Summary
Neisseria gonorrhoeae produces two transferrin binding proteins, TbpA and TbpB, which together enable efficient iron transport from human transferrin. We demonstrate that expression of the tbp genes is controlled by MisR, a response regulator in the two-component regulatory system that also includes the sensor kinase MisS. The tbp genes were up-regulated in the misR mutant under iron-replete conditions but were conversely down-regulated in the misR mutant under iron-depleted conditions. The misR mutant was capable of transferrin-iron uptake at only 50% of wild-type levels, consistent with decreased tbp expression. We demonstrate that phosphorylated MisR specifically binds to the tbpBA promoter and that MisR interacts with five regions upstream of the tbpB start codon. These analyses confirm that MisR directly regulates tbpBA expression. The MisR binding sites in the gonococcus are only partially conserved in Neisseria meningitidis, which may explain why tbpBA was not MisR-regulated in previous studies using this related pathogen. This is the first report of a trans-acting protein factor other than Fur that can directly contribute to gonococcal tbpBA regulation.
Graphical Abstract
Introduction
Neisseria gonorrhoeae is the causative agent of the sexually transmitted infection, gonorrhea. This common infection afflicts both genders but causes significant morbidity among women, with post infection sequelae including ectopic pregnancy, pelvic inflammatory disease (Paavonen et al., 2008), infertility (Westrom et al., 1992) and even disseminated infections. The WHO estimated that there were 78 million cases of gonorrhea worldwide in 2012 (Newman et al., 2015). The CDC estimates that the actual incidence of gonococcal infections in the U.S. is 820,000 new cases per year (CDC, 2015). Annual medical costs related to N. gonorrhoeae infections and related sequelae exceed $1 billion in the US alone (Aledort et al., 2005). Infection with N. gonorrhoeae does not elicit protective immunity (Hedges et al., 1999, Hedges et al., 1998), so repeat infections are common. Despite decades of research on the pathogenesis of gonococcal disease, no vaccine is available to prevent infections caused by this stealth pathogen. Even more concerning is the evolution of antimicrobial drug resistance among N. gonorrhoeae isolates (Barbee, 2014, Unemo & Nicholas, 2012, Hook & van Der Pol, 2013). Penicillin and tetracycline were abandoned years ago as appropriate empiric therapies to treat gonorrhea. More recently, resistance to fluoroquinolones increased to such a degree that this class of drugs was likewise removed from the chemotherapeutic arsenal (CDC, 2007). The currently-recommended therapy to treat uncomplicated gonococcal infections is the combination of ceftriaxone and azithromycin (CDC, 2012). Unfortunately, resistance to both of these drugs has emerged and these resistant strains are becoming increasingly prevalent worldwide (CDC, 2011, Soge et al., 2012, Unemo & Nicholas, 2012).
With increasing prevalence of disease and evolution of antimicrobial resistance, the specter of untreatable gonorrhea is increasingly likely (Barbee, 2014). This situation makes the search for gonococcal vaccine candidates that much more urgent and compelling. The gonococcus has been referred to as “chameleon-like” (Robinson et al., 1989) due to its ability to vary the antigenic character of surface antigens at high frequency. In contrast to most outer membrane proteins produced by N. gonorrhoeae, the components of the transferrin iron acquisition system are not subject to high-frequency antigenic or phase variation. The proteins that enable the gonococcus to use human transferrin as the sole source of iron include transferrin binding proteins A and B (TbpA and TbpB). These proteins are ubiquitously expressed by all strains and they are relatively well-conserved in sequence (for reviews see (Cornelissen, 2008, Cornelissen & Sparling, 1994)). Importantly, we demonstrated that a mutant unable to express the tbp genes was unable to elicit signs and symptoms of urethritis in a human male infection model (Cornelissen et al., 1998). These results indicate that expression of the transferrin receptor components is necessary to initiate mucosal infection in human males.
TbpA is an integral outer membrane protein in the TonB-dependent transporter family (Cornelissen et al., 1992), while TbpB is a surface-exposed lipoprotein (DeRocco & Cornelissen, 2007). While TbpA is absolutely essential for iron internalization from human transferrin (Cornelissen et al., 1992), TbpB only enhances the efficiency of iron uptake by approximately 50% (Anderson et al., 1994). While TbpA forms the essential conduit for iron through the outer membrane, TbpB enhances the iron internalization process by specifically recognizing the ferrated form of transferrin (Cornelissen & Sparling, 1996) and by enhancing deferrated transferrin release (DeRocco et al., 2008). Moreover, structural studies indicate that TbpB completes an iron chamber in the three-part protein complex comprised of TbpA, TbpB and transferrin (Noinaj et al., 2012b), thereby preventing iron diffusion after the metal has been extracted from transferrin (for review see (Noinaj et al., 2012a)).
Like most proteins involved in iron acquisition, the genes encoding the transferrin binding proteins are regulated by the internal concentration of iron. The mechanism by which this occurs is quite well understood (Bagg & Neilands, 1987). Ferrous iron, when at high concentration inside the cell, complexes with the Fur protein, which subsequently dimerizes for interaction with target DNA. The Fur repressor protein binds to a specific motif (the Fur box or binding site) within iron-regulated promoters, thereby repressing transcription. When internal iron pools decline, Fur is no longer an effective repressor and it therefore disassociates from promoter regions allowing transcription to ensue (Bagg & Neilands, 1987). Expression of the gonococcal tbp genes is further modulated by the expression of an upstream RNA, which is transcribed in the direction opposite to that of the tbp genes (Velez Acevedo et al., 2014). We recently demonstrated that inactivation of this upstream RNA alters both transcription and translation of the tbp locus (Velez Acevedo et al., 2014).
The transferrin binding proteins, TbpA and TbpB, are encoded by a bicistronic operon, with the tbpB gene located upstream of the tbpA gene (Ronpirin et al., 2001). We recently mapped the transcription start site and Fur binding site in the tbpB promoter region (Velez Acevedo et al., 2014). Furthermore, we demonstrated that under iron stress and at steady-state, the tbpB transcripts outnumber tbpA transcripts by 2:1, suggesting that processivity throughout the bicistronic transcript is incomplete (Ronpirin et al., 2001). This differential in tbpB:tbpA transcripts may be manifested as greater numbers of TbpB proteins relative to TbpA proteins under iron stress as we have also shown that TbpB-specific binding sites outnumber TbpA-specific binding sites on the cell surface by at least 2:1 (Cornelissen & Sparling, 1996).
In the current study, we sought to define additional conditions and regulators that impact expression of the tbp genes. Employing RNA-Seq, we characterized the MisR regulon in N. gonorrhoeae for the first time. MisR is the response regulator of the MisR-MisS two-component regulatory system, and its cognate sensor kinase is MisS (Tzeng et al., 2004, Tzeng et al., 2006). To date, the signal to which MisS responds in the pathogenic Neisseria species has not been identified. Among the genes regulated by gonococcal MisR, the tbpB gene demonstrated greater than five-fold up-regulation in the misR mutant. Meningococcal MisR has been demonstrated to impact the expression of iron- and metal-storage and transport genes, including hmbR, bfrAB, and tdfH (Tzeng et al., 2008). However, in contrast with the data shown here for gonococci, the meningococcal MisR regulon does not appear to include the tbp genes (Newcombe et al., 2005, Tzeng et al., 2008). In this report, we investigated the phenotype of the gonococcal misR mutant with respect to expression of the tbp genes, production of the Tbp proteins, and iron uptake from transferrin. Under iron-replete conditions, MisR down-regulates both tbpA and tbpB. Interestingly, under iron-depleted conditions, MisR appears to up-regulate both tbp genes, as evidenced by decreased tbp transcript and Tbp protein levels in the misR mutant when grown in low iron conditions. Consistent with this observation, the misR mutant is impaired for iron uptake from human transferrin relative to the wild-type strain. Furthermore, using DNA-binding experiments, we found that purified, phosphorylated MisR binds to defined sites in the promoter region upstream of the tbpBA operon.
Results
Expression of tbp genes is controlled by MisR
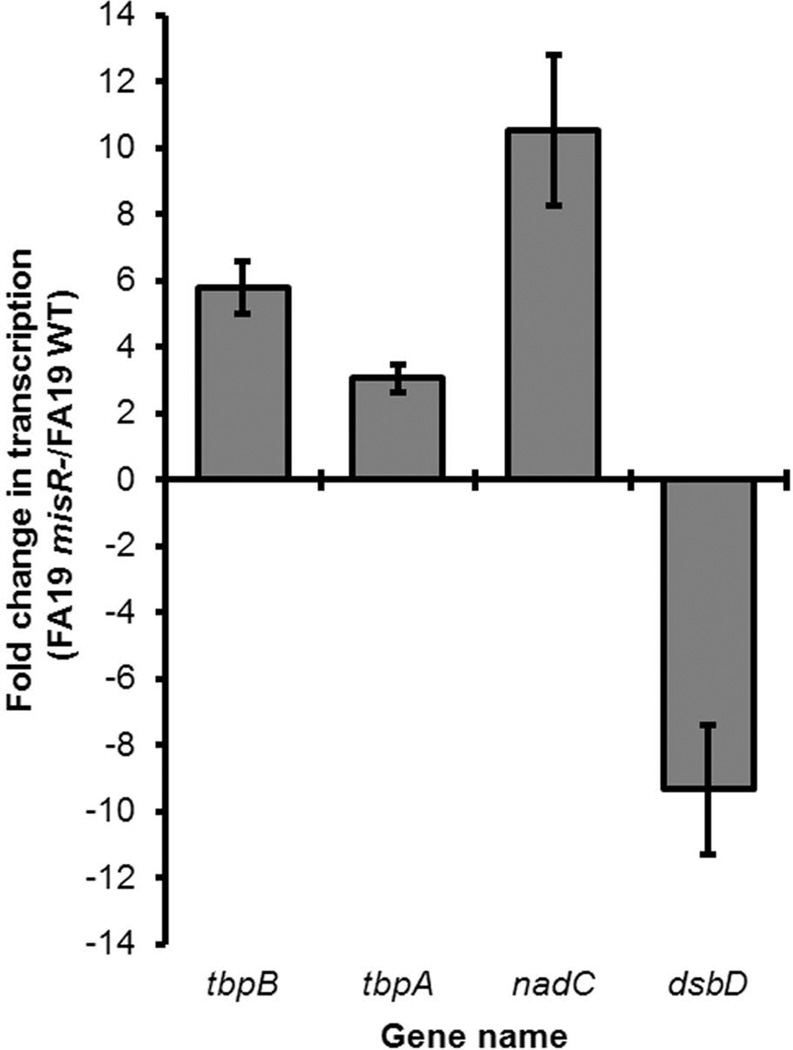
We employed RNA-Seq to evaluate the changes in gene expression in a misR::kan mutant compared to the wild-type strain FA19. As shown in Fig. 1 and Supplemental Table S1, tbpA and tbpB gene expression was increased in the misR mutant compared to the wild-type strain, indicating that these genes are MisR-repressed under the conditions tested. Quantitatively, tbpA was 3-fold and tbpB 5.8-fold down-regulated by MisR in the RNA-Seq analysis (Fig. 1). Also shown in Fig. 1 are the fold-changes in expression for the previously-reported meningococcal MisR target genes, nadC (Newcombe et al., 2005) and dsbD (Tzeng et al., 2008), which, as expected, were repressed and activated, respectively, by gonococcal MisR. These genes served as internal controls.
Fig. 1. RNA-Seq demonstrated that the tbp genes are among those regulated by MisR.
Fold change in gene expression (FA19 misR-/FA19 WT) is shown on the Y-axis. Shown are the average fold changes in transcription of tbpB, tbpA, and 2 control genes nadC and dsbD, in the presence or absence of MisR for three independent experiments. Error bars represent the standard deviation from the mean for each gene. Similar to our results shown here, control genes nadC and dsbD were repressed and activated, respectively by MisR in N. meningitidis (see Table S1 in this work and also Table S1 in (Newcombe et al., 2005) and Table S1 in (Tzeng et al., 2008)). These genes serve to validate our findings in the gonococcus. See Table S1 for Bonferroni corrected values demonstrating statistical significance.
Quantitative RT-PCR (qRT-PCR) confirms that tbp transcription is influenced by MisR
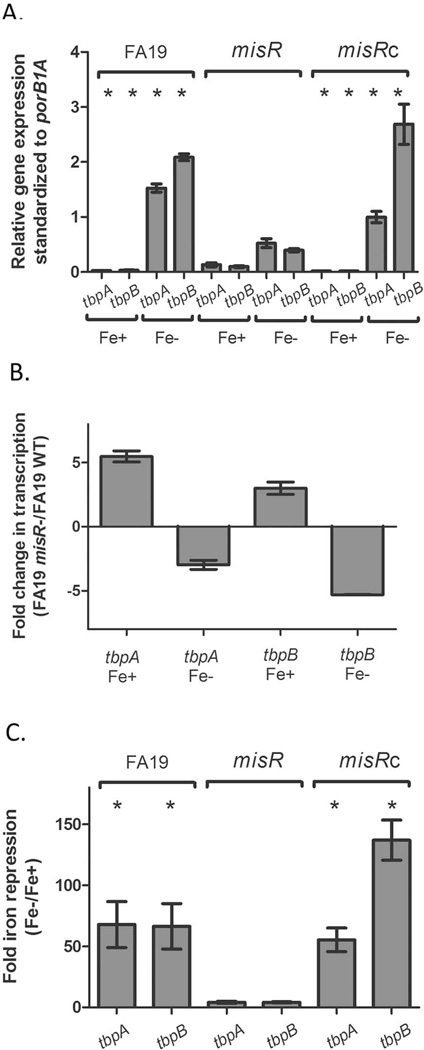
To validate the RNA-Seq results, we utilized qRT-PCR with gene specific primers for detection of tbpA and tbpB. We assessed gene expression with 10 mM Mg2+ (as was employed for the RNA-Seq analysis; Fig. S1) and without additional Mg2+ (Fig. 2), under both iron-replete and iron-depleted conditions. Expression of tbpBA expression is maximized when grown in iron-depleted media (Anderson et al., 1994, Cornelissen et al., 1992). Consistent with the RNA-Seq data, tbpA and tbpB expression under iron-replete conditions was up-regulated in the misR mutant (Fig. 2A and B). Expression of tbpA was five-fold upregulated in the mutant compared to the wild-type strain (Fig. 2A) while tbpB expression was upregulated approximately three-fold in the misR mutant relative to the wild-type strain (Fig. 2B). However, when iron was chelated by the addition of Desferal, both tbpB and tbpA were down-regulated in the misR mutant. These observations are supported whether the expression data are presented as relative gene expression compared to an internal standard (Fig. 2A) or as fold change in expression, mutant compared to wild-type (Fig. 2B). All pairwise comparisons with the misR mutant were statistically different (p < 0.05). The reversal in regulatory trend was important, since expression of tbpBA is nearly undetectable in the presence of iron (Velez Acevedo et al., 2014) and transcriptional differences under iron-depleted conditions are far more biologically relevant.
Fig. 2. qRT-PCR demonstrates differential expression of tbp genes depending on the iron status of the growth conditions.
Cultures were grown in GCB medium under iron replete (plus ferric nitrate, Fe+) or iron depleted (plus Desferal, Fe-) conditions. qRT-PCR experiments were conducted in triplicate. Panel A. Relative gene expression for tbpA and tbpB genes under iron-replete and iron-depleted conditions for WT, misR and misRc strains. Shown are the average relative gene expression values (standardized to the porB1A gene); error bars represent the standard deviation from the mean. Asterisks demonstrate those values that were significantly different (P < 0.05) as compared to the misR mutant in pairwise comparisons using an unpaired Student’s t-test. Panel B. The average fold change in expression of the tbp genes is shown on the Y-axis and represents the ratio of FA19 misR-/FA19 WT gene expression (as shown in Fig. 1). Error bars represent standard deviation from the mean for each gene and condition. Panel C. Fold iron repression is shown on the Y-axis and represents the relative expression of tbp genes under iron-depleted/iron-replete conditions. The error bars represent the standard deviation from the mean. Asterisks demonstrate those values that were significantly different (P < 0.01) as compared to the misR mutant in pairwise comparisons using an unpaired Student’s t-test.
To test the impact of MisR on the derepression of tbpBA, we next compared the transcription of tbpB and tbpA under iron-depleted vs. iron-replete conditions for all three strains. As shown in Fig. 2C, the fold repression of tbpBA was decreased from 40–60 fold in WT FA19 to less than 10 fold in the misR mutant. These results indicate that the degree to which the tbp genes can be derepressed under iron-restricted conditions is dramatically reduced by loss of misR. Complementation with the wild-type copy of misR reversed the effect on regulation of the tbp genes. All pairwise comparisons with the misR mutant were statistically different (p < 0.01). Taken together, these data indicate that MisR differentially regulates transcription of tbpBA depending on the availability of iron, and is required for maximal derepression of tbpBA transcription during iron stress.
Production of the Tbp proteins is also influenced by MisR
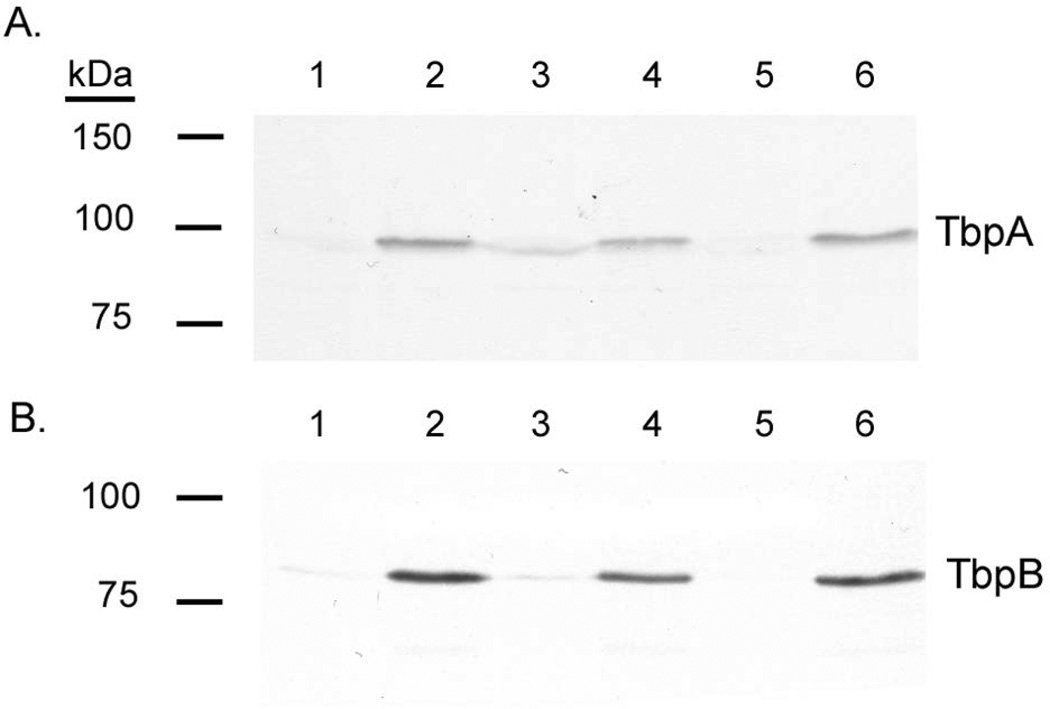
We next evaluated TbpA and TbpB protein production under iron-depleted and iron-replete conditions by Western blot. Protein levels under each condition were quantified using ImageJ (Schneider et al., 2012). All lanes were loaded equivalently as assessed by solubilizing the strains grown to the same culture density, loading equivalent protein amounts, and finally staining the nitrocellulose membrane with Ponceau S to confirm equivalent protein loading (data not shown). TbpA and TbpB proteins were upregulated 2.0 to 4.8 fold in the misR mutant under iron-replete conditions (Fig. 3), consistent with the RNA-Seq data. This suggested that efficient Fur+Fe2+-mediated repression of tbpBA requires MisR input. In contrast, under iron-depleted conditions, protein levels were down-regulated by approximately 1.5 fold in the misR mutant (Fig. 3). Complementation reversed the effects on both proteins. Fold iron repression of TbpA was decreased from 13 fold in the wild-type to 1.8 fold in the misR mutant. Fold iron repression of TbpB was similarly reduced from 40 fold in the wild-type to 13.7 fold in the misR mutant.
Fig. 3. Western blotting demonstrates that the Tbp proteins are influenced by expression of misR.
Panel A. Western blot probed for TbpA. Panel B. Western blot probed for TbpB. Lanes contain whole cell lysates, standardized by culture density, of the following strains grown under the described conditions: 1: wild-type FA19 +Fe; 2: wild-type FA19 -Fe; 3: misR mutant +Fe; 4: misR mutant -Fe; 5: misRc strain +Fe; 6: misRc strain -Fe. Approximate positions of molecular mass standards are shown to the left of the blots. The blots are representative of at least 10 independent experiments.
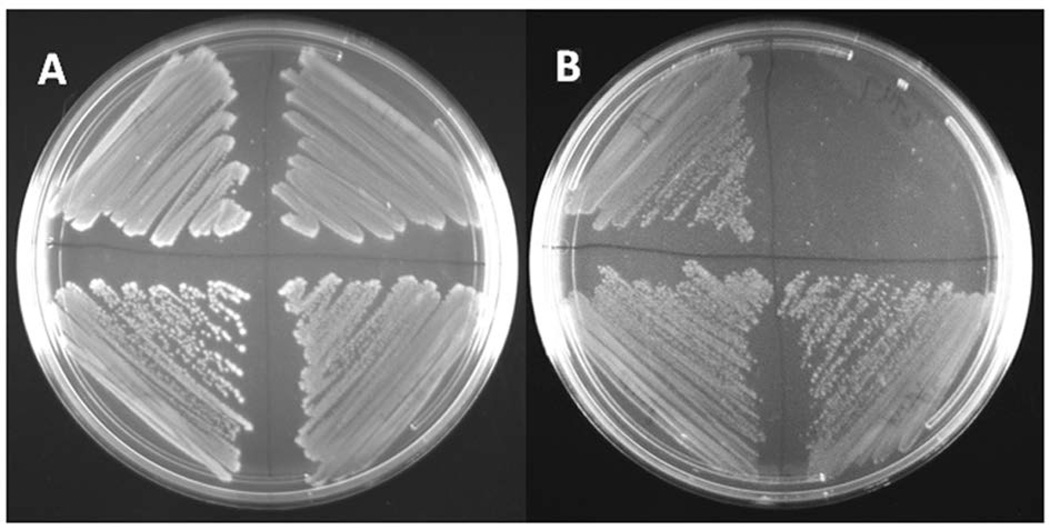
Growth of misR mutant and complemented strain on media containing ferric nitrate or human transferrin
Since MisR clearly impacted tbpBA expression, we next evaluated the ability of the mutant strains to grow on low-iron, solid media with defined iron sources in order to test whether MisR regulation was physiologically relevant. Regardless of the iron source [ferric nitrate or partially iron-saturated human transferrin], the misR mutant grew normally on solid GC medium, when provided either ferric nitrate (Fig. 4A) or human transferrin (Fig. 4B) as the sole iron source. As a control, a tbpA mutant strain (FA6747; (Cornelissen et al., 1992)) grew on GCB with ferric nitrate but could not grow when transferrin was provided as the sole iron source. We have previously demonstrated that even very low functionality of the TbpA transporter still enables growth on transferrin as a sole iron source (Cash et al., 2015). This observation is consistent with our data showing that down-regulation of tbp genes by MisR apparently has no effect on growth when transferrin is the only source of iron. Thus, we quantified the functionality of the transferrin transporter in the misR mutant employing a more sensitive measure of iron internalization from transferrin.
Fig. 4. Growth of the misR mutant and complemented strain on solid medium containing transferrin or ferric nitrate.
Gonococcal strains were streaked in quadrants on solid medium in the following orientation: upper left, WT FA19; upper right, tbpA mutant; lower left: misR mutant; lower right: misRc. Plate A contains ferric nitrate and plate B contains transferrin as the sole iron source.
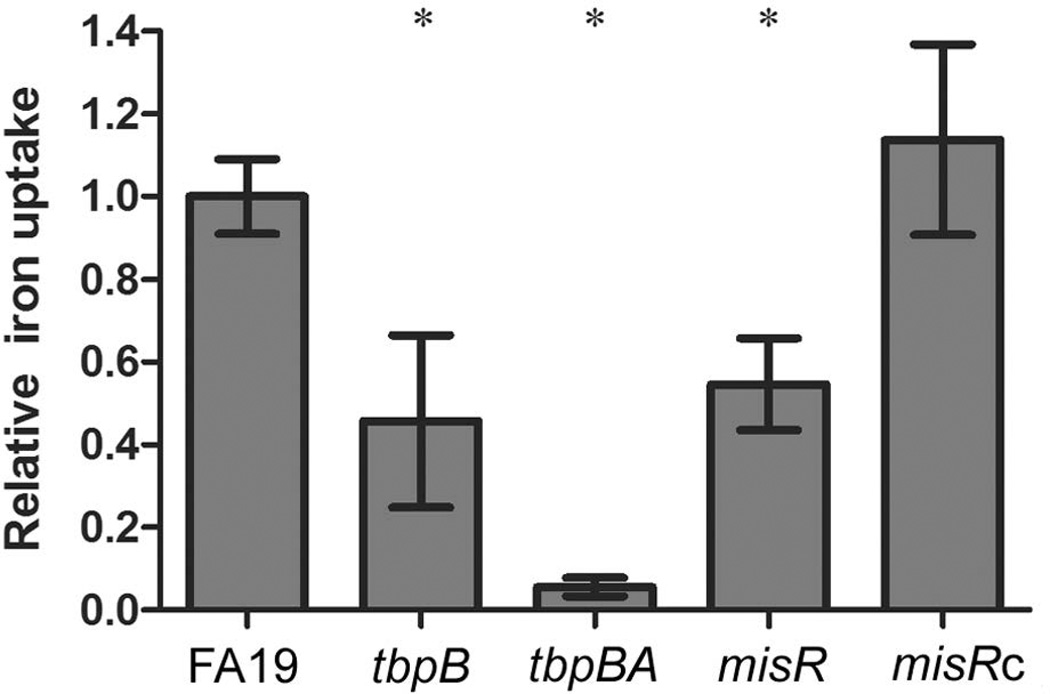
The misR mutant internalizes less iron from transferrin than the wild-type strain
We grew the wild-type strain, misR mutant and misRc strain under iron-depleted conditions and then measured the amount of transferrin-iron internalized by each strain in a 30-min iron internalization assay (Cash et al., 2015, DeRocco & Cornelissen, 2007, Anderson et al., 1994). We determined that the misR mutant only internalized about 50% of the iron that can be internalized by the wild-type strain in the 30 min assay (Fig. 5). The tbpB deletion strain (FA6905; (Cornelissen & Sparling, 1996)) is similarly able to internalize only about 50% of wild-type levels of transferrin-derived iron, by the function of TbpA alone, consistent with our previous results (Anderson et al., 1994). The negative control strain (FA6815), has a polar Ω cassette in tbpB and cannot express tbpA, which is absolutely required for iron uptake from transferrin, while expression of tbpB is not (Anderson et al., 1994). As expected, the negative control showed negligible iron uptake from transferrin. The results shown in Fig. 5 are consistent with a decrease in tbp gene expression in the misR mutant under iron-depleted growth conditions, leading to a decrease in the ability of the TbpBA proteins to accomplish iron uptake. Transferrin-iron internalization was restored to wild-type levels in the misR complemented strain.
Fig. 5. Transferrin-iron uptake by the wild-type and mutant gonococcal strains.
Gonococcal strains were grown in iron-depleted conditions, standardized to culture density and then incubated with transferrin-55Fe for 30 min. Unincorporated 55Fe was removed by filtration, followed by scintillation counting of extracted filters containing gonococcal strains. The specific amount of iron internalized by each strain was standardized to the wild-type strain, resulting in the relative iron uptake values shown on the y-axis. The each bar represents the mean and standard deviation from the mean generated from three independent assays. Asterisks represent those comparisons in which statistical significance (P-value <0.05) was reached in pairwise comparisons between wild-type and mutant strains using an unpaired student’s t-test.
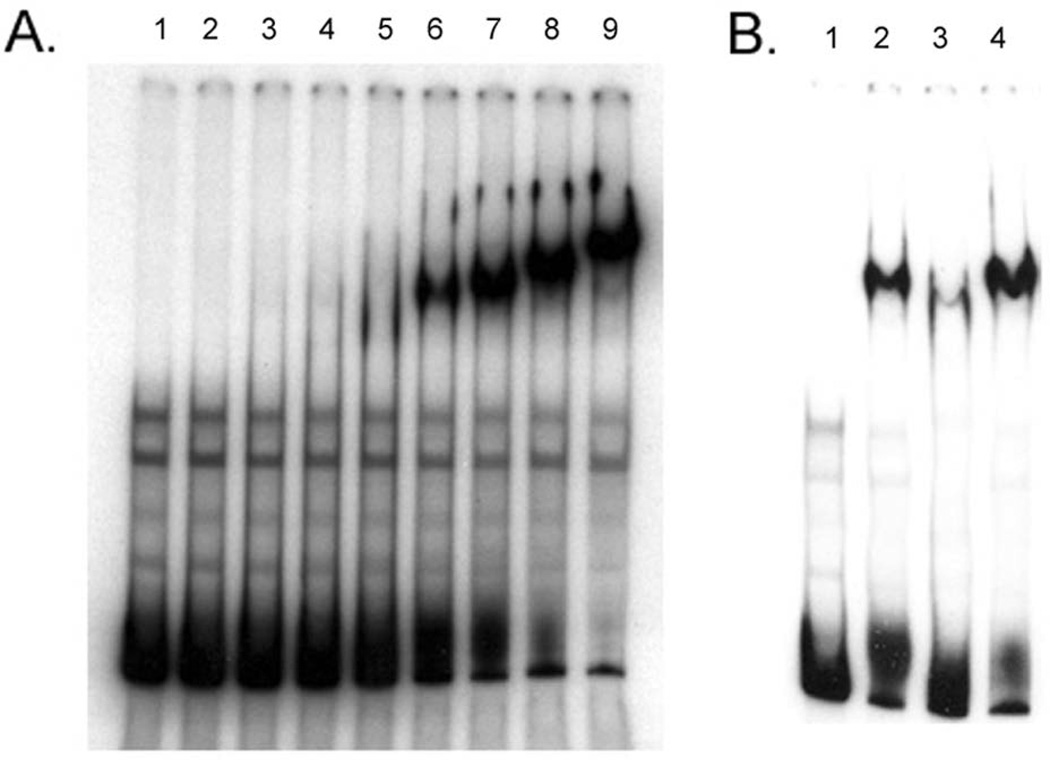
Phosphorylated MisR (MisR~P) specifically binds to the promoter region upstream of tbpBA
In order to determine if MisR directly regulates expression of tbpBA, we performed electrophoretic mobility shift assays (EMSA) using purified MisR-His6x and a PCR-generated DNA probe from upstream of the tbpBA operon through the 5’ end of the tbpB gene. In Fig. 6A, a titration EMSA demonstrated that MisR~P can efficiently shift the labeled tbpBA promoter probe. In contrast, non-phosphorylated MisR did not shift the tbpBA promoter probe (data not shown). As shown in Fig. 6B, we next performed a competitive EMSA, which demonstrated that MisR~P binding to tbpBA promoter probe was significantly diminished in the presence of unlabeled specific competitor (Fig. 7B, lane 3). In contrast, MisR~P binding was not impacted in the presence of unlabeled nonspecific competitor rnpB (Fig. 7B, lane 4). These results demonstrate that MisR~P binds specifically to the tbpBA promoter region.
Fig. 6. Mobility shift assays demonstrate that MisR binds directly to the tbpB A promoter region.
Panel A. Titration EMSA showing binding of MisR~P to the tbpBA promoter region. Lane 1: 2 ng labeled probe alone; lane 2: 2 ng labeled probe + 0.5 µg MisR~P; lane 3: 2 ng labeled probe + 0.75 µg MisR~P; lane 4: 2 ng labeled probe + 1.0 µg MisR~P; lane 5: 2 ng labeled probe + 1.5 µg MisR~P; lane 6: 2 ng labeled probe + 2.0 µg MisR~P; lane 7: 2 ng labeled probe + 2.5 µg MisR~P; lane 8: 2 ng labeled probe + 3.0 µg MisR~P; lane 9: 2 ng labeled probe + 4.0 µg MisR~P. Panel B. Competitive EMSA showing specificity of MisR~P binding to the tbpBA promoter region. Lane 1: 2 ng labeled probe alone; lane 2: 2 ng labeled probe + 2.5 µg MisR~P; lane 3: 2 ng labeled probe + 1 µg unlabeled specific competitor (unlabeled probe) + 2.5 µg MisR~P; lane 4: 2 ng labeled probe + 1 µg unlabeled nonspecific competitor (rnpB) + 2.5 µg MisR~P. Note that the addition of unlabeled specific competitor (unlabeled probe), but not unlabeled nonspecific competitor (rnpB) greatly reduces the amount of MisR~P shifted labeled probe.
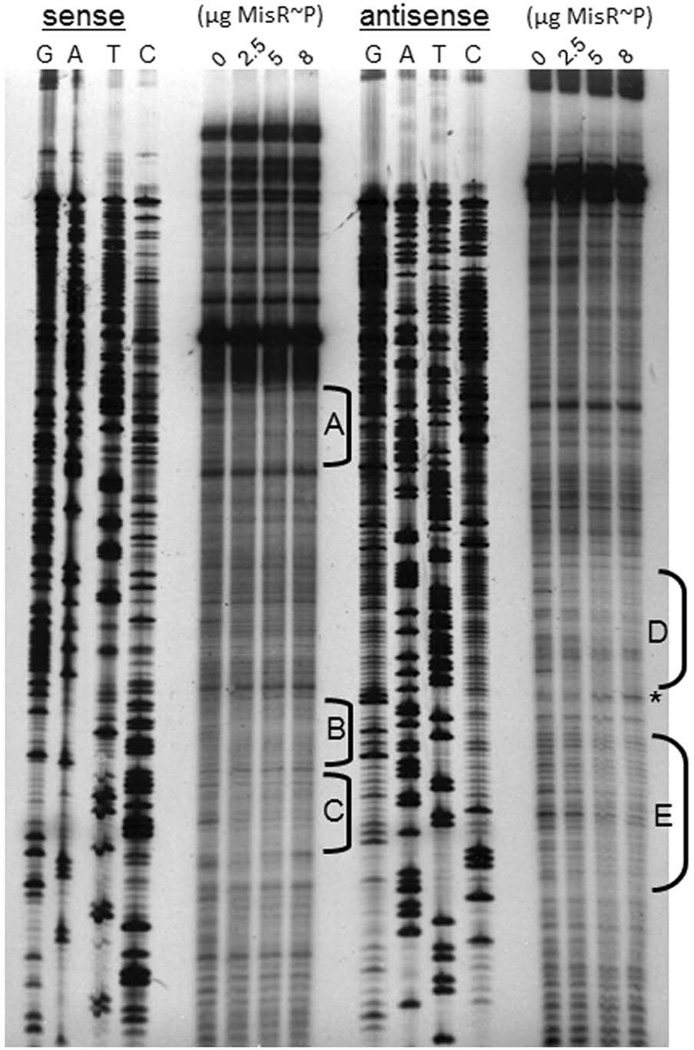
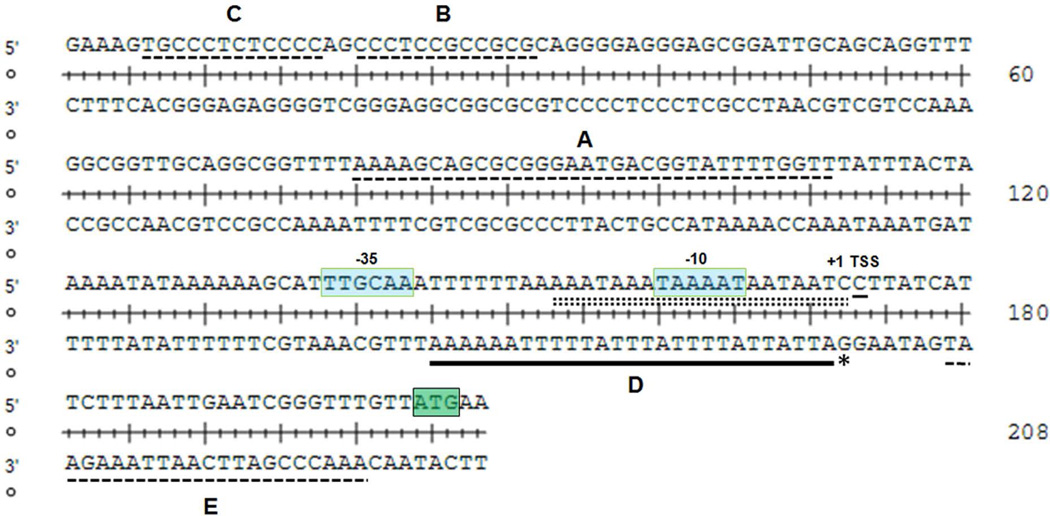
Fig. 7. Footprint analysis demonstrates the specific binding sites occupied by MisR~P in the tbpB promoter region.
DNase I footprinting used EMSA components described in Figure 6. Areas of protection are highlighted by black brackets (brackets A, B, and C correspond to protection on the sense strand, and brackets D and E correspond to protection on the antisense strand). A DNase I hypersensitivity site is shown by the asterisk.
Footprint analysis demonstrates that MisR~P binds to distinct regions of the tbpBA promoter
Using DNase I footprinting, we next sought to determine where in the tbpBA upstream region MisR~P bound. As shown in Fig. 7, footprint analysis revealed that MisR~P protected five regions (brackets labeled A through E). One region of the tbpBA upstream region (labeled bracket D) demonstrated a DNase I hypersensitivity site (asterisk). Region D also corresponds to a stretch of 27 nucleotides on the antisense strand that overlaps both the tbpBA −10 promoter element and the Fur binding site (Fig. 8).
Fig. 8. The promoter region upstream of tbpB with MisR~P binding sites identified relative to known promoter elements.
The sequence shown corresponds to the region of the tbpBA promoter analyzed by footprint assay in Figure 7. MisR~P protected sites are indicated on the appropriate strand by dashed lines or a black bar and are labeled corresponding to brackets in Figure 7. A DNase I hypersensitivity site is shown by the asterisk. The Fur binding site (Velez Acevedo et al., 2014) is shown as a double dashed line. Promoter elements and the transcriptional start site are indicated with text. The ATG start codon for tbpB is highlighted in green.
The tbpBA promoter and MisR binding sites are partially conserved between N. gonorrhoeae and N. meningitidis
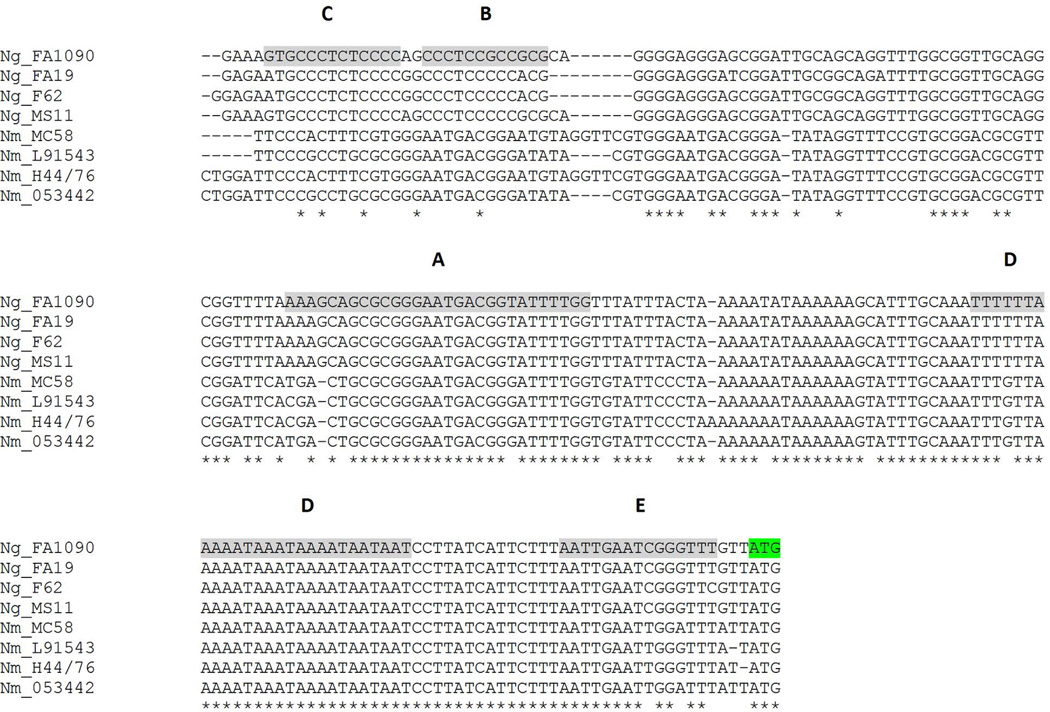
To explore whether MisR binding site differences might contribute to the lack of apparent MisR regulation of meningococcal tbpBA (Tzeng et al., 2008; Newcombe et al., 2005), we aligned the upstream tbpBA promoter regions of four gonococcal strains with those of four meningococcal strains. While the sequences upstream of tbpB in the gonococcal strains are nearly identical, the upstream sequences from the N. meningitidis strains diverge significantly from the gonococcal sequences (Fig. 9). Conservation between N. gonorrhoeae and N. meningitidis sequences was considerably higher near the start of the tbpB structural gene (green shading, Fig. 9) compared to sequences near the 5’ end of the aligned region. All 5 MisR binding sites were well-conserved among these N. gonorrhoeae strains but only partial conservation was detected in sites A, D and E in the N. meningitidis sequences. These observations suggest that MisR binding site conservation is maintained among gonococcal tbpBA promoters but the sequence diverges significantly among the N. meningitidis promoters.
Fig. 9. Sequence alignment of tbpBA promoter regions comparing N. meningitidis with N. gonorrhoeae strains.
The promoter regions of tbpBA from N. gonorrhoeae strains FA1090, FA19, F62 and MS11 were aligned with those of N. meningitidis strains MC58 (serogroup B), L91543 (serogroup C), H44/76 (serogroup B) and 053442 (serogroup C). Gray shading represents the MisR binding sites identified in Fig. 8. The tbpB start codon is highlighted in green.
Discussion
MisR is the OmpR-family response regulator in a gonococcal two-component regulatory system which also includes the sensor kinase, MisS (Tzeng et al., 2008). In the meningococcus, loss of MisR prevents the addition of phosphoethanolamine (PEA) to the inner core structure of lipooligosaccharide, thus leading to the gene acronym mis (meningococcal inner core structure) (Tzeng et al., 2004). The pathogenic Neisseriae encode far fewer (4 have been described) two-component regulatory systems than other Proteobacteria (which encode between 10 and 35 on average; (Qi et al., 2010)), and thus MisR/S may have evolved to “multitask” to some degree. Johnson et al. (Johnson et al., 2001) proposed that the meningococcal misR/S genes instead be named phoP/Q due to their similarity to the homologous genes in Salmonella. This group also suggested that MisR/MisS (termed “PhoP/PhoQ” therein) responds to Mg2+ as a signal, similar to the Salmonella PhoP/Q system (Johnson et al., 2001) (Newcombe et al., 2005). However, subsequent direct testing of this hypothesis (Tzeng et al., 2006) demonstrated that meningococcal MisR/MisS is unresponsive to changing Mg2+ concentrations. In another study, Jamet et al. (Jamet et al., 2009) suggested that the meningococcal MisR/MisS combine the functions of both the PhoP/Q and CpxR/A systems in other bacteria. Regardless of the genetic heritage of misR/misS, we demonstrate here, for the first time, that the gonococcal transferrin binding genes tbpB and tbpA are regulated by MisR at the transcriptional level, and that this regulation scheme is associated with a 50% decrease in iron uptake efficiency from human transferrin during iron starvation.
As mentioned, Fur is the primary, known regulator of tbpB and tbpA. Accessory cis-elements, such as the hairpin loop between tbpB and tbpA (Ronpirin et al., 2001) and an upstream region encoding various repeat units and a long, non-coding RNA (Velez Acevedo et al., 2014) can also modulate expression of the tbp genes between 2 to 7 fold. However, until now no trans-acting transcriptional regulatory protein other than Fur has been shown to directly regulate tbpBA in gonococci. After initial observations that tbpB and tbpA were controlled by MisR in the RNA-Seq experiment, we confirmed MisR regulation of this locus using qRT-PCR. However, since the media used in the RNA-Seq experiment were iron-replete, tbpBA expression would be very low under those conditions due to Fur+Fe2+-mediated repression (Velez Acevedo et al., 2014). Thus, we also tested transcription of the tbp genes under iron-restricted conditions and found that MisR again had a profound effect, but surprisingly in the opposite direction. Subsequent Western blot experiments recapitulated the results of our transcription assays, and together these expression studies demonstrate that MisR is necessary for wild-type repression under iron replete conditions but MisR is also important for wild-type de-repression during iron starvation. Consistent with these results, despite normal growth on plates containing partially iron-saturated transferrin as the only iron source, misR mutant gonococci displayed reduced uptake of iron from human transferrin similar to a gonococcal mutant completely lacking TbpB.
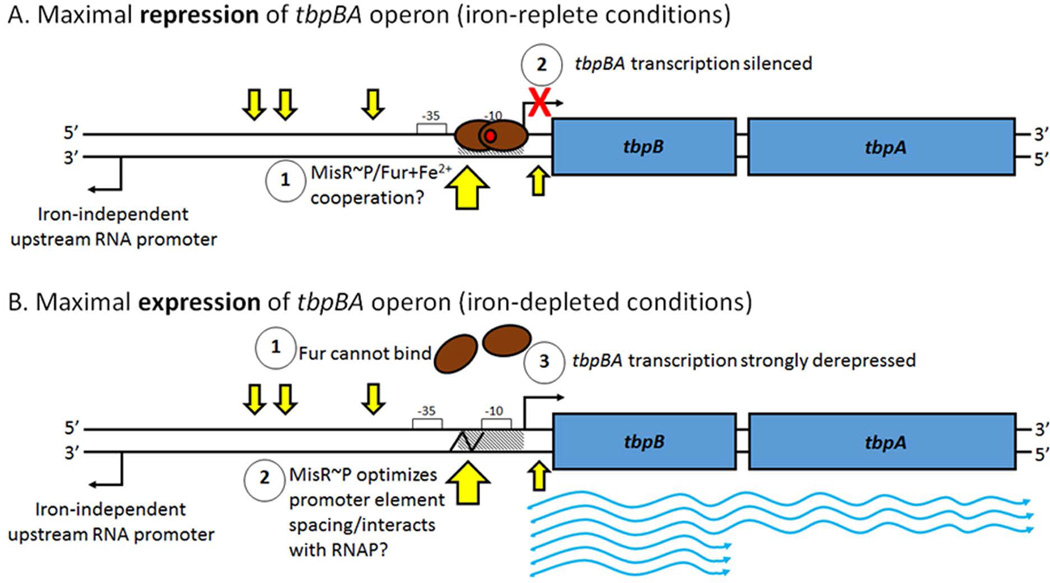
The binary nature of MisR’s regulatory role at the tbpBA locus may be explained by the location of its binding sites as determined by EMSA and DNase I footprinting analyses. We note that one MisR binding site (site “D”) occurred in the same place as the Fur box and −10 promoter element, but on the opposite strand. Recent structural studies have shown that metal-bound Fur protein can complex with dsDNA at recognition sequences either as a dimer on one side of the double-helix, or as two dimers on opposite sides (Deng et al., 2015). If this behavior holds true for neisserial Fur+Fe2+, we hypothesize that MisR might improve the affinity of Fur+Fe2+ for the tbpBA promoter without causing stearic hindrance (Fig. 10), although we have no direct evidence of a MisR-Fur interaction. On the other hand, positioning of a transcriptional regulator between the −10 and −35 promoter elements can compensate for impaired RNA polymerase binding and open complex formation at promoters with non-optimal spacing between these two cis-elements (Lee et al., 2012). We note that the spacing between the −35 and −10 elements of the gonococcal tbpBA promoter is indeed a sub-optimal 16 bp (Velez Acevedo et al., 2014), and as was shown for a mutated mtrR promoter (Hagman & Shafer, 1995), small spacing differences can greatly alter transcription in gonococci. Thus, MisR may help to improve tbpBA transcription during iron-starvation by compensating for the spacing between the −10 and −35 promoter elements (Fig. 10). Additionally, the nearby, undefined promoter driving divergent transcription of the long noncoding RNA is ~220 bp upstream of the tbpBA transcriptional start site (Velez Acevedo et al., 2014), and MisR-protected sites A-C were located between these two promoters (Fig. 10). While we did not detect any impact of MisR on expression of the upstream RNA in the RNA-Seq data, we have not yet explored the possibility that MisR might modulate expression of this RNA species under iron-depleted conditions, which could in turn affect tbpBA expression. Future work is needed to determine if any interplay between Fur, iron, MisR, the long non-coding upstream RNA, and the tbpBA transcript exist.
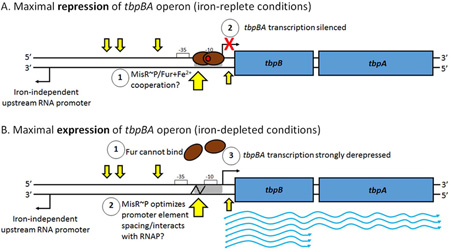
Fig. 10. Iron and MisR~P coordinately regulate expression of the tbpBA operon.
A) Under iron-replete conditions, the ferric uptake regulator, Fur (brown ovals), binds Fe2+ (red circle) and dimerizes, allowing strong binding of Fur+Fe2+ to the Fur box (hatched) at the tbpBA promoter, occluding the −10 element and silencing transcription. We hypothesize that MisR~P might cooperate with Fur+Fe2+ to ensure tbpBA silencing. B) Under iron-depleted conditions, Fur does not bind to iron, cannot dimerize, and releases the tbpBA promoter. MisR~P binding may enhance RNA polymerase binding or transcription initiation either by optimizing spacing between the −35 and −10 promoter elements (indicated by the jagged black line) or by making upstream contacts with the holoenzyme. tbpBA transcription is strongly de-repressed. tbpB transcription is approximately twice that of tbpA at steady state (Ronpirin et al., 2001). Un-phosphorylated MisR cannot bind the tbpBA promoter region. Four MisR~P binding sites are shown as small yellow arrows, and a fifth MisR~P binding site, associated with a DNase I hypersensitivity site, is shown as a large yellow arrow. RNA polymerase holoenzyme and MisR~P are omitted for clarity. The impact of MisR~P binding on the long, noncoding upstream RNA, which is unresponsive to iron and transcribed divergently from tbpBA (Velez Acevedo et al., 2014), is currently unknown.
MisR regulates a large number of genes in both Neisseria species; however, reports of the MisR regulons are not completely consistent with one another (this study; (Newcombe et al., 2005, Tzeng et al., 2008)). This inconsistency may be due to differences between the growth conditions used for culturing prior to microarray and RNA-Seq analysis. Diverse conditions included growth on blood agar plates, growth to mid- vs. late-log phase in liquid GC medium, and differences in MgCl2 concentration ((Newcombe et al., 2005, Tzeng et al., 2008) and the current study). Nevertheless, some findings were consistently reported and were likewise noted in the current N. gonorrhoeae study. For example, expression of bacterioferritin, encoded by bfrA and bfrB, and dsbD encoding a membrane bound thiol:disulfide interchange protein, was clearly activated by MisR in all three studies. With respect to the gonococcal MisR regulon, genes encoding chaperones, heat shock proteins, protein quality control factors, redox factors, and envelope-localized proteins were prominently represented.
Sequence divergence between N. meningitidis and N. gonorrhoeae in the region upstream of tbpB may explain why the tbp genes were not identified as being misR regulated in previous studies of the meningococcal MisR regulon (Tzeng et al., 2008; Newcombe et al., 2005). Other factors that could explain the inconsistent results between the current study and the previous study in N. meningitidis include the fact that our study used a more sensitive RNA-Seq approach to identify the gonococcal MisR regulon, as compared to employing microarray analysis in the previous studies (Tzeng et al., 2008; Newcombe et al., 2005). In addition, cultures were harvested for RNA isolation at different time points in the growth curve in these two studies, perhaps leading to the disparate results. Regardless of the methodologies and potential species and sequence differences, our cumulative results clearly indicate that the tbp genes are part of the gonococcal MisR regulon and that MisR~P binds specifically to the gonococcal tbpBA promoter region.
Analysis of the RNA-Seq data indicates that MisR regulates other metal-homeostasis genes in addition to bfrAB and tbpBA in the gonococcus. For example, MisR repressed the AraC-family regulator, MpeR, which is an activator of the xenosiderophore uptake system encoded by the fet operon (Hollander et al., 2011). Furthermore, MisR activated the laz (azurin) and NGO1215 copper-binding proteins; laz mutants are more sensitive to copper and H2O2 (Wu et al., 2005). While both gonococci and meningococci clearly use MisR to regulate metal-related genes, as we noted, meningococcal tbpBA was not a MisR target, even during iron starvation (Tzeng et al., 2008, Zhao et al., 2010). We propose that this regulatory divergence between gonococci and meningococci is related to the sequence variations between MisR binding sites upstream of tbpBA.
While this is the first report characterizing a functional interplay between iron stress and MisR-MisS in gonococci, others have found that Fur and CpxR-CpxA co-regulate expression of the EfeUOB ferrous iron transport system in E. coli (Cao et al., 2007), and recent work on V. cholerae demonstrates that CpxR-CpxA regulates several iron-responsive genes and helps this pathogen adapt to iron-scarce conditions (Acosta et al., 2015). Additionally, MisR regulation at the gonococcal tbpBA promoter described in this report is reminiscent of MisR regulation of the hemO-hmbR hemoglobin receptor locus in meningococci (Zhao et al., 2010). While gonococci are thought to primarily use the HpuBA hemoglobin receptor (Cornelissen & Hollander, 2011) and seem to encode only pseudogenes for hmbR (Harrison et al., 2013) the striking parallels between these two regulatory schemes suggest that pathogenic Neisseriae may deploy both Fur and MisR to balance their nutritional requirements for iron and their need to limit oxidative damage and the presentation of conserved antigens. In agreement with this hypothesis, misR mutant meningococci were more sensitive to oxidative stress (Tzeng et al., 2008) and generated broad, cross-reactive immunity in an intraperitoneal mouse model of infection (Newcombe et al., 2004). Future work is needed to assess the importance of gonococcal MisR for resistance to oxidative stress and modulation of the host immune response.
The N. gonorrhoeae MisR regulon also includes genes that encode adhesins (e.g., opcA, ompA), an efflux pump, permeases (e.g., macAB, pitB, putP), proteins involved in basic metabolism (e.g., ppk2, GAPDH, glnA, nrdAB), DNA repair enzymes (e.g., exoIII, recN), and cell division factors (e.g., scpA, nlpC, pbp2, maf). The MisR regulons from both Neisseria species also include many genes encoding conserved hypothetical proteins of unknown function, but it is important to note that most of these regulatory targets remain unconfirmed by secondary methods. Additionally, the activating signal for MisR/MisS remains undefined (Kumar et al., 2011). Irrespective of these experimental caveats, we propose that MisR control of gonococcal gene expression is important for the survival of gonococci during infection as it would help this human pathogen to respond to a number of stresses and changing environmental conditions.
In summary, this is the first report to characterize the MisR regulon in N. gonorrhoeae, which differs significantly from that published for N. meningitidis. This is also the first demonstration that the tbp genes are regulated through direct interaction with any other protein regulatory factor besides Fur. We defined the molecular parameters for MisR interaction with the tbp promoter region, demonstrating that MisR~P specifically binds to five regions in the DNA upstream of the tbpB start codon, and quantitatively showed that MisR is required for optimal iron uptake from transferrin. Finally, differences between the Neisseria species in the promoter regions upstream of tbpB may explain why the tbp operon was not previously recognized as a target of MisR-dependent regulation in N. meningitidis. The data presented in this study suggest that N. gonorrhoeae fine-tunes tbpBA expression depending upon both iron status and the activation state of the two-component regulatory system comprised of MisR and MisS. The many inputs for tbpBA regulation may allow the gonococcus to rapidly adapt to in vivo signals and remodel the bacterial cell surface for optimum growth and survival.
Experimental Procedures
Bacterial strains and growth conditions
Neisseria gonorrhoeae strains were maintained on GC medium base (Difco) containing Kellogg’s supplement I (Kellogg et al., 1963) and 12 µM ferric nitrate. Plates and liquid cultures were incubated at 37°C in an atmosphere supplemented with 5% CO2. Liquid cultures of the wild type and mutant strains were grown in GC broth (GCB) in acid-washed glassware. Iron-replete conditions were generated by the addition of ferric nitrate at inoculation and after one mass doubling. Iron-depleted conditions were generated by addition of 10 µM Desferal (deferoxamine) after one doubling of the population of cells. To induce expression of misR in the misRc strain, 1 mM IPTG was added to liquid growth cultures. Addition of IPTG to the wild-type and mutant strains had no effects on growth or assay outcomes. For some experiments, cultures were additionally supplemented with 10 mM MgCl2 as noted in the results.
Construction of misR and misRc strains
The misR gene was inactivated using the non-polar aphA3 kanamycin resistance cassette (Menard et al., 1993). FA19 misR::kan was constructed by transforming FA19 with meningococcal genomic DNA from the misR-deficient N. meningitidis mutant SZT1001 constructed previously (Zhao et al., 2010). Plate transformations were performed as described previously (Gunn & Stein, 1996). In general, completely misR-deficient transformants could be obtained with 3–4 days of incubation on GC agar containing kanamycin at 50 µg/mL, and we often found that kanamycin resistant transformants arising 1–2 days after plating on selection were merodiploid (data not shown). The FA19 misR::kan mutant was complemented with a wild-type (WT) copy of misR cloned into pGCC4 using primers misR3PacI (5’-CAAACAATTAATTAACGCCACGCCCG-3’) and misR4PmeI (5’-GTTGGAACAGTTTAAACTGCCCG-3’) and methods described previously (Lewis et al., 2009). Loss of misR was confirmed by PCR across the misRS operon using primers misR1 (5’-CCATGAGCCGCGTATTACTC-3’) and MisSR (5’-GCGGACGGTATGTGCGTGA-3’). Complementation in pGCC4 was confirmed by PCR using primers lctP (5’-GCGCGATCGGTGCGTTCT-3’) and aspC1 (5’-GCCGGATGCGTCTTTGTAC-3’). Mutant and complement strains were further verified by Western blot (anti-MisR antisera kindly provided by Yih-Ling Tzeng, Emory University), which demonstrated the complete loss of MisR protein in true misR::kan mutants and recovery of MisR protein production in the complemented strain upon induction with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG).
RNA-Seq experiments
RNA-Seq fold change (≥2-fold was the reporting cutoff) was calculated by averaging the normalized RPKM (reads-per-kilobase-per-million-reads) values from three independent experiments. RNA-Seq raw data (and methods) for the results shown herein have been published (Velez Acevedo et al., 2014) (GEO accession number GSE50184; SRA accession number SRP029218). All average fold changes satisfied a statistical significance cutoff value of <0.05 as determined using the Bonferroni correction.
Assessment of mutant growth on solid medium containing transferrin, lactoferrin and ferric nitrate
Wild-type and mutant strains were grown on GC medium or chemically defined, chelexed medium (CDM) (West & Sparling, 1987) supplemented with human transferrin (2.5 µM, 20% saturated with iron), human lactoferrin (2.5 µM, 30% saturated with iron) or ferric nitrate (12 µM). Plates were incubated for 48 hours at 37°C in an atmosphere supplemented with 5% CO2 before imaging (Alpha Innotech Imager).
Iron internalization assays
Transferrin-iron internalization assays were performed as described previously (DeRocco & Cornelissen, 2007). Human transferrin was saturated to 20% with 55Fe (Perkin-Elmer). Gonococcal strains, grown overnight on GC medium plates containing 10 µM Desferal, were standardized to the same optical density and then mixed with 3 µM 55Fe labeled transferrin in a final volume of 150 µl of CDM plus BSA (bovine serum albumin) as a nonspecific protein blocker. Duplicate samples contained 215 µM KCN to metabolically poison N. gonorrhoeae cells, allowing for the assessment of the amount of 55Fe bound to but not internalized into cells. Following a 30 min incubation period, Millipore multiscreen microtiter dishes containing the labeled gonococcal cells were filtered, washed and the nitrocellulose filters at the bottom of the dishes were allowed to air dry. The filters were removed from each plate and radioactive iron associated with or internalized by the gonococcal cells was detected using a Beckman LS6500 scintillation counter. Triplicate counts were averaged and surface-associated counts were subtracted from total counts to determine the counts internalized during the 30 min experiment. The data are presented as values normalized to the positive control (wild-type FA19).
Western blotting to detect TbpA and TbpB (including ImageJ analysis)
N. gonorrhoeae strains were grown under iron-depleted and iron-replete conditions (as described above) in GCB. Strains were grown for 4 hours after one doubling, standardized to cell density by Klett reading, and pelleted by centrifugation. Standardized cell pellets were resuspended in Laemmli solubilizing buffer (Laemmli, 1970) followed by protein assay by BCA. Standardized amounts of protein from each strain were supplemented with 5% beta-mercaptoethanol and then loaded into lanes of an SDS polyacrylamide gel (Laemmli, 1970). Separated proteins were transferred to nitrocellulose membrane in a submerged transfer apparatus (BioRad) by the method of Towbin (Towbin et al., 1979). Nitrocellulose membranes were stained with Ponceau S to ensure that each lane was loaded equivalently with whole-cell lysate proteins from each strain. To detect TbpA, nitrocellulose blots were blocked with 5% BSA in high salt tris-buffered saline (TBS). Subsequently a polyclonal anti-TbpA antibody (Cornelissen et al., 1992) was used to probe the blots, followed by washing with high-salt TBS, addition of the secondary goat-anti-rabbit alkaline phosphatase conjugate, additional washing, and finally development with the NBT/BCIP system (Sigma). To detect TbpB, nitrocellulose filters were blocked with 5% non-fat skim milk in low salt TBS, followed by the addition of a polyclonal anti-TbpB serum (Thomas et al., 2006), washing and then addition of secondary antibody and detection system as described above for TbpA. Blots were imaged using ImageJ software (Schneider et al., 2012).
qRT-PCR
N. gonorrhoeae was grown in GCB under iron-depleted and iron-replete conditions as described above. Four hours after addition of the iron chelator (Desferal), supplemental iron or IPTG, cells were mixed with 10 ml of RNAprotect (Qiagen), vortexed for 5 sec and incubated at RT for 5 min. The cell suspension was centrifuged, the supernatant decanted and the pellets stored at −20°C until further processing. Total RNA was isolated using the RNAeasy Mini Kit (Qiagen) according to manufacturer’s instructions. Purified RNA was treated with RNAse-free DNAse twice, followed by addition of Superase-In (Ambion) and finally storage at −80°C. Reverse transcription of RNA into cDNA was accomplished using the Accuscript high-fidelity 1st strand cDNA synthesis kit (Agilent Technologies) and random hexamers as primers. cDNA was amplified with gene specific primers and the SensiMix SYBR no-ROX kit (Bioline) employing the CFX96 real-time system (Bio-Rad). Expression of target genes (tbpA and tbpB) was normalized to porB1A expression as an internal control. Each assay was performed at least in triplicate. Relative expression values for each gene were calculated using the 2−ΔΔCt method (Livak & Schmittgen, 2001).
Purification and phosphorylation of MisR-His6x protein
His-tagged MisR protein was purified using methods adapted from (Tzeng et al., 2006). Two 5 mL cultures of E. coli BL21 (DE3) cells harboring the pYT298 plasmid were grown overnight in LB broth with kanamycin selection (50 µg/mL) in a 37°C water bath, shaking at 200 rpm. These cultures were used as an inoculum for a 1 L culture grown under the same conditions as above. At mid-log (OD600 of ~0.35) the culture was induced with 1 mM IPTG. IPTG-induced expression of MisR-His6x was allowed to continue for approximately 3 hours. These stationary phase cells were harvested by centrifugation at 7,500 rpm for 10 mi at 4°C, then pellets were stored at −70°C. Frozen pellets were thawed on ice and resuspended in a total of 30 mL of lysis buffer + 1 mM PMSF (Tzeng et al., 2006). BL21 (DE3) lysates were obtained using 3 rounds of disruption by a French Press Cell Disruptor (Thermo Electron Corporation) at 600 psi with 10 min cooling intervals on ice. Cell debris was removed by centrifugation at 10,000 rpm for 15 min at 4°C. Lysate supernatant was passed through 0.2 µm sterile filters to remove any remaining intact cells. 3 mL of packed Ni-NTA agarose (Qiagen) were equilibrated on a 20 mL Econo-Pac® chromatography column (Biorad) using two 10 mL washes with lysis buffer. MisR-His6x filtrate was added to the equilibrated Ni-NTA agarose and rotated on column overnight at 4°C. Washes, elution, and validation by SDS-PAGE/Coomassie stain were performed as described previously (Tzeng et al., 2006). The MisR-containing fractions were pooled, loaded into prequilibrated dialysis tubing (Spectra/Por Dialysis Membrane, MWCO:12–14 kDa, cat. # 132 676) and dialyzed in dialysis buffer + 1 mM dithiothreitol (DTT) for 2 hours with 2 buffer changes at 4°C. Dialyzed protein MisR-His6x (molecular weight ~25 kDa) was concentrated to ~0.9 µg/µL at 4°C using an Amicon Ultra-4 filter and stored in 10% (v/v) glycerol at −70°C. Purified MisR-His6x protein was phosphorylated by incubation with 50 mM acetyl phosphate for 30 min at 37°C.
Electrophoretic mobility shift assay
Primers tbpBA_1090F (5’-CTTGTGTTTTAGAAGACTCAGGG-3’) and tbpBA_1090R (5’-CACAGGCAACACCATAGCAGC-3’) and chromosomal DNA were used to amplify a 297 bp product corresponding to the promoter region (−221 to +75) of the tbpBA operon from N. gonorrhoeae strain FA1090, which was subsequently purified by QIAquick Gel Extraction Kit (Qiagen). Nonspecific competitor rnpB DNA was similarly prepared using primers RnpB1F (5’-CGGGACGGGCAGACAGTCGC-3’) and RnpB1R (5’-GGACAGGCGGTAAGCCGGGTTC-3’). Electrophoretic mobility shift assays (EMSA) were performed as described previously (Tzeng et al., 2006).
DNase I footprint analysis
DNase I footprinting assays were performed as described previously (Tzeng et al., 2006). Briefly, the location of MisR-His6x binding sites was assessed by incubating dsDNA (labeled on either the sense or antisense strand with γ-32P–ATP) with MisR-His6x protein as described above for EMSAs. Note that the binding buffer for DNaseI footprinting does not contain salmon sperm DNA. Bound DNA was digested with 0.0625 ng/µL DNaseI for 50 seconds, then stopped using a phenol:chloroform:isoamyl alcohol (25:24:1) and ethanol extraction. Pelleted, digested, labeled DNA was resuspended in TE (pH 8.0) and loading dye, then normalized by counts per min on a scintillation counter. Sanger sequencing reactions (Epicentre, Madison, WI) were run alongside the sense and antisense footprinting reactions for all four bases. All samples were heated to 95°C for 2 min then iced prior to loading on an 8% denaturing polyacrylamide gel, which was run and imaged using autoradiography as described previously (Johnson et al., 2011).
Sequence alignment
The extended promoter regions upstream of the tbpB genes from N. gonorrhoeae and N. meningitidis strains were aligned using T-Coffee (http://tcoffee.crg.cat/apps/tcoffee/all.html. Sequence data was aligned from the following sources: N. gonorrhoeae FA 1090 (http://www.ncbi.nlm.nih.gov/nuccore/AE004969); N. gonorrhoeae FA19 (http://www.ncbi.nlm.nih.gov/nuccore/KJ579425); N. gonorrhoeae F62 (http://www.ncbi.nlm.nih.gov/nuccore/KJ579423); N. gonorrhoeae MS11 (http://www.ncbi.nlm.nih.gov/nuccore/KJ579424); N. meningitidis MC58 (http://www.ncbi.nlm.nih.gov/nuccore/AE002098); N. meningitidis L91543 (http://www.ncbi.nlm.nih.gov/nuccore/JTJE01000016); N. meningitidis H44/76 (http://www.ncbi.nlm.nih.gov/nuccore/CP002420); N. meningitidis 053442 (http://www.ncbi.nlm.nih.gov/nuccore/CP000381).
Supplementary Material
Acknowledgments
Funding for this work was provided to C.N.C. by Public Health Service grants R01 AI047141, R01 AI065555, R01 AI084400, and U19 AI31496 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. R.V.A. was supported by a diversity supplement to R01 AI047141. D.R.C. was supported by fellowship grant F30 AI112199 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Additional support was provided to W.M.S. by Public Health Service grants U19 AI 113170, R37 AI21150, and U19 AI31496 all from the National Institute of Allergy and Infectious Diseases, National Institutes of Health and a VA Merit Award (510 1BX000112-07) from the Biomedical Laboratory Research and Development Service of the Department of Veterans Affairs. W.M.S. was also supported by a Senior Research Career Award from the Biomedical Laboratory Research and Development Service of the Department of Veterans Affairs. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We thank Abena Watson-Siriboe and Virginia Stringer for excellent technical assistance. We also acknowledge the participation of two summer interns, Tiffany Wang and Kathryn Hebert, in the execution of the studies described in this report.
REFERENCES
- Acosta N, Pukatzki S, Raivio TL. The Vibrio cholerae Cpx envelope stress response senses and mediates adaptation to low iron. J. Bacteriol. 2015;197:262–276. doi: 10.1128/JB.01957-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aledort JE, Hook EW, 3rd, Weinstein MC, Goldie SJ. The cost effectiveness of gonorrhea screening in urban emergency departments. Sex. Transm. Dis. 2005;32:425–436. doi: 10.1097/01.olq.0000154501.22566.fa. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Sparling PF, Cornelissen CN. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J. Bacteriol. 1994;176:3162–3170. doi: 10.1128/jb.176.11.3162-3170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagg A, Neilands JB. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli . Biochemistry. 1987;26:5471–5477. doi: 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- Barbee LA. Preparing for an era of untreatable gonorrhea. Curr. Opin. Infect. Dis. 2014;27:282–287. doi: 10.1097/QCO.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Woodhall MR, Alvarez J, Cartron ML, Andrews SC. EfeUOB (YcdNOB) is a tripartite, acid-induced and CpxAR-regulated, low-pH Fe2+ transporter that is cryptic in Escherichia coli K-12 but functional in E. coli O157:H7. Mol. Microbiol. 2007;65:857–875. doi: 10.1111/j.1365-2958.2007.05802.x. [DOI] [PubMed] [Google Scholar]
- Cash DR, Noinaj N, Buchanan SK, Cornelissen CN. Beyond the crystal structure: Insight into the function and vaccine potential of TbpA expressed by Neisseria gonorrhoeae . Infect. Immun. 2015;83:4438–4449. doi: 10.1128/IAI.00762-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. Morb. Mortal. Wkly. Rep. 2007;56:332–336. [PubMed] [Google Scholar]
- CDC. Cephalosporin Susceptibility Among Neisseria gonorrhoeae Isolates --- United States, 2000--2010. Morb. Mortal. Wkly. Rep. 2011;60:873–877. [PubMed] [Google Scholar]
- CDC. Update to CDC’s Sexually Transmitted Diseased Treatment Guidelines, 2010: Oral Cephalosporins No longer a recommended treatment for gonococcal infections. Morb. Mortal. Wkly. Rep. 2012;61:590–954. [PubMed] [Google Scholar]
- CDC. 2015 Sexually transmitted diseases treatment guidelines. Morb. Mortal. Wkly. Rep. 2015;64:1–137. [Google Scholar]
- Cornelissen CN. Identification and characterization of gonococcal iron transport systems as potential vaccine antigens. Future Microbiol. 2008;3:287–298. doi: 10.2217/17460913.3.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen CN, Biswas GD, Tsai J, Paruchuri DK, Thompson SA, Sparling PF. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J. Bacteriol. 1992;174:5788–5797. doi: 10.1128/jb.174.18.5788-5797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen CN, Hollander A. TonB-dependent transporters expressed by N. gonorrhoeae . Front. Microbio. 2011;2:1–13. doi: 10.3389/fmicb.2011.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen CN, Kelley M, Hobbs MM, Anderson JE, Cannon JG, Cohen MS, Sparling PF. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol. Microbiol. 1998;27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- Cornelissen CN, Sparling PF. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol. Microbiol. 1994;14:843–850. doi: 10.1111/j.1365-2958.1994.tb01320.x. [DOI] [PubMed] [Google Scholar]
- Cornelissen CN, Sparling PF. Binding and surface exposure characteristics of the gonococcal transferrin receptor are dependent on both transferrin-binding proteins. J. Bacteriol. 1996;178:1437–1444. doi: 10.1128/jb.178.5.1437-1444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Wang Q, Liu Z, Zhang M, Machado AC, Chiu TP, Feng C, Zhang Q, Yu L, Qi L, Zheng J, Wang X, Huo X, Qi X, Li X, Wu W, Rohs R, Li Y, Chen Z. Mechanistic insights into metal ion activation and operator recognition by the ferric uptake regulator. Nat Commun. 2015;6:7642. doi: 10.1038/ncomms8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRocco AJ, Cornelissen CN. Identification of transferrin-binding domains in TbpB expressed by Neisseria gonorrhoeae . Infect. Immun. 2007;75:3220–3232. doi: 10.1128/IAI.00072-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRocco AJ, Yost-Daljev MK, Kenney CD, Cornelissen CN. Kinetic analysis of ligand interaction with the gonococcal transferrin-iron acquisition system. Biometals. 2008;22:439–451. doi: 10.1007/s10534-008-9179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn JS, Stein DC. Use of a non-selective transformation technique to construct a multiply restriction/modification-deficient mutant of Neisseria gonorhoeae . Mol. Gen. Genet. 1996;251:509–517. doi: 10.1007/BF02173639. [DOI] [PubMed] [Google Scholar]
- Hagman KE, Shafer WM. Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae . J. Bacteriol. 1995;177:4162–4165. doi: 10.1128/jb.177.14.4162-4165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison OB, Bennett JS, Derrick JP, Maiden MC, Bayliss CD. Distribution and diversity of the haemoglobin-haptoglobin iron-acquisition systems in pathogenic and non-pathogenic Neisseria . Microbiol. 2013;159:1920–1930. doi: 10.1099/mic.0.068874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges SR, Mayo MS, Mestecky J, Hook EW, Russell MW. Limited local and systemic antibody responses to Neisseria gonorrheae during uncomplicated genital infections. Infect. Immun. 1999;67:3937–3946. doi: 10.1128/iai.67.8.3937-3946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges SR, Sibley DA, Mayo MS, Hook EW, Russell MW. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J. Infect. Dis. 1998;178:742–751. doi: 10.1086/515372. [DOI] [PubMed] [Google Scholar]
- Hollander A, Mercante AD, Shafer WM, Cornelissen CN. The iron-repressed, AraC-like regulator MpeR activates expression of fetA in Neisseria gonorrhoeae . Infect. Immun. 2011;79:4764–4776. doi: 10.1128/IAI.05806-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook E, van Der Pol B. Evolving gonococcal antimicrobial resistance: research priorities and implications for management. Sex. Transm. Infect. 2013;89:v60–iv62. doi: 10.1136/sextrans-2013-051021. [DOI] [PubMed] [Google Scholar]
- Jamet A, Rousseau C, Monfort JB, Frapy E, Nassif X, Martin P. A two-component system is required for colonization of host cells by meningococcus. Microbiol. 2009;155:2288–2295. doi: 10.1099/mic.0.027755-0. [DOI] [PubMed] [Google Scholar]
- Johnson CR, Newcombe J, Thorne S, Borde HA, Eales-Reynolds LJ, Gorringe AR, Funnell SG, McFadden JJ. Generation and characterization of a PhoP homologue mutant of Neisseria meningitidis . Mol. Microbiol. 2001;39:1345–1355. doi: 10.1111/j.1365-2958.2001.02324.x. [DOI] [PubMed] [Google Scholar]
- Johnson PJ, Stringer VA, Shafer WM. Off-target gene regulation mediated by transcriptional repressors of antimicrobial efflux pump genes in Neisseria gonorrhoeae . Antimicrob. Agents Chemother. 2011;55:2559–2565. doi: 10.1128/AAC.00010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DS, Jr, Peacock WL, Jr, Deacon WE, Brown L, Pirkle CI. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Sannigrahi S, Scoullar J, Kahler CM, Tzeng YL. Characterization of DsbD in Neisseria meningitidis . Mol. Microbiol. 2011;79:1557–1573. doi: 10.1111/j.1365-2958.2011.07546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Minchin SD, Busby SJ. Activating transcription in bacteria. Annu. Rev. Microbiol. 2012;66:125–152. doi: 10.1146/annurev-micro-092611-150012. [DOI] [PubMed] [Google Scholar]
- Lewis LA, Choudhury B, Balthazar JT, Martin LE, Ram S, Rice PA, Stephens DS, Carlson R, Shafer WM. Phosphoethanolamine substitution of lipid A and resistance of Neisseria gonorrhoeae to cationic antimicrobial peptides and complement-mediated killing by normal human serum. Infect. Immun. 2009;77:1112–1120. doi: 10.1128/IAI.01280-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods (San Diego, Calif. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Menard R, Sansonetti P, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe J, Eales-Reynolds LJ, Wootton L, Gorringe AR, Funnell SG, Taylor SC, McFadden JJ. Infection with an avirulent phoP mutant of Neisseria meningitidis confers broad cross-reactive immunity. Infect. Immun. 2004;72:338–344. doi: 10.1128/IAI.72.1.338-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe J, Jeynes JC, Mendoza E, Hinds J, Marsden GL, Stabler RA, Marti M, McFadden JJ. Phenotypic and transcriptional characterization of the meningococcal PhoPQ system, a magnesium-sensing two-component regulatory system that controls genes involved in remodeling the meningococcal cell surface. J. Bacteriol. 2005;187:4967–4975. doi: 10.1128/JB.187.14.4967-4975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, Temmerman M. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS ONE. 2015;10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noinaj N, Buchanan SK, Cornelissen CN. The transferrin-iron import system from pathogenic Neisseria species. Mol. Microbiol. 2012a;86:246–257. doi: 10.1111/mmi.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noinaj N, Easley NC, Oke M, Mizuno N, Gumbart J, Boura E, Steere AN, Zak O, Aisen P, Tajkhorshid E, Evans RW, Gorringe AR, Mason AB, Steven AC, Buchanan SK. Structural basis for iron piracy by pathogenic Neisseria . Nature. 2012b;483:53–58. doi: 10.1038/nature10823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavonen J, Westrom L, Eschenback D. Pelvic Inflammatory Disease. In: Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, Cohen MS, Watts DH, editors. Sexually Transmitted Diseases. 4th. New York, NY: McGraw Hill; 2008. pp. 1017–1050. [Google Scholar]
- Qi M, Sun FJ, Caetano-Anolles G, Zhao Y. Comparative genomic and phylogenetic analyses reveal the evolution of the core two-component signal transduction systems in enterobacteria. J Mol Evol. 2010;70:167–180. doi: 10.1007/s00239-009-9318-2. [DOI] [PubMed] [Google Scholar]
- Robinson EN, Clemens CM, Schoolnik GK, McGee ZA. Probing the surface of Neisseria gonorrhoeae: immunoelectron microscopic studies to localize cyanogen bromide fragment 2 in gonococcal pili. Mol. MIcrobiol. 1989;3:57–64. doi: 10.1111/j.1365-2958.1989.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Ronpirin C, Jerse AE, Cornelissen CN. The gonococcal genes encoding transferrin binding proteins (Tbp) A and B are arranged in a bicistronic operon but are subject to differential expression. Infect. Immun. 2001;69:6336–6347. doi: 10.1128/IAI.69.10.6336-6347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soge OO, Harger D, Schafer S, Toevs K, Raisler KA, Venator K, Holmes KK, Kirkcaldy RD. Emergence of increased azithromycin resistance during unsuccessful treatment of Neisseria gonorrhoeae infection with azithromycin (Portland, OR, 2011) Sex. Transm. Dis. 2012;39:877–879. doi: 10.1097/OLQ.0b013e3182685d2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CE, Zhu W, Dam CNV, Davis NL, Johnson RE, Sparling PF. Vaccination of mice with gonococcal TbpB expressed in vivo from Venezuelan Equine Encephalitis viral replicon particles. Infect. Immun. 2006;74:1612–1620. doi: 10.1128/IAI.74.3.1612-1620.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc. Nat. Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng YL, Datta A, Ambrose K, Lo M, Davies JK, Carlson RW, Stephens DS, Kahler CM. The MisR/MisS two-component regulatory system influences inner core structure and immunotype of lipooligosaccharide in Neisseria meningitidis . J. Biol. Chem. 2004;279:35053–35062. doi: 10.1074/jbc.M401433200. [DOI] [PubMed] [Google Scholar]
- Tzeng YL, Kahler CM, Zhang X, Stephens DS. MisR/MisS two-component regulon in Neisseria meningitidis . Infect. Immun. 2008;76:704–716. doi: 10.1128/IAI.01007-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng YL, Zhou X, Bao S, Zhao S, Noble C, Stephens DS. Autoregulation of the MisR/MisS two-component signal transduction system in Neisseria meningitidis . J. Bacteriol. 2006;188:5055–5065. doi: 10.1128/JB.00264-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unemo M, Nicholas RA. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol. 2012;7:1401–1422. doi: 10.2217/fmb.12.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez Acevedo RV, Ronpirin C, Kandler J, Shafer WM, Cornelissen CN. Identification of regulatory elements that control expression of the tbpBA operon in Neisseria gonorrhoeae . J. Bacteriol. 2014;196 doi: 10.1128/JB.01693-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West SEH, Sparling PF. Aerobactin utilization by Neisseria gonorrhoeae and cloning of a genomic DNA fragment that complements Escherichia coli fhuB mutations. J. Bacteriol. 1987;169:3414–3421. doi: 10.1128/jb.169.8.3414-3421.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex. Transm. Dis. 1992;19:185–192. [PubMed] [Google Scholar]
- Wu HJ, Seib KL, Edwards JL, Apicella MA, McEwan AG, Jennings MP. Azurin of pathogenic Neisseria spp. is involved in defense against hydrogen peroxide and survival within cervical epithelial cells. Infection and immunity. 2005;73:8444–8448. doi: 10.1128/IAI.73.12.8444-8448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Montanez GE, Kumar P, Sannigrahi S, Tzeng YL. Regulatory role of the MisR/S two-component system in hemoglobin utilization in Neisseria meningitidis . Infect. Immun. 2010;78:1109–1122. doi: 10.1128/IAI.00363-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.