Abstract
Lymph node involvement in pancreatic adenocarcinoma (PAC) predicts postresection survival, but early lymph node metastasis detection is not easily accomplished. We assessed a panel of microRNAs (miRNAs) in a common hepatic artery lymph node (station 8) that is readily accessible during pancreatoduodenectomy (PD) to determine if increased miRNA levels correlate with postresection recurrence. Station 8 lymph nodes overlying the common hepatic artery collected during PD were assayed for miRNA-10b, miRNA-30c, miRNA-21, and miRNA-155 and cytokeratin-19 (CK19), an epithelial cell marker, using quantitative PCR. Expression was correlated with disease recurrence, recurrence-free survival (RFS), and overall survival (OS). Station 8 lymph nodes from 37 patients (30 periampullary carcinomas (PCs), 2 chronic pancreatitis, 5 other cancers) exhibited increased miRNA-10b levels in 14/30 PCs, and in 10 of these 14 patients, cancer recurred during the study period (2012–2015). High miRNA-10b was also associated with shorter RFS (42.5 vs. 92.4 weeks, p < 0.05) but not OS, whereas miRNA-30c, miRNA-21, and miRNA-155 levels and CK19 mRNA levels in station 8 nodes were variable and did not correlate with RFS or OS. We conclude that elevated miRNA-10b levels in station 8 lymph nodes could be utilized to assess risk for early disease progression in patients with periampullary tumors.
Keywords: Pancreatic cancer, Lymph node, MicroRNA
Introduction
Periampullary adenocarcinomas comprise a heterogeneous group of neoplasms at the head of the pancreas and denotes dismal prognosis with 5-year survival ranging from 7 to 20% depending on the stage and tumor type.1,2 Pancreatic adenocarcinoma (PAC), distal cholangiocarcinoma, and ampullary carcinoma collectively, termed periampullary cancer (PC), account for more than 50,000 cancer-related deaths in the USA annually.1–3 Curative pancreaticoduodenectomy (PD) remains the treatment of choice.1,3 Unfortunately, the majority of these cancers are not resectable at presentation, and other therapeutic modalities only lead to modest improvements in mortality.1,3 This dire situation is due, in part, to the absence of screening tests and early detection methods for these neoplasms.
To date, the extent of peripancreatic lymph node involvement is the best prognostic factor for resectable periampullary carcinoma.1,3–5 The utilization of biochemical markers to diagnose and assess prognosis for periampullary tumors is limited. Serum CA 19-9 levels had been used in the clinical settings to detect disease recurrence after resection. However, the preoperative diagnostic and prognostic capability of CA19-9 is restricted due to marginal sensitivity and specificity and falsely elevated readings in the setting of biliary obstruction.6,7 Other studies have attempted to identify additional tumor markers but none has proven to be superior to CA 19-9.
MicroRNAs (miRNAs) are short, non-coding RNAs that are expressed in many tissues where they participate in the regulation of numerous cellular processes by modulating mRNA stability and translation.8 In addition, miRNAs are released into the circulation and other bodily fluids.9 We previously reported that miRNA-10b is upregulated in the cancer cells in PAC, and high miRNA-10b levels are associated with poor neoadjuvant radiochemotherapy response, as well as shorter time to metastasis.10 Moreover, elevated plasma levels of miRNA-10b, miRNA-30c, miRNA-106b, and miRNA-155 can differentiate PAC from chronic pancreatitis.9 It is not known, however, whether miRNAs are also present in metastatic lymph nodes in PC. Therefore, in the present study, we measured the levels of these four miRNAs in the common hepatic artery lymph node, termed station 8 lymph node,11 to determine if they are present within this lymph node and whether their levels correlate with recurrence-free and/or overall survival in PC. We focused on studying miRNA expression in this particular lymph node because it is a common site for early metastasis11,12 and is accessible for excisional biopsy during PD. The station 8 lymph node also lends itself for tissue sampling during preoperative endoscopic ultrasound.
Methods
Patients
Patient information and specimens were collected at Indiana University Health University Hospital, Indianapolis, IN, USA, under the approval of primary investigators and the Institutional Review Board (IRB) at Indiana University School of Medicine. All patients signed informed consent. No patient identifying information were collected for this particular study or utilized during data analyses. Patients who underwent PD with collection of the common hepatic artery lymph node (station 8) were identified. Demographics including age and gender, date of recurrence and/or death, +/− adjuvant chemotherapy, and final pathology of both the lymph nodes and pancreatic specimens were recorded. Tumors with pathology other than periampullary adenocarcinoma were excluded from the study. Recurrence was determined by standard imaging studies (e.g. CT, PET) and/or by the presence of biopsy-proven lesions. Survival was calculated from the date of operation to the date of death (overall survival (OS)) or date of first recurrence (recurrence-free survival (RFS)). Patients lost to follow-up had their recurrence-free period calculated based on the last documented note in medical records.
miRNA Analysis
Station 8 lymph node samples were collected, flash-frozen in liquid nitrogen, and stored at −80 °C. RNA extraction was performed using the guanidine thiocyanate method as described.13 Total RNA was converted to cDNA using a high-capacity RNA-to-cDNA kit as per the manufacturer’s recommendations (Life Technologies). Assays for miRNA-10b, miRNA-21, miRNA-30c, and miRNA-155 were performed as described.9 Briefly, 10 ng of RNA was used with miRNA-specific reverse transcription primers and a miRNA reverse transcription kit, and quantitative PCR (qPCR) was performed for each miRNA using Taqman assay reagents (Life Technologies). miRNA levels were normalized to snoRNA135 which served as the endogenous control. The same approach was used to assess CK19 levels but RPS6 was the endogenous control. Fold changes were calculated relative to chronic pancreatitis patients using the 2−ΔΔCt method.14
Statistical Analysis
miRNA-10b levels in PC were dichotomized based on a cutoff value of ≥1.5-fold above chronic pancreatitis controls and compared to patient recurrence status that was determined as described above. Given the non-parametric (non-normal) distribution of miRNA values, the Mann-Whitney U test was used to assess for significant differences between high (≥1.5-fold) and low (≤1.5-fold) miRNA-10b levels. A p < 0.05 was considered statistically significant. Patient grouping for the miRNA-10b analysis was maintained in assessing the levels of miRNA-21, miRNA-30c, and miRNA-155 and CK19 mRNA levels. In order to compare the validity of utilizing lymph node histology versus miRNA levels to predict cancer recurrence, sensitivity and specificity were calculated for the above variables. We determined the differences in survival (RFS, OS) between the low and high miRNA groups over the follow-up time period using Kaplan-Meier log-rank survival analyses to factor in the effects of time on survival. Cox-regression analysis was used to determine if miRNA levels are independent predictors of RFS and OS. This method allowed us to analyze the relative risks of multiple variables (i.e., age, gender, miRNA levels, staging) on specific outcomes (i.e., RFS, OS).
Results
Patient cohort
Station 8 lymph nodes were collected from 37 patients who underwent PD from 2012 to 2015. Patient demographics are shown in Table 1. Review of pathology reports indicated that 30 of these cases were PC (27 PAC; 3 cholangiocarcinomas), and 2 were chronic pancreatitis, whereas 5 were neither PC nor chronic pancreatitis and were therefore excluded from further analysis. The median age in the PC group was ~64, and there were 20 males and 10 females (Table 1). Moreover, 80 % of patients had stage IIB tumors, and 83 % received adjuvant radiochemotherapy (Table 1). Resection margin status, perineural invasion, lymphovascular invasion, and tumor size were analyzed, and no differences were found in these parameters between the comparison groups (Table 2). However, there was a statistically significant difference in the number of positive lymph nodes in recurrent versus non-recurrent cohort (28.36 versus 13.44 % respectively) (Table 2).
Table 1.
Periampullary carcinoma patient cohort
| Adjuvant therapy (yes), n (%) |
||
|---|---|---|
| Gender | ||
| Male | 20 | 16 (80) |
| Female | 10 | 9 (90) |
| Stage, n (%) | ||
| IA | 1 (3.33) | 1 (3.33) |
| IB | 1 (3.33) | 1 (3.33) |
| IIA | 4 (13.34) | 1 (3.33) |
| IIB | 24 (80.0) | 22 (73.3) |
| Total | 30 (100) | 25 (83.3) |
| Age (years) | ||
| Range | 47.9–84.5 | |
| Median | 64.4 |
Shown are the demographics and tumor staging for the PC patient cohort and the number of patients who received adjuvant radiochemotherapy
Table 2.
Recurrent versus non-recurrent patients
| Recurrent | Non-recurrent | p value | |
|---|---|---|---|
| Gender | |||
| Male | 10 | 10 | 0.7739 |
| Female | 3 | 7 | 0.7110 |
| Total | 13 | 17 | 0.4404 |
| Stage, n (%) | |||
| IA | 1 (3.33) | 0 (0) | 0.3182 |
| IB | 0 (0) | 1 (3.33) | 0.3182 |
| IIA | 0 (13.34) | 4 (13.34) | 0.1445 |
| IIB | 12 (50) | 12 (50) | 0.7843 |
| Age (years) | |||
| Range | 47.9–84.5 | 50.4 – 82.6 | 0.8220 |
| Median | 61.6 | 64.6 | |
| Adjuvant therapy (yes), n (%) | 12 (92.3) | 13 (76.5) | 0.7899 |
| Tumor size (cm) | |||
| Range | 1.8–5.2 | 1.4–5.5 | 0.6021 |
| Mean | 3.1 | 3.35 | |
| R0 resection (yes), n (%) | 12 (92.31) | 16 (94.12) | 1.0000 |
| Perineural invasion (yes), n (%) | 2 (15.38) | 2 (11.76) | 1.0000 |
| Lymphovascular invasion (yes), n (%) | 9 (69.23) | 10 (58.82) | 0.7080 |
| Lymph node status | |||
| No. of positive | 6 | 3.18 | |
| No. of examined | 22.38 | 21.08 | |
| % positive (mean) | 28.36 | 13.44 | 0.0247 |
Comparison of recurrent with non-recurrent patients shows that there are no differences in gender, age, or tumor stage between these groups, and that a similar number of patients in each group received adjuvant radiochemotherapy. No differences were detected in tumor size, perineural or lymphovascular invasion, or R0 resection, but there is a statistically significant difference in the number of positive lymph nodes in the recurrent versus non-recurrent group
miRNA Analysis
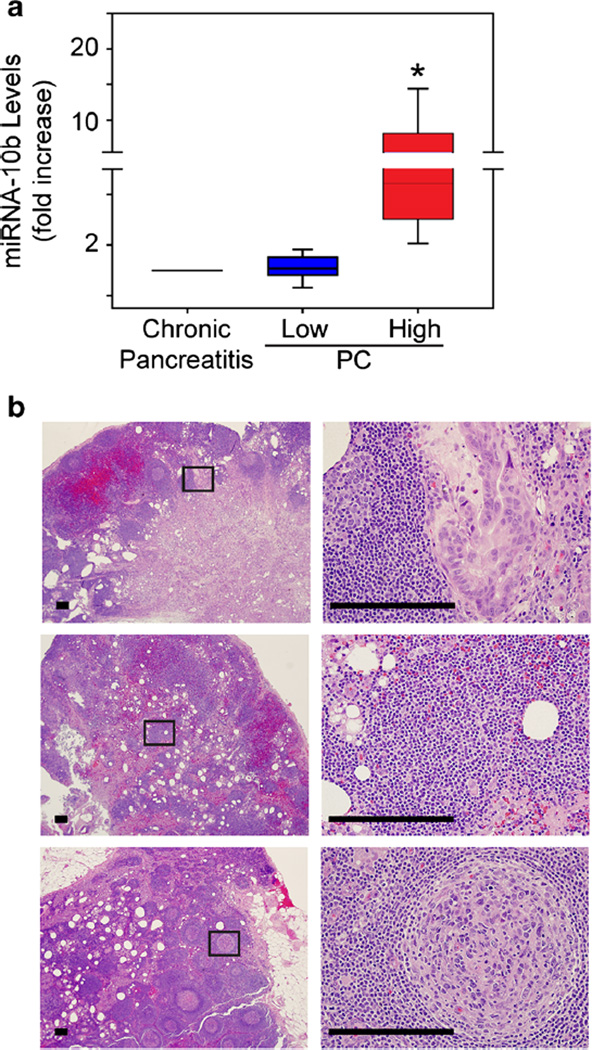
From the miRNA panel, we first measured miRNA-10b levels in station 8 lymph nodes because it is expressed at high levels in pancreatic cancer cells and is the most sensitive and specific circulating miRNA to distinguish pancreatic ductal adenocarcinomas from chronic pancreatitis.7,9 In a subset of PC nodes (n = 14), miRNA-10b was increased by >1.5-fold (p < 0.05), but in the other PC nodes (n = 16), it did not differ from chronic pancreatitis (p = 0.795) (Fig. 1a). PC patients in the miRNA-10b-high group (n = 10) developed recurrent disease, whereas only three patients with low miRNA-10b levels developed recurrence (p < 0.01, Fisher’s exact test).
Fig. 1.
Elevated miRNA-10b levels in a subset of PC station 8 nodes. a Quantitative PCR for miRNA-10b in station 8 lymph nodes from chronic pancreatitis or PC patients shows that this miRNA is significantly increased in nodes from some PC patients (high, red bar; *p < 0.05), whereas other nodes have levels that are similar to chronic pancreatitis (low, blue bar). Data are presented as interquartile range (IQR). b H&E staining of station 8 lymph nodes from PC patients with high miRNA-10b. Shown are representative images from a non-recurrent patient with moderately differentiated PC (top) and recurrent patients with poorly differentiated PC (middle) or without cancer cells (bottom) in the station 8 lymph node. Panels on the right are magnified images of boxed areas. Scale bars, 50 µm
There were no differences in age or gender between recurrent and non-recurrent patients or the number of patients in each group who received adjuvant therapy (Table 2). Analysis for cancer cells in station 8 lymph nodes revealed that 5/14 (~38.5 %) patients in the recurrent group had obvious microscopic metastases compared with only 1/16 (~5.9 %) patients in the non-recurrent group (Fig. 1b). Based on histological analysis, sensitivity to predict recurrence was 66.7 %, whereas miRNA-10b levels predicted recurrence with 77 % sensitivity.
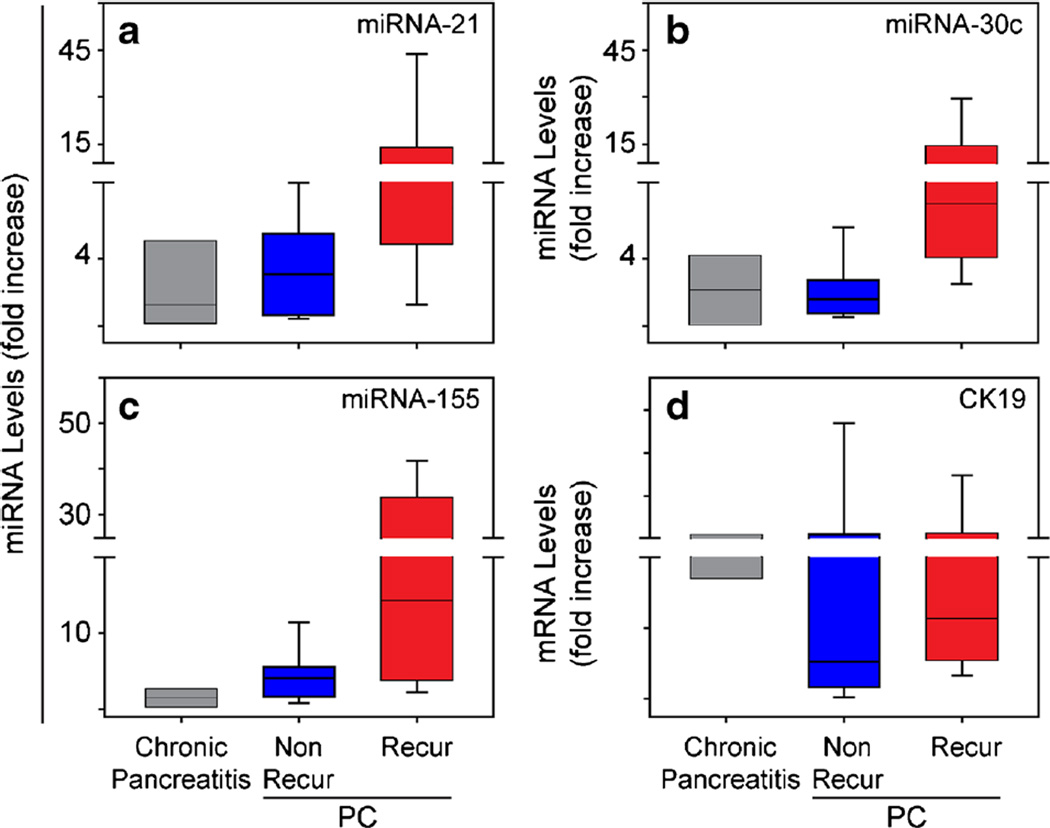
There was a tendency for the other three miRNAs in the panel (miRNA-21, miRNA-30c, and miRNA-155) to be upregulated in station 8 nodes in the recurrent group (Fig. 2a–c). However, these differences were not significant (p > 0.05). Moreover, the levels of CK-19 mRNA, which is a marker denoting cells of the ductal epithelial origin and should reflect the presence of metastatic pancreatic cancer cells in lymph nodes, were similar in both PC groups (Fig. 2d).
Fig. 2.
The levels of three other miRNAs and CK 19 are similar in recurrent and non-recurrent patients. Quantitative PCR reveals that there are no significant differences in miRNA-21 (a), miRNA-30c (b), miRNA-155 (c), or CK19 (d) levels in station 8 lymph nodes from recurrent (red bars) or non-recurrent (blue bars) PC patients compared with chronic pancreatitis. Data are presented as IQR
Recurrence-Free and Overall Survival
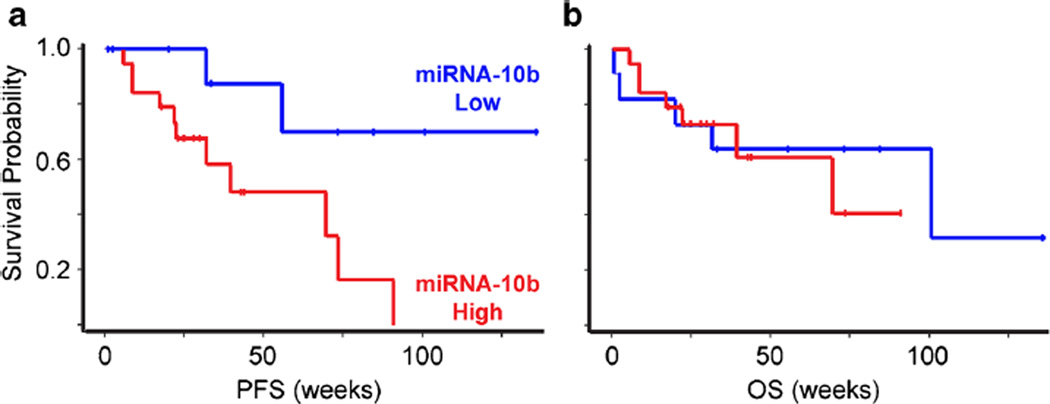
The median follow-up for all the patients in this study was 48.4 weeks (range, 2.4 to 146.8 weeks). For survivors, the median follow-up was 49.4 weeks (range, 28.4 to 146.8 weeks). Kaplan-Meier survival analyses indicated that non-recurrent patients who had low miRNA-10b had a significantly longer RFS (p < 0.05) (Fig. 3a). However, there was no correlation between miRNA-10b levels and OS (Fig. 3b). Moreover, there were no differences in either RFS or OS based on a similar analysis of the other miRNAs examined (not shown). When a separate subanalysis was performed for PAC samples without the three cholangiocarcinomas, the correlation between high miRNA-10b levels and RFS just missed achieving statistical significance (p = 0.053).
Fig. 3.
High miRNA-10b levels are associated with decreased PFS. a Kaplan-Meier plots show that PC patients with high miRNA-10b (red lines) have shorter PFS (p < 0.05) than do patients with low miRNA-10b (blue lines). b By contrast, the OS between these groups is similar.
Station 8 miRNA-10b Levels as an Independent Predictor of Disease-Free Versus Overall Survival
Cox regression analysis of age, gender, staging, and miRNA-10b was performed to determine if any of these factors are independent predictors of RFS versus of OS. High miRNA-10b was associated with a significantly increased hazard ratio (13.147) for RFS but not OS (Table 3). No other factors including age, gender, or staging showed a statistically significant increase or decrease in hazard ratio (Table 3).
Table 3.
miRNA-10b predicts progression free survival
| PFS (Hz ratio) | p value | OS (Hz ratio) | p value | |
|---|---|---|---|---|
| miRNA-10b high group | 13.147 | 0.03a | 1.095 | >0.05 |
| Age | 0.988 | >0.05 | 1.043 | >0.05 |
| Gender | 1.262 | >0.05 | 0.990 | >0.05 |
| TNM Staging | 0.102 | >0.05 | 0.9959 | >0.05 |
Comparison of the miRNA-10b high and low groups shows that there is a statistically significant increase in the PFS hazard (Hz) ratio in PC patients with elevated miRNA-10b (lowercase letter), but no differences in OS, age, gender, or stage
Discussion
Lymph node metastasis is common in many cancers, but in contrast to carcinomas such as breast or melanoma, PAC and PC lack a single draining or sentinel lymph node that can be biopsied preoperatively to detect early metastasis. Instead, these cancers have multiple routes for lymphatic dissemination that vary depending on the tumor mass, its effects on lymphatic flow, and its location within the pancreatic parenchyma.15 Several studies have shown that the degree of pancreatic lymphadenopathy rather than one specific lymph node in the examined specimen correlates with poor prognosis.16,17 Therefore, the status of lymph node metastases in PAC and PC remains the most important predictor of mortality as also demonstrated in our study (Table 2).18,19 Unfortunately, preoperative visualization of these peripancreatic nodes by current imaging modalities is limited and cannot detect the presence of micrometastases within the lymph nodes. Moreover, preoperative endoscopic lymph node biopsy for histological examination may yield false-negative results due to sampling error.20 Hence, PD is required for complete lymph node assessment and extensive lymph node resection may be associated with enhanced morbidity.21,22 The common hepatic artery lymph node is readily accessible during PD. Its location is also amenable to preoperative endoscopic biopsy. In the present study, we have isolated RNA from frozen station 8 lymph nodes collected at the time of PD and assayed the levels of CK19 mRNA and several miRNAs. In breast cancer and colorectal cancer, CK19 mRNA levels were shown to be useful for identifying lymph nodes that harbored metastatic cells.23,24 While the expression of CK19 in Station 8 lymph nodes implies the presence of pancreatic cancer cells in these nodes, there was no difference in CK19 mRNA levels between patients with recurrent versus non-recurrent disease. By contrast, among the miRNAs examined in the current study, high levels of miRNA-10b, but not the other miRNAs currently investigated, were significantly increased in patients who developed cancer recurrence. Moreover, high levels of miRNA-10b correlated with RFS, but not OS, in patients with PC. In addition, Cox regression analysis indicated that elevated miRNA-10b levels were significantly correlated with shorter disease-free intervals independent of other factors including age, gender, and TNM staging. Given that traditional histologic examinations of lymph nodes only have a sensitivity of 66.7 % and specificity of 87 % in predicting recurrence, our findings raise the possibility that miRNA-10b could be a novel, more sensitive marker for metastatic lymph node involvement and disease recurrence in patients with PC. Moreover, a subanalysis of PAC indicated that there was a tendency for miRNA-10b to be elevated in patients who developed recurrent PAC. However, due to the small sample size, and potential for false-negative detection rates from sampling errors, these elevated values barely missed achieving statistical significance.
There are three potential sources for the presence of PC-derived miRNA-10b in the station 8 lymph node. First, metastatic pancreatic cancer cells likely express miRNA-10b. Indeed, miRNA-10b was initially reported as a metastasis promoter in breast cancer25,26 and subsequently implicated in enhancing metastasis in pancreatic, gastric, hepatic, renal, thyroid (follicular), and bladder carcinomas.10,27–31 Moreover, we previously reported that, first, the presence of high miRNA-10b levels in pancreatic cancer cells within PAC samples obtained by fine needle aspiration (FNA) during endoscopic ultrasonography (EUS) correlated with earlier disease recurrence following neoadjuvant radiochemotherapy10 and provided mechanistic evidence that miRNA-10b can promote epithelial-to-mesenchymal transition and pancreatic cancer cell invasion.32 Second, miRNAs are present in the circulation in PAC patients,9 where they are often bound to the Agonaute2 protein or to high-density lipoprotein particles,33,34 and these miRNAs may get trapped within the lymph node. Third, miRNA-10b is abundant in exosomes,35 which may similarly localize to the lymph nodes. Previously, it has been demonstrated that miRNA-203 and miRNA-205 may serve as markers for detecting lymph node metastases in patients with head and neck squamous cell cancer.36 Moreover, we recently reported that elevated miRNA levels in serum, including miRNA-10b, can help detect the presence of microscopic PAC.37 Thus, our ability to detect microRNA in the common hepatic lymph node provides an excellent adjunct to EUS/FNA in preoperative staging and prognostication for periampullary tumors but does not replace the need for EUS/FNA in preoperative planning. Our current findings, by highlighting the utility of common hepatic artery lymph nodes as sentinel nodes in studying periampullary cancer micrometastasis through the monitoring of miRNA-10b levels in these nodes, may therefore provide a biomarker of metastatic disease in such lymph nodes even in cases of early PAC. Additional studies are therefore urgently needed to explore this possibility.
Conclusions
Our findings suggest that the presence of miRNA-10b in station 8 lymph nodes may allow for the rapid and early detection of lymph node micrometastases in PC, especially since these lymph nodes can be sampled by EUS/FNA prior to surgical resection. However, our study is retrospective and is based on a small number of samples with limited duration of follow-up. It must therefore be viewed as a pilot study. Additional longer-term studies are required to determine whether miRNA-10b levels in station 8 lymph nodes may be used as a biomarker for directing surgical and neoadjuvant/adjuvant therapy in periampullary tumors, as well as for early PAC detection, and to assess the potential utility of other miRNAs in this regard.
Acknowledgments
Funding This work was supported in part by a grant from the National Cancer Institute (NCI) of the National Institutes of Health under award number CA-075059 to M. Korc and by the Indiana Economic Development Fund (IEDF) to M. Korc.
Footnotes
Author Contributions Study conception and design: Nguyen, Gore, House, Korc
Acquisition of data: Nguyen, Gore, Zhong, Savant, Deitz-McElyea, Schmidt, House
Analysis and interpretation of data: Nguyen, Gore, House, Korc
Drafting of manuscript: Nguyen, Gore, Korc
Critical revision: Gore, House, Schmidt, Korc
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict of interest.
Abstract Presentation American College of Surgeons, Chicago, IL, October 2015.
References
- 1.Riall TS, Cameron JL, Lillemoe KD, Campbell KA, Winter JM, Hruban RH, Yeo CJ. Resected periampullary adenocarcinoma: 5-year surVIVOrs and their 6 to 10-year follow-up. Journal of Surgical Research. 2006;130(2):310. doi: 10.1016/j.surg.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: A Cancer Journal for Clinicians. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Lillemoe KD, Pitt HA. Periampullary adenocarcinoma: analysis of 5-year survivors. Annals of Surgery. 1998;227(6):821–831. doi: 10.1097/00000658-199806000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buc E, Couvelard A, Kwiatkowski F, Dokmak S, Ruszniewski P, Hammel P, Belghiti J, Sauvanet A. Adenocarcinoma of the pancreas: Does prognosis depend on mode of lymph node invasion? European Journal of Surgical Oncology. 2014;40(11):1578–1585. doi: 10.1016/j.ejso.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Valsangkar NP, Bush DM, Michaelson JS, Ferrone CR, Wargo JA, Lillemoe KD, Castillo CF-d, Warshaw AL, Thayer SP. N0/N1, PNL, or LNR? The Effect of Lymph Node Number on Accurate Survival Prediction in Pancreatic Ductal Adenocarcinoma. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2013;17(2):257–266. doi: 10.1007/s11605-012-1974-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poruk KE, Gay DZ, Brown K, Mulvihill JD, Boucher KM, Scaife CL, Firpo MA, Mulvihill SJ. The Clinical Utility of CA 19–9 in Pancreatic Adenocarcinoma: Diagnostic and Prognostic Updates. Current molecular medicine. 2013;13(3):340–351. doi: 10.2174/1566524011313030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osayi SN, Bloomston M, Schmidt CM, Ellison EC, Muscarella P. Biomarkers as Predictors of Recurrence following Curative Resection for Pancreatic Ductal Adenocarcinoma: A Review. Bio Med Research International. 2014;2014:10. doi: 10.1155/2014/468959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15(6):321–333. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cote GA, Gore AJ, McElyea SD, Heathers LE, Xu H, Sherman S, Korc M. A Pilot Study to Develop a Diagnostic Test for Pancreatic Ductal Adenocarcinoma Based on Differential Expression of Select miRNA in Plasma and Bile. Am J Gastroenterol. 2014 doi: 10.1038/ajg.2014.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Preis M, Gardner TB, Gordon SR, Pipas JM, Mackenzie TA, Klein EE, Longnecker DS, Gutmann EJ, Sempere LF, Korc M. microRNA-10b Expression Correlates with Response to Neoadjuvant Therapy and Survival in Pancreatic Ductal Adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(17):5812–5821. doi: 10.1158/1078-0432.CCR-11-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Japan Pancreas Society: Classification of Pancreatic Carcinoma. Tokyo: Kanehara: 2003. [Google Scholar]
- 12.Kayahara M, Nagakawa T, Kobayashi H, Mori K, Nakano T, Kadoya N, Ohta T, Ueno K, Miyazaki I. Lymphatic flow in carcinoma of the head of the pancreas. Cancer. 1992;70(8):2061–2066. doi: 10.1002/1097-0142(19921015)70:8<2061::aid-cncr2820700808>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Chang S-C, Brannon PM, Korc M. Effects of Dietary Manganese Deficiency on Rat Pancreatic Amylase mRNA Levels. J Nutr. 1990;120(10):1228–1234. doi: 10.1093/jn/120.10.1228. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Tol JAMG, Gouma DJ, Bassi C, Dervenis C, Montorsi M, Adham M, Andrén-Sandberg A, Asbun HJ, Bockhorn M, Büchler MW, Conlon KC, Fernández-Cruz L, Fingerhut A, Friess H, Hartwig W, Izbicki JR, Lillemoe KD, Milicevic MN, Neoptolemos JP, Shrikhande SV, Vollmer CM, Yeo CJ, Charnley RM. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: A consensus statement by the International Study Group on Pancreatic Surgery (ISGPS) Surgery. 2014;156(3):591–600. doi: 10.1007/978-1-4939-1726-6_59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, Wolfgang C, Hruban RH, Schulick RD, Yeo CJ, Choti MA. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141(5):610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Slidell MB, Chang DC, Cameron JL, Wolfgang C, Herman JM, Schulick RD, Choti MA, Pawlik TM. Impact of Total Lymph Node Count and Lymph Node Ratio on Staging and Survival after Pancreatectomy for Pancreatic Adenocarcinoma: A Large, Population-Based Analysis. Ann Surg Oncol. 2007;15(1):165–174. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 18.Sohn TA, Yeo CJ, Lillemoe KD, Koniaris L, Kaushal S, Sauter PK, Coleman J, Hruban RH, Cameron JL. Resected adenocarcinoma of the pancreas - 616 patients: Results, outcome and prognostic indicators. Gastroenterology. 2000;118(4):A1059. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 19.Maithel SK, Khalili K, Dixon E, Guindi M, Callery MP, Cattral MS, Taylor BR, Gallinger S, Greig PD, Grant DR, Vollmer CM. Impact of Regional Lymph Node Evaluation in Staging Patients With Periampullary Tumors. Ann Surg Oncol. 2007;14(1):202–210. doi: 10.1245/s10434-006-9041-9. [DOI] [PubMed] [Google Scholar]
- 20.Kocher HM, Sohail M, Benjamin IS, Patel AG. Technical limitations of lymph node mapping in pancreatic cancer. European Journal of Surgical Oncology (EJSO) 2007;33(7):887–891. doi: 10.1016/j.ejso.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 21.Cordera F, Arciero CA, Li T, Watson JC, Hoffman JP. Significance of Common Hepatic Artery Lymph Node Metastases During Pancreaticoduodenectomy for Pancreatic Head Adenocarcinoma. Ann Surg Oncol. 2007;14(8):2330–2336. doi: 10.1245/s10434-006-9339-7. [DOI] [PubMed] [Google Scholar]
- 22.Michalski CW, Kleeff J, Wente MN, Diener MK, Büchler MW, Friess H. Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. British Journal of Surgery. 2007;94(3):265–273. doi: 10.1002/bjs.5716. [DOI] [PubMed] [Google Scholar]
- 23.Tsujimoto M, Nakabayashi K, Yoshidome K, Kaneko T, Iwase T, Akiyama F, Kato Y, Tsuda H, Ueda S, Sato K, Tamaki Y, Noguchi S, Kataoka TR, Nakajima H, Komoike Y, Inaji H, Tsugawa K, Suzuki K, Nakamura S, Daitoh M, Otomo Y, Matsuura N. One-step Nucleic Acid Amplification for Intraoperative Detection of Lymph Node Metastasis in Breast Cancer Patients. Clinical Cancer Research. 2007;13(16):4807–4816. doi: 10.1158/1078-0432.CCR-06-2512. [DOI] [PubMed] [Google Scholar]
- 24.Croner RS, Geppert CI, Bader FG, Nitsche U, Späth C, Rosenberg R, Zettl A, Matias-Guiu X, Tarragona J, Güller U, Stürzl M, Zuber M. Molecular staging of lymph node-negative colon carcinomas by one-step nucleic acid amplification (OSNA) results in upstaging of a quarter of patients in a prospective, European, multicentre study. British Journal of Cancer. 2014;110(10):2544–2550. doi: 10.1038/bjc.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 26.Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson E, Teruya-Feldstein J, Bell GW, Weinberg RA. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nature biotechnology. 2010;28(4):341–347. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z, Zhu J, Cao H, Ren H, Fang X. miR-10b promotes cell invasion through RhoC-AKT signaling pathway by targeting HOXD10 in gastric cancer. International Journal of Cancer. 2012;40(5):1553–1560. doi: 10.3892/ijo.2012.1342. [DOI] [PubMed] [Google Scholar]
- 28.Li Q-j, Zhou L, Yang F, Wang G-x, Zheng H, Wang D-s, He Y, Dou K-f. MicroRNA-10b promotes migration and invasion through CADM1 in human hepatocellular carcinoma cells. Tumor Biol. 2012;33(5):1455–1465. doi: 10.1007/s13277-012-0396-1. [DOI] [PubMed] [Google Scholar]
- 29.Wotschofsky Z, Liep J, Meyer H-A, Jung M, Wagner I, Disch AC, Schaser KD, Melcher I, Kilic E, Busch J, Weikert S, Miller K, Erbersdobler A, Mollenkopf H-J, Jung K. Identification of Metastamirs as Metastasis-associated MicroRNAs in Clear Cell Renal Cell Carcinomas. International Journal of Biological Sciences. 2012;8(10):1363–1374. doi: 10.7150/ijbs.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jikuzono T, Kawamoto M, Yoshitake H, Kikuchi K, Akasu H, Ishikawa H, Hirokawa M, Miyauchi A, Tsuchiya S, Shimizu K, Takizawa T. The miR-221/222 cluster, miR-10b and miR-92a are highly upregulated in metastatic minimally invasive follicular thyroid carcinoma. International Journal of Oncology. 2013;42(6):1858–1868. doi: 10.3892/ijo.2013.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao H, Li H, Yu G, Xiao W, Hu J, Tang K, Zeng J, He W, Zeng G, Ye Z. MicroRNA-10b promotes migration and invasion through KLF4 and HOXD10 in human bladder cancer. Oncology reports. 2014;31(4):1832–1838. doi: 10.3892/or.2014.3048. [DOI] [PubMed] [Google Scholar]
- 32.Ouyang H, Gore J, Deitz S, Korc M. microRNA-10b enhances pancreatic cancer cell invasion by suppressing TIP30 expression and promoting EGF and TGF-[beta] actions. Oncogene. 2013 doi: 10.1038/onc.2013.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 34.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(12):5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joshi GK, Deitz-McElyea S, Liyanage T, Lawrence K, Mali S, Sardar R, Korc M. Label-Free Nanoplasmonic-Based Short Noncoding RNA Sensing at Attomolar Concentrations Allows for Quantitative and Highly Specific Assay of MicroRNA-10b in Biological Fluids and Circulating Exosomes. ACS Nano. 2015;9(11):11075–11089. doi: 10.1021/acsnano.5b04527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Carvalho AC, Scapulatempo-Neto C, Maia DCC, Evangelista AF, Morini MA, Carvalho AL, Vettore AL. Accuracy of microRNAs as markers for the detection of neck lymph node metastases in patients with head and neck squamous cell carcinoma. BMC Medicine. 2015;13(1):1–14. doi: 10.1186/s12916-015-0350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muratore S, Zeng X, Korc M, McElyea S, Wilhelm J, Bellin M, Beilman G. Metastatic Pancreatic Adenocarcinoma after Total Pancreatectomy Islet Autotransplantation for Chronic Pancreatitis. American Journal of Transplantation. 2016 doi: 10.1111/ajt.13851. [DOI] [PMC free article] [PubMed] [Google Scholar]