Abstract
The goals of resuscitation in hemorrhagic shock are to correct oxygen deficit and to maintain perfusion pressure to the vital organs. We created liposome-encapsulated hemoglobin (LEH) as a nanoparticulate oxygen carrier (216 ± 2 nm) containing 7.2 g/dL hemoglobin, and examined its ability to prevent the systemic manifestations of hemorrhagic shock (45% blood loss) in a rat model. We collected plasma after 6 h of shock and LEH resuscitation, and determined the circulating biomarkers of systemic inflammation and functions of liver, gut, heart, and kidney. As is typical of the shock pathology, a significant increase in the plasma levels of cardiac troponin, liver function enzymes, soluble CD163 (macrophage activation), and creatinine, and the liver/gut myeloperoxidase activity was observed in the hemorrhaged rats. The plasma levels of TNF-α, IL-6, IL-1α, CINC-1, and IL-22 also increased after hemorrhagic shock. LEH administration prevented the hemorrhagic shock-induced accumulation of the markers of injury to the critical organs and pro-inflammatory cytokines. LEH also decreased the plasma levels of stress hormone corticosterone in hemorrhaged rats. Although saline also reduced the circulating corticosterone and a few other tissue injury markers, it was not as effective as LEH in restraining the plasma levels of creatinine, alanine transaminase, CD163, TNF-α, IL-6, and IL-1α. These results indicate that resuscitation with nanoparticulate LEH creates a pro-survival phenotype in hemorrhaged rats, and because of its oxygen-carrying capacity, LEH performs significantly better than saline in hemorrhagic shock.
Keywords: Nanoparticle, Liposome-encapsulated hemoglobin, Resuscitation, Hemorrhagic shock, Organ injury, Inflammation
Graphical abstract
1. Introduction
Liposome-encapsulated hemoglobin (LEH, alternatively known as hemoglobin vesicles) is being investigated as a universal O2-carrier of nanometric dimensions which mimics red blood cells (RBCs) in oxygen diffusivity (Awasthi, 2005; Sakai et al., 2009). In the last two decades of LEH research, the particle characteristics and other issues related to the stability of encapsulated hemoglobin and large-scale production technology have been gradually addressed. The major breakthroughs in its development came with the application of polyethylene glycol (PEG)-phospholipid to enhance circulation t1/2 of LEH (Phillips et al., 1999) and the use of a non-phospholipid anionic lipid to increase the hemoglobin encapsulation and reduce the host immune response to liposomes containing anionic phospholipids (Sou and Tsuchida, 2008). Although the current size of LEH (∼250 nm) is not exactly congruent to the conventional definition of nanoparticles (< 100 nm), LEH is one preparation where the size factor has been reconciled with the loading capacity, surface modification, and circulation t1/2 of liposomes (Awasthi et al., 2003; Awasthi et al., 2004). In addition to the size, the surface properties are important because they determine the circulation persistence of liposome particles and the host immune response to their administration. The choice of a surface modifier is critical particularly for LEH, because it is infused in large amounts and is expected to persist in circulation for a prolonged time. Earlier, we established that 275 nm is the largest size of PEG-modified liposomes which retains a significant level of stealthiness; the ability of PEG as a stealth polymer drops drastically above this optimal size (Awasthi et al., 2003). We adapted these advances to a high-shear method of manufacturing LEH containing a non-phospholipid anionic lipid called hexadecylcarbamoylmethylhexadecanoate (HDAS). We also employed HDAS-conjugated PEG to surface-modify the LEH particles to further improve their tolerance against host immune response (Agashe and Awasthi, 2009; Agashe et al., 2010; Nag et al., 2013). By virtue of these continuous improvements, LEH has now acquired a potential to become a feasible product as an oxygen carrying resuscitation fluid for use in hemorrhagic shock.
According to the current guidelines, the inadequate tissue perfusion in the victims of trauma and hemorrhagic shock is normally corrected by resuscitation with crystalloid fluids such as 0.9% saline and lactated Ringer's solution, or oncotic colloids such as hydroxyethyl starch and albumin; for the remediation of oxygen deficit, whole blood or packed RBCs are used on need basis. The crystalloids or colloids can restore salt balance and increase cardiac output, but their aggressive use may worsen shock because of the resultant cardiac overload and hemodilution. Tissue oxygenation can only be enhanced by increasing the arterial O2 tension, for which whole blood or packed RBCs are the best choice. However, the availability of safe and clinically-matched blood products at the place and time of need is still a significant barrier, which continues to threaten the optimal management of trauma and shock victims (Stollings and Oyen, 2006). According to a national survey about 72,000 transfusion-related adverse reactions and 11,000 severe adverse donor reactions were documented in 2006 (AABB). These issues have catalyzed the research on the development of artificially assembled O2-carriers such as LEH.
An innate physiological response to acute blood loss is the diversion of blood flow from the splanchnic network to the brain. This compensatory mechanism is meant to preserve the brain function, but it aggravates the volume deficit and ischemia in splanchnic organs and induces debilitating injuries in the gut, liver, and kidney. Since any investigational product for resuscitation should prevent the multi-organ injuries and systemic inflammatory response syndrome (SIRS) caused by hypoxia and hypoperfusion, the goal of this study was to determine the effect of resuscitation with test LEH on the plasma levels of pro-inflammatory cytokines and indicators of organ injury in a rat model of hemorrhagic shock. Earlier, we have shown that the test LEH preparation was effective in restoring the perturbed cerebral energy metabolism in the same model (Rao et al., 2015). More recently, we reported that this LEH preparation prevents deterioration of proteostatic mechanisms in the gut tissue (Rao et al., 2016). In the present work, the gut, liver, hear, kidney and plasma samples were also sourced from these two reported study.
2. Materials & Methods
Phospholipids were purchased from Lipoid (Ludwigshafen, Germany), Avanti Polar Lipids (Alabaster, AL), or NOF Corporation (Tokyo, Japan). Cholesterol was obtained from Calbiochem (Gibbstown, NJ). Stroma-free hemoglobin was isolated from outdated RBC units and characterized by following the previously described methods (Agashe et al., 2010). The outdated RBC units were sourced from Oklahoma Blood Institute (Oklahoma City, OK). The anionic lipid HDAS and stealth lipo-polymer HDAS-PEG2k were synthesized using methods described elsewhere (Nag et al., 2013). All the stock and working solutions were prepared in endotoxin-free water or reagents, and the procedures were performed under strict aseptic conditions.
2.1. LEH preparation
LEH was manufactured by a high pressure homogenization method as reported previously (Agashe et al., 2010). Briefly, a mixture of lipid phase, consisting of a lyophilized powder of pro-liposomes, and stroma-free hemoglobin was homogenized at 20,000 psi for 4 cycles in an Emulsiflex-C3 homogenizer (Avestin, Ottawa, ON, Canada). The fluid temperature was maintained at about 20° C during homogenization by passing the homogenate through a cooling coil. The pro-liposomes were made from dipalmitoylphosphatidylcholine (∼38 mol%), cholesterol (∼38 mol%), HDAS (∼20 mol%), HDAS-PEG2K (0.3 mol%), and α-tocopherol (∼2.4 mol%). The method of pro-liposome preparation from raw lipids and its lyophilization has been described in our previous articles (Agashe et al., 2010; Awasthi et al., 2003; Nag et al., 2013). After homogenization, the unencapsulated hemoglobin was separated from LEH particles by a process of tangential-flow filtration (TFF) through a 50 nm hollow fiber filter using PBS (pH 7.4) as the diluting solvent.
The crude LEH was surface-modified with HDAS-PEG2K using a post-insertion method described elsewhere (Nag et al., 2013). Briefly, HDAS-PEG2k (10 mol% of total lipid) was dissolved in 50 mL of water and filtered through a 0.2 µm membrane. The solution was gradually added over a period of 2 h to a stirred diluted suspension of LEH at room temperature. The concentration of HDAS-PEG2k was maintained below its critical micelle concentration (4.25 µM). The stirring was continued overnight at 4° C and the preparation was washed free of any unincorporated HDAS-PEG2k by TFF using 10 volumes of PBS (pH 7.4) in a 2 sq ft hollow fiber 50 nm filter (Minntech, Minneapolis, MN). The same TFF technique was employed to concentrate the LEH preparation. To the concentrated LEH, sufficient volume of sterile 25% w/v solution of human serum albumin was added to achieve a final concentration of 5% w/v of albumin. The final LEH preparation was stored at 4° C in darkness.
2.2. Laser scanning confocal microscopy (LSCM)
In order to substantiate the presence of HDAS-PEG on the surface of LEH particles, we performed LSCM by immunostaining LEH with an Alexa Fluor® 488 Dye-conjugated anti-PEG antibody. First, a small sample of LEH was coated on glass slides for 16 h at 4° C. After thorough washing with PBS, the nonspecific sites were blocked with 0.25% w/v bovine serum albumin for 30 min. Next, the slides were incubated with a 1:2,000 dilution of goat anti-PEG antibody (Epitomics, Burlingame, CA) for 2 h. The slides were washed again and allowed another 2 h-incubation with a 1:1,000 diluted Alexa Fluor® 488-conjugated donkey anti-goat IgG antibody (Invitrogen, Carlsbad, CA). Finally, the stained slides were imaged using a Zeiss LSM-710 Multiphoton Laser Microscope (Ex495nm/Em500-700nm).
2.3. LEH characterization
The LEH preparation was characterized for the hemoglobin content, methemoglobin, size, oxygen affinity (p50), and the phospholipid concentration. The phospholipid concentration was determined by the method of Stewart (Stewart, 1980). Oxygen affinity (p50) was measured by a Hemox-analyzer (TCS Scientific Corp., New Hope, PA). For estimation of hemoglobin and methemoglobin in LEH, hemoglobin was quantitatively released from liposomes by using phosphate-buffered (0.1M, pH 6.8) 25% dimethylsulfoxide (PB-25%DMSO). Briefly, about 50 µl of LEH was diluted with 200 µl of PB-25%DMSO. The dilution was vortexed vigorously for 1 min, diluted to 1 ml with phosphate buffer, and probe-sonicated (50 Sonic Dismembrator, Fisher Scientific) for 30 seconds on ice at 45% power output. The mixture was spun at 14,000 rpm for 10 min to remove any debris, and the supernatant was used for the hemoglobin and methemoglobin estimation. The amount of encapsulated hemoglobin was determined by monitoring the absorbance of LEH at 540 nm (Tomita et al., 1968). Methemoglobin content was measured in SFH by the method of Matsuoka (Matsuoka, 1997).
The particle size was determined by photon correlation spectroscopy using a Brookhaven particle size analyzer equipped with Mas Option software (Brookhaven Instruments Corp., Holtsville, NY). To document the formation and integrity of liposome, we performed electron microscopy (EM) at the imaging core facility of Oklahoma Medical Research Foundation (OK, USA).
2.4. Hemorrhagic shock and resuscitation
All animal work was performed according to the NIH Animal Use and Care Guidelines and was approved by the institutional IACUC. Male Sprague Dawley rats (250-300 g) were purchased from Harlan (now Envigo, Indianapolis, IN), housed in regular light/dark cycles, and allowed to acclimatize for at least 5 days prior to the experiments. The left femoral artery was surgically implanted with an indwelling catheter by a method described elsewhere (Awasthi et al., 2007). The rats were allowed at least 2 days to recover from the catheter surgery. The cannulated rats were clustered in four groups: control (Ctrl), hemorrhagic shock (HS), HS+Saline (Sal), and HS+LEH. On the day of the experiment, the rats were anesthetized with isoflurane (2-3%) in a stream of medical air (2 L per min, 21% oxygen and 78% nitrogen). Approximately 100 U of heparin was injected through the catheter to maintain patency of the indwelling catheter. Hemorrhagic shock was induced by withdrawing 0.027 ml of blood/g bodyweight at the rate of 1.0 ml/min. After allowing the animals to remain in the constant-volume shock for 15 min, LEH resuscitation was administered via a tail vein at 1 ml/min using a syringe pump. The volume of LEH administered was equal to the volume of blood withdrawn for the induction of shock and the preparation was warmed to approximately 35° C before infusion. The resuscitated rats were allowed to wake up and roam freely in their cages. After collection of blood samples at 6 h of shock, the rats were killed by administering an overdose of SOMNASOL (Butler Schein, Dublin, OH). During the procedure, the hemodynamic parameters were recorded at baseline, after shock, after resuscitation, and at 6 h before termination of the study. As a comparative control, we used 0.9% saline. The choice of saline over the other crystalloids, such as Ringer's solution, was based on a report that normal saline is preferred over Ringer's lactate for the pre-hospital intervention in a combat zone (Lairet et al., 2012). Moreover, we employed isovolemic infusion because it has been shown that compared to aggressive fluid overloading, mildly hypotensive resuscitation increases the survival rates in a rat model of hemorrhagic shock (Li et al., 2012). It also eliminates the possibility of hyperchloremic metabolic acidosis, fluid overloading, and tissue edema which become a cause of concern in clinical management of hemorrhagic shock (Besen et al., 2015).
2.5. Plasma samples
Plasma was isolated from heparinized blood collected at the baseline and 6 h after the shock. The samples were stored at -80 °C until the time of the assays. As described below, the plasma samples were subjected to the assays of the biomarkers indicative of the functional status of heart, liver, intestine, and kidney. We also measured the general markers of SIRS and stress to determine the overall health of the organism. The scope of these examinations was limited by the availability of sufficient plasma and the reagents specific for the biomarkers of rat origin. In general, we followed the protocols supplied by the various vendors of the assay kits.
2.6. Corticosterone
Plasma corticosterone was measured using an enzyme-linked immunoassay (ELISA) kit from Cayman Chemicals (Ann Arbor, Michigan).
2.7. Creatinine
Plasma creatinine was estimated by a BioVision kit (Milpitas, CA) containing creatininase and creatinase enzymes. In this assay, creatinine present in the samples was first converted into creatine by creatininase. The creatine was further converted into sarcosine by the enzymatic activity of creatinase. The sarcosine was allowed to react with a probe to develop a color which was read at 570 nm.
2.8. Creatine kinase
Creatine kinase was determined by a kit from BioVision (Milpitas, CA). Briefly, the sample-bound creatine kinase was allowed to catalyze the conversion of creatine into phosphocreatine in an assay buffer containing ATP. The resultant phosphocreatine and ADP generated an intermediate which was allowed to react with a probe to produce a color for quantitation at 450 nm. The creatine kinase activity (mU/ml) was calculated based on a standard curve plotted from the readings of standard solutions of NADH (0-10 nmol/µl) treated identically.
2.9. Troponin I (TnI)
We determined the levels of cardiac TnI (cTnI) and skeletal muscle TnI (sTnI) in plasma by using the rat-specific ELISA kits obtained from Life Diagnostics (West Chester, PA).
2.10. Interleukin (IL)-22, Tumor necrosis factor (TNF)-α, IL-6, and IL-1α
We employed a sandwich ELISA kit for IL-22 (R& D Systems, Minneapolis, MN). For TNF-α, IL-6 and IL-1α, a custom sandwich ELISA kit was designed to carry out three cytokines in a single 96-well plate (Signosis, Santa Clara, CA).
2.11. Growth-regulated gene product/cytokine-induced neutrophil chemoattractant (GRO/CINC-1)
We estimated GRO/CINC-1 in the rat plasma samples as well as in the lysates of intestinal and liver tissues by using an ELISA kit (Enzo Life Sciences, Plymouth Meeting, PA). The tissue lysates were made by mincing the frozen tissues in 0.5 ml of ice-cold buffer consisting of 10% Nonidet P-40, 5 M NaCl, 1 M HEPES, 0.1 M ethylene glycol tetra-acetic acid, 0.5 M ethylenediaminetetraacetic acid, 0.1 M phenylmethylsulfonyl fluoride, 0.2 M sodium orthovanadate, 1 M NaF, 2 μg/ml aprotinin, and 2 μg/ml leupeptin. The mixture was incubated on ice for 30 min, homogenized using a disposable microtube-pestle, and centrifuged at 14,000 rpm at 4° C for 10 min to obtain the test supernatants.
2.12. Myeloperoxidase (MPO) assay
MPO activity in the ileal and liver tissue samples was estimated by a method described elsewhere (Yadav et al., 2013). Briefly, the frozen ileal and liver tissue samples were thawed, diced with a razor blade, and homogenized in a potassium phosphate buffer (50 mM, pH 6.0). The homogenate was centrifuged at 10,000 rpm for 15 min and the pellet was resuspended in 0.3 ml of a phosphate buffer containing 50 mM hexadecyltrimethylammonium bromide (HTAB). The mixture was subjected to 3-cycles of bath sonication (20 s), snap-freezing in liquid nitrogen, and thawing to the room temperature. The supernatants, collected by centrifugation (10,000 rpm for 10 min), were diluted 1:2 and 1:10 with the HTAB-phosphate buffer and added with 30 times the volume of a phosphate buffer containing 0.167 mg/ml O-dianisidine dihydrochloride and 0.0005% H2O2. The absorbance was monitored at 450 nm. For MPO activity in the heart tissue, we employed a colorimetric assay kit which uses tetramethylbenzidine as a chromogenic substrate (Cayman Chemicals, Ann Arbor, Michigan).
2.13. Citrulline
The concentration of citrulline in plasma samples was determined by using a sandwich ELISA kit purchased from MyBiosource (San Diego, CA).
2.14. Aspartate transaminase (AST), alanine transaminase (ALT), and CD163
The plasma levels of the AST and ALT were measured by using kits from Biovision (Milpitas, CA). The plasma concentration of soluble CD163 (sCD163) was estimated by using an ELISA kit from MyBioSource (San Diego, CA).
2.15. Data analysis
The data are reported as the mean ± standard error of mean (sem). For comparison among the four groups (Control, HS, SAL, and LEH), ordinary one-way analysis of variance (ANOVA) with Bonferroni correction (single-pooled variance) was employed using Prism 6 software (GraphPad, San Diego, CA, USA). A p value < 0.05 was considered as statistically significant.
3. Results
3.1. LEH characteristics
The structural characteristics of LEH are shown in Fig. 1. The average effective diameter (DLS) of LEH was measured to be 215.6 nm with a polydispersity of approximately 0.187 units (Table 1). The ƺ potential of LEH was determined to be -30.92 ± 0.16 mV, and the osmolality was 331.7 ± 2.9 mOsmol/kg. The hemoglobin content of the LEH preparation was 7.2 ± 0.2 g/dL, with methemoglobin concentration of less than 5%. On weight basis, the amount of encapsulated hemoglobin was 1.5 mg for each mg of phospholipid (distearoylphosphatidylcholine equivalents). The EM images showed that the particles maintained the typical spherical liposome structure with relatively dense core, perhaps owing to the presence of encapsulated hemoglobin (Fig. 1b).
Figure 1.
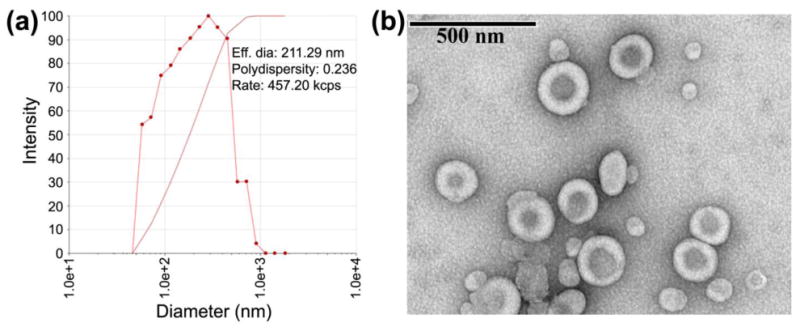
The particle characteristics of LEH. (a) A DLS pictogram showing particle size distribution. (b) EM of LEH particles.
Table 1. LEH characteristics.
| Parameter | Value (± sem) |
|---|---|
| Particle diameter (DLS) | 215.6 ± 2.16 nm |
| Polydispersity | 0.187 |
| Zeta potential | -30.62 ± 0.16 mV |
| [Hemoglobin] | 7.2 ± 0.2 g/dL |
| Methemoglobin content | < 5% |
| Oxygen affinity (p50) | 31.9 ± 2.0 mm Hg |
| pH | 7.2 |
| Osmolality | 331.7 ± 2.9 mOsmol/kg |
| Phospholipid | 47.7 ± 0.4 mg/ml |
| Cholesterol | 10.8 mg/ml |
| Endotoxin (gel clot assay) | < 0.25 EU/ml |
| Sterility (bacterial broth) | Pass (no growth in 7 d culture) |
To confirm the presence of HDAS-PEG2k modification on the liposome surface, a dilute suspension of LEH was immunolabeled with fluorescent antibody and observed by confocal microscopy. The green fluorescence emanating from the LEH particles shows the presence of surface PEG (Fig. 2a). The control preparation of unmodified plain liposomes did not show any fluorescence (not shown). The oxygen equilibrium curve showed that the partial pressure of oxygen for 50% hemoglobin saturation (p50) in LEH was approximately 30 mm Hg (Fig. 2b). The low oxygen affinity of hemoglobin implies that LEH will readily deliver oxygen to the perfused tissues; the p50 value of hemoglobin in normal blood is approximately 27 mm Hg. The LEH preparation was tested to be sterile and endotoxin-free (less than 0.25 EU/ml).
Figure 2.
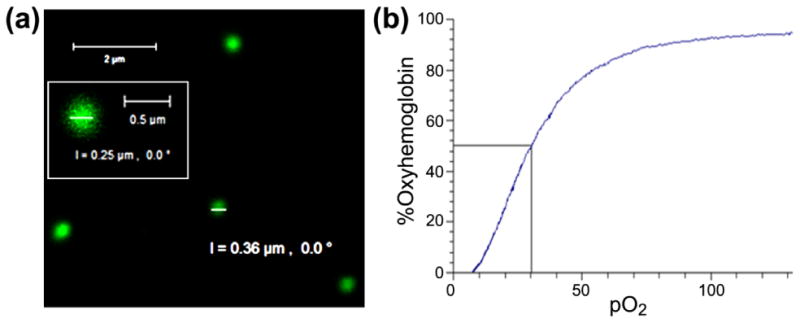
LEH properties. (a) A confocal micrograph of HDAS-PEG2K-LEH labeled with anti-PEG antibody, showing the presence of PEG on the liposome surface. (b) The oxygen equilibrium curve of LEH preparation showing oxygen affinity of encapsulated hemoglobin.
3.2. Hemodynamics and systemic stress after shock and LEH resuscitation
The physicochemically-characterized LEH preparation was employed for resuscitation of rats with hemorrhagic shock. The sequence of events during the animal experiments is illustrated in Fig. 3a. After 45% hemorrhage, the hematocrit decreased commensurate to the amount of blood lost; the average hematocrit at baseline was 41 as compared to 25.3 measured after 6 h of shock in the HS group (p < 0.05, Fig. 3b). There was a slight drop in hematocrit in rats resuscitated with LEH and saline, perhaps because of the dilution effect of the administered fluids (p > 0.05 LEH or Sal vs. HS). The heart rate immediately after hemorrhage dropped from the baseline value of 317 bpm to 192 bpm. After 6 h, all the groups, including the HS group, showed significant increase in the heart rate as compared to that noted immediately after shock (Fig. 3b). The blood withdrawal also caused a considerable drop in the mean arterial pressure (MAP) from 109 mm Hg at baseline to 49 mm Hg after blood withdrawal. Immediately after LEH infusion, the MAP significantly improved to 83.3 mm Hg and this improvement was maintained through the duration of the study. The improvement in MAP after saline infusion was also quantitatively similar to that after LEH infusion. However, the MAP in both the LEH and saline groups remained significantly lower than the baseline MAP (p < 0.05, Fig. 3b). This is characteristic of hypotensive resuscitation afforded by an isovolemic fluid infusion (Li et al., 2012). The HS group showed a spontaneous improvement in the MAP to approximately 61 mm Hg (p < 0.05 vs. HS). A representative trace of the blood pressure recording in the hemorrhaged and resuscitated rats is shown in Fig. 3c.
Figure 3.
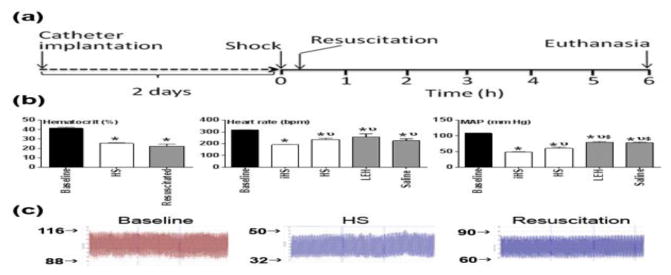
Experimental design. (a) Hemorrhagic shock was induced by withdrawing 45% of blood through an indwelling femoral artery catheter. The catheter surgery was performed at least 2 days before the shock study. The hemorrhaged rats were resuscitated with saline or LEH in volumes equal to the shed blood. The untreated hemorrhaged rats (HS group) received no treatment. The control group consisted of normal rats subjected to catheter implantation, but no hemorrhage or resuscitation was provided. At 6 h, the rats were euthanized and plasma samples were collected for evaluations described in this article. (b) Hemodynamic parameters (hematocrit, heart rate, and mean arterial pressure) recorded at baseline, immediately after blood withdrawal (iHS), after 6 h of shock (HS), and saline or LEH resuscitation (c) A representative trace of raw blood pressure readings. p < 0.05 vs. Ctrl*, iHSν, and HS$
To further characterize shock and resuscitation in our model, we measured the plasma levels of corticosterone, which is the only glucocorticoid in rats as an indicator of systemic stress. An increase in plasma glucocorticoids, secondary to the activation of hypothalamic-pituitary-adrenal axis, is a stress response and is a good indicator of overall physiological stress in shock (Rushing et al., 2006). We found that the plasma concentration of corticosterone was approximately 7 times more in the HS rats than in the control rats (117.8 pg/ml versus 17.5 pg/ml, respectively; Fig. 4). Both saline and LEH resuscitation averted this rise in plasma corticosterone levels, suggesting that just by replenishing the lost volume the systemic stress of hemorrhagic shock could be reduced.
Figure 4.
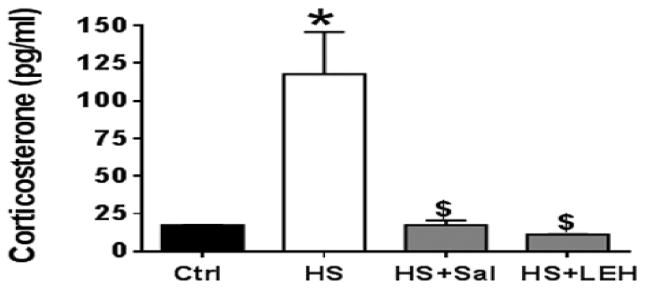
Plasma corticosterone levels (n = 4 per group). p < 0.05 vs. Ctrl* and HS$
3.3. Effect of LEH on the circulating markers reflective of systemic inflammation
To assess the effect of LEH resuscitation on the SIRS, we determined the concentrations of TNF-α, IL-1α, IL-6, and IL-22 in plasma. TNF-α, IL-1α, IL-6 could be regarded as the canonical proinflammatory cytokines with a well known contribution in shock pathology, whereas the role of IL-22 in hemorrhagic shock is not clear. As shown in Table 2, the profile displayed was similar for the TNF-α, IL-6, and IL-1α cytokines. Hemorrhagic shock significantly increased the levels of TNF-α, IL-1α, and IL-6 in plasma and resuscitation with LEH or saline prevented this increase. However, LEH resuscitation was significantly more effective than saline in this respect (p < 0.05, HS+Sal vs. HS+LEH). In fact, the saline-transfused rats exhibited significantly higher concentrations of the plasma TNF-α, IL-1α, and IL-6 as compared to their concentrations in the control rats (p < 0.05, HS+Sal vs. Ctrl). We extended our inquiry into the shock-induced SIRS by determining the plasma levels of IL-22 which has been implicated in the ischemia-reperfusion injury of liver (Chestovich et al., 2012). In contrast to the moderate effect of shock on the canonical pro-inflammatory cytokines, we found that hemorrhagic shock resulted in a large increase (28-fold) in the circulating levels of IL-22 (Table 2). Both saline and LEH resuscitation were able to avert this rise in the plasma IL-22 levels.
Table 2. Plasma levels of cytokines and citrulline.
| Plasma conc. | Sample size | Ctrl | HS | HS+Sal | HS+LEH |
|---|---|---|---|---|---|
| TNF-α (A.U.) | 4/group | 0.72 ± 0.03 | 0.96 ± 0.03* | 0.88 ± 0.05* | 0.68 ± 0.01$Φ |
| IL-1α (A.U.) | 4/group | 0.67 ± 0.02 | 1.09 ± 0.03* | 0.97 ± 0.01*$ | 0.70 ± 0.02$Φ |
| IL-6 (A.U.) | 4/group | 0.71 ± 0.00 | 0.97 ± 0.04* | 0.87 ± 0.02*$ | 0.73 ± 0.01$Φ |
| IL-22 (pg/ml) | 5/group | 15 ± 3.33 | 421.9 ± 63.98* | 51.48 ± 12.48$ | 177.4 ± 56.74$ |
| Citrulline (µg/ml) | 4/group | 2.36 ± 0.11 | 3.92 ± 0.11* | 2.93 ± 0.22$ | 3.08 ± 0.17*$ |
A.U. = Arbitrary units; p < 0.05
HS vs HS+Sal,
HS+Sal vs. Ctrl,
HS+LEH vs. HS+Sal
We further investigated the effect of shock and resuscitation on the plasma levels of an unconventional amino acid citrulline (Table 2). Systemic citrulline almost exclusively originates from the intestine where enterocytes synthesize it from either arginine or glutamine. It is a surrogate marker for estimating the production nitric oxide catalyzed by the activity of nitric oxide synthase (NOS). We found that hemorrhagic shock significantly increased the plasma citrulline levels. Both saline and LEH infusions prevented the hemorrhage-induced increase in plasma citrulline concentration; however, the LEH group, but not the saline group, remained significantly different from the control group.
3.4. Effect of LEH resuscitation on heart and kidney
As a measure of cardiac dysfunction, we estimated the concentration of cTnI and creatine kinase in plasma. Cardiac TnI is an inhibitor of ATP-ase activity of acto-myosin and is a sensitive and specific marker of cardiac injury. We found that hemorrhagic shock significantly increased the plasma levels of cTnI. Both saline and LEH resuscitation were able to prevent the rise in cTnI levels in a statistically significant manner (p < 0.05, Fig. 5a). However, in contrast to the cTnI, there was no change in the levels of creatine kinase among various groups (Fig. 5b).
Figure 5.
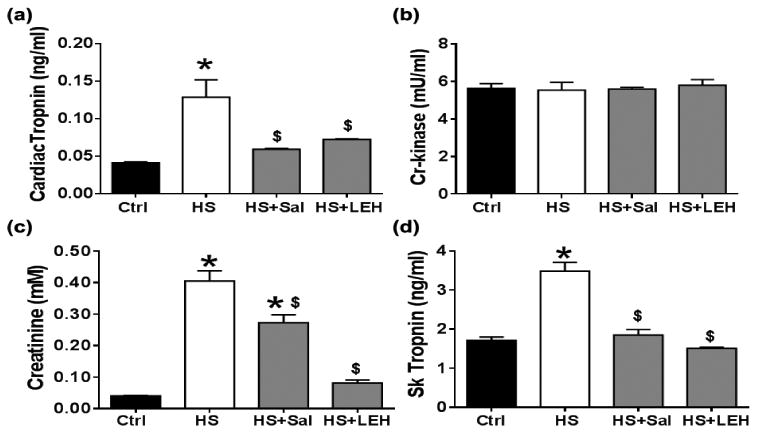
Cardiac, renal, and skeletal muscle injuries after shock and LEH resuscitation. Plasma levels of (a) cardiac troponin (n ≥ 6 per group), (b) creatine kinase (n = 6 per group), (c) creatinine (n = 6 per group), and (d) skeletal muscle troponin (n≥ 6 per group). p < 0.05 vs. Ctrl* and HS$.
A failing heart is frequently associated with renal insufficiency. The plasma creatinine, which is primarily removed from the circulation by glomerular filtration, is a clinical indicator of renal function. We found that hemorrhagic shock increased the plasma creatinine by approximately 10 fold (Fig. 5c). Although saline infusion reduced the creatinine levels (p < 0.05, HS+SAL vs. HS), it was still significantly higher as compared to the control levels (p < 0.05, HS+SAL vs. Ctrl). On the other hand, LEH resuscitation efficiently prevented the increase in plasma creatinine; the concentration of creatinine in HS+LEH group (0.08 mM) was not significantly different from the control levels (0.04 mM).
The creatinine circulating in blood is largely the product of skeletal muscle metabolism. We measured an isoform of TnI specific to the skeletal muscle (sTnI), which is generated only in the presence of skeletal muscle injury. The sTnI is highly homologous to the cTnI, but it lacks a 32-residue N-terminal extension which is present in the cTnI isoform (Gomes et al., 2002). Hemorrhagic shock increased the plasma sTnI concentration 2-fold (Fig. 5d), which indicates that the skeletal muscle injury contributes to the rise in plasma creatinine in hemorrhagic shock.
3.5. Effect of LEH on the markers of liver injury
The plasma levels of AST and ALT are recognized as the clinical markers of injury to the hepatic parenchyma. Likewise, the plasma levels of sCD163, which is a hemoglobin-heptaglobin scavenger receptor, are indicative of the activation status of the Kupffer cells (Gronbaek et al., 2012). As shown in Fig. 6a and 6b, there was a significant increase in the activity of ALT and AST in hemorrhaged rats (2.2-and 1.7-fold increase, respectively). Both saline and LEH resuscitation were able to prevent the activation of ALT and AST, but only LEH infusion could significantly alter the status of these enzymes in the hemorrhaged rats.
Figure 6.
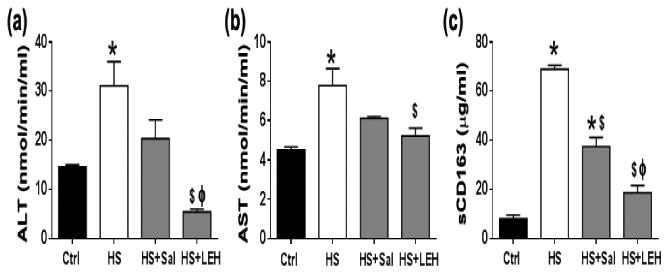
Effect of LEH resuscitation on liver function and Kupffer cell activation. (a) ALT (n = 6 per group), (b) AST (n = 6 per group), and (c) Plasma levels of sCD163 (n = 5 per group), an indicator of macrophage activation. p < 0.05 vs. Ctrl*, HS$ and Salϕ.
In contrast to the moderate effect of hemorrhagic shock on the hepatocyte function, we found that hemorrhagic shock increased the plasma sCD163 levels by more than 8-fold (Fig. 6c). This indicated that the severe loss of blood resulted in the activation of the liver-resident macrophages. Resuscitation with saline or LEH significantly prevented the shock-induced increase in the plasma levels of sCD163, but the saline-infused rats still showed significantly higher plasma sCD163 as compared to the control group (Fig. 6c). The difference in the plasma sCD163 between the LEH-transfused and the saline-transfused rats was statistically significant (p < 0.05).
3.6. Effect of LEH on the tissue neutrophil infiltration and CINC-1 (IL-8)
The influx of neutrophils in liver, small gut, and heart was determined by estimating the activity of MPO, which is an enzyme released during the degranulation of neutrophils (Fig. 7). Hemorrhagic shock caused a 3- and 2-fold increase in the MPO activity associated with the liver and gut tissues, respectively. This increased MPO activity in the liver and gut was restored to the basal levels in the saline or LEH resuscitated rats (Fig. 7a and 7b, respectively). Although LEH was slightly more effective than saline, the differences were not statistically significant (p = 0.07 and p = 0.18, Sal vs. LEH for liver and gut, respectively). In contrast to the MPO activity in the gut and liver tissues, the MPO activity in the heart tissue was not altered by hemorrhagic shock or saline and LEH resuscitation (Fig. 7c).
Figure 7.
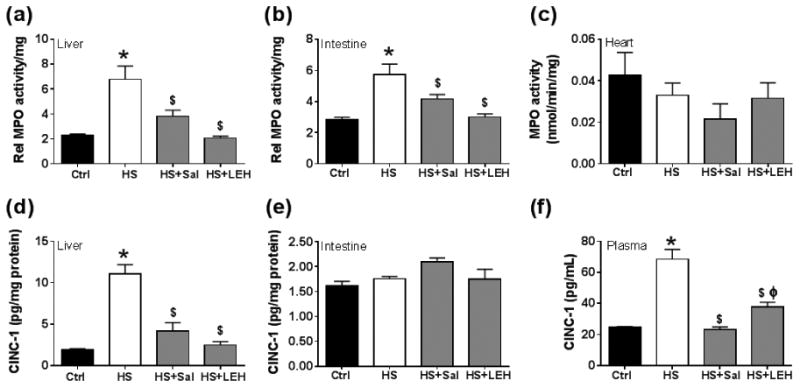
Tissue neutrophil infiltration and IL-8 (CINC-1) expression after shock and LEH resuscitation. Relative MPO activity in (a) liver (n = 6 per group), (b) small intestine (ileum, n = 6 per group), and (c) heart (n ≥ 5 per group). The tissue levels of GRO/CINC-1 (also known as IL-8) levels in (a) liver tissue (n= 4 per group), (b) gut tissue (n = 4 per group), and (c) plasma (n = 6 per group). p < 0.05 vs. Ctrl*, HS$ and Salϕ.
Since CINC-1, the rat equivalent of IL-8, is a powerful neutrophil chemoattractant and is produced by the injured tissues, next we measured CINC-1 in the liver, gut, and plasma samples. As shown in Fig.7d, hemorrhagic shock increased the concentration of CINC-1 by almost 6-fold in the liver tissue. Resuscitation with LEH as well as saline was able to prevent this shock-induced increase in the liver CINC-1 concentration. On the other hand, we found no change in the gut tissue-associated CINC-1 levels after hemorrhage or resuscitation (Fig. 7e). The production of CINC-1 in the inflamed tissues contributes to the increase in the plasma levels of CINC-1, from where it is capable of initiating the pro-inflammatory signaling in a systemic manner (MacDermott, 1999; Masumoto et al., 1998). As shown in Fig. 7f, we found that the plasma CINC-1 concentration increased from 24.8 pg/ml at the basal level to 68.5 pg/ml after 6 h of hemorrhagic shock (2.8-fold increase, p < 0.05 HS vs. Ctrl). Saline and LEH infusions were able to prevent this increase in the plasma CINC-1 levels in the HS rats.
4. Discussion
The concept of hemoglobin encapsulation within an artificial membrane was first investigated by Chang in 1964 (Chang, 1964). Since then several nano-capsular products using human or bovine hemoglobin have been investigated as oxygen-carrying substitutes for RBCs (Sakai et al., 2010). LEH also belongs to this lineage which could overcome several of the drawbacks associated with the oxygen carriers presenting naked hemoglobin in polymerized or PEGylated form. Compared to these acellular hemoglobin-based products, encapsulated hemoglobin can provide longer circulation persistence without any vasoactivity and renal toxicity (Awasthi et al., 2006; Cabrales et al., 2012). Moreover, LEH can also carry hemoglobin stabilizers and allosteric modifiers of oxygen affinity, which can be co-encapsulated, to enhance the functional efficacy of the product (Kettisen et al., 2015). LEH resuscitation has been shown to maintain the hemodynamics and microvascular circulation after acute blood loss (Sakai et al., 2004), and improve cerebral oxygenation (Kaneda et al., 2014; Kawaguchi et al., 2014) in pre-clinical models. The LEH in these earlier studies was largely constituted of saturated and acyl-symmetric phosphatidylcholines, an anionic phospholipid, cholesterol, and a PEG-linked phosphatidylethanolamine. Based on the phosphatidylcholine and cholesterol foundation, the anionic phospholipid such as dimyristroyl phosphatidylglycerol served as a charge-imparting stabilizer against coalescence and a significant enhancer of hemoglobin encapsulation. PEG-linked phosphatidylethanolamine, on the other hand, afforded stealth characteristics to the LEH for a prolonged circulation t1/2.
Although our LEH formulation is also mostly based on phospholipid and cholesterol, it contains two novel non-phospholipid constituents, namely HDAS and HDAS-PEG, as replacements of the anionic phospholipid and PEG-linked phosphatidylethanolamine, respectively. HDAS is an anionic lipid which enhances hemoglobin encapsulation inside the liposomes by enabling closer interaction between hemoglobin and lipid phase (Agashe et al., 2010), whereas HDAS-PEG delays the clearance of LEH by the monocyte phagocytic system and increases the mean residence time in circulation. Compared to the conventional phospholipids, HDAS and HDAS-PEG do not contain the phosphoryl moiety. The phosphoryl linkage has been shown to participate in complement activation and anaphylotoxin production by the PEGylated liposomes (Moghimi et al., 2006). Any untoward effect of a resuscitation fluid on the complement system has a potential to further complicate the pathology of shock, because the complement system also contributes to the SIRS (Szebeni et al., 2003). By virtue of their non-phospholipid nature, the HDAS-lipids reduce the complement activation induced by the liposome particles (Nag et al., 2013; Yadav et al., 2014).
The SIRS in hemorrhagic shock is determined by the immune cells via a balance of several cytokines and chemokines in circulation (Cai et al., 2010). Upon stimulation, these cells initiate a molecular cascade culminating in the secretion of effector cytokines, such as TNF-α and IL-6. In our model, we found only a moderate increase in the plasma TNF-α, IL-6, and IL-1α levels after hemorrhagic shock; LEH was effective in preventing the rise in the levels of these cytokines (Table 2). As compared to these canonical cytokines, the effect of hemorrhagic shock and resuscitation on the plasma levels of IL-22 was more pronounced. Incidentally, the pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α stimulate IL-22 production by the Th1, Th17, and Th22 lymphocytes (Pan et al., 2014). IL-22 is a member of the IFN/IL-10 cytokine family, which exerts its effect mainly on the non-immune epithelial cells and hepatocytes expressing high-affinity IL-22 receptor (Savan et al., 2011). However, except for its role in the liver ischemia-reperfusion injury (Chestovich et al., 2012), the importance of IL-22 in hemorrhagic shock has not been investigated. Yet another indicator of the SIRS is the plasma level of citrulline which serves as a product of arginine consumption in reactions catalyzed by inducible nitric oxide synthase or arginase I. In inflammatory conditions the activity of these enzymes increases (Ochoa et al., 2001). The normal lymphocytic function is dependent on the availability of adequate amounts of arginine (Angele et al., 1999). The catabolism of arginine into citrulline, makes plasma citrulline a negative indicator of lymphocyte population (Marik and Flemmer, 2012). In trauma, a decrease in plasma arginine and an increase in citrulline is commonly seen (Chiarla et al., 2006). LEH prevented the hemorrhage-induced increase in plasma citrulline levels (Table 2), suggesting that it improves the lymphocytic health by enabling an adequate supply of arginine.
The manifestation of SIRS is in the multi-organ failure (Keel and Trentz, 2005). We recently reported that resuscitation with LEH was able to restore the brain metabolism in the rats with hemorrhagic shock (Rao et al., 2015). Others have also reported similar recovery of cerebral function by LEH infusion (Kakehata et al., 2010; Kaneda et al., 2014; Tiwari et al., 2010). However, the brain is protected, to a certain extent, against hypoxia and hypoperfusion by virtue of a physiologic diversion of blood flow from peripheral vascular network into the cerebral supply. Like the brain, the heart microcirculation is also fairly well preserved during mild and moderate shock. Although others have shown a mild increase in creatine kinase, an indicator of muscle cell injury, after hemorrhagic shock (Ronn et al., 2011), we could not find any change in creatine kinase. Instead, we found a significant increase in the plasma levels of cTnI (Fig. 5), which is regarded as a more reliable and sensitive indicator of heart injury (Pervaiz et al., 1997). LEH resuscitation was able to prevent the rise in plasma cTnI in hemorrhagic shock. However, these results, and the unaltered myocardial MPO activity (Fig. 7c), suggest that the acute myocardial injury, if any, was mild in our model.
The acute damage from hemorrhagic shock actually occurs in splanchnic organs. Thus, the resuscitation efficacy of LEH should correlate with the prevention of injury in these organs. According to the current paradigm of multi-organ dysfunction in shock pathology, the primary target after trauma and shock is the gut. We recently reported the LEH infusion prevented the hemorrhagic shock-induced perturbations in gut proteostasis (Rao et al., 2016). The ischemic conditions cause a rapid deterioration of the gut barrier, which results in a leakage of inflammatory mediators into the portal circulation. The systemic effects of this barrier dysfunction include hepatic impairment, pulmonary injury and a generalized inflammatory response (Moore, 1999). We found that hemorrhagic shock increased the neutrophil infiltration in the intestinal tissue (Fig. 7b). Since this was not associated with a corresponding increase in the tissue levels of CINC-1 (Fig. 7e), we speculate that some other chemoattractant may be responsible for the neutrophil accumulation in the gut tissue after hemorrhagic shock. In the liver, on the other hand, the increase in tissue-associated CINC-1and the MPO activity paralleled each other (Fig. 7a and 7d); LEH resuscitation was able to reduce neutrophil infiltration as well as liver CINC-1 (Fig. 7a and 7d). LEH resuscitation also effectively protected the liver function and suppressed the activation of resident macrophages (Fig. 6). In comparison, saline was relatively less effective in reducing the markers of gut and liver injuries.
The multi-organ dysfunction syndrome in hemorrhagic shock also involves the renal system where a decline in perfusion results in an acute renal insufficiency. Based on the levels of plasma creatinine, it could be assessed that LEH resuscitation, but not saline resuscitation, was able to prevent the kidney dysfunction in hemorrhagic shock (Fig. 5c). The major contributor to the plasma creatinine is the skeletal muscle, and the deranged skeletal muscle metabolism is indicated by the plasma levels of sTnI. Both LEH and saline resuscitation were able to prevent the shock-induced increase in the plasma sTnI, and the relative efficacy of these fluids was similar (Fig. 5d). These results suggest that LEH resuscitation has salutary effects on the skeletal muscle injury and kidney function, but saline infusion cannot prevent the kidney damage despite reducing the skeletal muscle injury.
5. Conclusions
Overall, the results suggest that LEH, by providing volume replacement as well as oxygenation, was able to prevent the systemic inflammation and multi-organ injuries caused by hemorrhagic shock. As indicated by the reduction in corticosterone levels (Fig. 4), LEH resuscitation reduced the physiological stress, creating a pro-survival phenotype. Although we did not intently looked for survival statistics in this study, the findings described in this work also corroborate our recent reports that the correction of volume and oxygen deficits by LEH recovers the cerebral glucose metabolism, oxygen metabolism, and tissue energetics in hemorrhagic shock (Awasthi et al., 2010; Awasthi et al., 2007; Rao et al., 2015). Notwithstanding the salutary effects of LEH described in this work and despite the report that the use of non-phospholipid anionic lipid reduces the host immune response to liposomes containing anionic phospholipids (Sou and Tsuchida, 2008), the reported constitution of LEH should to be evaluated for its immune response in a repeat injection trial of longer duration. However, it is noteworthy that Taguchi et al found that the circulation kinetics of a large dose of PEGylated hemoglobin vesicles was not altered in mice with an IgM response to previous exposure to the same preparation (Taguchi et al., 2009).
Acknowledgments
The work was supported by a grant from National Heart, Lung & Blood Institute [R01HL104286].
Footnotes
Disclosures: The authors do not have any conflict of interest to disclose.
Author's Contributions: VRY and GR contributed equally to this work. VRY prepared LEH and assisted in animal experiments. GR conducted the biochemical assays and data analyses, and contributed in manuscript writing. HH assisted in the biochemical assays involving the tissue samples. AH was integrally involved in the induction of hemorrhagic shock and resuscitation in rats. SA and PRR contributed in manuscript writing, editing, and data interpretation. VA conceptualized the study, conducted the animal experiments, wrote the manuscript text and led the team in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AABB. AABB survey. http://www.aabb.org/Documents/Programs_and_Services/Data_Center/07nbcusrpt.pdf.
- Agashe H, Awasthi V. Current perspectives in liposome-encapsulated hemoglobin as oxygen carrier. In: Liu AL, editor. Advances in Planar Lipid Bilayers and Liposomes. First. Academic Press (Elsevier); Oxford, UK: 2009. pp. 1–28. [Google Scholar]
- Agashe H, Lagisetty P, Awasthi S, Awasthi V. Improved formulation of liposome-encapsulated hemoglobin with an anionic non-phospholipid. Colloids and surfaces B, Biointerfaces. 2010;75:573–583. doi: 10.1016/j.colsurfb.2009.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angele MK, Smail N, Knoferl MW, Ayala A, Cioffi WG, Chaudry IH. L-Arginine restores splenocyte functions after trauma and hemorrhage potentially by improving splenic blood flow. Am J Physiol. 1999;276:C145–151. doi: 10.1152/ajpcell.1999.276.1.C145. [DOI] [PubMed] [Google Scholar]
- Awasthi V. Pharmaceutical aspects of hemoglobin-based oxygen carriers. Curr Drug Deliv. 2005;2:133–142. doi: 10.2174/1567201053586029. [DOI] [PubMed] [Google Scholar]
- Awasthi V, Agashe H, Doblas S, Towner R. Magnetic resonance spectroscopy for evaluation of liposome-encapsulated hemoglobin as a resuscitation fluid. Artificial cells, blood substitutes, and immobilization biotechnology. 2010;38:69–78. doi: 10.3109/10731191003634638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi V, Yee SH, Jerabek P, Goins B, Phillips WT. Cerebral oxygen delivery by liposome-encapsulated hemoglobin: a positron-emission tomographic evaluation in a rat model of hemorrhagic shock. J Appl Physiol. 2007;103:28–38. doi: 10.1152/japplphysiol.00136.2006. [DOI] [PubMed] [Google Scholar]
- Awasthi VD, Garcia D, Goins BA, Phillips WT. Circulation and biodistribution profiles of long-circulating PEG-liposomes of various sizes in rabbits. Int J Pharm. 2003;253:121–132. doi: 10.1016/s0378-5173(02)00703-2. [DOI] [PubMed] [Google Scholar]
- Awasthi VD, Garcia D, Klipper R, Goins BA, Phillips WT. Neutral and anionic liposome-encapsulated hemoglobin: effect of postinserted poly(ethylene glycol)-distearoylphosphatidylethanolamine on distribution and circulation kinetics. J Pharmacol Exp Ther. 2004;309:241–248. doi: 10.1124/jpet.103.060228. [DOI] [PubMed] [Google Scholar]
- Awasthi VD, Goins EA, Phillips WT. Liposome-encapsulated hemoglobin: History, preparation and evaluation. In: Winslow RM, editor. Blood Substitutes. Acadmic Press; London: 2006. pp. 501–513. [Google Scholar]
- Besen BA, Gobatto AL, Melro LM, Maciel AT, Park M. Fluid and electrolyte overload in critically ill patients: An overview. World J Crit Care Med. 2015;4:116–129. doi: 10.5492/wjccm.v4.i2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrales P, Rameez S, Palmer AF. Hemoglobin encapsulated poly(ethylene glycol) surface conjugated vesicles attenuate vasoactivity of cell-free hemoglobin. Curr Drug Discov Technol. 2012;9:224–234. doi: 10.2174/157016312802650760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai B, Deitch EA, Ulloa L. Novel insights for systemic inflammation in sepsis and hemorrhage. Mediators Inflamm. 2010;2010:642462. doi: 10.1155/2010/642462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TM. Semipermeable Microcapsules. Science. 1964;146:524–525. doi: 10.1126/science.146.3643.524. [DOI] [PubMed] [Google Scholar]
- Chestovich PJ, Uchida Y, Chang W, Ajalat M, Lassman C, Sabat R, Busuttil RW, Kupiec-Weglinski JW. Interleukin-22: implications for liver ischemia-reperfusion injury. Transplantation. 2012;93:485–492. doi: 10.1097/TP.0b013e3182449136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarla C, Giovannini I, Siegel JH. Plasma arginine correlations in trauma and sepsis. Amino Acids. 2006;30:81–86. doi: 10.1007/s00726-005-0211-z. [DOI] [PubMed] [Google Scholar]
- Gomes AV, Potter JD, Szczesna-Cordary D. The role of troponins in muscle contraction. IUBMB Life. 2002;54:323–333. doi: 10.1080/15216540216037. [DOI] [PubMed] [Google Scholar]
- Gronbaek H, Sandahl TD, Mortensen C, Vilstrup H, Moller HJ, Moller S. Soluble CD163, a marker of Kupffer cell activation, is related to portal hypertension in patients with liver cirrhosis. Aliment Pharmacol Ther. 2012;36:173–180. doi: 10.1111/j.1365-2036.2012.05134.x. [DOI] [PubMed] [Google Scholar]
- Kakehata J, Yamaguchi T, Togashi H, Sakuma I, Otani H, Morimoto Y, Yoshioka M. Therapeutic potentials of an artificial oxygen-carrier, liposome-encapsulated hemoglobin, for ischemia/reperfusion-induced cerebral dysfunction in rats. J Pharmacol Sci. 2010;114:189–197. doi: 10.1254/jphs.10115fp. [DOI] [PubMed] [Google Scholar]
- Kaneda S, Ishizuka T, Sekiguchi A, Morimoto K, Kasukawa H. Efficacy of liposome-encapsulated hemoglobin in a rat model of cerebral ischemia. Artificial organs. 2014;38:650–655. doi: 10.1111/aor.12358. [DOI] [PubMed] [Google Scholar]
- Kawaguchi AT, Endo H, Aikawa H, Yamano M, Kawaguchi Y, Haida M, Watanabe T. Effects of liposome-encapsulated hemoglobin on learning ability in tokai high-avoider rat after total brain ischemia and reperfusion. Artificial organs. 2014;38:667–674. doi: 10.1111/aor.12352. [DOI] [PubMed] [Google Scholar]
- Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36:691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Kettisen K, Bulow L, Sakai H. Potential electron mediators to extract electron energies of RBC glycolysis for prolonged in vivo functional lifetime of hemoglobin vesicles. Bioconjug Chem. 2015;26:746–754. doi: 10.1021/acs.bioconjchem.5b00076. [DOI] [PubMed] [Google Scholar]
- Lairet JR, Bebarta VS, Burns CJ, Lairet KF, Rasmussen TE, Renz EM, King BT, Fernandez W, Gerhardt R, Butler F, DuBose J, Cestero R, Salinas J, Torres P, Minnick J, Blackbourne LH. Prehospital interventions performed in a combat zone: a prospective multicenter study of 1,003 combat wounded. J Trauma Acute Care Surg. 2012;73:S38–42. doi: 10.1097/TA.0b013e3182606022. [DOI] [PubMed] [Google Scholar]
- Li T, Zhu Y, Fang Y, Liu L. Determination of the optimal mean arterial pressure for postbleeding resuscitation after hemorrhagic shock in rats. Anesthesiology. 2012;116:103–112. doi: 10.1097/ALN.0b013e31823de24f. [DOI] [PubMed] [Google Scholar]
- MacDermott RP. Chemokines in the inflammatory bowel diseases. J Clin Immunol. 1999;19:266–272. doi: 10.1023/a:1020583306627. [DOI] [PubMed] [Google Scholar]
- Marik PE, Flemmer M. The immune response to surgery and trauma: Implications for treatment. J Trauma Acute Care Surg. 2012;73:801–808. doi: 10.1097/TA.0b013e318265cf87. [DOI] [PubMed] [Google Scholar]
- Masumoto T, Ohkubo K, Yamamoto K, Ninomiya T, Abe M, Akbar SM, Michitaka K, Horiike N, Onji M. Serum IL-8 levels and localization of IL-8 in liver from patients with chronic viral hepatitis. Hepatogastroenterology. 1998;45:1630–1634. [PubMed] [Google Scholar]
- Matsuoka T. Determination of methemoglobin and carboxyhemoglobin in blood by rapid colorimetry. Biological & pharmaceutical bulletin. 1997;20:1208–1211. doi: 10.1248/bpb.20.1208. [DOI] [PubMed] [Google Scholar]
- Moghimi SM, Hamad I, Andresen TL, Jorgensen K, Szebeni J. Methylation of the phosphate oxygen moiety of phospholipid-methoxy(polyethylene glycol) conjugate prevents PEGylated liposome-mediated complement activation and anaphylatoxin production. FASEB J. 2006;20:2591–2593. doi: 10.1096/fj.06-6186fje. [DOI] [PubMed] [Google Scholar]
- Moore FA. The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg. 1999;178:449–453. doi: 10.1016/s0002-9610(99)00231-7. [DOI] [PubMed] [Google Scholar]
- Nag OK, Yadav VR, Hedrick A, Awasthi V. Post-modification of preformed liposomes with novel non-phospholipid poly(ethylene glycol)-conjugated hexadecylcarbamoylmethyl hexadecanoic acid for enhanced circulation persistence in vivo. Int J Pharm. 2013;446:119–129. doi: 10.1016/j.ijpharm.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa JB, Bernard AC, O'Brien WE, Griffen MM, Maley ME, Rockich AK, Tsuei BJ, Boulanger BR, Kearney PA, Morris Jr SM., Jr Arginase I expression and activity in human mononuclear cells after injury. Ann Surg. 2001;233:393–399. doi: 10.1097/00000658-200103000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan CX, Tang J, Wang XY, Wu FR, Ge JF, Chen FH. Role of interleukin-22 in liver diseases. Inflamm Res. 2014;63:519–525. doi: 10.1007/s00011-014-0727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervaiz S, Anderson FP, Lohmann TP, Lawson CJ, Feng YJ, Waskiewicz D, Contois JH, Wu AH. Comparative analysis of cardiac troponin I and creatine kinase-MB as markers of acute myocardial infarction. Clin Cardiol. 1997;20:269–271. doi: 10.1002/clc.4960200316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips WT, Klipper RW, Awasthi VD, Rudolph AS, Cliff R, Kwasiborski V, Goins BA. Polyethylene glycol-modified liposome-encapsulated hemoglobin: a long circulating red cell substitute. J Pharmacol Exp Ther. 1999;288:665–670. [PubMed] [Google Scholar]
- Rao G, Hedrick AF, Yadav VR, Xie J, Hussain A, Awasthi V. The brain metabolic activity after resuscitation with liposome-encapsulated hemoglobin in a rat model of hypovolemic shock. J Cereb Blood Flow Metab. 2015;35:1528–1536. doi: 10.1038/jcbfm.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao G, Yadav VR, Awasthi S, Roberts PR, Awasthi V. Effect of liposome-encapsulated hemoglobin resuscitation on proteostasis in small intestinal epithelium after hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol. 2016 doi: 10.1152/ajpgi.00157.2016. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronn T, Lendemans S, de Groot H, Petrat F. A new model of severe hemorrhagic shock in rats. Comp Med. 2011;61:419–426. [PMC free article] [PubMed] [Google Scholar]
- Rushing GD, Britt RC, Britt LD. Effects of hemorrhagic shock on adrenal response in a rat model. Ann Surg. 2006;243:652–654. doi: 10.1097/01.sla.0000216759.36819.1b. discussion 654-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Masada Y, Horinouchi H, Yamamoto M, Ikeda E, Takeoka S, Kobayashi K, Tsuchida E. Hemoglobin-vesicles suspended in recombinant human serum albumin for resuscitation from hemorrhagic shock in anesthetized rats. Crit Care Med. 2004;32:539–545. doi: 10.1097/01.CCM.0000109774.99665.22. [DOI] [PubMed] [Google Scholar]
- Sakai H, Sou K, Horinouchi H, Kobayashi K, Tsuchida E. Review of hemoglobin-vesicles as artificial oxygen carriers. Artificial organs. 2009;33:139–145. doi: 10.1111/j.1525-1594.2008.00698.x. [DOI] [PubMed] [Google Scholar]
- Sakai H, Sou K, Horinouchi H, Kobayashi K, Tsuchida E. Hemoglobin-vesicle, a cellular artificial oxygen carrier that fulfils the physiological roles of the red blood cell structure. Adv Exp Med Biol. 2010;662:433–438. doi: 10.1007/978-1-4419-1241-1_62. [DOI] [PubMed] [Google Scholar]
- Savan R, McFarland AP, Reynolds DA, Feigenbaum L, Ramakrishnan K, Karwan M, Shirota H, Klinman DM, Dunleavy K, Pittaluga S, Anderson SK, Donnelly RP, Wilson WH, Young HA. A novel role for IL-22R1 as a driver of inflammation. Blood. 2011;117:575–584. doi: 10.1182/blood-2010-05-285908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sou K, Tsuchida E. Electrostatic interactions and complement activation on the surface of phospholipid vesicle containing acidic lipids: effect of the structure of acidic groups. Biochimica et biophysica acta. 2008;1778:1035–1041. doi: 10.1016/j.bbamem.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Stewart JC. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Analytical biochemistry. 1980;104:10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- Stollings JL, Oyen LJ. Oxygen therapeutics: oxygen delivery without blood. Pharmacotherapy. 2006;26:1453–1464. doi: 10.1592/phco.26.10.1453. [DOI] [PubMed] [Google Scholar]
- Szebeni J, Baranyi L, Savay S, Gotze O, Alving CR, Bunger R, Mongan PD. Complement activation during hemorrhagic shock and resuscitation in swine. Shock. 2003;20:347–355. doi: 10.1097/01.shk.0000082444.66379.17. [DOI] [PubMed] [Google Scholar]
- Taguchi K, Urata Y, Anraku M, Watanabe H, Kadowaki D, Sakai H, Horinouchi H, Kobayashi K, Tsuchida E, Maruyama T, Otagiri M. Hemoglobin vesicles, polyethylene glycol (PEG)ylated liposomes developed as a red blood cell substitute, do not induce the accelerated blood clearance phenomenon in mice. Drug Metab Dispos. 2009;37:2197–2203. doi: 10.1124/dmd.109.028852. [DOI] [PubMed] [Google Scholar]
- Tiwari VN, Kiyono Y, Kobayashi M, Mori T, Kudo T, Okazawa H, Fujibayashi Y. Automatic labeling method for injectable 15O-oxygen using hemoglobin-containing liposome vesicles and its application for measurement of brain oxygen consumption by PET. Nucl Med Biol. 2010;37:77–83. doi: 10.1016/j.nucmedbio.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Tomita S, Enoki Y, Santa M, Yoshida H, Yasumitsu Y. A simple spectrophotometric method for determination of met-hemoglobin in dilute solution. J Nara Med Assoc. 1968;19:6. [Google Scholar]
- Yadav VR, Hussain A, Sahoo K, Awasthi V. Remediation of Hemorrhagic Shock-Induced Intestinal Barrier Dysfunction by Treatment with Diphenyldihaloketones EF24 and CLEFMA. J Pharmacol Exp Ther. 2014;351:413–422. doi: 10.1124/jpet.114.217331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VR, Sahoo K, Roberts PR, Awasthi V. Pharmacologic Suppression of Inflammation by a Diphenyldifluoroketone, EF24, in a Rat Model of Fixed-Volume Hemorrhage Improves Survival. J Pharmacol Exp Ther. 2013;347:346–356. doi: 10.1124/jpet.113.208009. [DOI] [PMC free article] [PubMed] [Google Scholar]


