocytes that either lack an identifiable spindle or contain an impaired spindle (I210V, T238M and N348S). Some of the oocytes morphologically identifiable as MII but with an impaired spindle can be fertilized and cleave, but these embryos later become developmentally arrested. Typically, in the context of clinical IVF/ICSI, only morphological MII oocytes identified by light microscopy are selected for fertilization. In view of our discovery that oocytes morphologically recognizable as MII can be defective, determination of genetic variations in TUBB8 is likely to prove useful as a future additional criterion for evaluating the quality of patients’ MII oocytes. We note that Dokshin et al. demonstrated that stra8-deficient mouse oocytes can produce a polar body without premeitoic chromosomal replication or recombination, suggesting that oocyte growth and differentiation are genetically dissociable from meiosis in mice.44 Our observations provide direct evidence that as in mouse oocytes, meiosis and oocyte differentiation make independent contributions to the generation of a functional human egg.
Supplementary Material
S1 Fig: Location of TUBB8 mutations and their conservation among primate species
S2 Fig: Structural implications of TUBB8 amino acid substitutions
S3 Fig: Examples of microtubule architecture in Hela cells expressing wild type or mutant (missense) formsTUBB8
Table S1: Clinical oocytes characteristics of oocyte retrieved from maturation arrest patients
Acknowledgments
We thank Dr. Eva Nogales and Dr. Rui Zhang for great help on structural analysis of TUBB8 mutations. We thank Ke Qiao from Key Laboratory of Medical Molecular Virology, Ministry of Education and Public Health, School of Basic Medical Sciences, Fudan University for help with technical expertise in confocal microscopy.
Funding
This work was supported by the National Basic Research Program of China (2015CB943300), the National Natural Science Foundation of China (81270747, 81270749, 81571397, 81571501, 81300485, 81300550), the 111 Project (B13016) and by a National Institutes of Health grant (5R01GM097376 to Dr. Nicholas Cowan).
Footnotes
Contributors
Nicolas J. Cowan, Guoling Tian and Lei Wang conceived and designed the experiments. Min Yu, Zheng Yan, Xiaoxi Sun, Yanping Kuang and Bin Li collected the samples. Ruizhi Feng, Qing Sang, Guoling Tian, Yao Xu, Biaobang Chen, Ronggui Qu performed the experiments and analyzed the data. Zhaogui Sun, Li Jin and Lin He analyzed the data. Nicolas J. Cowan and Lei Wang wrote the paper. All authors reviewed the manuscript.
Competing interests
The authors have no conflict of interest to declare.
Patient consent
Obtained.
Ethics approval
Fudan University Institutional Medical Review Board, Shanghai, China. Reproductive Study Ethics Committee, the Ninth Hospital affiliated with Shanghai Jiao Tong University, Shanghai, China.
References
- 1.Li R, Albertini DF. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol. 2013;14:141–52. doi: 10.1038/nrm3531. [DOI] [PubMed] [Google Scholar]
- 2.Edwards RG, Bavister BD, Steptoe PC. Early stages of fertilization in vitro of human oocytes matured in vitro. Nature. 1969;221:632–5. doi: 10.1038/221632a0. [DOI] [PubMed] [Google Scholar]
- 3.Coticchio G, Dal Canto M, Mignini RM, Guglielmo MC, Brambillasca F, Turchi D, Novara PV, Fadini R. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update. 2015;21:427–54. doi: 10.1093/humupd/dmv011. [DOI] [PubMed] [Google Scholar]
- 4.Eppig JJ, Bivens CM, Viveiros MM, de la Fuente R. Chapter 7 - Regulation of Mammalian Oocyte Maturation. In: Adashi PCKL, editor. The Ovary (Second Edition) San Diego: Academic Press; 2003. p. 113. [Google Scholar]
- 5.Vogt E, Kirsch-Volders M, Parry J, Eichenlaub-Ritter U. Spindle formation, chromosome segregation and the spindle checkpoint in mammalian oocytes and susceptibility to meiotic error. Mutat Res. 2008;651:14–29. doi: 10.1016/j.mrgentox.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Eppig JJ. Regulation of mammalian oocyte maturation. In: Adash PCKL, editor. The Ovary. San Diego: Elsevier/Academic Press; 1993. pp. 185–208. [Google Scholar]
- 7.Choi T, Fukasawa K, Zhou R, Tessarollo L, Borror K, Resau J, Vande WG. The Mos/mitogen-activated protein kinase (MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes. Proc Natl Acad Sci USA. 1996;93:7032–5. doi: 10.1073/pnas.93.14.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huo LJ, Fan HY, Zhong ZS, Chen DY, Schatten H, Sun QY. Ubiquitin-proteasome pathway modulates mouse oocyte meiotic maturation and fertilization via regulation of MAPK cascade and cyclin B1 degradation. Mech Dev. 2004;121:1275–87. doi: 10.1016/j.mod.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Lee B, Vermassen E, Yoon SY, Vanderheyden V, Ito J, Alfandari D, De Smedt H, Parys JB, Fissore RA. Phosphorylation of IP3R1 and the regulation of [Ca2+]i responses at fertilization: a role for the MAP kinase pathway. Development. 2006;133:4355–65. doi: 10.1242/dev.02624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi T, Morrow JD, Wang H, Dey SK. Cyclooxygenase-2-derived prostaglandin E(2) directs oocyte maturation by differentially influencing multiple signaling pathways. J Biol Chem. 2006;281:37117–29. doi: 10.1074/jbc.M608202200. [DOI] [PubMed] [Google Scholar]
- 11.Kang HJ, Feng Z, Sun Y, Atwal G, Murphy ME, Rebbeck TR, Rosenwaks Z, Levine AJ, Hu W. Single-nucleotide polymorphisms in the p53 pathway regulate fertility in humans. Proc Natl Acad Sci USA. 2009;106:9761–6. doi: 10.1073/pnas.0904280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Cell Biol. 2002;4(Suppl):s41–9. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- 13.Kimura N, Hoshino Y, Totsukawa K, Sato E. Cellular and molecular events during oocyte maturation in mammals: molecules of cumulus-oocyte complex matrix and signalling pathways regulating meiotic progression. Soc Reprod Fertil Suppl. 2007;63:327–42. [PubMed] [Google Scholar]
- 14.Schmitt A, Nebreda AR. Signalling pathways in oocyte meiotic maturation. J Cell Sci. 2002;115:2457–9. doi: 10.1242/jcs.115.12.2457. [DOI] [PubMed] [Google Scholar]
- 15.Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–41. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–4. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 17.Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306:1947–50. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- 18.Mehlmann LM, Jones TL, Jaffe LA. Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science. 2002;297:1343–5. doi: 10.1126/science.1073978. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:366–9. doi: 10.1126/science.1193573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudak E, Dor J, Kimchi M, Goldman B, Levran D, Mashiach S. Anomalies of human oocytes from infertile women undergoing treatment by in vitro fertilization. Fertil Steril. 1990;54:292–6. doi: 10.1016/s0015-0282(16)53706-6. [DOI] [PubMed] [Google Scholar]
- 21.Eichenlaub-Ritter U, Schmiady H, Kentenich H, Soewarto D. Recurrent failure in polar body formation and premature chromosome condensation in oocytes from a human patient: indicators of asynchrony in nuclear and cytoplasmic maturation. Hum Reprod. 1995;10:2343–9. doi: 10.1093/oxfordjournals.humrep.a136297. [DOI] [PubMed] [Google Scholar]
- 22.Hartshorne G, Montgomery S, Klentzeris L. A case of failed oocyte maturation in vivo and in vitro. Fertil Steril. 1999;71:567–70. doi: 10.1016/s0015-0282(98)00505-6. [DOI] [PubMed] [Google Scholar]
- 23.Bergere M, Lombroso R, Gombault M, Wainer R, Selva J. An idiopathic infertility with oocytes metaphase I maturation block: case report. Hum Reprod. 2001;16:2136–8. doi: 10.1093/humrep/16.10.2136. [DOI] [PubMed] [Google Scholar]
- 24.Levran D, Farhi J, Nahum H, Glezerman M, Weissman A. Maturation arrest of human oocytes as a cause of infertility: case report. Hum Reprod. 2002;17:1604–9. doi: 10.1093/humrep/17.6.1604. [DOI] [PubMed] [Google Scholar]
- 25.Schmiady H, Neitzel H. Arrest of human oocytes during meiosis I in two sisters of consanguineous parents: first evidence for an autosomal recessive trait in human infertility: Case report. Hum Reprod. 2002;17:2556–9. doi: 10.1093/humrep/17.10.2556. [DOI] [PubMed] [Google Scholar]
- 26.Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell. 2007;130:484–98. doi: 10.1016/j.cell.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Manandhar G, Schatten H, Sutovsky P. Centrosome reduction during gametogenesis and its significance. Biol Reprod. 2005;72:2–13. doi: 10.1095/biolreprod.104.031245. [DOI] [PubMed] [Google Scholar]
- 28.Vainberg IE, Lewis SA, Rommelaere H, Ampe C, Vandekerckhove J, Klein HL, Cowan NJ. Prefoldin, a chaperone that delivers unfolded proteins to cytosolic chaperonin. Cell. 1998;93:863–73. doi: 10.1016/s0092-8674(00)81446-4. [DOI] [PubMed] [Google Scholar]
- 29.Hansen WJ, Cowan NJ, Welch WJ. Prefoldin-nascent chain complexes in the folding of cytoskeletal proteins. J Cell Biol. 1999;145:265–77. doi: 10.1083/jcb.145.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y, Thomas JO, Chow RL, Lee GH, Cowan NJ. A cytoplasmic chaperonin that catalyzes beta-actin folding. Cell. 1992;69:1043–50. doi: 10.1016/0092-8674(92)90622-j. [DOI] [PubMed] [Google Scholar]
- 31.Tian G, Vainberg IE, Tap WD, Lewis SA, Cowan NJ. Quasi-native chaperonin-bound intermediates in facilitated protein folding. J Biol Chem. 1995;270:23910–3. doi: 10.1074/jbc.270.41.23910. [DOI] [PubMed] [Google Scholar]
- 32.Cowan NJ, Lewis SA. Type II chaperonins, prefoldin, and the tubulin-specific chaperones. Adv Protein Chem. 2001;59:73–104. doi: 10.1016/s0065-3233(01)59003-8. [DOI] [PubMed] [Google Scholar]
- 33.Janke C. The tubulin code: molecular components, readout mechanisms, and functions. J Cell Biol. 2014;206:461–72. doi: 10.1083/jcb.201406055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammond JW, Cai D, Verhey KJ. Tubulin modifications and their cellular functions. Curr Opin Cell Biol. 2008;20:71–6. doi: 10.1016/j.ceb.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luduena RF. Multiple forms of tubulin: different gene products and covalent modifications. Int Rev Cytol. 1998;178:207–75. doi: 10.1016/s0074-7696(08)62138-5. [DOI] [PubMed] [Google Scholar]
- 36.Luduena RF. Are tubulin isotypes functionally significant. Mol Biol Cell. 1993;4:445–57. doi: 10.1091/mbc.4.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breuss M, Keays DA. Microtubules and Neurodevelopmental Disease: The Movers and the Makers. In: Nguyen L, Hippenmeyer S, editors. Cellular and Molecular Control of Neuronal Migration. Springer Science+Busimess Media; Dordrecht: 2014. pp. 75–96. [DOI] [PubMed] [Google Scholar]
- 38.Bahi-Buisson N, Poirier K, Fourniol F, Saillour Y, Valence S, Lebrun N, Hully M, Bianco CF, Boddaert N, Elie C, Lascelles K, Souville I, Beldjord C, Chelly J. The wide spectrum of tubulinopathies: what are the key features for the diagnosis? Brain. 2014;137:1676–700. doi: 10.1093/brain/awu082. [DOI] [PubMed] [Google Scholar]
- 39.Tischfield MA, Cederquist GY, Jr, Gupta ML, Engle EC. Phenotypic spectrum of the tubulin-related disorders and functional implications of disease-causing mutations. Curr Opin Genet Dev. 2011;21:286–94. doi: 10.1016/j.gde.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, Shi J, Tian G, Luchniak A, Fukuda Y, Li B, Yu M, Chen J, Xu Y, Guo L, Qu R, Wang X, Sun Z, Liu M, Shi H, Wang H, Feng Y, Shao R, Chai R, Li Q, Xing Q, Zhang R, Nogales E, Jin L, He L, Gupta ML, Cowan NJ, Wang L. Mutations in TUBB8 Cause Human Oocyte Meiotic Arrest. New Engl J Med. 2016;374:223–32. doi: 10.1056/NEJMoa1510791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang R, Alushin GM, Brown A, Nogales E. Mechanistic Origin of Microtubule Dynamic Instability and Its Modulation by EB Proteins. Cell. 2015;162:849–59. doi: 10.1016/j.cell.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shang Z, Zhou K, Xu C, Csencsits R, Cochran JC, Sindelar CV. High-resolution structures of kinesin on microtubules provide a basis for nucleotide-gated force-generation. Elife. 2014;3:e4686. doi: 10.7554/eLife.04686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breuss M, Heng JI, Poirier K, Tian G, Jaglin XH, Qu Z, Braun A, Gstrein T, Ngo L, Haas M, Bahi-Buisson N, Moutard ML, Passemard S, Verloes A, Gressens P, Xie Y, Robson KJ, Rani DS, Thangaraj K, Clausen T, Chelly J, Cowan NJ, Keays DA. Mutations in the beta-tubulin gene TUBB5 cause microcephaly with structural brain abnormalities. Cell Rep. 2012;2:1554–62. doi: 10.1016/j.celrep.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dokshin GA, Baltus AE, Eppig JJ, Page DC. Oocyte differentiation is genetically dissociable from meiosis in mice. Nat Genet. 2013;45:877–83. doi: 10.1038/ng.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1 Fig: Location of TUBB8 mutations and their conservation among primate species
S2 Fig: Structural implications of TUBB8 amino acid substitutions
S3 Fig: Examples of microtubule architecture in Hela cells expressing wild type or mutant (missense) formsTUBB8
Table S1: Clinical oocytes characteristics of oocyte retrieved from maturation arrest patients