Abstract
Epoxyeicosatrienoic acids (EETs) are formed from arachidonic acid by the action of P450 epoxygenases (CYP2C and CYP2J). Effects of EETs are limited by hydrolysis by soluble epoxide hydrolase to less active dihydroxyeicosatrienoic acids. Studies in rodent models provide compelling evidence that epoxyeicosatrienoic acids exert favorable effects on glucose homeostasis, either by enhancing pancreatic islet cell function or by increasing insulin sensitivity in peripheral tissues. Specifically, the tissue expression of soluble epoxide hydrolase appears to be increased in rodent models of obesity and diabetes. Pharmacological inhibition of epoxide hydrolase or deletion of the gene encoding soluble epoxide hydrolase (Ephx2) preserves islet cells in rodent models of type 1 diabetes and enhances insulin sensitivity in models of type 2 diabetes, as does administration of epoxyeicosatrienoic acids or their stable analogues. In humans, circulating concentrations of epoxyeicosatrienoic acids correlate with insulin sensitivity, and loss-of-function genetic polymorphisms in EPHX2 are associated with insulin sensitivity.
Keywords: Epoxyeicosatrienoic acids, soluble epoxide hydrolase, diabetes, insulin, glucose
Introduction
Epoxyeicosatrienoic acids (EETS) are formed from arachidonic acid by the action of P450 epoxygenases (CYP2C and CYP2J) (Figure 1).[1] EETs act as potent vasodilators and have been identified as endothelium-derived hyperpolarizing factor.[2] In the kidney, EETs promote sodium excretion by inhibiting the translocation of the Na+/H+ exchanger (NHE3) in the proximal tubule.[3] EETs decrease inflammation by decreasing the activation of NFκB.[4] It follows that increasing the actions of EETs in rodent models protects against hypertension, endothelial dysfunction, cardiovascular remodeling and renal injury.[1] The effects of EETs are limited by hydrolysis by soluble epoxide hydrolase (sEH) to the less active dihydroxyepoxyeicosatrienoic acids (DHET)s and strategies to increase the action of EETs include both increasing the expression of epoxygenases and decreasing the activity of sEH.[5]
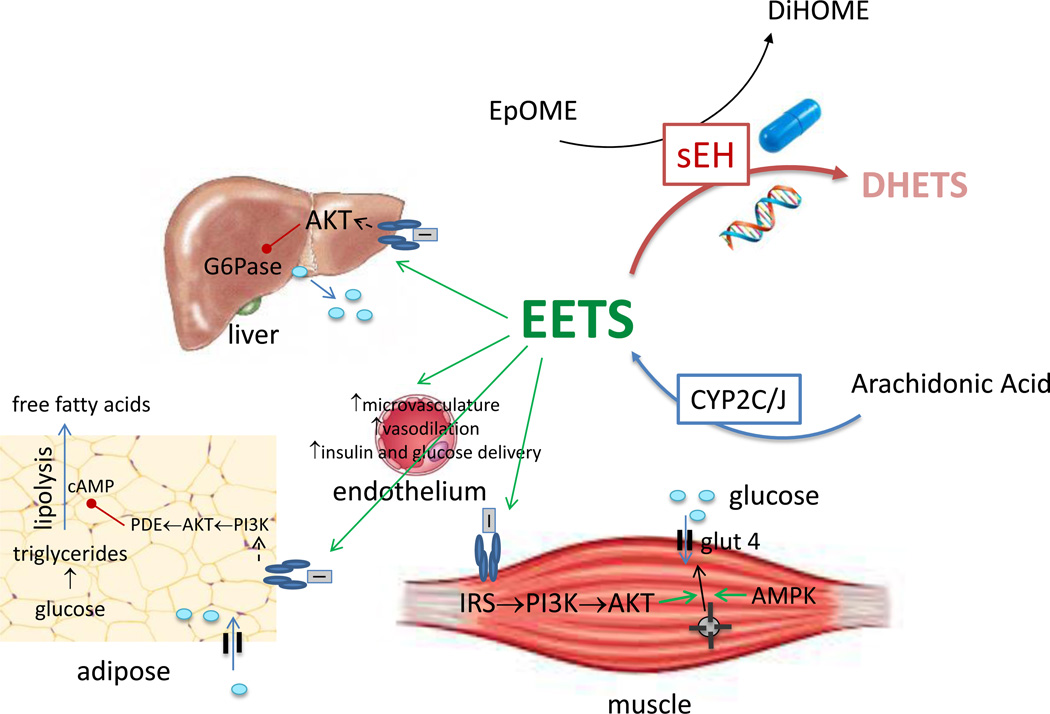
Figure 1.
Possible mechanisms through which epoxyeicosatrienoic acids (EETs) improve glucose homeostasis. EETs are produced by the actions of epoxygenases CYP2C and CYP2J on arachidonic acid. EETs improve insulin signaling in muscle, liver and adipose tissue. Genetic deletion of Ephx2 or pharmacologic inhibition of soluble epoxide hydrolase increases insulin-induced tyrosyl phosphorylation of the insulin receptor and tyrosyl phosphorylation of (IRS)-1 as well as AKT phosphorylation in insulin-sensitive tissues.[13, 15, 22–33] EETs also improve insulin sensitivity by increase capillary volume and microvascular blood flow in insulin sensitive tissues such as muscle.[33] The increase in capillary blood volume appears to be nitric oxide-independent, whereas increases in microvascular blood flow are nitric oxide-dependent. Not shown, EETs also preserve islet cell function and increase insulin secretion, particularly in rodent models of type 1 diabetes. The actions of EETs are limited by hydrolysis by soluble epoxide hydrolase (sEH) to dihydroxyeicosatrienoic acids (DHETs). sEH activity is often measured as the ratio of dihydroxyoctadecenoic acid (DiHOME) to epoxy-9Z-octadecenoic acid (EpOME). AKT, protein kinase B; IRS, insulin receptor substrate; G6Pase, glucose 6 phosphatase; glut 4, glucose transporter type 4; PDE, phosphodiesterase; PI3K, phosphoinositide 3-kinase
Over the last decade, studies in rodent models have provided compelling evidence that EETs also exert favorable effects on glucose homeostasis either by enhancing pancreatic islet cell function or by increasing insulin sensitivity in peripheral tissues. More recently, studies measuring insulin secretion and insulin sensitivity in individuals with functional polymorphisms in the gene encoding for sEH (EPHX2) provide support for the concept that EETs modulate glucose homeostasis in humans. We review here the evidence from rodent models and human studies suggesting that EETs contribute to metabolic regulation and the implications for the development of pharmacologic strategies to increase EETs.
Type 2 diabetes results when insulin resistance exceeds the ability of the pancreas to secrete insulin
Type 2 diabetes (T2DM) affects an estimated 366 million adults worldwide and this number is predicted to grow to 552 million by 2030 due to a worldwide increase in the prevalence of obesity.[6] In obesity, increased inflammation and circulating free fatty acids, as well as endothelial dysfunction lead to insulin resistance in muscle, liver and adipose tissue. T2DM results when the insulin secretory capacity can no longer compensate for the degree of insulin resistance.[7] Studies in rodent models suggest that EETs affect both insulin sensitivity in peripheral tissues and the capacity of the islets to respond to insulin resistance.
Expression of sEH is increased in rodent models of glucose intolerance
Tissue-specific sEH expression and/or activity appears to be increased in rodent models of both T1DM and T2DM. For example, sEH activity was increased more than two-fold in microsomes prepared from the livers of Fisher rats treated with alloxan or streptozotocin (STZ) to induce T1DM.[8] sEH expression was also increased in the heart and gastrocnemius muscle of Akita mice, a murine model of T1DM, but not in the liver; the investigators did not assess sEH activity.[9] Contradicting these findings, Oguro et al. reported that STZ treatment decreased sEH mRNA and protein expression in liver and kidney of mice, but the investigators did not measure sEH activity.[10] Insulin restored sEH expression in this model. The same investigators reported that high glucose concentrations reduced sEH expression in a hepatocarcinoma cell line, an effect that was mediated by Sp1.[11]
In a model of T2DM, Liu et al. reported that sEH expression was increased 2.6-fold in the liver of C57Bl6 mice after long-term (16 weeks) high-fat feeding, but not after a shorter duration (8 week) of high fat feeding; sEH activity was increased approximately 35% in the liver regardless of the duration of high fat feeding.[12] Schäfer et al. reported that high fat diet significantly decreased protein expression of epoxygenases, as well as the ω-hydroxylase Cyp4a12, in the liver by 21 days and up-regulated soluble epoxide hydrolase three-fold.[13] sEH activity has also been reported to be increased in epididymal fat in mice fed a high-fat diet.[14] In this study, sEH was expressed in cultured adipocytes and expression increased with differentiation of preadipocytes (3T3-L1 cells) into mature adipocytes.[14] Conversely, EET concentrations have been reported to be higher in preadipocytes or mesenchymal stem cells (MSCs) compared to mature adipocytes.[14,15]
Effects of EETs on insulin secretion and islet cell apoptosis
Epoxygenases of both the CYP2C and CYP2J families have been reported to be present in rodent and human islets.[16,17] Studies in isolated islets or beta cells provide conflicting data on the effect of EETs on insulin secretion. In 1983, Falck et al. reported that P450 inhibition decreased arginine-stimulated insulin and glucagon release and glucose-stimulated insulin release from isolated rat pancreatic islets.[18] The group reported that 5,6-EET stimulated insulin-secretion from islets, whereas 8,9-, 11,12-, and 14,15 EET stimulated glucagon secretion. Turk did not find an effect of EETs on glucose-stimulated insulin secretion in isolated islets.[19] In contrast, Klett et al. reported that unesterified EETs decrease insulin secretion in isolated INS 832/13 beta cells.[20] In this model, esterification of EETs by acyl-CoA synthetase 4 resulted in their sequestration into glycophospholipd and increased glucose-stimulated-insulin secretion.[20]
Luo et al. reported that Ephx2-null mice demonstrated preserved glucose-stimulated insulin secretion and were protected against islet cell apoptosis, measured by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) staining, following STZ treatment.[21] The group also found that treatment with the sEH inhibitor trans-4-[4-(3-adamantan-1-ylureido)-cyclohexyloxy]-benzoic acid (t-AUCB) prevented apoptosis in pancreatic islets from rats, measured five days after treatment with low-dose STZ.[16] Insulin release in response to glucose or potassium channel inhibition was increased in isolated islets from Ephx2-null mice and mice treated with t-AUCB.[21] There was no effect of t-AUCB on the ratio of EETs to DHETs measured in the pancreas or in plasma of the STZ-treated mice.[16]
EETS also appear to influence pancreatic islet cell number or size in models of T2DM. In high fat-fed mice, for example, targeted deletion of Ephx2 or selective epoxide hydrolase inhibition using 1-(1-methanesulfonyl-piperidin-4-yl)-3-(4-trifluoro methoxy-phenyl)-urea (TUPS) increased islet size and vascular density measured by staining for CD31.[22] In another study, t-AUCB increased the number of β cells without increasing islet size in high carbohydrate-, high fat-fed rats.[23]
EETs and insulin signaling in peripheral tissues
Luria examined the effect of genetic deletion of Ephx2 and pharmacologic epoxide hydrolase inhibition on insulin signaling in liver and in epididymal adipose tissue after high fat diet.[22] Insulin-induced tyrosyl phosphorylation of the insulin receptor was increased, as was tyrosyl phosphorylation of (IRS)-1 (Figure 1). Similarly, insulin-stimulated association of IRS-1 and the P85 subunit of PI3K was enhanced in Ephx2-null and inhibitor-treated mice resulting in increased phosphorylation of Ser473 and mitogen-activated protein kinase (MAPK). Genetic Ephx2 deficiency and pharmacological sEH inhibition also reduced high-fat feeding-induced endoplasmic reticulum (ER) stress in the liver and subcutaneous adipose tissue.[24] Iyer et al. also reported that epoxide hydrolase inhibition with t-AUCB decreased glucose after an oral glucose load, decreased non-esterified fatty acids and total cholesterol, and decreased steatosis in the liver of high carbohydrate-, high fat-fed rats; there was no effect on inflammation either in adipose or in the liver.[23]
In heme oxygenase (HO)-2 deficient mice, a model of obesity and insulin resistance, administration of an EET agonist (S)-2-(11-(nonyoxyl)undec-8(Z)-enamido)succinic acid (NUDSA) reduced weight gain, subcutaneous and visceral fat, and glucose.[25] Treatment with NUDSA also increased vascular expression of adiponectin and phosphorylation of AMPK and eNOS, and improved endothelial function.[25] NUDSA treatment increased circulating adiponectin concentrations, while decreasing tissue necrosis factor (TNF)-α and monocyte chemotactic protein (MCP)-1.
Endothelial-targeted overexpression of the human epoxygenase CYP2J2 resulted in decreased body weight, blood glucose and insulin, and blood pressure in high fat-fed mice, as well as decreased inflammation in adipose tissue and improved vascular function.[26] Delivery of CYP2J2 to db/db mice using a viral vector resulting in CYP2J2 expression primarily in liver increased circulating EETs, increased insulin sensitivity, decreased hepatic inflammation and increased expression of PPARγ.[27] Similar results have been observed with the delivery of CYP2J3 to fructose- or high fat-fed rats and db/db mice.[28–30]
In vitro in HepG2 cells, Bettaieb et al. reported that sEH inhibition prevents palmate-induced ER stress; sEH inhibition or administration of EETs and EpOME increased insulin-stimulated IR and AKT phosphorylation, whereas DHET and dihydroxyoctadecenoic acid (DiHOME) reduced insulin signaling.[24] Skepner et al. found that treatment with EETs prevented inactivating phosphorylation of IRS-1 at S312 and increased insulin-stimulated AKT phosphorylation and G6P expression in HepG2 cells.[31] Schäfer et al. also observed an effect of EETs on insulin-stimulated phosphorylation of AKT in cultured primary mouse hepatocytes but did not detect an effect on phosphorylation of IR or IRS-1.[13]
In cultured MSCs, Kim reported that EETs suppressed hypertrophy and increased hyperplasia during adipogenesis, and increased phosphorylation of AKT as well as adiponectin concentrations.[15] These effects were enhanced by sEH inhibition with 12-(3-adamantan-1-ylureido)-dodecanoic acid (AUDA). Administration of the EET agonist 12-(3-hexylureido)dodec-8(X)-enoic acid decreased adipocyte generation. The EET agonist also increased HO-1 and adiponectin and glucose uptake in adipocytes. Suppression of adipogenesis by EETs was prevented by pharmacological inhibition of either HO-1 activity or AKT. MSCs derived from HO-2 null mice demonstrate decreased HO-1 activity and increased adipogenesis.[32] Treatment of MSCs from HO-2 mice with an EET agonist 13-(2-(butylamino)-2-oxaacetamido) tridec-8(Z)-eonic acid increased HO-1, reduced adipogenesis, increased adiponectin and decreased inflammatory cytokine production.
In vivo, EETs can enhance insulin sensitivity not only through direct effects on insulin signaling, but also by increasing blood flow and insulin and glucose delivery (Figure 1). In wild-type and obese insulin-resultant mice (db/db), administration of the sEH inhibitor t-AUCB, increased muscle capillary blood volume and microvascular blood flow as measured by contrast enhanced ultrasound; in db/db mice this is accompanied by a decrease in glucose.[33] Conversely, insulin-induced increases in muscle capillary blood volume and microvascular blood flow were prevented by administration of an epoxygenase inhibitor N-methylsulfonyl-2-(2-propynyloxy)-benzenehexanamide (MS-PPOH). The increase on muscle capillary blood volume after sEH inhibition was nitric oxide-independent, whereas the microvascular blood flow appeared to be nitric oxide-dependent.
The EET pathway and insulin sensitivity in humans
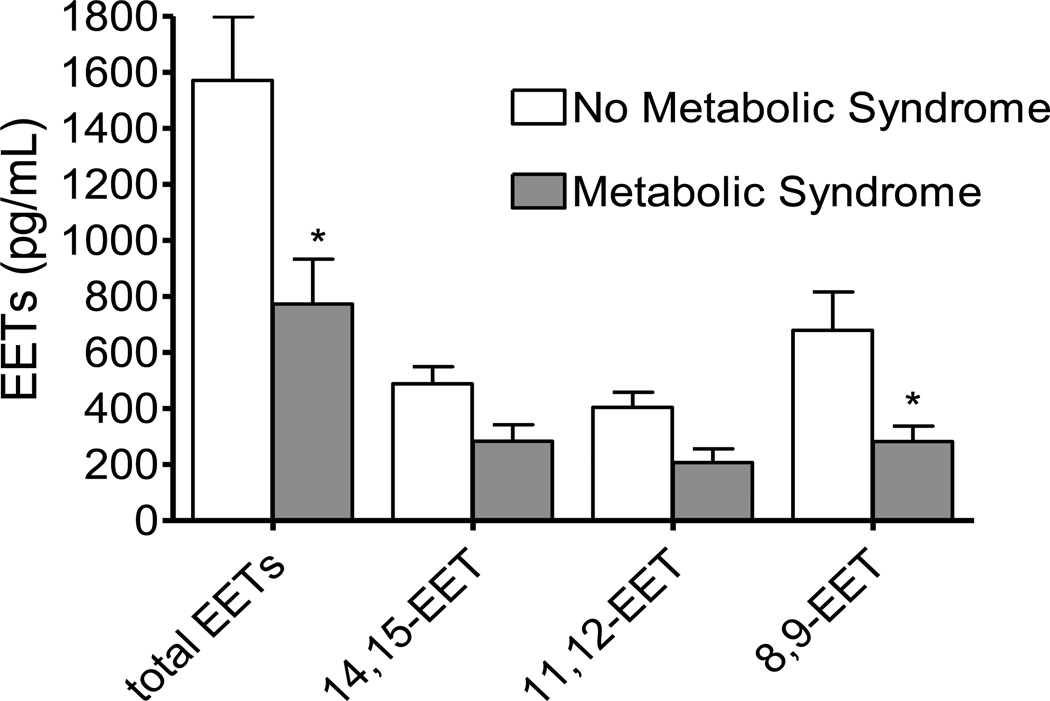
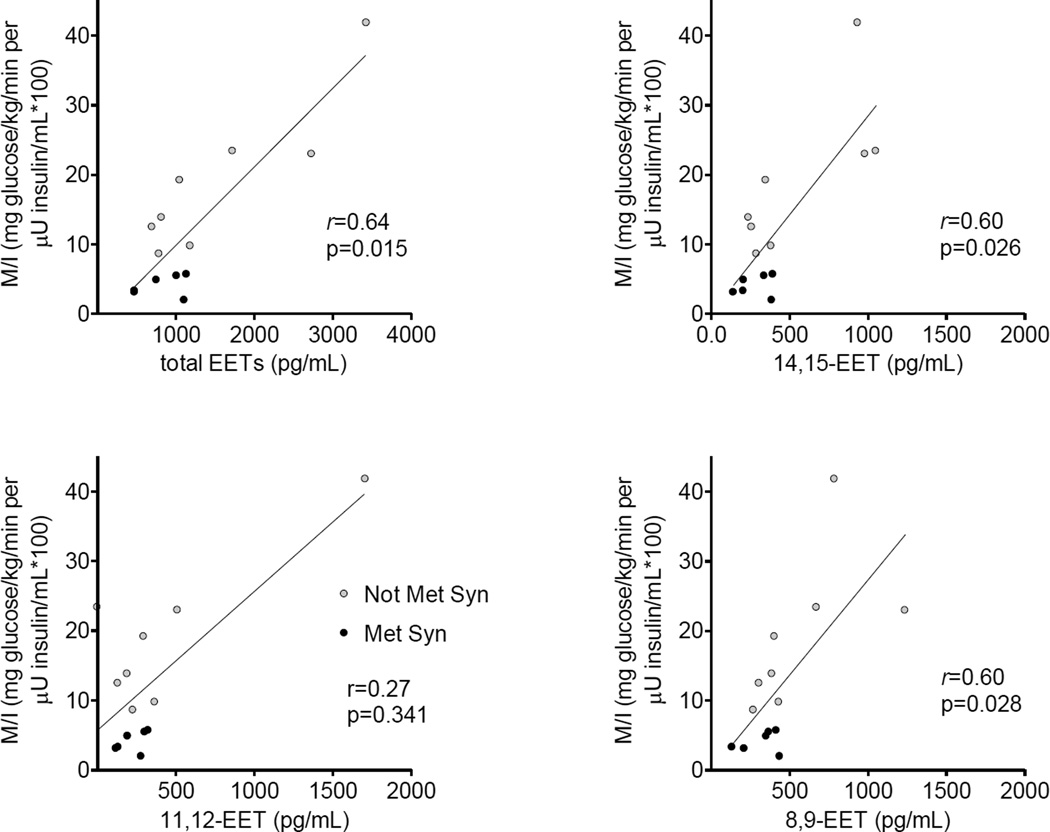
At least two groups have observed that circulating EET concentrations are decreased in obesity or insulin resistance. Theken et al. reported that EET concentrations are decreased in obese patients with coronary artery disease compared to non-obese patients with coronary artery disease, and that there is a significant inverse correlation between body mass index and plasma EETs.[34] We have found that plasma EET concentrations are lower in individuals with the metabolic syndrome than in those without the metabolic syndrome (Figure 2).[35] Similarly, EET concentrations correlate with insulin sensitivity in individuals assessed during hyperglycemic clamps (Figure 3).
Figure 2.
Plasma epoxyeicosatrienoic acids (EETs) in individuals without (N=28) and with (N=12) metabolic syndrome.[35] EETs were measured using UPLC/MS/MS. *P<0.05 versus without metabolic syndrome. Reprinted with permission.
Figure 3.
Spearman’s correlation between plasma epoxyeicosatrienoic acid (EET) concentrations and insulin sensitivity index (ISI) calculated from hyperglycemic clamp.[35] Solid circles depict individuals with metabolic syndrome (MetSyn) and open circles those without MetSyn. Reprinted with permission.
In the absence of EET agonists or sEH inhibitors that have been approved for use in humans, one strategy to investigate the role of EETS in glucose homeostasis in humans is to determine whether functional genetic polymorphisms in genes encoding for epoxygenases or EPHX2 are associated with obesity, diabetes, or measures of insulin sensitivity and secretion. Wang et al. examined the association between a functional G-50T polymorphism in the gene encoding the epoxygenase CYP2J2 that results in loss of expression and activity and diabetes in a Chinese population.[36] There was no association between the genotype and T2DM. Among patients with T2DM, however, the T allele was significantly over-represented among those with an early age of disease onset. In addition, circulating DHET concentrations were lower in carriers of the T allele.
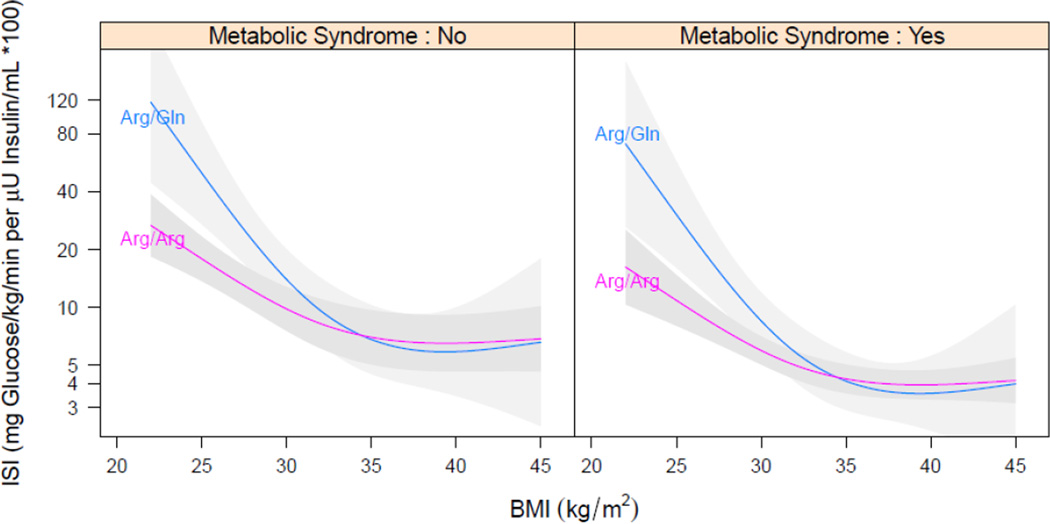
Our group and others have studied the relationship between functional polymorphisms in the gene encoding for sEH, EPHX2, and obesity, insulin sensitivity, or diabetes. The Arg287Gln variant encodes for a sEH enzyme with lower hydrolase activity, while Lys55Arg encodes for an sEH enzyme with increased hydrolase activity.[37] Both polymorphisms have lower phosphatase activity.[38] We have observed an association between the loss-of-function EPHX2 287Gln variant and increased insulin sensitivity.[35] Interestingly, the relationship between the EPHX2 287Gln variant and insulin sensitivity varied with body mass index (BMI), such that at an overweight BMI of 25 kg/M2, insulin sensitivity was 180% higher (95% CI: 45–439%, p=0.002) in EPHX2 287Gln carriers compared to Arg/Arg homozygotes, whereas at an obese BMI of 30 kg/M2 insulin sensitivity was 40% higher (95% CI: 0–130%, p=0.05) in carriers of the EPHX2 287Gln, and at higher BMI there was no relationship between insulin sensitivity and genotype (Figure 4). These data suggest that decreasing sEH activity in patients at risk for T2DM could enhance insulin sensitivity.
Figure 4.
Linear regression model fitted for log-transformed insulin sensitivity index (ISI) on genotype with adjustment for body mass index (BMI), as well as the interaction between genotype and BMI, in patients with or without metabolic syndrome. A smooth relationship was assumed for BMI using restricted cubic regression splines with three knots. Presented are predicted ISI as a function of BMI for Arg/Gln and Arg/Arg with metabolic syndrome set to overall mean; shaded area are 95% confidence intervals.
Contrary to these findings, Ohtoshi reported that the EPHX2 287Gln variant was associated with insulin resistance in Japanese patients with T2DM, although not with insulin resistance in healthy controls.[39] The group found no association between EPHX2 Arg287Gln genotype and T2DM.
Soluble epoxide hydrolase inhibitors are under development in humans
Among the pharmacological inhibitors of soluble epoxide hydrolase tested in rodents, the first to be tested in humans was 1-(1-acetyl-piperidin-4-yl)-3-adamantan-1-yl-urea or AR9281. AR9281 selectively inhibited human sEH with an IC50 of 8 nM, and improved glucose tolerance following an intraperitoneal glucose tolerance test in high-fat, high-fructose fed mice after AR9281 administration for 12 weeks.[40] Results from single- and multiple-dosing studies of AR9281 have been reported in humans.[41] AR9281 caused a dose-dependent decrease in sEH activity.[41] The single 500 mg dose inhibited sEH at 90% over a twelve-hour period. Multiple doses of AR9281 from 100 to 400 mg every eight hours resulted in a sustained 90% or greater inhibition of sEH at the trough. A placebo-controlled study of the effects of AR9281 in patients with mild-to-moderate hypertension and impaired glucose tolerance (NCT00847899) has been completed, but not published.
The sEH inhibitor GSK2256294 [(1R,3S)-N-(4-cyano-2-(trifluoromethyl)benzyl)-3-((4-methyl-6-(methylamino)-1,3,5-triazin-2-yl)amino)cyclohexanecarboxamide] has been studied safely in Phase I and II studies.[42] The tmax is 0.5 hours and t1/2 is 48 hours. In multiple dosing studies in overweight smokers, steady-state was achieved within seven days. A dose of 6 mg GSK2256294 achieved more than 90% inhibition of sEH throughout the dosing interval with repeated dosing.
Enthusiasm about the potential for pharmacological sEH inhibitors in the prevention of T2DM must be tempered by concerns about potential adverse effects. In pre-clinical cancer models, EETs increase proliferation and survival of endothelial cells and promote angiogenesis, leading to increased tumor metastasis.[43] The effect of sEH inhibition on angiogenesis and tumor growth is complex, however. While EETs promote angiogenesis, other fatty acid substrates of sEH, such as the epoxydocosapentaenoic acids (EpDPEs) reduce angiogenesis. Thus while in some rodent models overexpression of EETs or inhibition of sEH can promote growth and metastasis of existing tumors,[43] in others dual cyclooxygenase and sEH inhibition decreases angiogenesis and tumor growth.[44]
Summary
Studies in rodent models of T1DM indicate that EETs contribute to the preservation of islet function and, in models of obesity and diabetes, improved insulin sensitivity through effects on local insulin signaling as well as on capillary recruitment. Preventing the hydrolysis of EETs by sEH accentuates these beneficial effects. EET analogs are not currently available for administration to humans whereas sEH inhibitors are under development. Studies in humans suggesting that carriers of a loss-of-function polymorphism in EPHX2 demonstrate increased insulin sensitivity suggest that pharmacological sEH inhibition could provide benefit in humans at risk for T2DM.
Highlights.
Genetic deletion of Ephx2 or specific inhibition of soluble epoxide hydrolase prevents islet apoptosis in streptozotocin-treated rodents
Genetic deletion of Ephx2 or specific inhibition of soluble epoxide hydrolase enhances insulin signaling in mouse models of insulin resistance such as high fat-feeding, heme oxygenase-2 deficient mice and db/db mice, as well as in cultured hepatocytes and adipocytes
Circulating epoxyeicosatrienoic acids are decreased in obese humans and correlate with insulin sensitivity
Inhibitors of soluble epoxide hydrolase are under clinical development
Acknowledgments
Dr. Luther was supported by grants DK096994 and DK081662, while Dr. Brown was supported by grants DK038226 and HL125426, from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Imig JD. Epoxyeicosatrienoic acids, hypertension, and kidney injury. Hypertension. 2015;65:476–482. doi: 10.1161/HYPERTENSIONAHA.114.03585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 3.Harris RC, Homma T, Jacobson HR, Capdevila J. Epoxyeicosatrienoic acids activate Na+/H+ exchange and are mitogenic in cultured rat glomerular mesangial cells. J. Cell Physiol. 1990;144:429–437. doi: 10.1002/jcp.1041440310. [DOI] [PubMed] [Google Scholar]
- 4.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu. Rev. Pharmacol. Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 8.Thomas H, Schladt L, Knehr M, Oesch F. Effect of diabetes and starvation on the activity of rat liver epoxide hydrolases, glutathione S-transferases and peroxisomal beta-oxidation. Biochem. Pharmacol. 1989;38:4291–4297. doi: 10.1016/0006-2952(89)90528-5. [DOI] [PubMed] [Google Scholar]
- 9.Dewey S, Lai X, Witzmann FA, Sohal M, Gomes AV. Proteomic analysis of hearts from Akita mice suggests that increases in soluble epoxide hydrolase and antioxidative programming are key changes in early stages of diabetic cardiomyopathy. J Proteome. Res. 2013;12:3920–3933. doi: 10.1021/pr4004739. [DOI] [PubMed] [Google Scholar]
- 10.Oguro A, Fujita N, Imaoka S. Regulation of soluble epoxide hydrolase (sEH) in mice with diabetes: high glucose suppresses sEH expression. Drug Metab Pharmacokinet. 2009;24:438–445. doi: 10.2133/dmpk.24.438. [DOI] [PubMed] [Google Scholar]
- 11.Oguro A, Oida S, Imaoka S. Down-regulation of EPHX2 gene transcription by Sp1 under high-glucose conditions. Biochem. J. 2015;470:281–291. doi: 10.1042/BJ20150397. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Dang H, Li D, Pang W, Hammock BD, Zhu Y. Inhibition of soluble epoxide hydrolase attenuates high-fat-diet-induced hepatic steatosis by reduced systemic inflammatory status in mice. PLoS. One. 2012;7:e39165. doi: 10.1371/journal.pone.0039165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schafer A, Neschen S, Kahle M, Sarioglu H, Gaisbauer T, Imhof A, Adamski J, Hauck SM, Ueffing M. The Epoxyeicosatrienoic Acid Pathway Enhances Hepatic Insulin Signaling and is Repressed in Insulin-Resistant Mouse Liver. Mol. Cell Proteomics. 2015;14:2764–2774. doi: 10.1074/mcp.M115.049064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Taeye BM, Morisseau C, Coyle J, Covington JW, Luria A, Yang J, Murphy SB, Friedman DB, Hammock BB, Vaughan DE. Expression and regulation of soluble epoxide hydrolase in adipose tissue. Obesity. (Silver. Spring) 2010;18:489–498. doi: 10.1038/oby.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DH, Vanella L, Inoue K, Burgess A, Gotlinger K, Manthati VL, Koduru SR, Zeldin DC, Falck JR, Schwartzman ML, Abraham NG. Epoxyeicosatrienoic acid agonist regulates human mesenchymal stem cell-derived adipocytes through activation of HO-1-pAKT signaling and a decrease in PPARgamma. Stem Cells Dev. 2010;19:1863–1873. doi: 10.1089/scd.2010.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Fan C, Zhang Y, Bakri M, Dong H, Morisseau C, Maddipati KR, Luo P, Wang CY, Hammock BD, Wang MH. Beneficial effects of inhibition of soluble epoxide hydrolase on glucose homeostasis and islet damage in a streptozotocin-induced diabetic mouse model. Prostaglandins Other Lipid Mediat. 2013;104–105:42–48. doi: 10.1016/j.prostaglandins.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeldin DC, Foley J, Boyle JE, Moomaw CR, Tomer KB, Parker C, Steenbergen C, Wu S. Predominant expression of an arachidonate epoxygenase in islets of Langerhans cells in human and rat pancreas. Endocrinology. 1997;138:1338–1346. doi: 10.1210/endo.138.3.4970. [DOI] [PubMed] [Google Scholar]
- 18.Falck JR, Manna S, Moltz J, Chacos N, Capdevila J. Epoxyeicosatrienoic acids stimulate glucagon and insulin release from isolated rat pancreatic islets. Biochem. Biophys. Res. Commun. 1983;114:743–749. doi: 10.1016/0006-291x(83)90843-4. [DOI] [PubMed] [Google Scholar]
- 19.Turk J, Wolf BA, Comens PG, Colca J, Jakschik B, McDaniel ML. Arachidonic acid metabolism in isolated pancreatic islets. IV. Negative ion-mass spectrometric quantitation of monooxygenase product synthesis by liver and islets. Biochim. Biophys. Acta. 1985;835:1–17. doi: 10.1016/0005-2760(85)90023-2. [DOI] [PubMed] [Google Scholar]
- 20.Klett EL, Chen S, Edin ML, Li LO, Ilkayeva O, Zeldin DC, Newgard CB, Coleman RA. Diminished acyl-CoA synthetase isoform 4 activity in INS 832/13 cells reduces cellular epoxyeicosatrienoic acid levels and results in impaired glucose-stimulated insulin secretion. J Biol. Chem. 2013;288:21618–21629. doi: 10.1074/jbc.M113.481077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo P, Chang HH, Zhou Y, Zhang S, Hwang SH, Morisseau C, Wang CY, Inscho EW, Hammock BD, Wang MH. Inhibition or deletion of soluble epoxide hydrolase prevents hyperglycemia, promotes insulin secretion, and reduces islet apoptosis. J Pharmacol. Exp. Ther. 2010;334:430–438. doi: 10.1124/jpet.110.167544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luria A, Bettaieb A, Xi Y, Shieh GJ, Liu HC, Inoue H, Tsai HJ, Imig JD, Haj FG, Hammock BD. Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc. Natl. Acad. Sci. U. S. A. 2011;108:9038–9043. doi: 10.1073/pnas.1103482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iyer A, Kauter K, Alam MA, Hwang SH, Morisseau C, Hammock BD, Brown L. Pharmacological inhibition of soluble epoxide hydrolase ameliorates diet-induced metabolic syndrome in rats. Exp. Diabetes Res. 2012;2012:758614. doi: 10.1155/2012/758614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bettaieb A, Nagata N, Aboubechara D, Chahed S, Morisseau C, Hammock BD, Haj FG. Soluble Epoxide Hydrolase Deficiency or Inhibition Attenuates Diet-induced Endoplasmic Reticulum Stress in Liver and Adipose Tissue. J. Biol. Chem. 2013;288:14189–14199. doi: 10.1074/jbc.M113.458414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sodhi K, Inoue K, Gotlinger KH, Canestraro M, Vanella L, Kim DH, Manthati VL, Koduru SR, Falck JR, Schwartzman ML, Abraham NG. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J. Pharmacol. Exp. Ther. 2009;331:906–916. doi: 10.1124/jpet.109.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abraham NG, Sodhi K, Silvis AM, Vanella L, Favero G, Rezzani R, Lee C, Zeldin DC, Schwartzman ML. CYP2J2 targeting to endothelial cells attenuates adiposity and vascular dysfunction in mice fed a high-fat diet by reprogramming adipocyte phenotype. Hypertension. 2014;64:1352–1361. doi: 10.1161/HYPERTENSIONAHA.114.03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li R, Xu X, Chen C, Wang Y, Gruzdev A, Zeldin DC, Wang DW. CYP2J2 attenuates metabolic dysfunction in diabetic mice by reducing hepatic inflammation via the PPARgamma. Am J Physiol Endocrinol. Metab. 2015;308:E270–E282. doi: 10.1152/ajpendo.00118.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X, Zhao CX, Wang L, Tu L, Fang X, Zheng C, Edin ML, Zeldin DC, Wang DW. Increased CYP2J3 expression reduces insulin resistance in fructose-treated rats and db/db mice. Diabetes. 2010;59:997–1005. doi: 10.2337/db09-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X, Tu L, Wang L, Fang X, Wang DW. CYP2J3 gene delivery reduces insulin resistance via upregulation of eNOS in fructose-treated rats. Cardiovasc. Diabetol. 2011;10:114. doi: 10.1186/1475-2840-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, Tu L, Feng W, Ma B, Li R, Zheng C, Li G, Wang DW. CYP2J3 gene delivery up-regulated adiponectin expression via reduced endoplasmic reticulum stress in adipocytes. Endocrinology. 2013;154:1743–1753. doi: 10.1210/en.2012-2012. [DOI] [PubMed] [Google Scholar]
- 31.Skepner JE, Shelly LD, Ji C, Reidich B, Luo Y. Chronic treatment with epoxyeicosatrienoic acids modulates insulin signaling and prevents insulin resistance in hepatocytes. Prostaglandins Other Lipid Mediat. 2011;94:3–8. doi: 10.1016/j.prostaglandins.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Burgess AP, Vanella L, Bellner L, Gotlinger K, Falck JR, Abraham NG, Schwartzman ML, Kappas A. Heme oxygenase (HO-1) rescue of adipocyte dysfunction in HO-2 deficient mice via recruitment of epoxyeicosatrienoic acids (EETs) and adiponectin. Cell Physiol Biochem. 2012;29:99–110. doi: 10.1159/000337591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shim CY, Kim S, Chadderdon S, Wu M, Qi Y, Xie A, Alkayed NJ, Davidson BP, Lindner JR. Epoxyeicosatrienoic acids mediate insulin-mediated augmentation in skeletal muscle perfusion and blood volume. Am J Physiol Endocrinol. Metab. 2014;307:E1097–E1104. doi: 10.1152/ajpendo.00216.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theken KN, Lee CR. Genetic variation in the cytochrome P450 epoxygenase pathway and cardiovascular disease risk. Pharmacogenomics. 2007;8:1369–1383. doi: 10.2217/14622416.8.10.1369. [DOI] [PubMed] [Google Scholar]
- 35.Ramirez CE, Shuey MM, Milne GL, Gilbert K, Hui N, Yu C, Luther JM, Brown NJ. Arg287Gln variant of EPHX2 and epoxyeicosatrienoic acids are associated with insulin sensitivity in humans. Prostaglandins Other Lipid Mediat. 2014;113–115:38–44. doi: 10.1016/j.prostaglandins.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang CP, Hung WC, Yu TH, Chiu CA, Lu LF, Chung FM, Hung CH, Shin SJ, Chen HJ, Lee YJ. Genetic variation in the G-50T polymorphism of the cytochrome P450 epoxygenase CYP2J2 gene and the risk of younger onset type 2 diabetes among Chinese population: potential interaction with body mass index and family history. Exp. Clin. Endocrinol. Diabetes. 2010;118:346–352. doi: 10.1055/s-0029-1243604. [DOI] [PubMed] [Google Scholar]
- 37.Przybyla-Zawislak BD, Srivastava PK, Vazquez-Matias J, Mohrenweiser HW, Maxwell JE, Hammock BD, Bradbury JA, Enayetallah AE, Zeldin DC, Grant DF. Polymorphisms in human soluble epoxide hydrolase. Mol. Pharmacol. 2003;64:482–490. doi: 10.1124/mol.64.2.482. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava PK, Sharma VK, Kalonia DS, Grant DF. Polymorphisms in human soluble epoxide hydrolase: effects on enzyme activity, enzyme stability, and quaternary structure. Arch. Biochem. Biophys. 2004;427:164–169. doi: 10.1016/j.abb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Ohtoshi K, Kaneto H, Node K, Nakamura Y, Shiraiwa T, Matsuhisa M, Yamasaki Y. Association of soluble epoxide hydrolase gene polymorphism with insulin resistance in type 2 diabetic patients. Biochem. Biophys. Res. Commun. 2005;331:347–350. doi: 10.1016/j.bbrc.2005.03.171. [DOI] [PubMed] [Google Scholar]
- 40.Anandan SK, Webb HK, Chen D, Wang YX, Aavula BR, Cases S, Cheng Y, Do ZN, Mehra U, Tran V, Vincelette J, Waszczuk J, White K, Wong KR, Zhang LN, Jones PD, Hammock BD, Patel DV, Whitcomb R, MacIntyre DE, Sabry J, Gless R. 1-(1-acetyl-piperidin-4-yl)-3-adamantan-1-yl-urea (AR9281) as a potent, selective, and orally available soluble epoxide hydrolase inhibitor with efficacy in rodent models of hypertension and dysglycemia. Bioorg. Med. Chem. Lett. 2011;21:983–988. doi: 10.1016/j.bmcl.2010.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen D, Whitcomb R, MacIntyre E, Tran V, Do ZN, Sabry J, Patel DV, Anandan SK, Gless R, Webb HK. Pharmacokinetics and pharmacodynamics of AR9281, an inhibitor of soluble epoxide hydrolase, in single- and multiple-dose studies in healthy human subjects. J Clin. Pharmacol. 2012;52:319–328. doi: 10.1177/0091270010397049. [DOI] [PubMed] [Google Scholar]
- 42.Lazaar AL, Yang L, Boardley RL, Goyal NS, Robertson J, Baldwin SJ, Newby DE, Wilkinson IB, Tal-Singer R, Mayer RJ, Cheriyan J. Pharmacokinetics, pharmacodynamics and adverse event profile of GSK2256294, a novel soluble epoxide hydrolase inhibitor. Br. J Clin. Pharmacol. 2015 doi: 10.1111/bcp.12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panigrahy D, Edin ML, Lee CR, Huang S, Bielenberg DR, Butterfield CE, Barnes CM, Mammoto A, Mammoto T, Luria A, Benny O, Chaponis DM, Dudley AC, Greene ER, Vergilio JA, Pietramaggiori G, Scherer-Pietramaggiori SS, Short SM, Seth M, Lih FB, Tomer KB, Yang J, Schwendener RA, Hammock BD, Falck JR, Manthati VL, Ingber DE, Kaipainen A, D'Amore PA, Kieran MW, Zeldin DC. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J Clin. Invest. 2012;122:178–191. doi: 10.1172/JCI58128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang G, Panigrahy D, Hwang SH, Yang J, Mahakian LM, Wettersten HI, Liu JY, Wang Y, Ingham ES, Tam S, Kieran MW, Weiss RH, Ferrara KW, Hammock BD. Dual inhibition of cyclooxygenase-2 and soluble epoxide hydrolase synergistically suppresses primary tumor growth and metastasis. Proc. Natl. Acad. Sci. U. S. A. 2014;111:11127–11132. doi: 10.1073/pnas.1410432111. [DOI] [PMC free article] [PubMed] [Google Scholar]