Abstract
Impaired postural control is a cardinal symptom following concussion. Planned gait termination (GT) is a non-novel, dynamic task that challenges postural control in individuals with neurological deficits, and it could be an impactful measure for identifying dynamic postural control impairments following concussion. Therefore, the purpose of this study was to assess acute post-concussion dynamic postural control utilizing a planned GT task. The concussion participants (n= 19, age: 19.0 ± 0.8 years, height: 177.0 ± 10.1 cm, weight: 83.3 ± 20.0 kg) completed five planned GT trials during preseason baseline testing (Baseline) and on Day 1 post-concussion (Day-1). Healthy control participants (n=19, age: 20.4 ± 1.2 years, height: 173.8 ± 8.9 cm, weight: 80.2 ± 17.6 kg) completed the same trials a week apart. The dependent variables of interest included COP displacement and velocity in the mediolateral (ML) and anteroposterior (AP) axes during the three phases (braking, transitional, stabilization) of planned GT. There were significant interactions observed in both the braking ML and transitional AP displacement (p= 0.042, p= 0.030) and velocity (p= 0.027, p= 0.030). These results suggest a conservative post-concussion motor control strategy during planned GT. Further, these results support the use of dynamic postural control tasks as measures of post-concussion impairments.
Keywords: Mild traumatic brain injury, Motor control, Center of pressure displacements, Center of pressure velocities
1. Introduction
Impairments in postural control are a primary concussion symptom; thus, postural control testing is a recommended component of the multifaceted concussion assessment battery. [1,2] Current concussion assessments include both clinical (Balance Error Scoring System (BESS)) and experimental (Sensory Organization Test (SOT)) protocols. The most commonly utilized clinical assessment tool, BESS, is limited by low interrater and intrarater reliability scores, test administration environment, and low sensitivity (0.34) acutely post-concussion. [3-6] Despite its limitations, the BESS does have high content validity for identifying balance impairments following a concussion, and the modified version of the BESS, which is recommended by the 3rd edition of the Sport Concussion Assessment Tool, has demonstrated good reliability. [7-9] However, both the BESS and SOT are limited by substantial practice effects, potentially because these are novel tasks (e.g., standing barefoot on a foam surface with the eyes closed), and repeat administration has routinely identified improved performance. [10,11] Further, the BESS and SOT are static assessments that rely on feedback mechanisms to maintain upright posture on an unstable surface and do not evaluate transitional, dynamic movements, which are likely more challenging to the postural control systems. [12] These limitations may explain the surprising finding that post-concussion static postural control often recovers prior to both symptom resolution and cognitive deficits. [6] Therefore, the utilization of common dynamic motor activities of daily living (ADL), which are unlikely to be subjected to a practice effect, may be more appropriate for identifying post-concussion impairments. [13]
An acute post-concussion conservative gait strategy, consisting of reduced step velocity, step length, center of pressure (COP) and center of mass (COM) separation, as well as increased double support time and frontal plane COM sway, has been consistently identified. [14,15] These deficits appear to persist for up to two months post-injury, suggesting impairments in dynamic postural control persist well beyond BESS recovery. [16] Gait is an innate, or non-novel, dynamic task, and the parameters are not generally susceptible to the practice effects in otherwise healthy young adults. [17] Gait performance is highly consistent in healthy college-aged recreational and student-athletes. [18] Specifically, changes in gait parameters (e.g., velocity, stride length) are most pronounced up to age 10, after which there are minimal changes in gait pattern. [17] Unlike quiet standing, unobstructed gait is less reliant on sensory feedback as both supraspinal planning (motor cortex and pyramidal tract) and central pattern generators are largely responsible for feedforward control. [19,20] Further, transitional movements, such as initiating or terminating gait, are likely more challenging to dynamic postural control systems than gait, which likely increases the neurological resources required to safely complete the task. [21]
Planned gait termination (GT) is a transitional motor task that encompasses the shift from cyclical gait to quiet standing and requires the central nervous system to anticipate, control, and slow the forward momentum of the body while maintaining the COM within the base of support (BOS). [22] This transitional task is divided up into three phases: braking (S1), transitional (S2), and stabilization (S3). In fRMI studies, planned GT appears to be controlled supraspinally, with activation patterns identified within the right prefrontal area, specifically the right inferior frontal gyrus. [19] The planned GT task requires the participant be aware of the location or time to terminate gait and is comprised of a penultimate (second to last step before termination) and termination step. [22] Mechanically, planned GT requires two coupled braking mechanisms: 1) a reduction in the foot propulsive force during the penultimate step and 2) an increase in the braking force during the terminating step. [22] Thus, it is not surprising that GT has already identified both acute and lingering alterations in post-concussion propulsive and braking forces only; however, the COP trajectories have not been elucidated. [23] Further, individuals with compromised neurological systems (e.g., Parkinson’s disease, Cerebellar disease, moderate to severe traumatic brain injury) have noted planned GT deficits, including diminished COP displacements.
Planned GT is a stable, non-novel ADL task that challenges the postural control systems and that relies on active feedforward control. [20] Impaired postural control is a known consequence of concussion; however, most assessment protocols utilize novel static tasks that have not been associated with specific postural control mechanisms. Therefore, the purpose of this study was to evaluate planned GT performance between baseline and post-concussion individuals with comparison to healthy individuals. We hypothesize an interaction will be present wherein herein healthy control participants will demonstrate consistent task performance whereas the post-concussion participants will demonstrate an impairment during GT.
2. Methods
2.1 Participants
There were 38 participants in this study; 19 National Collegiate Athletic Association Division I student-athletes, from a single institution, diagnosed with a sports-related concussion and a control group consisting of 19 uninjured, physically active individuals from the same institution. (Table 1) All concussions were identified by a certified athletic trainer and subsequently diagnosed by the team physician. The inclusion criteria for the concussion participants was a diagnosed concussion with valid baseline data, and the control participants were intercollegiate or recreational athletes with no history of concussion within the past 6 months. The exclusion criteria included any self-reported neurological disorders, current lower extremity orthopedic injury, and metabolic, vestibular, vision disorders or other conditions that would impair gait performance. A current lower extremity orthopedic injury was classified as any ongoing or past orthopedic injury that would alter an individual’s normal gait pattern. Each participant provided oral and written informed consent in accordance with the University’s IRB.
Table 1.
Participant Demographics. There were no significant differences (P>0.05) between groups for demographics.
| Age (years) M ± SD | Height (cm) M ± SD | Weight (kg) M ± SD | Concussion History | |
|---|---|---|---|---|
| Concussion (n=19) | 19.0 ± 0.8 | 177.0 ± 10.1 | 83.3 ± 20.0 | 0.9 ± 1.0 |
| Control (n=19) | 20.4 ± 1.2 | 173.8 ± 8.9 | 80.2 ± 17.6 | 0.8 ± 1.2 |
1/19 concussion participants presented with loss of consciousness (LOC)
4/19 concussion participants presented with post-traumatic amnesia (PTA)
2.2 Instrumentation and Procedure
Kinetic data was collected at 1,000 Hz from four 400 mm × 600 mm force plates (AMTI, Model OR-6, Watertown, MA. USA) mounted flush with the walkway surface and COP was calculated with standard biomechanics formulas. [24] The concussion participants completed their first test during pre-participation physical examinations (Baseline), prior to any participation as an intercollegiate student-athlete, and were retested on the first day following their concussion (Day-1). The median time between baseline testing and Day-1 post-concussion was 118 days (range: 49 – 807 days). The control participants completed the trials on an initial day (Baseline) were retested one week later (Day-1) outside of their intercollegiate season. While gait termination, to our knowledge, has not been evaluated for stability across time, it has been established that there are minimal changes to an individual’s gait pattern after age 10 [17]; therefore, it is likely that any differences identified herein were associated with the concussion and not the testing interval. Furthermore, the feasibility of recruiting healthy student-athletes for additional testing sessions within season is logistically difficult.
Each participant performed 5 planned GT trials during each testing session. Participants were instructed that, in response to a verbal cue, they would traverse an 8-meter walkway and perform planned GT on the force plates. The penultimate step impacted either force plate #3 or #4, depending on footfall, and the terminating step occurred on force plates #1 and #2. (Figure 1) Practice trials were performed to ensure a natural footfall on the force plates and if errors, occurred during the test trials (e.g., irregular footfall pattern, falling to stop) it was repeated.
Figure 1.
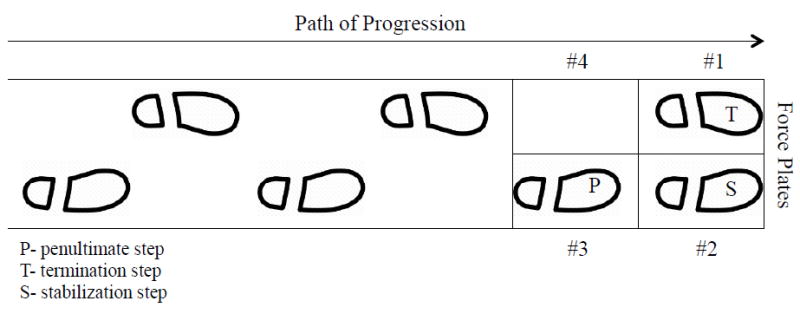
Gait termination progression. The planned GT trials required the participants to walk from the starting point to the force plates, with the penultimate step occurring on force plate #3 or #4 and the termination and stabilization steps landing on #1 or #2.
2.3 Data Analysis
This was a prospective longitudinal study. The independent variables included group (control or concussion) and time (Baseline, Day-1). The dependent variables measured from the GT trials included both the COP segment displacements and COP segment mean velocity. The COP displacements during GT are similar, but reversed, from gait initiation, thus similar terminology is applied herein. [25] (Figure 2) The COP displacements were calculated in both the ML and AP directions during three phases (S1, S2, S3) of COP shift. [26] The mean velocity was calculated from each segment (S1, S2, and S3) in both planes (APV and MLV). Similar to previous literature, the first phase of GT, S1, or the braking phase, occurred during a shift from the initial heel contact of the lead limb during the penultimate step to the ball of the terminating step foot contacting the ground. [26] During S2, the transitional phase, COP is transferred under the lead limb until the trailing limb completed the swing phase and resumed a bipedal stance on the force plate. [26] The final phase, S3 or the stabilization phase, is a final shift in the COP back to the midline once both feet were planted. [26] The heel strike was captured on the force plate when the ground reaction force exceeded 20N.
Figure 2.
Center of pressure trajectory. The shift of the COP occurs in three phases: braking (S1), transitional (S2) and stabilization (S3). The S1 phase represents the braking phase of GT, during which a shift occurs from the penultimate step to the heel of the lead limb as it hits the ground and finally toward the ball of the foot of the leading limb. The transitional phase, S2, occurs as the pressure is transferred fully under the lead limb, while the trailing limb is in swing phase, until the trailing limb strikes the force plate and bilateral stance is established. During the stabilization phase, S3, there is a shift in the COP back to the middle as both feet are planted.
2.4 Statistical Analysis
The mean of the 5 trials was evaluated in the statistical analysis. The dependent variables of interest were compared with a 2 (group) × 2 (time) mixed design analysis of variance (ANOVA). Significant interactions were followed up with a pairwise comparison, using Tukey’s procedure to examine the simple main effect of time for each group. A traditional level of significance (α = 0.05) was used for the COP displacements and velocities.
3. Results
All participants completed the five trials without incident.
3.1 S1 ML and S1 MLV
There was a significant interaction between time and group during S1 for ML for both COP displacement (F= 4.425, p= 0.042, η2= 0.109) and velocity (F= 4.100, p= 0.050, η2= 0.129). The COP displacement in S1 ML was significantly reduced (Δ4.8 cm, p= 0.002) within the concussion group at Day-1, whereas the control group remained largely unchanged from baseline (Δ0.4 cm, p = 0.753). (Table 2) Similarly, the COP velocity in S1 MLV was significantly reduced (Δ47.8 cm/s, p= 0.004) within the concussion group at Day-1, whereas the control group remained largely unchanged from baseline (Δ3.1 cm/s, p= 0.844). (Table 3)
Table 2.
Center of Pressure Displacement Results. There was a significant interaction observed in S1 ML and S2 AP. A significant decrease in S1 ML and a significant increase in S2 AP displacements were observed in the concussion group, compared to the controls.
| COP Phase | Control (M±SD) (cm) | Concussion (M±SD) (cm) | Mixed Design ANOVA Interaction (p-value) | ||
|---|---|---|---|---|---|
| Baseline | Day 1 | Baseline | Day 1 | ||
|
| |||||
| Braking (S1AP) | 64.0 ± 5.6 | 61.2 ± 6.4 | 60.3 ± 9.5 | 50.6 ± 19.7 | 0.188 |
|
| |||||
| Braking (S1ML) | 16.8 ± 5.4 | 16.4 ± 5.9 | 18.0 ± 6.1 | 13.2 ± 6.7 | 0.042* |
|
| |||||
| Transitional (S2AP) | 3.9 ± 4.8 | 4.0 ± 4.2 | 9.5 ± 5.6 | 18.6 ± 16.7 | 0.030* |
|
| |||||
| Transitional (S2ML) | 12.6 ± 3.6 | 12.4 ± 3.7 | 17.8 ± 4.8 | 16.5 ± 4.2 | 0.475 |
|
| |||||
| Stabilization (S3AP) | 2.1 ± 1.1 | 1.7 ± 0.9 | 2.6 ± 1.0 | 2.7 ± 1.7 | 0.441 |
|
| |||||
| Stabilization (S3ML) | 2.3 ± 2.0 | 2.5 ± 2.4 | 7.1 ± 3.9 | 7.9 ± 4.9 | 0.620 |
denotes significant interaction
Table 3.
Center of Pressure Velocity Results. There was a significant interaction observed in S1 ML and S2 AP. A significant decrease in S1 ML and a significant increase in S2 AP velocities were observed in the concussion group, compared to the controls.
| COP Phase | Control (M±SD) (cm/s) | Concussion (M±SD) (cm/s) | Mixed Design ANOVA Interaction (p-value) | ||
|---|---|---|---|---|---|
| Baseline | Day 1 | Baseline | Day 1 | ||
|
| |||||
| Braking (S1 APV) | 812.1 ± 102.1 | 815.6 ± 127.1 | 760.8 ± 127.4 | 689.6 ± 202.8 | 0.203 |
|
| |||||
| Braking (S1 MLV) | 213.7 ± 69.8 | 216.8 ± 81.8 | 223.9 ± 73.1 | 176.1 ± 71.9 | 0.027* |
|
| |||||
| Transitional (S2 APV) | 68.5 ± 92.5 | 80.2 ± 94.6 | 194.3 ± 147.7 | 438.6 ± 424.0 | 0.030* |
|
| |||||
| Transitional (S2 MLV) | 214.4 ± 78.4 | 227.7 ± 75.3 | 313.5 ± 110.5 | 323.5 ± 127.8 | 0.926 |
|
| |||||
| Stabilization (S3 APV) | 28.6 ± 8.9 | 26.3 ± 16.1 | 40.4 ± 25.4 | 36.2 ± 26.3 | 0.842 |
|
| |||||
| Stabilization (S3 APV) | 31.3 ± 20.2 | 30.3 ± 22.2 | 99.5 ± 71.4 | 112.5 ± 86.3 | 0.604 |
denotes significant interaction
3.2 S2 AP and S2 APV
There was also a significant interaction between time and group during S2 for AP for both displacement (F= 5.123, p= 0.030, η2= 0.125) and velocity (F= 6.182, p= 0.030, η2= 0.124). The COP displacement in S2 AP was significantly increased (Δ9.1 cm, p= 0.003) within the concussion group on Day-1, whereas the control group was nearly identical to baseline (Δ0.1 cm, p= 0.973). (Table 2) Similarly, the COP velocity in S2 APV was significantly increased (Δ244.3 cm/s, p= 0.002) within the concussion group on Day-1, whereas the control group remained largely unchanged from baseline (Δ11.7 cm/s, p= 0.873). (Table 3)
4. Discussion
This investigation utilized a mixed design analysis for the concussion participants, incorporating baseline data in the identification of post-concussion impairments in dynamic postural control. The main finding of this study was an altered COP displacement and velocity in the braking and transitional phases of planned GT, within the post-concussion group when compared to both their own baseline values and to matched controls. Specifically, a decreased COP displacement and velocity in the ML direction during the braking phase (S1) and an increased COP displacement and velocity in the AP direction during the transitional phase (S2) were noted during planned GT following concussion. There were no significant main effects or interactions in the stabilization phase (S3) of COP for either displacement or velocity. Thus, it would appear concussion acutely alters the motor component, but not the stabilization component, of planned GT.
The altered COP displacements during the braking and transitional phases suggest the adoption of a conservative locomotor strategy, which is consistent with previous post-concussion GT and gait studies; however, herein we compared within subjects to healthy baseline data, as opposed to prior studies, which were cross-sectional in nature. [23] The alterations identified herein may reflect changes in anticipatory postural adjustments (APAs) during planned GT. During a planned action, such as planned GT, the APAs are likely responsible for feedforward neurological control to maintain postural control during the transition from rhythmic gait through movement termination. [27] The post-concussion COP displacements (reduced braking and increased transitional COP displacements) likely reflect an altered movement strategy to preserve postural control by arresting the forward momentum of the body while maintaining the COM within the BOS. Specifically, by reducing the braking anterior COP displacement by ~17% and subsequently doubling the anterior COP displacement during the transitional phase, when the lead limb has arrested movement, the individual likely restricts the separation of the COM and BOS. (Table 3) The overall anterior COP displacement, combined between the braking and transitional phases totaling ~70cm, stays nearly identical between BL and D1 (<1cm total difference), and thus the individual is able to successfully complete the task, but utilized an altered strategy.
The braking (S1) phase alterations in ML displacement and velocity further argue the presence of an altered movement strategy following concussion. The change in the control group was minimal for both displacement (-2.7%) and velocity (1%), which indicates a consistent GT pattern in healthy individuals. Conversely, the concussion group demonstrated significantly greater adjustments during this phase, with a -26.5% change in displacement, and a 21% decrease in velocity. Thus, there is a clear shift in strategy for planned GT by the concussion group, and it would appear that the changes in displacement are driving the changes in velocity.
Similarly, the transitional (S2) phase demonstrated a coupled alteration in AP COP displacement and velocity. As observed in the braking phase, the healthy controls did not display a large variation in S2 AP displacement (2.5%) or velocity (17%), further supporting the notion that healthy individuals maintain a consistent GT pattern, regardless of time. The concussed individuals presented nearly a 100% increase in S2 AP displacement (include pre and post) and more than doubled their velocity (include pre and post). Although the underlying mechanism for these alterations requires further exploration, it is clear that, acutely following concussion, a conservative motor control strategy (e.g. braking and transitional phase alterations) is adopted in order to successfully complete a planned GT task.
The control pathways of planned GT remain to be fully elucidated, however fMRI studies have suggested that supraspinal activation patterns in the right prefrontal cortex/inferior frontal gyrus are responsible. [19] These areas play a fundamental role in response inhibition and successfully stopping movement potentially by applying a “brake” to the basal ganglia motor loop and subsequent suppression of the primary motor cortex. [28] Indeed, in a preliminary study, Buckley et al identified an altered propulsive and braking force motor strategy following concussion during planned GT. [23] Specifically, this strategy presented independent of gait velocity and persisted beyond clinical recovery (e.g., self-reported symptoms, computerized neurocognitive testing, and clinical cognitive/balance exams), suggesting a persistent deficit at least 10 days post-concussion; however that study was cross-sectional as baseline data was not presented. [23] Conversely, herein these findings identify alterations both within participants, as compared to healthy baseline performance, as well as to a healthy athletic population. Importantly, while there was differences in the timeline between group testing sessions, the healthy participant should high levels of consistency in the task. Finally, impaired planned GT has been reported in elderly and Parkinson disease patients who demonstrated altered soleus-tibialis anterior muscular activation patterns consistent with an overall conservative postural control strategy. [29]
Impaired postural control is a cardinal symptom of concussion and clinical measures typically suggest static postural control recovers within 3 – 5 days of injury. However, instrumented measures of dynamic postural control, utilizing common ADL tasks, have frequently reported deficits well beyond clinical recovery. [23,30] While this study only investigated the acute effects of concussion on planned GT, these findings offer further support to the utilization of common ADLs in the assessment of post-concussion postural control. Indeed, common clinical tests such as the BESS demonstrate substantial practice effects with repeat administration, even months apart following a single administration, whereas gait related ADLs typically stabilize before adolescence, these tasks (e.g., gait, GT) are likely an effective stable measure of dynamic motor and postural control. [10,17] Clearly, access to sophisticated instrumented biomechanics laboratories is limited; however, recent advances in more cost effective inertial sensors and accelerometers may offer improved clinical access to postural control assessment. [30] The results herein are laboratory, rather than clinical, outcomes, and future studies should assess these findings across clinical time points and investigate incorporating smart phone and tablet technologies during this task.
This investigation was limited to kinetic data and therefore did not include either kinematic or electromyographic data, which could have further explored the specific neuromuscular approaches and can be incorporated into future studies. Additionally, there was a difference in the time that testing took place between the control and concussion group. Gait patterns are generally stable beyond adolescence and herein the control participants demonstrated highly consistent GT performance and varying time points have been utilized post-concussion. Nonetheless, this potential limitation must be considered when extrapolating these results. We are also speculating alterations in central postural control mechanisms from behavioral outcomes, which can be targeted in future research. There were participants in this study with a concussion history prior to that of the 6 months before the study began; however, there were no differences in the dependent variables between the control and concussion groups at baseline, thus, there did not appear to be any lingering effects from prior concussions.
The results of this study identify an altered motor control strategy during planned GT acutely post-concussion. These results further suggest that planned GT is an effective tool for investigating motor control alterations acutely post-concussion. Future studies may also utilize planned GT to identify lingering deficits in post-concussion motor control that are not currently identified by current clinical assessments. Although the specific mechanism behind these alterations yields further exploration, the present results elucidate more understanding of post-concussion postural control, which is suggested to be controlled by supraspinal structures.
Highlights.
Dynamic postural tasks can identify impairments post-concussion better than static
Concussion acutely alters motor control strategy during planned GT
COP displacements were altered during planned GT in concussed individuals
COP velocities were altered during planned GT acutely post-concussion
Planned GT is an effective tool for analyzing postural control post-concussion
Acknowledgments
This study was supported by the National Institute of Health/National Institute of Neurological Disorders and Stroke (1R15NS070744-01A1). The funding source had no role in the study design, data collection and interpretation, or decision to submit this manuscript for publication.
Footnotes
Conflict of Interest Statement
There are no conflicts of interest associated with the present study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jessie R. Oldham, Email: Jroldham@udel.edu.
Barry A. Munkasy, Email: Bmunkasy@georgiasouthern.edu.
Kelsey M. Evans, Email: Kelsey.evans@emory.edu.
Erik A. Wikstrom, Email: Wikstrom@unc.edu.
References
- 1.Broglio SP, Cantu RC, Gioia GA, Guskiewicz KM, Kutcher J, Palm M. National Athletic Trainers’ Association position statement: Management of sport concussion. J Athl Train. 2014;49:245–65. doi: 10.4085/1062-6050-49.1.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCrory P, Meeuwisse WH, Aubry M, Cantu B, Dvorak J, Echemendia RJ, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250–8. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- 3.Finnoff JT, Peterson VJ, Hollman JH, Smith J. Intrarater and interrater reliability of the balance error scoring system (BESS) PM&R. 2009;1:50–4. doi: 10.1016/j.pmrj.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Rahn C, Munkasy BA, Joyner AB, Buckley TA. Sideline performance of the balance error scoring system during a live sporting event. Clin J Sport Med. 2014 doi: 10.1097/JSM.0000000000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly K, Jordan E, Joyner A, Burdette G, Buckley T. National Collegiate Athletic Association Division I athletic trainers’ concussion-management practice patterns. J Athl Train. 2014;49:665–73. doi: 10.4085/1062-6050-49.3.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. Jama. 2003;290:2556–63. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- 7.Hunt TN, Ferrara MS, Bornstein RA, Baumgartner TA. The reliability of the modified Balance Error Scoring System. Clin J Sport Med. 2009;19:471–5. doi: 10.1097/JSM.0b013e3181c12c7b. [DOI] [PubMed] [Google Scholar]
- 8.SCAT3. Br J Sports Med. 2013;47:259. [PubMed] [Google Scholar]
- 9.Guskiewicz KM, Ross SE, Marshall SW. Postural Stability and Neuropsychological Deficits After Concussion in Collegiate Athletes. J Athl Train. 2001;36:263–73. [PMC free article] [PubMed] [Google Scholar]
- 10.Burk J, Joyner A, Munkasy B, Buckley T. Balance error scoring system performance changes after a competitive athletic season. Clin J Sport Med. 2013;23:312–7. doi: 10.1097/JSM.0b013e318285633f. [DOI] [PubMed] [Google Scholar]
- 11.Wrisley DM, Stephens MJ, Mosley S, Wojnowski A, Duffy J, Burkard R. Learning effects of repetitive administrations of the sensory organization test in healthy young adults. Arch Phys Med Rehabil. 2007;88:1049–54. doi: 10.1016/j.apmr.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Parker TM, Osternig LR, Lee H, van Donkelaar P, Chou L. The effect of divided attention on gait stability folllowing concussion. Clin Biomech. 2005;20:389–95. doi: 10.1016/j.clinbiomech.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Chou L, Kaufman KR, Walker-Rabatin AE, Brey RH, Basford JR. Dynamic instability during obstacle crossing following traumatic brain injury. Gait Posture. 2004;20:245–54. doi: 10.1016/j.gaitpost.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Buckley TA. Concussion and Gait. Gait Biometrics. 2014:141–64. [Google Scholar]
- 15.Lee H, Sullivan SJ, Schneiders SG. The use of a dual-task paradigm in detecting gait performance deficits following a sports-related concussion: A systematic review and meta-analysis. J Sci Med Sport. 2012:1–6. doi: 10.1016/j.jsams.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Howell D, Osternig L, Chou L. Dual-task effect on gait balance control in adolescents with concussion. Arch Phys Med Rehabil. 2013;94:1513–20. doi: 10.1016/j.apmr.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Norlin R, Odenrick P, Sandlund B. Development of gait in the normal child. J Pediatr Orthop. 1981;1:261–6. doi: 10.1097/01241398-198111000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Buckley T, Oldham J, Caccese J. Postural control deficits identify lingering post-concussion neurological deficits. J Sport Heal Sci. 2016;5:61–9. doi: 10.1016/j.jshs.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Wai Y, Weng Y, Ng K, Huang Y, Ying L, et al. Functional MRI in the assessment of cortical activation during gait-related imaginary tasks. J Neural Transm. 2009;116:1087–92. doi: 10.1007/s00702-009-0269-y. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Li L. Feedforward and feedback control for gait and balance. Gait Biometrics. 2014:191–205. [Google Scholar]
- 21.Chang H, Krebs D. Dynamic balance control in elders: gait initiation assessment as a screening tool. Arch Phys Med Rehabil. 1999;80:490–4. doi: 10.1016/s0003-9993(99)90187-9. [DOI] [PubMed] [Google Scholar]
- 22.Sparrow WA, Tirosh O. Gait termination: a review of experimental methods and the effects of ageing and gait pathologies. Gait Posture. 2005;22:362–71. doi: 10.1016/j.gaitpost.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Buckley TA, Munkasy BA, Tapia-Lovler TG, Wikstrom EA. Altered gait termination strategies following a concussion. Gait Posture. 2013;38:549–51. doi: 10.1016/j.gaitpost.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckley TA, Pitsikoulis C, Hass CJ. Dynamic postural stability during sit-to-walk transitions in Parkinson disease patients. Mov Disord. 2008;23:1274–80. doi: 10.1002/mds.22079. [DOI] [PubMed] [Google Scholar]
- 25.Hass CJ, Buckley TA, Pitsikoulis C, Barthelemy EJ. Progressive resistance training improves gait initiation in individuals with Parkinson’s disease. Gait Posture. 2012;35:669–73. doi: 10.1016/j.gaitpost.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Ryckewaert G, Delval A, Bleuse S, Blatt JL, Defebvre L. Biomechanical mechanisms and centre of pressure trajectory during planned gait termination. Neurophysiol Clin. 2014;44:227–33. doi: 10.1016/j.neucli.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Massion J. Movement, Posture and Equilibrium: Interaction and Coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- 28.Coxon JP, Stinear CM, Byblow WD. Stop and Go: The Neural Basis of Selective Movement Prevention. J Cogn Neurosci. 2009;21:1193–203. doi: 10.1162/jocn.2009.21081. [DOI] [PubMed] [Google Scholar]
- 29.Bishop M, Brunt D, Marjama-Lyons J. Do people with Parkinson’s disease change strategy during unplanned gait termination? Neurosci Lett. 2006;397:240–4. doi: 10.1016/j.neulet.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 30.Howell D, Osternig L, Chou L. Monitoring recovery of gait balance control following concussion using an accelerometer. J Biomech. 2015 doi: 10.1016/j.jbiomech.2015.06.014. Epub ahead. [DOI] [PubMed] [Google Scholar]


