Abstract
Background
The six-minute walk (6MW) is a common walking outcome in multiple sclerosis (MS) thought to measure fatigability in addition to overall walking disability. However, direct evidence of 6MW induced gait deterioration is limited by the difficulty of measuring qualitative changes in walking.
Objectives
This study aims to (1) define and validate a measure of fatigue-related gait deterioration based on data from body-worn sensors; and (2) use this measure to detect gait deterioration induced by the 6MW.
Methods
Gait deterioration was assessed using the Warp Score, a measure of similarity between gait cycles based on dynamic time warping (DTW). Cycles from later minutes were compared to baseline cycles in 89 subjects with MS and 29 controls. Correlation, corrected (partial) correlation, and linear regression were used to quantify relationships to walking and fatigue outcomes.
Results
Warp Scores rose between minute 3 and minute 6 in subjects with mild and moderate disability (p < 0.001). Statistically significant correlations (p < 0.001) to the MS walking scale (MSWS-12), modified fatigue impact scale (MFIS) physical subscale, and cerebellar and pyramidal functional system scores (FSS) were observed even after controlling for walking speed. Regression of MSWS-12 scores on Warp Scores and walking speed explained 73.9% of response variance. Correlations to individual MSWS-12 and MFIS items strongly suggest a relationship to fatigability.
Conclusion
The Warp Score has been validated in MS subjects as an objective measure of fatigue-related gait deterioration. Progressive changes to gait cycles induced by the 6MW often appeared in later minutes, supporting the importance of sustained walking in clinical assessment.
Keywords: six-minute walk, multiple sclerosis, dynamic time warping, body-worn sensors, walking impairment
INTRODUCTION
Walking outcomes are central to multiple sclerosis (MS) care and research because of the pervasiveness of MS-related walking impairment and its negative consequences. Loss of mobility reduces functional independence1, and it is reported to be the most challenging aspect of the disease2.
The six-minute walk (6MW) has become a common walking outcome measure in MS3 thought to measure fatigability and overall walking disability4. It may be more sensitive to motor fatigue than other objective measures, particularly in mild disease3. The 6MW also induces physiologic changes in MS subjects, as shown by the increase in oxygen consumption occurring over the first three minutes5. Unfortunately, direct evidence of motor fatigue during the 6MW is limited by the inherent difficulty of measuring qualitative changes in walking.
Inertial sensors have enhanced gait and balance assessment in MS via extracted features such as sway and angular motion6–8. However, our experience suggests these features may not detect the subtle, progressive changes occurring during the 6MW. Instead, pairs of gait cycles from different portions of the walk can be directly compared using the dynamic time warping (DTW) algorithm. DTW has been applied to inertial gait data to recognize persons by their gait patterns9 and distinguish normal walking from simulated pathology10.
Some practitioners believe that a two-minute walk (2MW) is sufficient for clinical assessment, citing high correlation between 6MW and 2MW distances11. However, based on repeated observation of 6MW trials, we hypothesize that measurable gait deterioration sometimes appears beyond two minutes; and further, that the degree of deterioration correlates with subjectively reported walking difficulty. To test this, we measured progressive changes in gait by applying DTW to gait cycles from the 6MW. Our primary purpose was to discover and validate objective evidence of gait alteration, supporting the importance of sustained walking in clinical assessment. A secondary goal was to establish a device-based measure sensitive to aspects of walking disability reported by subjects, but not detected by other objective means. Such a measure would improve our understanding of gait dynamics in MS and compliment other walking outcomes in clinical trials and care evaluation.
METHODS
Data Collection
All study procedures were approved by the University of Virginia (UVa) Institutional Review Board for Health Sciences Research, and written consent was obtained from all participants. Eighty-six subjects with clinically definite MS12 and 29 healthy controls were recruited. Subjects were screened by chart review and self-report. All subjects were age 18 to 64 years and ambulatory. Subjects with neurological impairment from other diagnoses (e.g. stroke), orthopedic limitations of the lower extremity, morbid obesity, or known cardiac or respiratory disease were also excluded.
Subjects completed a six-minute walk (6MW) wearing an ActiGraph GT3X accelerometer on one hip. Neurological examination was conducted by Neurostatus-certified staff prior to the walk for Expanded Disability Status Scale (EDSS)13 assessment. The 6MW was conducted in a 75-foot hospital corridor using the instructions and script developed by Goldman et al3.
The MS Walking Scale (MSWS-12)14 and Modified Fatigue Impact Scale (MFIS)15 were the primary outcomes used to validate DTW results. These patient-reported outcomes (PROs) use five-point rating scales to assess MS-related walking impairment and fatigue, respectively, from the patient perspective. Both outcomes are scored by summing responses on all items. The MSWS-12 is then linearly normalized to a 100-point scale. Physical, cognitive, and psychosocial subscores of the MFIS are calculated by summing items pertaining to that domain.
The MFIS was collected in control subjects, but EDSS and MSWS-12 were not. Controls were assigned a 0 on the EDSS and MSWS-12 for statistical analysis, as subjects with any form of disability were excluded from the control group.
Gait Cycle Extraction
Raw 3-axis acceleration data at 30 samples per second were exported via ActiLife 6.1 and processed in Matlab R2015a. The data were separated into minute-long segments and multiple gait cycles were taken from each segment of interest. Gait cycles are typically identified by the heel strike or toe-off in healthy subjects, but in MS these features can be irregular or absent. Instead, each subject’s cycle frequency was identified by taking the fast Fourier transform (FFT) of the entire walk. The highest peak within the 0.2 – 2.0 Hz band was identified as the cycle rate, and non-overlapping segments of corresponding length were extracted as gait cycles. Importantly, this method does not guarantee that all cycles are in phase, as discussed in the next section.
Dynamic Time Warping (DTW)
DTW is a common algorithm for curve registration, in which two sequences are aligned to minimize the Euclidean distance between them16. The DTW algorithm takes two sequences as inputs, aligns them, and returns two outputs, the DTW distance and warping path (Figure 1). The warping path specifies the optimal alignment between cycles, and the DTW distance is the Euclidean distance between them once aligned. Cycle alignment is needed to prevent measured similarity between cycles from being unduly affected by small differences in timing16.
Figure 1.
The Dynamic Time Warping (DTW) algorithm aligns two gait cycles from a subject with mild disability (EDSS = 2.5). The asterisks show the same peak before and after phase correction. Two regions with warp insertions have been circled; warps may be identified by the horizontal segments indicating repeated data points. The DTW Distance is the Euclidean distance between the cycles on the right, and the warping length is the number of warps inserted.
Before running DTW, cycles were resampled to be 100 samples in length and normalized by subtracting the mean along each axis, then dividing all samples by the standard deviation of the vector magnitude. These steps mitigate the effects of cycle length and gait speed on the results. Our implementation of DTW operates on sequences of three-dimensional acceleration vectors, so the acceleration components (x, y, and z) are analyzed together, not individually.
Gait cycles from the first and second minutes of the 6MW were used as template cycles, serving as a baseline for comparison to later cycles. Cycles from subsequent minutes were used as test cycles. Template cycles were compared to test cycles from each minute, resulting in (m × n) DTW distances and warping paths per minute, where m is the number of template cycles and n the number of test cycles for that minute. Each warping path was reduced to a single number, the warping length, calculated as the difference in length between input cycles and DTW-aligned counterparts. The warping length measures how much cycles were stretched to achieve the optimal alignment.
The DTW distances and warping lengths from each minute were reduced to single values: the Distance Score and Warp Score, respectively. These were calculated by taking the best match for each cycle – i.e. the minimum over the columns and rows – then computing the median best match for both template and test sets. Finally, the two median values were averaged into a single score9. Using the median (rather than the mean) makes results less sensitive to falls, turns, stops, and other anomalies. Thus the Distance Score is a composite score based on many DTW distances, and the Warp Score is a composite score based on many warping lengths.
A phase-invariant implementation of DTW was needed to identify a common starting point between cycles. Given cycles A = (a1, …, an) and B = (b1, …, bn), all possible offsets were examined: DTW was run between Ai = (ai, …, an, a1, …, a(i−1)) and B for all i ∈ {1, …, n} This would have increased computation 100-fold – once for each starting point – but Keogh lower bounding and early DTW termination were used to pre-emptively abandon starting points that could not be optimal17. Sakoe-Chiba banding was also used to reduce computation, with the adjustment window set to 25 samples18.
Statistical Analysis
Statistical analysis was conducted in Matlab R2015a. Subjects were grouped by EDSS as mild (EDSS 0 – 2.5), moderate (EDSS 3.0 – 4.5), and severe (EDSS 5.0 – 6.5)3 to highlight differential DTW performance. Two-sample and paired t-tests were used to test between-group and within-group changes in DTW results, respectively, with p < 0.05 taken as significant. Effect sizes were calculated using Cohen’s d. Demographic characteristics were compared using chi-square test or t-test as appropriate for categorical and interval variables, respectively.
Correlations between DTW results and clinical outcomes were calculated using Spearman’s rho (rs), which is appropriate when working with ordinal data. The following outcomes were evaluated: EDSS, including individual functional system scores (FSS); MSWS-12, including all individual items; and MFIS, including the physical subscale and all individual items. Partial Spearman correlations (prs) were also calculated for all outcomes to correct for the effects of age, walking speed, and the cumulative time elapsed. Controlling for walking speed is necessary to demonstrate that DTW captures distinct information compared to traditional administration of the 6MW. Due to the large number of correlations tested, a conservative significance threshold (p < 0.001) was applied. Correlations refer to results from all minutes of the walk unless otherwise specified.
An exploratory linear model of MSWS-12 scores was fitted using the following predictors: walking speed, Distance Score, Warp Score, cumulative time elapsed, age, EDSS, and all interaction terms. Additional models with all possible subsets of these predictors were then fitted, and the model minimizing the Bayesian Information Criterion (BIC) was chosen as the final model.
RESULTS
Demographics
Participant demographics are summarized in Table 1. MS subjects and controls are similar in sex (p = 0.059) but significantly different in age, MFIS score, and 6MW distance (p < 0.001). All subjects had EDSS ≤ 7, and most had mild disability (median EDSS = 2.5). Of the 86 MS subjects, 48 had mild disability (EDSS 0 – 2.5), 27 moderate (EDSS 3.0 – 4.5), and 11 severe (EDSS 5.0 – 6.5).
Table 1.
Demographics and Outcomes Measures in MS Subjects and Controls
| MS | Control | |
|---|---|---|
| N (Female/Male) | 86 (73/13) | 29 (20/9) |
| Age, median (range) [IQR] | 46 (19 – 61) [38 – 52] | 40 (19 – 54) [32 – 48] |
| EDSS, median (range) [IQR] | 2.5 (0 – 7) [2 – 3.5] | NA |
| MSWS-12 Score, median (range) [IQR] | 14.6 (0 – 100) [0 – 45.8] | NA |
| MFIS Score, median (range) [IQR] | 29 (0 – 69) [10.5 – 45.5] | 9 (0 – 42) [2.5 – 25] |
| 6MW Distance*, median (range) [IQR] | 1574 (129 – 2281) [1352 – 1873] | 2009 (1529 – 2587) [1793 – 2166] |
IQR: Inter-Quartile Range; EDSS: Expanded Disability Status Scale; MSWS-12: MS Walking Scale; MFIS: Modified Fatigue Impact Scale; 6MW: Six-Minute Walk
distance reported in feet
Warp Score Progression and Variability
Best results were obtained using the Warp Score with minute 2 as the template set. For example, the correlation between the Warp Score and MSWS-12 was stronger with minute 2 as the template set (rs = 0.429) compared to minute 1 (rs = 0.385). Similarly, the Warp Score was more strongly correlated with the MSWS-12 than the Distance Score (rs = 0.429 and rs = 0.263, respectively). Thus in the sections to follow, we present Warp Score results with minute 2 cycles used as the template set.
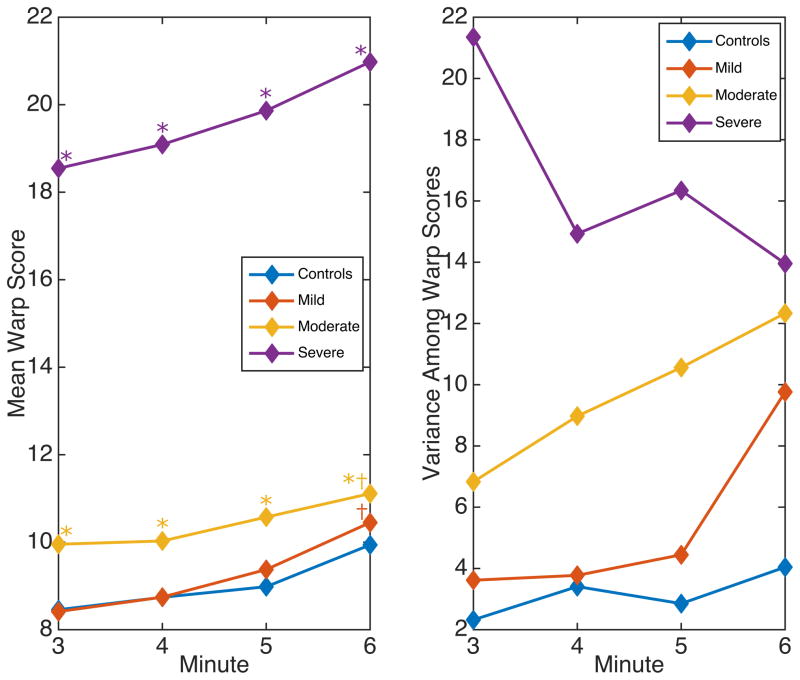
Figure 2 shows the mean Warp Score (left) and variance among Warp Scores (right) among subjects grouped by EDSS in minutes 3 to 6. Warp Scores were much higher in severe subjects (EDSS > 4.5) than in all other groups (p < 0.001). Variance decreased over time in the severe group, but increased in other groups. Warp Scores were higher in moderate subjects (EDSS 3 – 4.5) compared to controls even in minute 3 (d = 0.706, p = 0.01), with steadily increasing variance. Moderate subjects had significantly higher warp scores than mild subjects (EDSS 0 – 2.5) in minute 3 (d = 0.700, p = 0.006), but this gap narrowed by minute 6 (d = 0.202, p = 0.41). Mild subjects and controls had similar Warp Scores in minutes 3 and 6 (d = 0.024, p = 0.92 and d = 0.186, p = 0.44, respectively). Variance among mild subjects increased sharply between minutes 5 and 6 from just over 4 to almost 10. Warp Scores increased between minutes 3 and 6 in mild (d = 0.786, p < 0.001) and moderate subjects (d = 0.374, p < 0.001).
Figure 2.
Warp Score mean (left) and variance (right) in subjects grouped by EDSS from minutes 3 to 6.
Correlations between Warp Scores and Clinical Outcomes
Non-corrected correlations to Warp Scores were significant for 9 of 13 clinical outcomes, the only exceptions being the Sensory and Cerebral FSS. Corrected correlations between Warp Scores (all minutes) and the MSWS-12, MFIS physical subscale, EDSS, and the Pyramidal, Cerebellar, and Sensory FSS are statistically significant. The MFIS and Bowel and Bladder FSS almost reach significance (p = 0.0050 and p = 0.0091). Note that the corrected correlation to the Sensory FSS is significant and negative.
All MSWS-12 items had statistically significant corrected correlation to the Warp Score (p < 0.001) except Item 5 (“Limited your balance when standing or walking?”) (p = 0.011). Corrected correlation was strongest in Item 7 (“Increased the effort needed for you to walk?”) (prs = 0.383) and Item 6 (“Limited how far you are able to walk?”) (prs = 0.331). Corrected correlations were also significant for the following MFIS items: 4, 6, 9, 10, 17, 18, 20. Five of these items are on the MFIS physical subscale, with Item 20 (“I have limited my physical activities”) being strongest (prs = 0.261). Two items not on the physical subscale also reach significance: Item 9 (“I have been limited in my ability to do things away from home”) (prs = 0.161) and Item 18 (“My thinking has been slowed down”) (prs = 0.163). Correlations to all MSWS-12 and MFIS items may be found in the supplementary materials.
MSWS-12 Regression Model
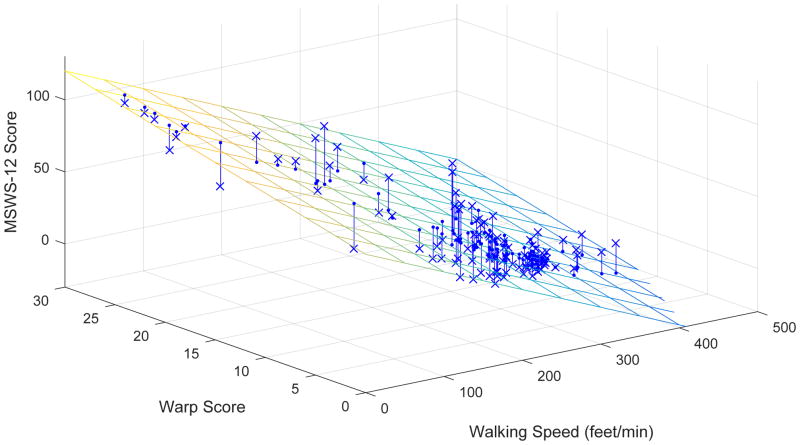
The MSWS-12 regression model with lowest BIC (Figure 3) included only walking speed and the Warp Score as predictors, both of which easily reached statistical significance (p < 10−10). This model explained almost 74% of total MSWS-12 variance (r2 = 0.739, adjusted r2 = 0.715). In contrast, simple linear regression on walking speed or Warp Scores alone explained less of the total variance (r2 = 0.683 and r2 = 0.480, respectively).
Figure 3.
Multiple regression of the MSWS-12 on walking speed and Warp Scores from minute 6. Residuals are shown as lines from observed scores (X) to predicted scores (on the plane). *significantly different from controls; †significantly different from minute 3
Warp Scores and MSWS-12 scores were more strongly correlated by minute 6 (prs = 0.322, p < 0.001) compared to minute 3 (prs = 0.215, p = 0.025). Consequently, the MSWS-12 model performed better in minute 6 (r2 = 0.753, adjusted r2 = 0.748) than in minute 3 (r2 = 0.720, adjusted r2 = 0.715).
In one notable mild subject (EDSS = 2.0), the Warp Score rose from 15.5 in minute 5 to 23.5 in minute 6. Simple regression on walking speed estimated this subject’s MSWS-12 as 12.4, higher than the average score among mild subjects (μ = 6.4). However, their true MSWS-12 score was 27.1, resulting in a large model error (14.7). Interestingly, they also had an unusually high MFIS response of 3 on two items: Item 6 (“I have had to pace myself in my physical activities”) and Item 21 (“I have needed to rest more often or for longer periods”). In contrast, the multiple regression model accurately estimated the MSWS-12 score (estimate = 26.9, error = 0.2).
DISCUSSION
We have used Dynamic Time Warping (DTW) to measure changes in gait dynamics induced by the 6MW. Gait deterioration from minute to minute has been quantified using the Warp Score, which measures the stretch needed to align gait cycles, and the Distance Score, which measures their similarity once aligned.
Warp Scores were more strongly correlated to clinical outcomes than Distance Scores. This may reflect a fundamental difference between the current work and other applications of DTW, as the Distance Score is commonly reported while the Warp Score is not. Typically, DTW is used to overcome signal distortions, but here the distortions themselves – the stretch captured by the Warp Score – are manifestations of gait pathology. Further work is needed to clarify the difference between these measures in walking data, and to study the Warp Score’s value in other applications.
As hypothesized, Warp Scores correlate with subjectively reported walking difficulty (MSWS-12) and physical fatigue (MFIS). Among individual items, the corrected correlation was strongest for MSWS-12 Item 7, which asks how much MS has “Increased the effort needed for you to walk”. On the MFIS, corrected correlations were strongest for items related to physical fatigue.
These findings can be partly explained by disease severity; but importantly, correlations persist even after controlling for walking speed. Thus the Warp Score tells a different story compared to other objective measures, one confirmed by subjective report. Critically, information is drawn out over the walk: Warp Scores rise in mild and moderate subjects between minutes 3 and 6, with a corresponding increase in correlation to the MSWS-12. As case in point, one subject’s Warp Score rose only in minute six. Their speed was steady throughout the walk, yet they had a high score on the MFIS physical subscale compared to other subjects with similar disability. Walking speed was not sensitive to this subject’s fatigue, but the Warp Score detected it.
Mild subjects’ Warp Scores were similar to controls on average, but the sharp increase in variance in minute 6 is important. Compared to controls, mild subjects’ Warp Scores were less consistent: some increased toward the end of the walk, while others were unchanged. The regression model shows that these differences are meaningful, as they improve prediction of MSWS-12 scores. In contrast, moderate subjects had higher Warp Scores throughout the walk, with steadily increasing variance. Evidently gait deterioration appeared earlier in these subjects, becoming more common and extreme over time. Severe subjects’ scores are much higher on average, and unlike the other groups, variance decreases. This occurs because some subjects’ Warp Scores are high in minute 3, but most are high by minute 6, increasing consistency within the group.
The negligible corrected correlations to the Vision, Cerebral, and Brainstem FSS provide assurance that Warp Scores measure gait deterioration, not something else. In contrast, strong correlation to the Bladder and Bowel FSS is expected, as bladder dysfunction correlates with spinal cord disease and pyramidal impairment19. Interestingly, the negative corrected correlation between Warp Scores and Sensory FSS suggests that for patients at similar pace, high Sensory FSS implies less gait deterioration during the walk.
Progressive increases in the Warp Score are evidence of gait deterioration elicited by sustained walking. Measurable deterioration can emerge in later minutes of the walk, as shown by statistically significant increases in Warp Scores among mild and moderate subjects. While 6MW and 2MW distances are highly correlated on a group level, the 2MW may not be long enough to produce similar results. Further work is needed to directly study the 2MW, which is distinct from the first two minutes of the 6MW.
The Warp Score is an objective measure of gait deterioration, a finding previously observed only by subjective means. Correlations to the MSWS-12 and MFIS validate the Warp Score as a real and meaningful feature of MS gait. This measure compares current walking patterns to a known baseline, an approach which may be applicable to a broader range of gait pathologies. Warp Scores can be collected at low cost, requiring only a single, hip-worn accelerometer; and unlike PROs, patient input and initiative are not required. Together with distance, this new measure could be used as a 6MW-based walking outcome. With continued study, device-derived measures have great potential to facilitate and improve MS care.
Supplementary Material
Table 2.
Spearman Correlations and Corrected Spearman Correlations between Clinical Outcomes and Warp Scores in All Subjects
| Spearman Correlation | Corrected Spearman Correlation | |||
|---|---|---|---|---|
| rs | p | prs | p | |
| MSWS | 0.429* | < 0.0001 | 0.363* | < 0.0001 |
| MFIS | 0.273* | < 0.0001 | 0.136 | 0.0050 |
| MFIS_p | 0.293* | < 0.0001 | 0.161* | 0.0008 |
| EDSS | 0.367* | < 0.0001 | 0.257* | < 0.0001 |
| Cerebellar FSS | 0.464* | < 0.0001 | 0.258* | 0.0001 |
| Sensory FSS | −0.052 | 0.4251 | −0.258* | 0.0001 |
| Pyramidal FSS | 0.471* | < 0.0001 | 0.241* | 0.0002 |
| Bowel and Bladder FSS | 0.458* | < 0.0001 | 0.170 | 0.0091 |
| Vision FSS | 0.227* | 0.0004 | 0.098 | 0.1347 |
| Cerebral FSS | 0.164 | 0.0108 | −0.063 | 0.3330 |
| Brainstem FSS | 0.254* | 0.0001 | 0.003 | 0.9666 |
MSWS: MS Walking Scale; MFIS: Modified Fatigue Impact Scale; MFIS-P: MFIS Physical Subscale; EDSS: Expanded Disability Status Scale; FSS: Functional System Score
statistically significant (α = 0.001)
HIGHLIGHTS.
A sensor-based measure of gait deterioration is validated in multiple sclerosis subjects.
This measure – the Warp Score – quantifies gait cycle “stretching” induced by the six-minute walk.
Warp scores correlate with patient-reported fatigue even after controlling for walking speed.
Measurable evidence of gait deterioration can emerge after several minutes of walking.
Acknowledgments
This work was partly supported by the University of Virginia Broadband Wireless Access and Applications Center (BWAC) and the ziMS Foundation. Dr. Goldman is supported by the National Institutes of Health – National Institute of Neurologic Disorders and Stroke (K23NS62898). Funders did not directly participate in research activities including study design, data collection, manuscript preparation, and manuscript submission.
We owe special thanks to Karen Schmidt, Josh Inouye, and Casey Engel for their helpful comments and revisions.
Footnotes
CONTRIBUTORS
Matthew M. Engelhard: study design; processing, analysis, and interpretation of the data; drafting the manuscript
Sriram Raju Dandu: processing, analysis, and interpretation of the data; revising the manuscript for intellectual content
Stephen D. Patek: revising the manuscript for intellectual content
John C. Lach: revising the manuscript for intellectual content
Myla D. Goldman: study design, interpretation of the data, revising the manuscript for intellectual content
All authors have approved the final version of this article.
CONFLICT OF INTEREST STATEMENT
Dr. Goldman reports grants from National Institutes of Health-National Institute of Neurologic Disorders and Stroke (K23NS62898), grants from Biogen Idec, grants from Novartis, other from Acorda, other from Biogen Idec, other from Novartis, personal fees from Questcor, and personal fees from Sarepta.
The remaining authors have no potential conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Matthew M. Engelhard, Email: mme@virginia.edu, Department of Systems and Information Engineering, University of Virginia, P.O. Box 400747, Charlottesville, VA, USA, 22904, 434-924-5393 (phone), 434-982-2972 (fax)
Sriram Raju Dandu, Email: sd9aj@virginia.edu, Department of Electrical and Computer Engineering, University of Virginia, P.O. Box 400743, Charlottesville, VA, USA, 22904.
Stephen D. Patek, Email: patek@virginia.edu, Department of Systems and Information Engineering, University of Virginia, P.O. Box 400747, Charlottesville, VA, USA, 22904
John C. Lach, Email: jlach@virginia.edu, Department of Electrical and Computer Engineering, University of Virginia, P.O. Box 400743, Charlottesville, VA, USA, 22904
Myla D. Goldman, Email: mdg3n@virginia.edu, Department of Neurology, University of Virginia, P.O. Box 800394, Charlottesville, VA, USA, 22908
References
- 1.Sutliff MH. Contribution of impaired mobility to patient burden in multiple sclerosis. Curr Med Res Opin. 2010;26:109–119. doi: 10.1185/03007990903433528. [DOI] [PubMed] [Google Scholar]
- 2.LaRocca DNG. Impact of Walking Impairment in Multiple Sclerosis. Patient Patient-Centered Outcomes Res. 2012;4:189–201. doi: 10.2165/11591150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Goldman MD, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. 2008;14:383–390. doi: 10.1177/1352458507082607. [DOI] [PubMed] [Google Scholar]
- 4.Kieseier BC, Pozzilli C. Assessing walking disability in multiple sclerosis. Mult Scler J. 2012;18:914–924. doi: 10.1177/1352458512444498. [DOI] [PubMed] [Google Scholar]
- 5.Motl RW, Suh Y, Balantrapu S, Sandroff BM, Sosnoff JJ, Pula J, Goldman MD, Fernhall B. Evidence for the different physiological significance of the 6- and 2-minute walk tests in multiple sclerosis. BMC Neurol. 2012;12:6. doi: 10.1186/1471-2377-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spain RI, Mancini M, Horak FB, Bourdette D. Body-worn sensors capture variability, but not decline, of gait and balance measures in multiple sclerosis over 18 months. Gait Posture. 2014;39:958–964. doi: 10.1016/j.gaitpost.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spain RI, St George RJ, Salarian A, Mancini M, Wagner JM, Horak FB, Bourdette D. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait Posture. 2012;35:573–578. doi: 10.1016/j.gaitpost.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritz NE, Newsome SD, Eloyan A, Marasigan RER, Calabresi PA, Zackowski KM. Longitudinal relationships among posturography and gait measures in multiple sclerosis. Neurology. 2015;84:2048–2056. doi: 10.1212/WNL.0000000000001580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulgouris NV, Plataniotis KN, Hatzinakos D. Gait recognition using dynamic time warping. IEEE 6th Workshop Multimed Signal Process; 2004; pp. 263–266. [Google Scholar]
- 10.Engelhard MM, Dandu SR, Lach JC, Goldman MD, Patek SD. Toward Detection and Monitoring of Gait Pathology Using Inertial Sensors Under Rotation, Scale, and Offset Invariant Dynamic Time Warping. Proc 10th EAI Int Conf Body Area Netw; 2015; pp. 269–275. [Google Scholar]
- 11.Gijbels D, Eijnde BO, Feys P. Comparison of the 2- and 6-minute walk test in multiple sclerosis. Mult Scler J. 2011;17:1269–1272. doi: 10.1177/1352458511408475. [DOI] [PubMed] [Google Scholar]
- 12.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–1444. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 14.Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: The 12-Item MS Walking Scale (MSWS-12) Neurology. 2003;60:31–36. doi: 10.1212/wnl.60.1.31. [DOI] [PubMed] [Google Scholar]
- 15.Ritvo P, Fischer JS, Miller DM, Andrews H, Paty DW, LaRocca NG. MSQLI—Multiple Sclerosis Quality of Life Inventory, Users Man. New York: Natl MS Soc; 1997. [Google Scholar]
- 16.Keogh E, Ratanamahatana CA. Exact indexing of dynamic time warping. Knowl Inf Syst. 2004;7:358–386. [Google Scholar]
- 17.Keogh E, Wei L, Xi X, Vlachos M, Lee S-H, Protopapas P. Supporting Exact Indexing of Arbitrarily Rotated Shapes and Periodic Time Series Under Euclidean and Warping Distance Measures. VLDB J. 2009;18:611–630. [Google Scholar]
- 18.Sakoe H, Chiba S. Dynamic programming algorithm optimization for spoken word recognition. IEEE Trans Acoust Speech Signal Process. 1978;26:43–49. [Google Scholar]
- 19.Betts CD, D’Mellow MT, Fowler CJ. Urinary symptoms and the neurological features of bladder dysfunction in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1993;56:245–250. doi: 10.1136/jnnp.56.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.