Abstract
Fibrous dysplasia (FD) is an uncommon and debilitating skeletal disorder resulting in fractures, deformity, functional impairment and pain. It arises from postzygotic somatic activating mutations in GNAS, in the cAMP-regulating transcript α-subunit, Gsα. Constitutive Gs signaling results in activation of adenylyl cyclase and dysregulated cAMP production. In the skeleton this leads to the development of FD lesions with abnormal bone matrix, trabeculae, and collagen, produced by undifferentiated mesenchymal cells. FD may occur in isolation or in combination with extraskeletal manifestations, including hyperfunctioning endocrinopathies and café-au-lait macules, termed McCune-Albright syndrome (MAS). This review summarizes current clinical and translational perspectives in FD/MAS, with an emphasis on FD pathogenesis, natural history, pre-clinical and clinical investigation, and future directions.
Keywords: fibrous dysplasia, McCune-Albright syndrome, GNAS gene, Gsα, FGF23
Introduction
Fibrous dysplasia (FD; OMIM 174800) is an uncommon skeletal disorder resulting in fractures, deformity, pain and functional impairment. First described by Lichtenstein in 1938, FD is an example of somatic mosaicism occurring along a broad clinical spectrum [1]. Disease may affect one bone (monstotic) or multiple (polyostotic), and may occur in isolation or in combination with café-au-lait spots and hyperfunctioning endocrinopathies, termed McCune-Albright syndrome (MAS) [2]. Any part or combination of features is possible, giving rise to a phenotype that is complex and striking in its diversity.
GNAS gene
FD/MAS is caused by somatic activating mutations in GNAS, located on the q arm of chromosome 20 at position 13.3. The GNAS locus is a complex, imprinted gene that uses several promoters to produce assorted gene products, including the ubiquitously expressed α-subunit of the Gs stimulatory protein (Gsα) [2]. The primary role of Gsα is to couple hormones and certain seven-transmembrane receptors to adenylyl cyclase, facilitating production of intracellular cyclic AMP [3]. Gsα is comprised in part by an intrinsic GTPase domain, which binds guanine nucleotide and interacts with specific receptors and effectors. The intrinsic GTPase activity of Gsα inactivates receptor signaling by hydrolyzing bound GTP to GDP [2, 4].
In FD/MAS, activating missense mutations in Gsα have been identified in two amino acid residues: Arg201 (>95% mutations, divided evenly between R201C and R201H) or Gln227 (<5%)[5]. R201 is an essential regulator of GTPase, and mutations in this domain result in disruption of GTPase activity and constitutive Gsα activation [2]. Intracellularly, this results in dysregulated production of cyclic AMP and other downstream messengers [6]. Constitutive receptor activation results in overproduction of hormones, manifesting clinically as hyperfunctioning endocrinopathies and increased skin pigmentation. The effects of Gsα activation on bone are complex, and are discussed in detail below.
Mosaicism in FD/MAS
FD/MAS arises sporadically, and there are no confirmed cases of vertical transmission. This has led to the unproven but generally accepted model of FD pathogenesis where post-zygotic GNAS mutations acquired early in embryogenesis lead to a somatic mosaic disease state [7]. Under this model, the lack of vertical transmission presumably reflects early lethality in germline mutations. This concept is also supported by reports of discordance of FD/MAS between monozygotic twins [8-10].
Mosaicism is apparent in the clinical features of FD/MAS, including patchy bone disease occurring along a broad spectrum of distribution, which can range from an isolated, trivial lesion to severe disease involving nearly the entire skeleton (fig 1A and B). Skin lesions additionally have characteristic jagged borders and reflect along the midline of the body, following patterns of embryonic cell migration (fig 1C and D). Under this model, the phenotype in FD/MAS is determined by a combination of factors; including the point in development in which the mutation occurs, the locations to which the mutated progeny cell migrate, and the specific effects of constitutive Gsα in the affected tissues. In cases where tissues originating from all three germ layers are involved (i.e. ectoderm, mesoderm, and endoderm, comprising portions of the skin, endocrine, and skeletal systems), it can be postulated that the mutation arose in a single pluripotent cell at the inner cell mass stage, prior to the derivation of the germ layers.
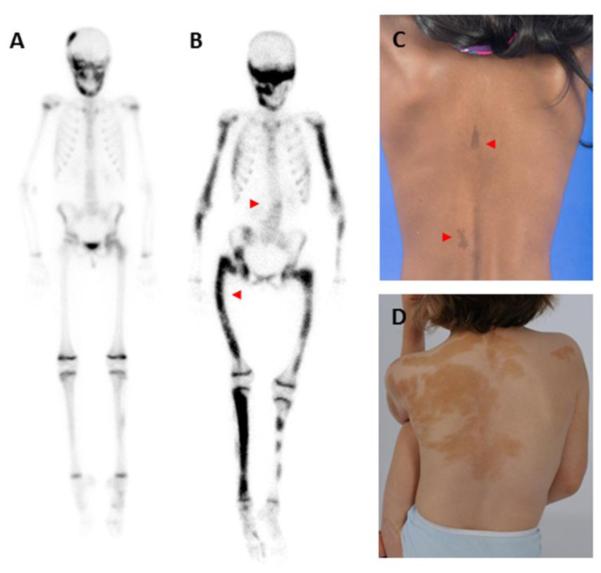
Figure 1. Mosaicism in fibrous dysplasia/McCune-Albright syndrome.
Panels A and B show 99technetium bone scintrigraphy studies. Note physiologic bilateral tracer uptake in growth plates in the extremities and ribs. Additional areas of tracer uptake correspond to FD lesions, which range from mild (panel A, limited to the skull and left femur) to severe (panel B, involving the majority of the skeleton). Note the presence of scoliosis and a right-sided coxa vara (“shepherd’s crook”) deformity in panel B (red arrowheads). Panels C and D demonstrate typical café-au-lait macules, which tend to have jagged borders and reflect along the midline of the body, following patterns of embryonic cell migration. Macules occur along a broad spectrum, which may range from small subtle lesions (panel C, red arrowheads) to large areas of pigmentation with extensive truncal involvement (panel D).
Animal studies provide further support for a mosaic model of FD. Bianco et al observed that cells with both normal and disease-associated genotype are found within an individual FD lesion [11]. In this study, transplantation of clonal populations of mutant cells into immunocompromised mice led to loss of transplanted cells, while transplantation of a mixture of normal and mutant cells resulted in formation of abnormal ectopic ossicles with histologic features consistent with human FD [11]. These findings suggest that somatic mosaicism at the tissue level is characteristic of FD, and the presence of both mutant and normal cells are required for the formation of dysplastic lesions.
Cellular and histologic features of fibrous dysplasia
FD is a disease of postnatal skeletal stem cells. In bone, constitutive Gsα activation gives rise to bone marrow stromal cells (BMSCs) with impaired capacity to differentiate towards mature osteoblasts, adipocytes, and hematopoiesis-supporting stroma. Bone and bone marrow are thus replaced by proliferating BMSCs, resulting in fibro-osseous tissue typically devoid of hematopoietic marrow [5]. The changes in the properties of skeletal progenitors mediate the pathological effect of mutant Gsα on bone.
FD is characterized by complex site-specific histologic changes falling broadly into three categories [12] (fig 2). In this classic form, low to moderately cellular fibrous stroma surround irregular, curvilinear trabeculae of woven bone, arranged in a discontinuous pattern commonly referred to as a “Chinese writing pattern”. The sclerotic/pagetoid histologic type is typically associated with cranial bones, and characterized by dense, sclerotic trabecular bone with complex systems of arrest/reversal (cement) lines. The sclerotic/hypercellular type is characteristically associated with gnathic and skull base bones, and includes discontinuous bony trabeculae distributed in an ordered, often parallel pattern. At all sites, subtle recurrent features can generally distinguish FD from other fibro-osseous lesions, including stellate-shaped osteoblasts, Sharpey fibers, and excess osteoid indicative of undermineralization [12, 13].
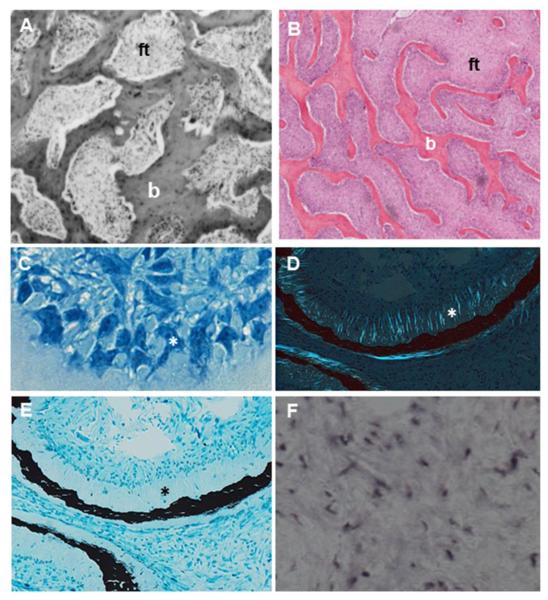
Figure 2. Representative histologic features of fibrous dysplasia (FD).
A. FD lesions in the calvarium demonstrate typical complexes of bone trabeculae (b) embedded in areas of fibrous tissue (ft). B. Bone trabeculae (b) within areas of fibrous tissue (ft) demonstrate a typical discontinuous and parallel pattern. C. Morphologically atypical osteoblasts located along bone surfaces (asterisk). D. Collagen fibers perpendicularly oriented along forming bone surfaces, also termed Sharpey fibers (asterisk). E. Severe undermineralization of FD bone, as evidenced by excess osteoid (asterisk). F. In situ hybridization demonstrates FGF23 production by activated FD osteogenic cells.
Clinical features of FD/MAS
FD/MAS has a broad phenotypic spectrum, with disease potentially involving any part or combination of the skin, bone, and endocrine systems. Café-au-lait skin macules are typically the first manifestation, and are apparent at or shortly after birth. These lesions characteristically reflect along the midline of the body and include jagged, irregular borders said to resemble the ‘coast of Maine’, in contrast to the smooth-bordered ‘coast of California’ macules seen in neurofibromatosis (fig 1A and B).
FD lesions are typically not apparent at birth, and begin to manifest clinically during the first few years of life [14]. In the appendicular skeleton fractures and bowing are common; in particular the proximal femur which may develop the classic “Shepherd’s crook” coxa vara deformity [15, 16] (fig 1B). Scoliosis resulting from spinal FD occurs commonly [17] (fig 1B), and in rare cases can be severe, progressive, leading to respiratory compromise and even death [17, 18]. FD in the craniofacial region may cause facial deformity and rarely functional deficits, including vision and hearing loss [19, 20].
Precocious puberty is frequently the presenting symptom in girls with FD/MAS, affecting nearly 80% of patients [21]. Recurrent ovarian cysts result in episodic estrogen production and intermittent vaginal bleeding, which may ultimately lead to bone age advancement and reduced adult height. Testicular involvement is common in boys, however precocious puberty develops only rarely [22]. Thyroid abnormalities affect approximately 2/3 of patients, half of which are associated with frank hyperthyroidism [23]. Growth hormone excess occurs less commonly, affecting 15-20% of patients [21]. Fibroblast growth factor-23 (FGF23) is overproduced by FD cells, which may lead to frank hypophosphatemia and rickets/osteomalacia in patients with high skeletal disease burden [24]. Hypercortisolism is the rarest endocrinopathy associated with MAS, and occurs exclusively during the neonatal period due to cortisol overproduction from the fetal adrenal gland. [25] Endocrinopathies typically develop during infancy or early childhood and persist into adulthood. Exceptions include neonatal hypercortisolism, which may undergo spontaneous resolution after involution of the fetal adrenal gland, and FGF23-mediated hypophosphatemia, which may wax and wane along with disease activity (discussed below).
Evaluation and treatment of endocrinopathies is an important component of management in FD/MAS, because endocrine dysfunction can exacerbate skeletal disease. In particular growth hormone excess is associated with expansion of craniofacial FD, leading to macrocephaly and increased risk of vision loss [26]. Hypophosphatemia has been associated with increased risk of fractures and bone pain [27].
Activating GNAS mutations in FD/MAS are considered weak oncogenes, and may infer a small increased risk of malignant transformation in affected tissues. Cancers reported in association with FD/MAS include bone [28], thyroid [29], breast [30, 31], and testicular [22]. Activating GNAS mutations have also been identified as early driver mutations for intraductal papillary mucinous neoplasms (IPMNs) [32], and these lesions along with rare cases of pancreatic cancer have been identified in patients with FD/MAS [33, 34].
Fibrous dysplasia and FGF23 processing
Studies of the role of FGF23 in FD/MAS have been informative in advancing understanding of mineral metabolism. The recognition that hypophosphatemia in FD/MAS results from overproduction of FGF23 by abnormal skeletal progenitor cells led to the observation that under physiologic conditions, normal bone is the tissue source of FGF23 [35]. While urinary phosphate wasting is one of the most common extraskeletal features of FD/MAS, frank hypophosphatemia occurs infrequently. Serum levels of FGF23 correlate with disease activity [35], and significant hypophosphatemia is typically seen only in patients with substantial FD burden. Hypophosphatemia is one of the few endocrinopathies that may wax and wane; it may be “unmasked” during periods of rapid linear growth, and may improve or resolve with age as disease activity declines [21, 24, 36].
The infrequency of hypophosphatemia in FD/MAS patients despite high levels of FGF23 production appears to be explained by alterations in FGF23 processing [37]. Under physiologic conditions, biologically active intact FGF23 is glycosylated by ppGalNAcT3 and cleaved by furin into inactive N- and C-terminal fragments. Total FGF23 levels are elevated in the serum of patients with FD/MAS, however there is a proportionally greater elevation in levels of the C-terminal fragment relative to intact FGF23 [24, 37]. An analysis of normal and mutation-bearing bone marrow stromal cells (BMSCs) demonstrated that cells harboring the causative Gsα mutation had higher cAMP levels, lower ppGalNAcT3, and higher furin activity [37]. These findings support a model where a cAMP-mediated decrease in glycosylation by ppGalNAcT3 results in enhanced FGF23 cleavage by furin, suggesting that regulation of FGF23 processing controls overall FGF23 activity in patients with FD/MAS. Clinically, hypophosphatemia is associated with early-onset and more frequent fractures, necessitating prompt identification and treatment [16].
Age-related changes in fibrous dysplasia
FD lesions are characterized by age-related histologic, radiographic and clinical changes. Kuznetsov et al. observed a decline in the number of mutated BMSCs and high levels of apoptosis in FD specimens from older individuals [36]. Lesions in older patients also demonstrated fewer histologic features characteristic of FD, and in some cases appeared to demonstrate normal bone and marrow histology, complete with restoration of hematopoiesis [36]. This suggests a model where, over time, mutation-bearing skeletal stem cells in the BMSC population fail to self-renew and are ultimately consumed by apoptosis, while co-existent normal skeletal stem cells survive and enable formation of normal marrow structures.
These findings are mirrored radiographically. In young children (<2 years of age), FD lesions often appear heterogeneous on radiographs and lack the classic “ground glass” appearance [38] (fig 3A). In later childhood, lesions typically develop a homogeneous “ground glass” radiographic density, and with aging appear denser and more sclerotic [38] (fig 3B and C). Craniofacial lesions in older individuals typically become less homogeneous on computed tomography, developing discrete radiolucent, “cystic” appearing areas over time [13] (fig 3D and E).
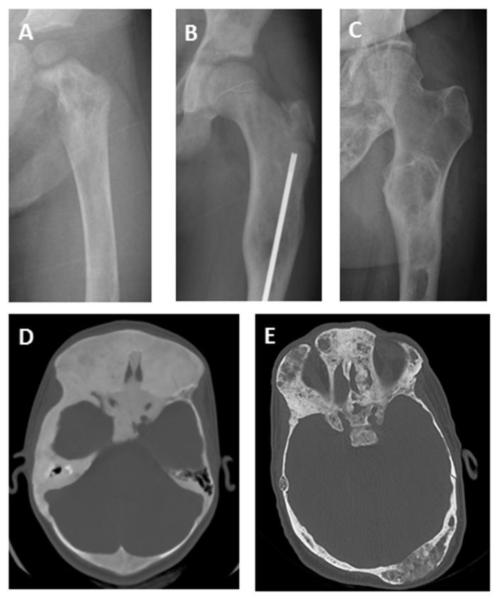
Figure 3. Age-related radiographic features of fibrous dysplasia (FD).
A. Radiograph from a 2-year-old demonstrates a typical heterogeneous-appearing FD lesion in the proximal femur. B. Radiograph from an 11-year-old shows a characteristic “ground glass” lesion, which appears homogeneous and radiolucent. C. Radiograph from a 57-year-old demonstrates the tendency of FD to become more heterogenous and sclerotic over time. D. Head computed tomography from an 11-year-old shows diffuse FD involvement with homogenous “ground glass” appearance. E. Head computed tomography from a 54-year-old demonstrates the tendency for FD to become more heterogeneous, with focal areas of lucency throughout.
The natural history of FD also supports a model of age-related changes in disease activity. Skeletal development appears to occur normally in utero, and FD lesions are typically not evident at birth. FD becomes apparent in early childhood, with the majority of skeletal lesions and their associated disability manifesting within the first decade of life [14]. In a retrospective series of 103 patients, 90% of craniofacial lesions were present by age 3.4 years, 90% of extremity lesions by 13.7 years, and 90% of axial lesions by 15.5 years [14]. Functional impairment was also established relatively early, with 92% of subjects who ultimately lost independent ambulation receiving ambulatory aids prior to age 17 [14]. Fracture rates in FD similarly peak relatively early in life, with the highest prevalence between ages 6 and 10 years and declining thereafter [16].
Taken together, these studies suggest an age-related pattern of disease activity, where skeletal lesions develop in the post-natal period, progress during childhood, and remain static or potentially improved in later adulthood. Therapeutic interventions for skeletal disease should therefore be tailored individually, with consideration given to the expected course and natural history of the disease.
Clinical Management
Management in FD/MAS should be individualized, and is best accomplished through the input of a multidisciplinary team. Treatment for FD lesions is mainly palliative, with an emphasis on optimizing function and minimizing morbidity related to deformities and fractures. There are no medications capable of altering the disease course. Optimizing conservative techniques is an important component of management, including controlling underlying endocrinopathies, physical therapy to maintain strength and range of motion, orthoses to correct limb length discrepancies and misalignment, and avoidance of prolonged non-weight bearing [39].
Surgical management is challenging, and there is little data to inform appropriate indications and techniques. Approaches such as curettage, grafting, plates and screws, and other external fixation devices are frequently ineffective and should generally be avoided [40, 41]. A retrospective analysis of a large cohort of patients who received femoral bone grafts showed a high rate of graft resorption, particularly in patients less than 18 years of age [40]. Intramedullary devices are generally preferred for management of fractures and deformity in the lower extremities [38, 41], and progressive scoliosis can generally be managed with standard instrumentation and fusion techniques [17, 41, 42]. Surgical management in the craniofacial skeleton is complicated by frequent post-operative FD regrowth, and should focus on correction of functional deformities [43, 44]. Optic nerve decompression may be required for patients with objective vision loss, however prophylactic decompression increases the risk of vision loss and is contraindicated [19, 45].
Antiresorptive therapy with bisphosphonates has been advocated due to high levels of bone resorption frequently seen in FD tissue [12, 46]. Early, uncontrolled series reported improvements in bone pain, with variable effects on FD radiographic appearance [47-49]. A 2-year randomized, placebo-controlled trial of alendronate did not demonstrate an improvement in FD-related bone pain, radiographic appearance or skeletal disease burden [46]. Given the data thus far, bisphosphonates are not likely to be effective in altering the disease course in FD, however more potent intravenous formulations may have a role in treating FD-related bone pain.
Pain is common in FD, and can significantly impact mobility and quality of life [50]. Interestingly, there is no association between the extent or location of the disease and the likelihood of having pain; patients with mild FD may have debilitating pain and patients with extensive disease may be pain-free [38]. FD pain is more common in adults, however it may be under-reported and under-treated in children [50]. Patients with FD pain should first be evaluated for underlying metabolic, functional, and orthopedic complications. In particular pain may be the presenting symptom in patients with untreated hypophosphatemia, leg-length discrepancies, and acute or impending fracture. When other causes have been excluded, intrinsic FD pain is generally treated with conservative measures and non-opioid analgesics. Intravenous bisphosphonates may be helpful for persistent, moderate to severe pain, given at the lowest dose and frequency needed [18].
Endocrinopathies are generally managed medically. Growth hormone excess responds to somatostatin analogues or pegvisomant in most cases [18, 51]. Precocious puberty in girls is managed by the aromatase inhibitor letrozole, which may be used in combination with leuprolide in patients with comorbid central puberty [18]. Precocious puberty in boys may be managed with a combination of an aromatase inhibitor and a testosterone receptor antagonist [22]. Similar to other disorders of FGF23 excess, hypophosphatemia is managed with oral phosphorus and calcitriol. Hyperthyroidism is effectively managed by methimazole, with total thyroidectomy as a potential option for long-term management [52].
In summary, clinical management in FD/MAS is complex and highly individualized depending upon the extent and severity of each patient’s presentation. A comprehensive compilation of evidence-based and expert opinion-based treatment for all aspects of FD/MAS is available in reference [18].
Animal models of fibrous dysplasia
The development of representative animal models in FD/MAS has been challenging. The first successful animal studies were reported by Bianco et al., where immunocompromised mice were given transplants of both normal and mutant BMSCs leading to the production of a human-like fibrous dysplastic ossicle [11]. This model provided valuable insights into FD pathogenesis by suggesting the necessity of both mutant and wild-type BMSCs to support formation of FD lesions. Limitations included the need for repeated transplantations, and the inability to replicate the age-related changes seen in human disease.
Hsiao et al. developed a mouse model with constitutive Gs signaling induced by expression of an engineered receptor, Rs1, in cells of the osteoblastic lineage [53]. Mice developed dramatic increases in bone volume, with histologic features reminiscent of FD [54]. Interestingly the phenotype was substantially attenuated when expression of the Rs1 receptor was delayed past the postnatal period, and was largely reversed when Rs1 signaling was suppressed in adult mice [53, 54]. While this model did not replicate the mosaic nature of the human disease phenotype, it provided important evidence for an age-dependent effect of Gs signaling on bone formation, and supported inhibition of Gs signaling as a potential therapeutic strategy to treat FD.
Saggio et al developed the first inherited, histopathologically similar replica of human FD by generating multiple lines of mice with constitutive Gsα R201C expression [55]. Similar to human disease, FD lesions in mice developed only in the postnatal period, however the lesions developed through a sequence of three distinct histopathological stages which do not appear to be characteristic of human disease. This included a primary phase of excess bone formation, followed by a secondary phase of excess remodeling with marked narrowing of the marrow cavity. The tertiary phase, which most resembled human FD, was not established in these mice until well into adulthood at age ≥ 1 year. This is notably different than the human phenotype, where lesions develop in early childhood. While animal studies have provided valuable insights in FD pathogenesis, further work is needed to develop a model that replicates key features of human disease, including mosaic gene expression and early postnatal lesion development.
Future Directions
There is a critical need to develop medical therapies to treat FD. Several lines of investigation are ongoing. Skeletal stem cells are an intuitive potential intervention, given that skeletal progenitors are the disease-causing cells in FD. These multipotential cells are self-renewing, and when transplanted in vivo have the ability to generate a miniature bone organ with appropriate histology, architecture and hematopoietic microenvironment [56]. Preclinical studies have demonstrated that when used in conjunction with appropriate carriers, in vivo transplantation of skeletal stem cell/BMSC may promote skeletal regeneration; however the quality of regeneration is greatly affected by the ability of transplanted cells [57, 58]. In contrast skeletal stem cell transfusion has been studied in a diversity of skeletal and immune-mediated disorders in an effort to harness purported paracrine, immunomodulatory and immunosuppressive effects of these cells, and results have been generally disappointing [59]. While it continues to hold promise as a potential therapeutic intervention, at present the clinical applicability of skeletal stem cells remains limited by knowledge gaps in their precise nature, function, and optimal handling and delivery [56, 59].
Another potential therapeutic strategy is to alter the activity of the mutant Gsα. Because greater than 90% of mutations in FD/MAS occur at the R201 position, this provides the opportunity to develop therapies that specifically target this mutated protein and modify its GTPase activity. In an ongoing project at the NIH, a small molecule library of >350,000 compounds was screened to identify modulators of Gsα activity [60]. Both inhibitors and activators were evaluated, based upon age-related changes that suggest further activation of the mutated Gsα might promote apoptosis of mutant BMSCs. Compounds have been subjected to secondary screens and are currently undergoing further investigation, with future studies including testing in preclinical in vivo models and early phase clinical studies.
Interest has recently emerged regarding a potential role for the receptor activator of nuclear kappa-B ligand (RANKL) pathway in treatment of FD. RANKL is a cell surface protein involved in many cellular processes, including osteoclastogenesis [61]. Denosumab is a humanized monoclonal antibody against RANKL, approved for treatment of osteoporosis and prevention of skeletal-related events from bone metastases [62, 63]. Denosumab interrupts the cycle of RANKL binding to osteoprotegerin and prevents the formation and function of osteoclasts [64]. Evidence suggests denosumab may be effective for treatment of giant cell tumors of bone, another disorder of BMSCs which is histologically similar to FD [65, 66]. RANKL is also reported to be overexpressed in a model of FD-like bone cells [6] and in FD tissue [67], suggesting this pathway may play a role in FD pathogenesis.
There are several reports of denosumab treatment in FD patients [68, 69], including a 9-year-old boy treated for a rapidly expanding femoral lesion [69]. In this patient denosumab treatment was associated with improved pain and decreased femoral expansion; however the patient developed hypophosphatemia and hypocalcemia while on treatment. Upon denosumab discontinuation, there was a marked rebound in bone turnover accompanied by life-threatening hypercalcemia, requiring hospitalization and bisphosphonate treatment [69]. Rebound hypercalcemia upon denosumab discontinuation has subsequently been reported in other disorders of high bone turnover, including giant cell tumor and juvenile Paget’s disease [70-72]. These findings suggest denosumab may hold promise as a potential treatment for FD, however given these safety concerns its use should be limited to research protocols.
Conclusions
Clinical and translational studies in FD/MAS have greatly advanced our understanding of the pathogenesis and natural history of this complex disorder. The development of therapeutic interventions has been relatively less robust, and current treatments are inadequate. There is a critical need to develop novel interventions capable of altering disease activity in FD, as well as pre-clinical and animal models in which to study them.
Acknowledgments
Funding: This research was supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research
Footnotes
Conflict of Interest
Cemre Robinson, Michael T Collins, and Alison M Boyce declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human subjects performed by any of the authors.
With regard to the authors’ research cited in this paper, all institutional and national guidelines for the care and use of laboratory animals were followed.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.Lichtenstein L. Polyostotic fibrous dysplasia. Arch Surg. 1938;36:874–898. [Google Scholar]
- 2.Weinstein LS, et al. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325(24):1688–95. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 3.Turan S, Bastepe M. GNAS Spectrum of Disorders. Curr Osteoporos Rep. 2015;13(3):146–58. doi: 10.1007/s11914-015-0268-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990;348(6297):125–32. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- 5.Riminucci M, et al. Fibrous dysplasia as a stem cell disease. J Bone Miner Res. 2006;21(Suppl 2):125–31. doi: 10.1359/jbmr.06s224. [DOI] [PubMed] [Google Scholar]
- 6.Piersanti S, et al. Transfer, analysis, and reversion of the fibrous dysplasia cellular phenotype in human skeletal progenitors. J Bone Miner Res. 2010;25(5):1103–16. doi: 10.1359/jbmr.091036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Happle R. The McCune-Albright syndrome: a lethal gene surviving by mosaicism. Clin Genet. 1986;29(4):321–4. doi: 10.1111/j.1399-0004.1986.tb01261.x. [DOI] [PubMed] [Google Scholar]
- 8.Endo M, et al. Monozygotic twins discordant for the major signs of McCune-Albright syndrome. Am J Med Genet. 1991;41(2):216–20. doi: 10.1002/ajmg.1320410217. [DOI] [PubMed] [Google Scholar]
- 9.Lemli L. Fibrous dysplasia of bone. Report of female monozygotic twins with and without the McCune-Albright syndrome. J Pediatr. 1977;91(6):947–9. doi: 10.1016/s0022-3476(77)80898-6. [DOI] [PubMed] [Google Scholar]
- 10.Peleg R, Treister-Goltzman Y. Images in clinical medicine: McCune-Albright syndrome. J Clin Endocrinol Metab. 2014;99(4):1105–6. doi: 10.1210/jc.2013-3647. [DOI] [PubMed] [Google Scholar]
- 11.Bianco P, et al. Reproduction of human fibrous dysplasia of bone in immunocompromised mice by transplanted mosaics of normal and Gsalpha-mutated skeletal progenitor cells. J Clin Invest. 1998;101(8):1737–44. doi: 10.1172/JCI2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riminucci M, et al. The histopathology of fibrous dysplasia of bone in patients with activating mutations of the Gs alpha gene: site-specific patterns and recurrent histological hallmarks. J Pathol. 1999;187(2):249–58. doi: 10.1002/(SICI)1096-9896(199901)187:2<249::AID-PATH222>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 13.Collins MT, Riminucci M, Bianco P. Fibrous dysplasia. In: Rosen C, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 8th American Society of Bone and Mineral Research; Washington, D.C: 2013. pp. 786–793. [Google Scholar]
- 14.Hart ES, et al. Onset, progression, and plateau of skeletal lesions in fibrous dysplasia and the relationship to functional outcome. J Bone Miner Res. 2007;22(9):1468–74. doi: 10.1359/jbmr.070511. [DOI] [PubMed] [Google Scholar]
- 15.Ippolito E, et al. Radiographic classification of coronal plane femoral deformities in polyostotic fibrous dysplasia. Clin Orthop Relat Res. 2014;472(5):1558–67. doi: 10.1007/s11999-013-3380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leet AI, et al. Fracture incidence in polyostotic fibrous dysplasia and the McCune-Albright syndrome. J Bone Miner Res. 2004;19(4):571–7. doi: 10.1359/JBMR.0301262. [DOI] [PubMed] [Google Scholar]
- 17.Leet AI, et al. Fibrous dysplasia in the spine: prevalence of lesions and association with scoliosis. J Bone Joint Surg Am. 2004;86-A(3):531–7. [PubMed] [Google Scholar]
- 18*.Boyce AM, Collins MT. Fibrous Dysplasia/McCune-Albright Syndrome. In: Pagon RA, et al., editors. GeneReviews(R) University of Washington, Seattle; Seattle WA: 1993. This review contains specific diagnostic and treatment algorithms compiled from the highest quality evidenced-based literature and expert opinion currently available. [Google Scholar]
- 19**.Amit M, et al. Surgery versus watchful waiting in patients with craniofacial fibrous dysplasia--a meta-analysis. PLoS One. 2011;6(9):e25179. doi: 10.1371/journal.pone.0025179. This meta-analysis establishes that prophylactic optic nerve decompression is contraindicated in patients with FD involving the optic canals without evidence of objective vision loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frisch CD, et al. Fibrous dysplasia of the temporal bone: a review of 66 cases. Laryngoscope. 2015;125(6):1438–43. doi: 10.1002/lary.25078. [DOI] [PubMed] [Google Scholar]
- 21.Collins MT, Singer FR, Eugster E. McCune-Albright syndrome and the extraskeletal manifestations of fibrous dysplasia. Orphanet J Rare Dis. 2012;7(Suppl 1):S4. doi: 10.1186/1750-1172-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyce AM, et al. Characterization and management of testicular pathology in McCune-Albright syndrome. J Clin Endocrinol Metab. 2012;97(9):E1782–90. doi: 10.1210/jc.2012-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celi FS, et al. The role of type 1 and type 2 5'-deiodinase in the pathophysiology of the 3,5,3'- triiodothyronine toxicosis of McCune-Albright syndrome. J Clin Endocrinol Metab. 2008;93(6):2383–9. doi: 10.1210/jc.2007-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyce AM, Bhattacharyya N, Collins MT. Fibrous dysplasia and fibroblast growth factor-23 regulation. Curr Osteoporos Rep. 2013;11(2):65–71. doi: 10.1007/s11914-013-0144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown RJ, Kelly MH, Collins MT. Cushing syndrome in the McCune-Albright syndrome. J Clin Endocrinol Metab. 2010;95(4):1508–15. doi: 10.1210/jc.2009-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyce AM, et al. Optic neuropathy in McCune-Albright syndrome: effects of early diagnosis and treatment of growth hormone excess. J Clin Endocrinol Metab. 2013;98(1):E126–34. doi: 10.1210/jc.2012-2111. ** This paper demonstrates that early diagnosis and treatment of growth hormone excess in FD/MAS prevents long-term craniofacial morbidity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leet AI, et al. The correlation of specific orthopaedic features of polyostotic fibrous dysplasia with functional outcome scores in children. J Bone Joint Surg Am. 2006;88(4):818–23. doi: 10.2106/JBJS.E.00259. [DOI] [PubMed] [Google Scholar]
- 28.Ruggieri P, et al. Malignancies in fibrous dysplasia. Cancer. 1994;73(5):1411–24. doi: 10.1002/1097-0142(19940301)73:5<1411::aid-cncr2820730516>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 29.Collins MT, et al. Thyroid carcinoma in the McCune-Albright syndrome: contributory role of activating Gs alpha mutations. J Clin Endocrinol Metab. 2003;88(9):4413–7. doi: 10.1210/jc.2002-021642. [DOI] [PubMed] [Google Scholar]
- 30.Tanabeu Y, et al. Breast Cancer in a Patient with McCune-Albright Syndrome. Breast Cancer. 1998;5(2):175–178. doi: 10.1007/BF02966691. [DOI] [PubMed] [Google Scholar]
- 31.Huston TL, Simmons RM. Ductal carcinoma in situ in a 27-year-old woman with McCune-Albright syndrome. Breast J. 2004;10(5):440–2. doi: 10.1111/j.1075-122X.2004.21490.x. [DOI] [PubMed] [Google Scholar]
- 32.Wu J, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3(92):92ra66. doi: 10.1126/scitranslmed.3002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaujoux S, et al. Hepatobiliary and Pancreatic neoplasms in patients with McCune-Albright syndrome. J Clin Endocrinol Metab. 2014;99(1):E97–101. doi: 10.1210/jc.2013-1823. [DOI] [PubMed] [Google Scholar]
- 34.Parvanescu A, et al. Lessons from McCune-Albright syndrome-associated intraductal papillary mucinous neoplasms: : GNAS-activating mutations in pancreatic carcinogenesis. JAMA Surg. 2014;149(8):858–62. doi: 10.1001/jamasurg.2014.535. [DOI] [PubMed] [Google Scholar]
- 35.Riminucci M, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112(5):683–92. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuznetsov SA, et al. Age-dependent demise of GNAS-mutated skeletal stem cells and "normalization" of fibrous dysplasia of bone. J Bone Miner Res. 2008;23(11):1731–40. doi: 10.1359/jbmr.080609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhattacharyya N, et al. Mechanism of FGF23 processing in fibrous dysplasia. J Bone Miner Res. 2012;27(5):1132–41. doi: 10.1002/jbmr.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leet AI, Collins MT. Current approach to fibrous dysplasia of bone and McCune-Albright syndrome. J Child Orthop. 2007;1(1):3–17. doi: 10.1007/s11832-007-0006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paul SM, et al. Disease severity and functional factors associated with walking performance in polyostotic fibrous dysplasia. Bone. 2014;60:41–7. doi: 10.1016/j.bone.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40**.Leet AI, et al. Bone-Grafting in Polyostotic Fibrous Dysplasia. J Bone Joint Surg Am. 2016;98(3):211–9. doi: 10.2106/JBJS.O.00547. This study demonstrates that the common surgical practice of bone grafting is frequently ineffective in patients with FD, particularly children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanton RP, Springfield D, Lindaman L, Wientroub S, Leet A. The surgical management of fibrous dysplasia of bone. Orphanet J Rare Dis. 2012;24(7)(Suppl 1):S1. doi: 10.1186/1750-1172-7-S1-S1. I.E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mancini F, et al. Scoliosis and spine involvement in fibrous dysplasia of bone. Eur Spine J. 2009;18(2):196–202. doi: 10.1007/s00586-008-0860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gabbay JS, et al. Fibrous dysplasia of the zygomaticomaxillary region: outcomes of surgical intervention. Plast Reconstr Surg. 2013;131(6):1329–38. doi: 10.1097/PRS.0b013e31828bd70c. [DOI] [PubMed] [Google Scholar]
- 44.Lee J, Chen Y, Kim H, Lustig L, Akintoye S, Collins M, Kaban L. Clinical guidelines for the management of craniofacial fibrous dysplasia. 2012;24(Suppl 1) doi: 10.1186/1750-1172-7-S1-S2. F.E. S2(7): p. Suppl 1:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JS, et al. Normal vision despite narrowing of the optic canal in fibrous dysplasia. N Engl J Med. 2002;347(21):1670–6. doi: 10.1056/NEJMoa020742. [DOI] [PubMed] [Google Scholar]
- 46.Boyce AM, et al. A randomized, double blind, placebo-controlled trial of alendronate treatment for fibrous dysplasia of bone. J Clin Endocrinol Metab. 2014;99(11):4133–40. doi: 10.1210/jc.2014-1371. * This study reports the first randomized controlled trial in FD, demonstrating that oral alendronate is ineffective in improving FD-related pain or the radiographic appearance of FD lesions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chapurlat RD, et al. Treatment of fibrous dysplasia of bone with intravenous pamidronate: long-term effectiveness and evaluation of predictors of response to treatment. Bone. 2004;35(1):235–42. doi: 10.1016/j.bone.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Liens D, Delmas PD, Meunier PJ. Long-term effects of intravenous pamidronate in fibrous dysplasia of bone. Lancet. 1994;343(8903):953–4. doi: 10.1016/s0140-6736(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 49.Plotkin H, et al. Effect of pamidronate treatment in children with polyostotic fibrous dysplasia of bone. J Clin Endocrinol Metab. 2003;88(10):4569–75. doi: 10.1210/jc.2003-030050. [DOI] [PubMed] [Google Scholar]
- 50.Kelly MH, Brillante B, Collins MT. Pain in fibrous dysplasia of bone: age-related changes and the anatomical distribution of skeletal lesions. Osteoporos Int. 2008;19(1):57–63. doi: 10.1007/s00198-007-0425-x. [DOI] [PubMed] [Google Scholar]
- 51.Salenave S, et al. Acromegaly and McCune-Albright syndrome. J Clin Endocrinol Metab. 2014:jc20133826. doi: 10.1210/jc.2013-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tessaris D, et al. Thyroid abnormalities in children and adolescents with McCune-Albright syndrome. Horm Res Paediatr. 2012;78(3):151–7. doi: 10.1159/000342641. [DOI] [PubMed] [Google Scholar]
- 53.Hsiao EC, et al. Osteoblast expression of an engineered Gs-coupled receptor dramatically increases bone mass. Proc Natl Acad Sci U S A. 2008;105(4):1209–14. doi: 10.1073/pnas.0707457105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsiao EC, et al. Gs G protein-coupled receptor signaling in osteoblasts elicits age-dependent effects on bone formation. J Bone Miner Res. 2010;25(3):584–93. doi: 10.1002/jbmr.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saggio I, et al. Constitutive expression of Gsalpha(R201C) in mice produces a heritable, direct replica of human fibrous dysplasia bone pathology and demonstrates its natural history. J Bone Miner Res. 2014;29(11):2357–68. doi: 10.1002/jbmr.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bianco P, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kagami H, et al. The use of bone marrow stromal cells (bone marrow-derived multipotent mesenchymal stromal cells) for alveolar bone tissue engineering: basic science to clinical translation. Tissue Eng Part B Rev. 2014;20(3):229–32. doi: 10.1089/ten.TEB.2013.0578. [DOI] [PubMed] [Google Scholar]
- 58.Robey PG, et al. Generation of clinical grade human bone marrow stromal cells for use in bone regeneration. Bone. 2015;70:87–92. doi: 10.1016/j.bone.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim N, Cho SG. New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int J Stem Cells. 2015;8(1):54–68. doi: 10.15283/ijsc.2015.8.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhattacharyya N, et al. A high throughput screening assay system for the identification of small molecule inhibitors of gsp. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0090766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dempster DW, et al. Role of RANK ligand and denosumab, a targeted RANK ligand inhibitor, in bone health and osteoporosis: a review of preclinical and clinical data. Clin Ther. 2012;34(3):521–36. doi: 10.1016/j.clinthera.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Prolia [package insert] Amgen, Inc.; Thousand Oaks, CA: 2010. [Google Scholar]
- 63.Xgeva [package insert] Amgen, Inc.; Thousand Oaks, CA: 2010. [Google Scholar]
- 64.Xu SF, et al. Denosumab and giant cell tumour of bone-a review and future management considerations. Curr Oncol. 2013;20(5):e442–7. doi: 10.3747/co.20.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh AS, Chawla NS, Chawla SP. Giant-cell tumor of bone: treatment options and role of denosumab. Biologics. 2015;9:69–74. doi: 10.2147/BTT.S57359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomas D, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11(3):275–80. doi: 10.1016/S1470-2045(10)70010-3. [DOI] [PubMed] [Google Scholar]
- 67.Wang HD, et al. Effects of denosumab treatment and discontinuation on human growth plates. J Clin Endocrinol Metab. 2014;99(3):891–7. doi: 10.1210/jc.2013-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benhamou J, Gensburger D, Chapurlat R. Transient improvement of severe pain from fibrous dysplasia of bone with denosumab treatment. Joint Bone Spine. 2014;81(6):549–50. doi: 10.1016/j.jbspin.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 69.Boyce AM, et al. Denosumab treatment for fibrous dysplasia. J Bone Miner Res. 2012;27(7):1462–70. doi: 10.1002/jbmr.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gossai N, et al. Critical hypercalcemia following discontinuation of denosumab therapy for metastatic giant cell tumor of bone. Pediatr Blood Cancer. 2015;62(6):1078–80. doi: 10.1002/pbc.25393. [DOI] [PubMed] [Google Scholar]
- 71.Grasemann C, et al. Effects of RANK-ligand antibody (denosumab) treatment on bone turnover markers in a girl with juvenile Paget's disease. J Clin Endocrinol Metab. 2013;98(8):3121–6. doi: 10.1210/jc.2013-1143. [DOI] [PubMed] [Google Scholar]
- 72.Setsu N, et al. Severe hypercalcemia following denosumab treatment in a juvenile patient. J Bone Miner Metab. 2016;34(1):118–22. doi: 10.1007/s00774-015-0677-z. [DOI] [PubMed] [Google Scholar]