Summary
Calcium (Ca2+) flux into the matrix is tightly controlled by the mitochondrial Ca2+ uniporter (MCU) due to vital roles in cell death and bioenergetics. However, the precise atomic mechanisms of MCU regulation remain unclear. Here, we solved the crystal structure of the N-terminal matrix domain of human MCU, revealing a β-grasp-like fold with a cluster of negatively charged residues that interacts with divalent cations. Binding of Ca2+ or Mg2+ destabilizes and shifts the self-association equilibrium of the domain toward monomer. Mutational disruption of the acidic face weakens oligomerization of the isolated matrix domain and full-length human protein similar to cation binding and markedly decreases MCU activity. Moreover, mitochondrial Mg2+ loading or blockade of mitochondrial Ca2+ extrusion suppresses MCU Ca2+ uptake rates. Collectively, our data reveal that the β-grasp-like matrix region harbors an MCU regulating acidic patch that inhibits human MCU activity in response to Mg2+ and Ca2+ binding.
Keywords: mitochondrial calcium uniporter, calcium binding, magnesium binding, structure, autoinhibition, oligomerization, stability, MCU regulating acidic patch
Graphical abstract
Mitochondria are organelles that synthesize adenosine triphosphate (ATP), establishing a large electrical gradient across the inner mitochondrial membrane (IMM) (i.e. ΔΨm~ −180 mV) (Marchi and Pinton, 2014) that is a major driving force for cation uptake (Carafoli, 2003). However, calcium (Ca2+) uptake must be precisely controlled by the mitochondrial Ca2+ uniporter (MCU) due to roles in vital signaling processes such as bioenergetics, cell death and shaping cytosolic Ca2+ transients. Under resting cytosolic Ca2+ levels, MCU is inactive; upon agonist stimulation of plasma membrane (PM) receptors that increase cytosolic Ca2+ levels, rapid and transient increases in matrix Ca2+ levels occur via MCU.
MCU (CCDC109A) was originally characterized as a Ca2+ selective ion channel which resides on the IMM (Kirichok et al., 2004) and an ion channel after self-association and arrangement of multiple TM domains into an ion pore (Baughman et al., 2011; De Stefani et al., 2011). Numerous binding partners that regulate MCU activity have been identified, suggesting that MCU functions as a heteromeric complex (Kamer and Mootha, 2015). Mitochondrial calcium uptake (MICU)1 and MICU2 are EF-hand containing proteins that serve as Ca2+-dependent gatekeepers of MCU activity (Csordas et al., 2013; Hoffman et al., 2013; Mallilankaraman et al., 2012b; Plovanich et al., 2013). Essential MCU regulator (EMRE) is a single IMM spanning protein of ~10 kDa; knockdown of EMRE in live cells attenuates mitochondrial Ca2+ uptake which cannot be rescued by MCU over-expression (Sancak et al., 2013). MCUb shares ~50% sequence similarity with MCU, but has no ability to constitute a Ca2+-permeable channel despite the presence of TM domains; rather, MCUb functions in a dominant-negative manner, inhibiting MCU activity (Raffaello et al., 2013). MCUR1 is a 40 kDa protein containing two putative TM domains; moreover, MCUR1 interacts with MCU, and over-expression greatly enhances while knockdown of MCUR1 decreases matrix Ca2+ levels in HeLa cells (Mallilankaraman et al., 2012a). Recently, we have revealed that MCUR1 is a vital scaffold factor that stabilizes the MCU complex (Tomar et al., 2016). SLC25A23, a magnesium (Mg2+)-ATP and PO43− transporter protein found on the IMM also interacts with MCU and MICU1, enhancing mitochondrial Ca2+ uptake (Hoffman et al., 2014).
Despite the realization that MCU functions as an oligomer in complex with a suite of proteins, an understanding of the molecular regulatory mechanisms remains outstanding. Many Ca2+ channel proteins exhibit Ca2+-dependent feedback mechanisms including inositol 1,4,5-trisphosphate receptors (Bezprozvanny et al., 1991), ryanodine receptors (Meissner et al., 1986) and Ca2+ release activated Ca2+ channels (Hoth and Penner, 1993). Further, while a crystal structure of the N-terminal domain of MCU has been elucidated (Lee et al., 2015), it is unclear how divalent cations regulate MCU. Here, we report the atomic resolution structure of a conserved MCU matrix domain, which adopts a β-grasp-like fold containing an MCU regulating acidic patch (MRAP) that binds Mg2+ and Ca2+. Interaction of these divalent cations with MRAP destabilizes the protein and shifts the self-association equilibrium toward monomer. Disruption of MRAP by variation of a single Asp destabilizes the domain coupled with monomerization similar to Ca2+ or Mg2+ binding. Mutational disruption of MRAP in full-length human MCU perturbs oligomerization, markedly decreases agonist-induced mitochondrial Ca2+ uptake and attenuates basal mitochondrial Ca2+ levels. Importantly, we show that sustained elevation of matrix Ca2+ or bathing with extramitochondrial Mg2+ suppresses mitochondrial Ca2+ uptake. Together, our data establish that MRAP within the β-grasp-like matrix domain acts as an important regulatory component of human MCU that is Ca2+ and Mg2+ dependent.
Results
MCU contains a conserved β-grasp-like matrix domain
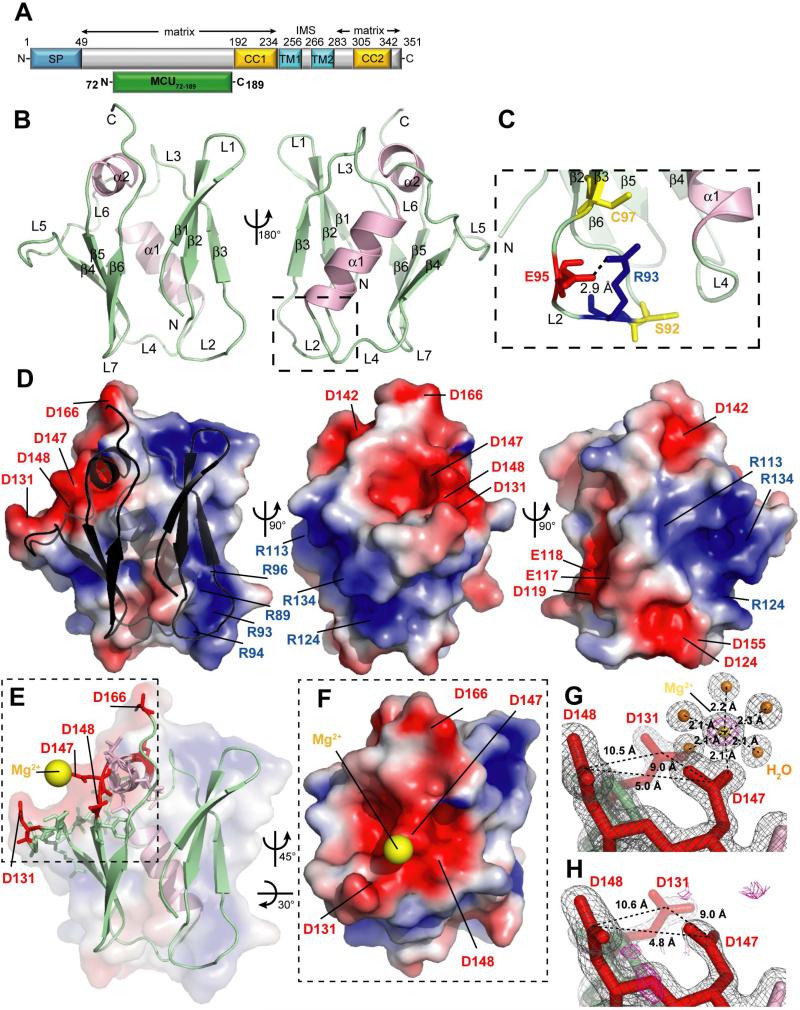
Human MCU is a ~40 kDa protein made up of 351 amino acids (NCBI, Q8NE86) (Fig. 1A). The mitochondrial signal peptide is localized in the first 49 N-terminal residues. MCU contains two predicted TM domains (TM1, residues 234-256; TM2, residues 266-283) close to the C-terminal region. Two putative coiled-coil (CC) domains can be identified prior to TM1 and after TM2, adjacent to the C-terminus. Both the N- and C-termini, which represents the bulk of the soluble regions and the overall majority of the protein, are localized within the matrix (Kamer and Mootha, 2015). Multiple sequence alignment of MCU proteins show a matrix-oriented region outside the CC and TM domains conserved among roundworm, fruit fly, fish, human and mouse sequences between residues 75 and 191 (Fig. S1). Thus, we engineered an MCU construct encompassing residues 72 to 189 (i.e. MCU72-189). Far-UV circular dichroism (CD) spectroscopy showed that MCU72-189 adopts an α-helix/β-strand mixture (Fig. S2A) and cooperatively unfolds with a high midpoint of temperature denaturation (Tm) > 60 °C with negligible ionic strength dependence (Fig. S2B), indicating a stably folded domain.
Fig. 1. Domain architecture and structural features of MCU.
(See also Fig. S1, Fig. S3, Fig. S7 and Table S1).
(A) Domain architecture of human MCU (NCBI, Q8NE86). Residue ranges and the prevailing topology are indicated at top. The relative location of the signal peptide (SP, blue), transmembrane domains (TM1, TM2, cyan), CC domains (CC1, CC2, yellow) and MCU72-189 (green) are indicated.
(B) Backbone structure of MCU72-189. The β-strands (β1-β6, arrows) and loop regions (L1-L6) are colored green while the α-helices (α1, α2) are pink. L2 is bounded by a dashed box.
(C) Zoomed view of L2. The R93 and E95 side chains are shown as red and blue sticks, respectively. The C97 and S92 residues are shown as yellow sticks.
(D) Electrostatic surface potential of MCU72-189. The gradient shown is from −3 (acidic, red) to +3 (basic, blue) kT/e. Locations of acidic and basic residues are indicated.
(E) Location of the Mg2+ atom (yellow sphere) relative to the backbone structure and acidic residues (red sticks). The dashed box bounds the acidic patch region.
(F) Zoomed view of the acidic patch shown in (E) and centrally located Mg2+ atom (yellow sphere).
(G) Mg2+ atom coordination geometry. The 2Fo-Fc (2.0 σ) electron density map (grey mesh), protein chain (sticks), water molecules (orange spheres) and Mg2+ atom (yellow sphere) are shown. The Fo-Fc (9.0 σ) omit map (magenta mesh) shows unaccounted for density in the absence of the Mg2+ atom. Distances between the Asp (red sticks) Cγ atoms are indicated. The coordination geometry of Mg2+ is shown with distances to oxygen atom indicated.
(H) Electron density map of MCU72-189 solved in the presence of LiSO4. The 2Fo-Fc (2.0 σ) map (grey mesh) of the same region shown in (G) does not exhibit evidence for a cation, and the Fo-Fc (2.5 σ) map (magenta mesh) shows no unaccounted for density.
We crystallized an I141M/L146M MCU72-189 double mutant to permit phasing by selenomethionine (Se-Met) incorporation. The human native MCU72-189 protein was subsequently crystalized and solved by molecular replacement using the Se-Met structure. Native MCU72-189 structures were crystalized under three different conditions (Table S1). The native structure (1.6 Å) revealed two central α-helices approximately perpendicular to one another, sandwiched between two 3-stranded β-sheets (Fig. 1B). The first β-sheet is formed with β1 (i.e. residues 76-80), β2 (i.e. residues 83-88) and β3 (i.e. residues 97-100) arranged in an antiparallel manner. One short loop (i.e. L1; residues 81-82) and one long loop (i.e. L2; residues 89-96) link the β-strands. The L2 conformation is stabilized by a salt bridge between the NH1 atom of R93 and the Oε1 atom of E95 (Fig. 1C). This salt bridge is in close proximity to a single Cys (i.e. C97) as well as a known phosphorylation site (i.e. S92) and may serve to stabilize the loop for post-translational modifications of these residues (Joiner et al., 2012; Lee et al., 2015). A centrally located α1 helix (i.e. residues 108-118) is connected to the N-terminal β-sheet through L3 (i.e. residues 101-107). The second β-sheet is located C-terminal to the central α1 and is comprised of β4 (i.e. residues 125-128), β5 (i.e. residues 149-153) and β6 (i.e. residues 156-160) strands arranged in an antiparallel orientation. The β4 and β5 strands are separated by a short α-helix (i.e. α2; residues 141-146) that is perpendicular to α1 and also centrally positioned relative to the two β-sheets. The α2 is linked to the β4 and β5 strands through one long 12-residue loop (i.e. L5; residues 129-140) and one short 2-residue loop (i.e. L6; residues 147-148). The C-terminal region of the MCU72-189 construct (i.e. residues 168-189) does not show any appreciable electron density, suggesting the C-terminal tail is dynamic under these conditions.
Overall, the tertiary structure buries 33 residues that are at least 85% solvent inaccessible. The majority of these side chains are directed toward the inner core of the structure; however, V85 and V152, which are centrally located on each of the β-sheets, respectively, are positioned away from the inner core (Fig. S3A) and may play roles in mediating protein-protein interactions and/or stabilizing the β-sheets at a more local level. The electrostatic surface of the protein reveals a prominent negative patch near the C-terminal face with D131, D142, D147, D148 and D166 contributing the bulk of the acidic charge in this region (Fig. 1D). A second negative patch closer to the N-terminal face of the protein is primarily made up of E117, E118, D119, D124 and D155. Two basic patches on the surface of the protein are made up of R89, R93, R94 and R96 as well as R113, R124 and R134, respectively (Fig. 1D). The large degree of surface charge polarity exhibited by MCU72-189 may facilitate a complementarity for higher order assembly of the domain.
A search for similar folds showed that the closest known structural homologue is ubiquitin-like protein 5 (ULP5) which adopts a β-grasp fold. Sequence identity between the crystallized region of ULP5 and MCU72-189 is ~5%, yet the backbone root mean square deviation (RMSD) between the two proteins is 3.0 Å (Fig. S3B). While MCU72-189 conforms to the core β-grasp structure consisting of a central α-helix that packs diagonally against a β-sheet with parallel and central N- and C-terminal β-strands (Burroughs et al., 2012), it also contains distinct divergences. First, MCU72-189 contains a discontinuous sheet split into two halves due to lack of hydrogen (h)-bonding between the central β1 and β6 strands; second, a third β-strand is inserted in the N-terminal sheet (i.e. β3) prior to the central helix changing the orientation of α1 by ~90°; third, a short α-helix is inserted between β4 and β5, capping one end of the structure. This observed fold MCU72-189 is not likely due to construct design since omission of the first two strands (i.e. leaving a 4-stranded core with the N- and C-terminal strands parallel) results in a destabilized conformation (Fig. S2C and S2D).
The β-grasp domain of MCU interacts with Mg2+ and Ca2+
MCU72-189 crystallized in the presence of MgCl2 revealing a Mg2+ atom near the negatively charged D131, D147 and D148 side chains that are primary contributors to the major acidic surface patch (Fig. 1E). Side chain OΔ1 and OΔ2 atoms of D147 are within 3.5 and 2.1 Å, respectively, of the Mg2+ ion which is centrally located in the acidic patch, while D131 and D148 side chains are positioned further away (i.e. minimum OΔ distance ~6.6 Å) (Fig. 1F). Mg2+ is coordinated by the D147 OΔ2 atom and 5 water molecules arranged in octahedral geometry (Fig. 1G). The arrangement of the 5 ligand water molecules and the D147 carboxylate around the Mg2+ is visible in the 2Fo-Fc electron density map contoured at 2.0σ; further, an Fo-Fc omit map contoured at 9.0σ clearly shows the unaccounted for density when the Mg2+ atom is removed (Fig. 1G). Within the crystal, two of the water molecules that coordinate the Mg2+ in the first shell are bridged by the D123 side chain of a symmetry mate in second shell coordination.
MCU72-189 also crystallized with either LiSO4 or Ca(CH3COO)2 (Table S1). In the presence of LiSO4 (1.5 Å), the Fo-Fc omit map contoured at 2.5σ shows no unaccounted for electron density in the acidic patch, demonstrating specificity for the Mg2+ atom found in the presence of MgCl2 (Fig. 1H). The structure crystallized with Ca(CH3COO)2 showed considerably lower resolution than the MgCl2 or LiSO4 structures (i.e. 2.7 Å versus 1.5-1.6 Å), possibly contributing to an inability to reliably locate a Ca2+ atom. A comparison of the Cα atom locations between the MgCl2, LiSO4 and Ca(CH3COO)2 structures reveals highly similar backbone conformations with only minor deviations at the N- and C-terminal ends; however, an all-atom comparison reveals larger variations for specific side-chains in the loop and structured regions (Fig. S3C and S3D). The precise significance of these differences requires further study.
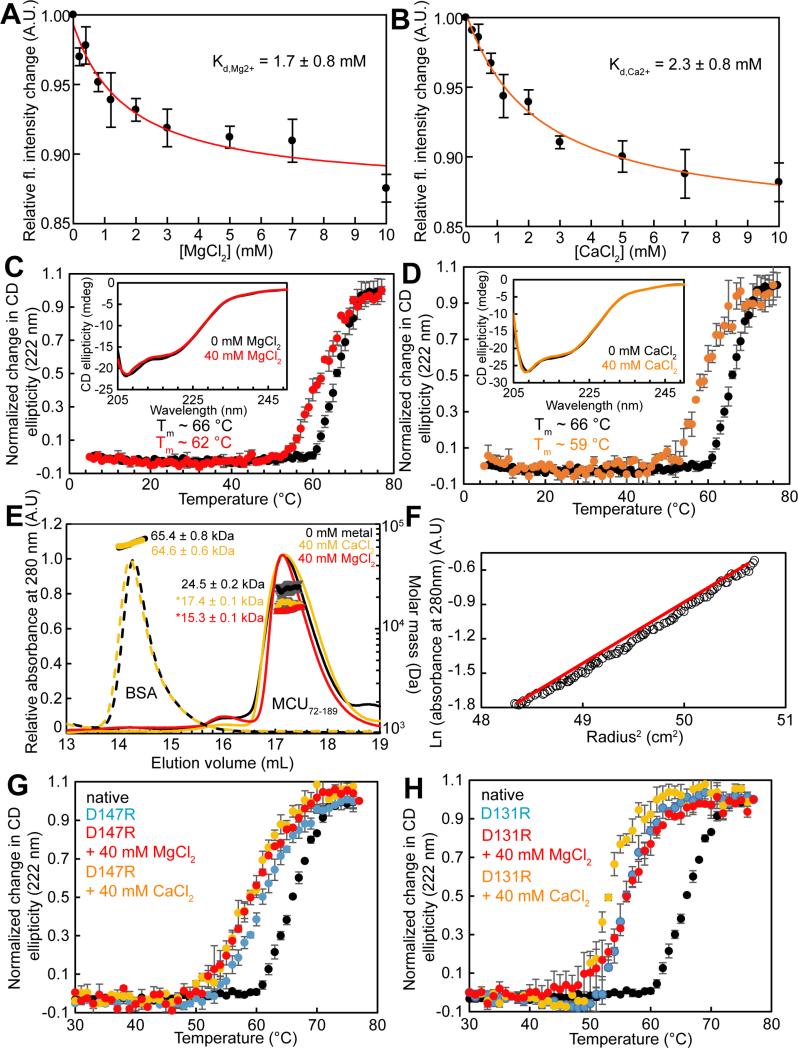
Given the discovery of the Mg2+ ion bound to MCU72-189 in the crystal state, we sought to characterize the cation binding in solution. We used changes in intrinsic fluorescence of MCU72-189 as a readout of structural changes in response to MgCl2 or CaCl2 titration. In divalent cation-free buffer and using an excitation wavelength (λex) of 280 nm, MCU72-189 shows an intrinsic fluorescence emission maximum at a wavelength (λem) of ~305 nm, consistent with Tyr fluorescence and the absence of Trp in the domain; moreover, increasing concentrations of CaCl2 or MgCl2 systematically decreased the intrinsic fluorescence (Fig. S2E and S2F). Guanidine-induced denaturation was also found to decrease the intrinsic fluorescence of MCU72-189 suggesting that the fluorescence change observed in our titrations may reflect a loss in structure associated with the cation (Fig. S2G). Plots of the changes in fluorescence intensity at the wavelength showing maximum fluorescence change (i.e. λex=280 nm; λem=302 nm) versus CaCl2 or MgCl2 revealed saturable binding. Fitting the binding curves to a one site binding model that takes into account protein concentration indicated equilibrium dissociation constants for Ca2+ (Kd,Ca2+) and Mg2+ (Kd,Mg2+) of ~2.3 and ~1.7 mM, respectively (Fig. 2A and 2B).
Fig. 2. Cation binding, oligomerization and stability of MCU72-189.
(See also Fig. S2, Fig. S4 and Tables S2-S4).
(A) and (B) Changes in intrinsic fluorescence (fl.) as a function of MgCl2 (A) and CaCl2 (B) concentration at 25 °C. Data were fitted to a one-site binding scheme (solid lines).
(C) and (D) MCU72-189 secondary structure and stability sensitivity to MgCl2 (C) and CaCl2 (D). The (C) and (D) insets show far-UV-CD spectra in the absence (black line) and presence of 40 mM MgCl2 (red line) or CaCl2 (orange line) at 4 °C. Thermal stability in the absence (black circles) and presence of 40 mM MgCl2 (red circles) or CaCl2 (orange circles).
(E) SEC-MALS data in the absence (black) or presence of 40 mM MgCl2 (red) or CaCl2 (orange). The elution peaks are representative (lines) and the molecular weights are means ± SE (circles) of n=3 experiments at 4 °C. *P<0.001. Injections of BSA are shown (dashed lines) with a negligible change in molecular weight (circles) in the absence and presence of 40 mM CaCl2.
(F) Equilibrium ultracentrifugation assessment of MCU72-189. Data (open circles) are shown for a 1.4 mg mL−1 sample acquired at 25,000 rpm and 25 °C. The red line shows the non-linearity in the data indicative of multiple species in solution.
(G) Thermal stability of native (black circles) and D147R MCU72-189 (blue circles) in the presence of 40 mM MgCl2 (red circles) or 40 mM CaCl2 (orange circles).
(H) Thermal stability of native (black circles) and D131R MCU72-189 (blue circles) in the presence of 40 mM MgCl2 (red circles) or 40 mM CaCl2 (orange circles). All data were acquired in 20 mM HEPES, 150 mM KCl, 2 mM DTT, pH 7.5 at 0.5 mg mL−1 (A), (B), (C), (D), (G), (H) or ~2.0 mg mL−1 (E). Mean ± SE, (n=3 independent experiments).
To test whether Mg2+ or Ca2+ binding to MCU72-189 influences secondary structure or stability in solution, we first assessed the far-UV-CD spectrum of the protein as a function of increasing Mg2+ or Ca2+ levels. Increasing concentrations of MgCl2 or CaCl2 nominally changed the far-UV CD ellipticity suggesting that cation interaction with MCU72-189 does not appreciably alter secondary structure (Fig. 2C and 2D, insets). Effects on domain stability were monitored by change in far-UV-CD signal at 222 nm as a function of temperature in the presence and absence of MgCl2 or CaCl2. In the absence of cations, MCU72-189 had an apparent midpoint of Tm of ~66 °C. Addition of 40 mM MgCl2 decreased the Tm of MCU72-189 to ~62 °C (Fig. 2C). Remarkably, addition of 40 mM CaCl2 destabilized the protein to a greater extent than MgCl2 with a Tm value of ~59 °C (Fig. 2D).
Taken together, results from crystal structures, solution far-UV-CD and intrinsic fluorescence suggest that MCU72-189 binds Mg2+ and Ca2+ with ~mM affinity on an acidic region of the protein surface resulting in destabilization of the domain.
Ca2+ and Mg2+ promote MCU72-189 monomerization
With relatively small secondary structural changes associated with Mg2+ or Ca2+ binding, we sought to determine if other factors might contribute to the modest destabilization caused by cation binding. Size exclusion chromatography (SEC) was used to assess the quaternary structure of MCU72-189. The protein eluted as a single major peak on a Superdex 200 10/300 column with an apparent molecular weight of ~24.0 kDa, equal to 1.7× the theoretical 13.9 kDa monomer mass suggesting a monomer-dimer equilibrium in fast exchange (Table S2). Consistent with this notion, we found that lower protein concentration shifted the apparent molecular weight toward monomer (Table S2). We next performed SEC with in-line multi-angle light scattering (MALS) to assess the effects of Mg2+ and Ca2+ ions on the self-association of MCU72-189. The SEC-MALS-determined molecular weight of MCU72-189 was 24.5 ± 0.2 kDa, consistent with the fast exchange equilibrium implied by the SEC experiments; moreover, the addition of 40 mM CaCl2 or MgCl2 shifted apparent molecular weight to 17.4 ± 0.1 or 15.3 ± 0.1 kDa, respectively (Fig. 2E). Thus, both Mg2+ and Ca2+ cations shift the self-association equilibrium of MCU72-189 toward monomer.
We performed analytical ultracentrifugation (AUC) to confirm the tendency for MCU72-189 dimerization and to quantify the strength of the self-association in the presence and absence of CaCl . A plot of ln(absorbance) versus radius2 showed a non-linearity indicative of multiple species in solution (Fig. 2F). Global fitting of the equilibrium data to a single ideal species revealed molecular weights of ~22.3, 21.5 and 18.5 kDa for samples prepared at 1.2, 0.8 and 0.4 mg mL−1, respectively (Fig. S4A, S4B and S4C; Table S3); moreover, these fitted molecular weights corresponded to 1.6, 1.5 and 1.3× the monomer mass, confirming the concentration dependence and fast exchange equilibrium as observed with SEC-MALS. The equilibrium dissociation constants for the monomer-dimer equilibrium (Kd,dimer) were also extracted using global fits at the three protein concentrations, and the Kd,dimer values were 58.6, 77.9 and 126 μM for the 1.2, 0.8 and 0.4 mg mL−1 datasets, respectively. Consistent with SEC-MALS, CaCl2 decreased the fitted molecular weights to ~20.0, 18.8 and 16.2 kDa, corresponding to 1.4, 1.4 and 1.2× the monomer mass for the 1.2, 0.8 and 0.4 mg/mL datasets, respectively (Fig. S4D, S4E and S4F; Table S3). Similarly, the globally fitted Kd,dimer values were 139, 197 and 369 μM, approximately ~2-3× higher (i.e. weaker affinity) than the Kd,dimer measured in the absence of the divalent cation.
Taken together, SEC-MALS and AUC data demonstrate that MCU72-189 exists in a self-association equilibrium with sub-mM affinity undergoing fast exchange. Importantly, CaCl2 weakens MCU72-189 multimerization by ~2-3-fold suggesting a potential feedback mechanism of regulation.
Acidic patch mutations destabilize and monomerize MCU72-189 similar to metal binding
To validate the notion that Ca2+ and Mg2+-induced destabilization is caused by binding to the acidic patch of MCU72-189, we introduced D131R or D147R substitutions and assessed the effects of CaCl2 and MgCl2 supplementation on thermal stability. D147 is adjacent to the C-terminal end of the α2 helix and D131 is found within ~9.0 Å of D147 (i.e. Cγ distance) on the loop immediately following the β4 strand (Fig. 1E). D147R and D131R mutant proteins exhibited Tm values of ~61 °C and ~56 °C, respectively, compared to ~66 °C for the native protein, indicating that introduction of a positively charged residue in the acidic patch markedly destabilizes the domain, despite the loop localization of these residues (Fig. 2G and 2H). Addition of 40 mM MgCl2 or CaCl2 decreased the Tm of the D147R protein by ~2 °C, in contrast to the ~4 °C and ~7 °C destabilization observed for native protein by MgCl2 and CaCl2, respectively (Fig. 2G and Table S4). A suppressed destabilization compared to the native protein was also observed for the D131R sample in the presence of 40 mM MgCl2 or CaCl2 (i.e. ~0 °C and 3 °C, respectively) (Fig. 2H and Table S4).
To confirm that the mutation-induced destabilization of MCU72-189 is associated with monomerization as observed with Mg2+ and Ca2+ binding, we performed SEC-MALS on the D147R protein. The SEC-MALS-determined molecular weight of the D147R protein with and without 40 mM CaCl2 was 15.6 ± 0.1 and 15.7 ± 0.2 kDa, respectively (Fig. S2H). Thus, our MCU72-189 mutations in the acidic patch caused a domain destabilization (i.e. ΔTm between ~−5 and −10 °C) similar to the loss in stability caused by Ca2+ and Mg2+ binding to native protein (i.e. ΔTm between ~−4 and −7 °C); further, these mutations suppressed the effects of cation binding on stability and promoted monomerization of the domain. These data suggest that the cation sensitivity may be an important regulatory feature of this domain.
MCU is functionally sensitive to acidic patch mutations
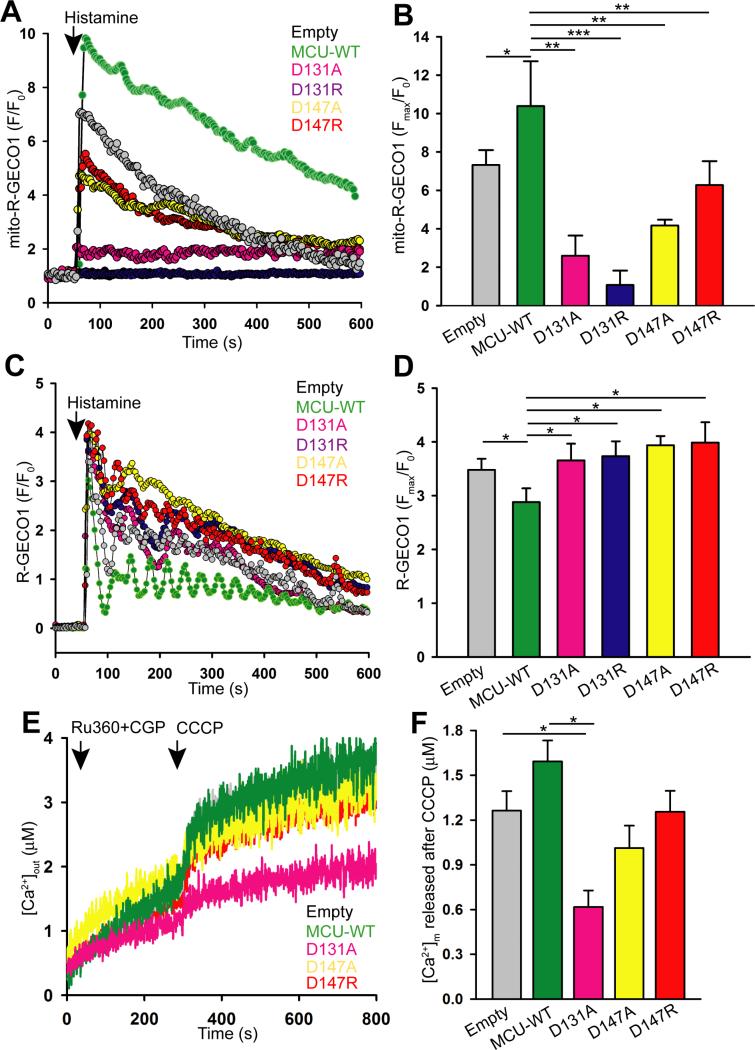
To test the idea that the acidic patch of MCU72-189 plays a regulatory role in MCU activity we used genetically encoded Ca2+ sensors targeted to the mitochondrial matrix or the cytosol (i.e. mito-R-GECO1 or R-GECO1, respectively). We overexpressed full-length wild-type (WT) as well as D131A, D131R, D147A and D147R mutant proteins in HeLa cells and assessed changes in Ca2+ levels in response to the GPCR agonist, histamine. Reconstitution of human WT MCU and mutants did not alter the protein expression and mitochondrial localization (Fig. S5). HeLa cells overexpressing WT MCU showed a significantly increased maximal mitochondrial Ca2+ uptake compared to empty vector transfected cells (Fig. 3A and 3B). Each of the D131 and D147 mutants showed significantly reduced mitochondrial Ca2+ uptake compared to endogenous MCU and WT MCU overexpression, revealing a dominant negative effect. Concomitantly, HeLa cells expressing WT MCU also showed a significant decrease in maximal cytosolic Ca2+ levels after histamine stimulation compared to control cells owing to increased MCU activity, while ectopic constitution of D131A, D131R, D147A and D147R did not significantly alter the maximal cytosolic Ca2+ levels (Fig. 3C and 3D). To verify that the observed functional differences for D131 and D147 mutants were not due to altered ΔΨm we loaded the WT and mutant MCU-overexpressing HeLa cells with the fluorescent ΔΨm indicator, tetramethylrhodamine methyl ester (TMRM). Under unstimulated conditions, TMRM fluorescence was measured before and after addition of the ΔΨm uncoupler, carbonyl cyanide m-chlorophenyl hydrazine (CCCP). The TMRM fluorescence change was similar for WT and mutant MCU expressing cells suggesting intact ΔΨm (Fig. S6A and S6B).
Fig. 3. Live cell MCU functional activity.
(See also Fig. S5 and Fig. S6).
HeLa cells were transiently transfected with mito-R-GECO1 or R-GECO1. Cells were stimulated with 100 μM histamine after one minute baseline recording (arrowheads).
(A) Representative mean traces of mito-R-GECO1 fluorescence (f.a.u.) in HeLa cells transfected with empty vector (grey), MCU-WT-GFP (green), MCU D131A-GFP (magenta), MCU D131RGFP (blue), MCU D147A-GFP (yellow), and MCU D147R-GFP (red).
(B) Quantification of peak mito-R-GECO1 fluorescence (Fmax/F0) (mean ± S.E.). *P <0.05; **P <0.01; ***P <0.001.
(C) Representative mean traces of cytosolic R-GECO1 fluorescence (f.a.u) in HeLa cells transfected with empty vector (grey), MCU-WT-GFP (green), MCU D131A-GFP (magenta), MCU D131R-GFP (blue), MCU D147A-GFP (yellow), and MCU D147R-GFP (red).
(D) Quantification of cytosolic Ca2+ elevation as a function of R-GECO1 fluorescence (Fmax/F0) (mean ± SE). *P <0.05.
(E) HEK293T cells stably expressing pBSD (empty vector), MCU-WT, MCU D131A, MCU D147A or MCU D147R were permeabilized with digitonin in intracellular-like medium containing Fura2FF and thapsigargin (2 μM). Ru360 (1 μM), CGP37157 (1 μM), and CCCP (2 μM) were added prior to thapsigargin addition (arrowheads). Traces are means of n=3 independent experiments.
(F) Quantification of mitochondrial Ca2+ release ([Ca2+]m) after CCCP addition for each stable cell line. Mean ± SE, (n=3 independent experiments). *P <0.05.
To assess mitochondrial Ca2+ uptake in a permeabilized system, we simultaneously measured ΔΨm and extramitochondrial Ca2+ clearance. HEK293T cells were suspended with JC-1 and Fura2FF for measuring ΔΨm and extramitochondrial Ca2+, respectively, in the presence of the SERCA pump blocker, thapsigargin to exclude ER Ca2+ uptake. As expected, the WT MCU expressing cells showed a rapid mitochondrial Ca2+ uptake after 20 μM external Ca2+ addition which is concomitant with loss of ΔΨm (Fig. S6C-S6F). Strikingly, cells harboring the MCU D131R mutation displayed a marked decrease in mitochondrial Ca2+ uptake with nominal ΔΨm change, fully consistent with the reduced MCU activity (Fig. S6C-S6F). These data reinforce the notion that acidic patch mutations of MCU decrease mitochondrial Ca2+ uptake.
To determine if the MCU acidic patch plays a role in basal mitochondrial Ca2+ homeostasis, we generated HEK293T cell lines stably expressing pBSD (i.e. Empty vector), WT, D131A, D147A or D147R MCU. Cells were bathed in Ca2+-free medium containing digitonin, Fura2FF and thapsigargin. To prevent mitochondrial MCU and Na+/Ca2+ exchanger (NCLX)-dependent Ca2+ flux, Ru360 and CGP37157 were added prior to permeabilization. Ca2+ released from the mitochondrial matrix was quantified by Fura2FF fluorescence after CCCP addition. HEK293T cells expressing WT MCU showed enhanced matrix Ca2+ release compared to empty vector expressing cells; however, each of the D131A, D147A and D147R expressing cells demonstrated similar or lower (i.e. D131A) mitochondrial Ca2+ release compared to control cells (Fig. 3E and 3F). The D131R substitution caused the greatest destabilization of MCU72-189 in vitro (i.e. ΔTm ~−10 °C) (Table S4), which is supported by maximal inhibition of agonist-induced mitochondrial Ca2+ uptake (Fig. 3B). Thus, depletion of basal mitochondrial Ca2+ in D131A-expressing cells may reflect greater destabilization of D131 mutants when compared to the D147 mutants and is correlated with an observed lethality for stably D131R-expressing cells.
Collectively, our live cell results suggest that D131 and D147 residues contribute to the regulatory region on the matrix domain (i.e. MRAP) that, if perturbed by mutation, inhibits MCU activity.
MCU is functionally sensitive to Ca2+ and Mg2+
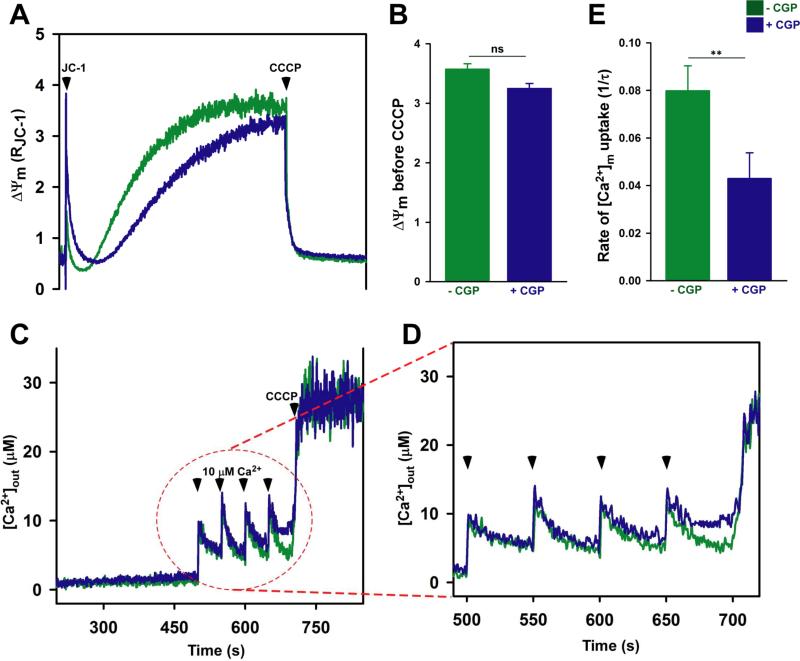
Having observed an inhibition of MCU activity by mutations which destabilize MCU72-189, we aimed to determine whether elevation of mitochondrial matrix Ca2+ can similarly effect MCU activity. Permeabilized HEK293T cells were treated with the NCLX blocker CGP37157 to attenuate Ca2+ efflux from the matrix and exposed to train of external Ca2+ pulses. The ΔΨm remained intact after four pulses of Ca2+ addition in control and CGP treated cells (Fig. 4A and 4B). Remarkably, the mitochondrial Ca2+ uptake rate was significantly reduced in cells treated with CGP when compared to control (Fig. 4C, 4D and 4E), which is consistent with Ca2+-dependent MCU inhibition mechanism.
Fig. 4. Matrix Ca2+-dependent MCU inactivation.
(See also Fig. S6).
Digitonin-permeabilized HEK293T cells bathed in intracellular-like solution containing thapsigargin were loaded with the Ca2+ indicator Fura2FF (1 μM) and the ΔΨm indicator JC-1, to which pulses of 10 μM extracellular Ca2+ were delivered before the addition of the mitochondrial uncoupler CCCP (2 μM) (arrowheads). Simultaneous measurements of ΔΨm and changes in bath Ca2+ ([Ca2+]out) were monitored in the presence and absence of the NCLX blocker, CGP37157 (10 μM).
(A) Representative traces of JC-1 report ΔΨm from n=3 independent experiments.
(B) Quantification of the JC-1 ΔΨm before addition of CCCP. Mean ± SE, (n=3 independent experiments). ns, not significant.
(C) Representative traces of [Ca2+]out from n=3 independent experiments.
(D) Magnified view of the kinetic changes in [Ca2+]out representing mitochondrial Ca2+ uptake.
(E) Quantification of the [Ca2+]m uptake rate taken from the reduction in bath Ca2+ after 3 pulses of 10 μM [Ca2+]out addition. Mean ± SE. **P <0.01.
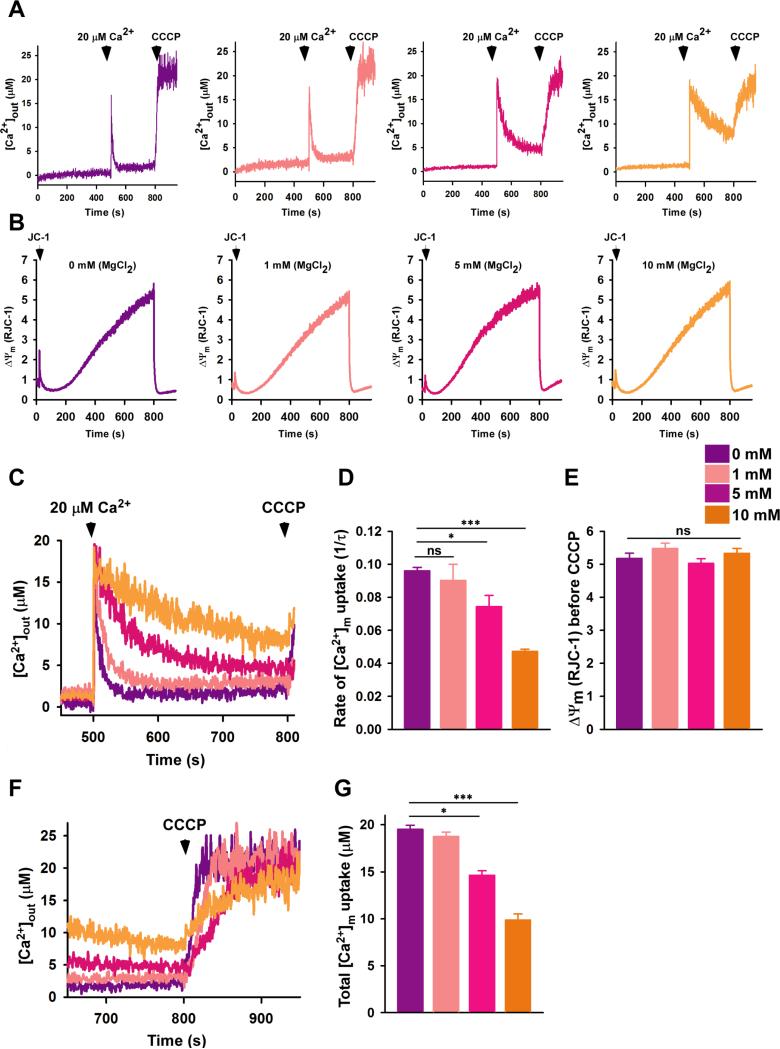
To test whether Ca2+ uptake via MCU can be regulated by Mg2+, we adapted our permeabilized cell system. HEK293T permeabilized cells were bathed with varying concentrations of MgCl2 (i.e. 0, 1, 5 or 10 mM). A Mg2+ concentration-dependent decrease in the rate of mitochondrial Ca2+ uptake was observed after addition of 20 μM external Ca2+ (Fig. 5A). Importantly, no differences in ΔΨm were observed while simultaneously monitoring bath Ca2+ clearance (Fig. 5B and 5E). Significantly suppressed rates of mitochondrial Ca2+ uptake were observed in the presence of 5 mM and 10 mM extramitochondrial MgCl2 compared to cells bathed in medium nominally free of MgCl2 (Fig. 5C and 5D); moreover, even 1 mM Mg2+ significantly suppresses the slow MCU Ca2+ uptake rate assessed over a longer time frame (Fig. S6G amd S6H). As expected, after CCCP addition, reduced release of matrix Ca2+ was observed in the presence of 5 and 10 mM Mg2+ (Fig. 5F and 5G). These functional data show that Mg2+ reduces mitochondrial Ca2+ uptake in a concentration-dependent manner without altering ΔΨm. Although we cannot exclude the possibility that Mg2+ inhibits MCU from outside the matrix, consistent with the Mg2+ and Ca2+-dependent destabilization and monomerization of MCU72-189 that we observed in vitro, increases in matrix Ca2+ as well as Mg2+ have an inhibitory effect on mitochondrial Ca2+ uptake.
Fig. 5. Mg2+-dependent MCU inactivation in live cells.
(See also Fig. S6).
(A) Permeabilized HEK293T cells were pulsed with 20 μM Ca2+ in the presence of MgCl2 as indicated. Representative traces show changes in extramitochondrial Ca2+ concentrations ([Ca2+]out); (n=3 independent experiments).
(B) Representative traces show ΔΨm (i.e. JC-1 ratio) in permeabilized HEK293T cells in response to Ca2+ and CCCP. The [Ca2+]out and ΔΨm traces were acquired simultaneously; (n=3 independent experiments).
(C) Overlay of kinetic changes in [Ca2+]out during 300 s in the presence of different MgCl2 concentrations.
(D) The rate of mitochondrial Ca2+ uptake was calculated as 1/τ. Mean ± SE. *P < 0.05, ***P < 0.001; (n=3 independent experiments).
(E) Quantification of ΔΨm before the addition of CCCP. Mean ± SE. n.s., not significant; (n=3 independent experiments).
(F) and (G) Quantification of [Ca2+]m after CCCP addition. Mean ± SE. *P < 0.05, ***P < 0.001; (n=3 independent experiments).
MCU72-189 regulates higher order oligomerization of full-length human MCU
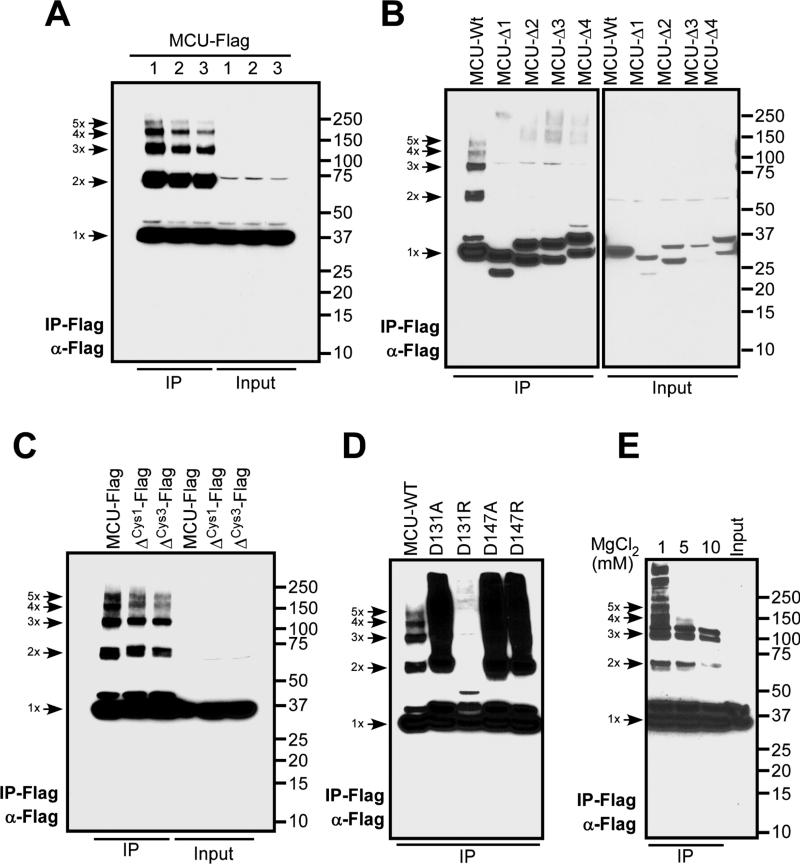
Our recent study using SEC and native PAGE suggested that human MCU forms complexes with molecular masses between ~44 and 670 kDa (Tomar et al., 2016). To elucidate whether ectopically expressed full-length human MCU forms oligomers, we used HEK293T cells expressing Flag-tagged MCU protein. The enrichment of human MCU-Flag protein by immunopurification resulted in both low and higher order oligomers that includes monomer, dimer, trimer, tetramer, and pentamers (Fig. 6A). We tested whether the conserved cysteines that reside in the N-terminal soluble region are essential for MCU oligomer formation by generating a single (i.e. C97A) and triple (i.e. C67A, C97A, C191A) MCU mutant constructs. Elimination of these cysteine residues did not affect oligomerization indicating that human MCU could form multimers independent of cysteine crosslinking (Fig. 6C).
Fig. 6. Assembly of human MCU multimers.
(A) HEK293T cells were transfected with MCU-Flag and lysates were subjected to immunopurification. The eluent was subjected to SDS-PAGE separation under reducing conditions followed by probing with anti-Flag antibody.
(B) HEK293T cells were transfected with MCU-Flag truncation constructs [i.e. MCU-Δ1 (residues 150-200), MCU-Δ2 (residues 291-320), MCU-Δ3 (residues 321-351) and MCU-Δ4 (residues 225-231)], immunopurified and detected for MCU complex formation as described above.
(C) MCU cysteine mutants (ΔCys1: C97A, and ΔCys3: C67A, C97A, C191A) were transfected in HEK293T cells, immunopurified and detected for MCU complex formation as described above.
(D) MCU N-terminal mutants (D131A, D131R, D147A and D147R) were transfected in HEK293T cells, immunopurified and detected for MCU complex formation as described above.
(E) HEK293T cells were transfected with MCU-Flag and lysates were subjected to immunopurification by Flag antibody in the presence of varying concentrations of MgCl2. The eluent was subjected to SDS-PAGE separation under reducing condition followed by probing with anti-Flag antibody.
Images are representative of n=2-3 independent experiments.
Having observed full-length human MCU oligomerization in the absence of crosslinking, we next sought to identify regions of MCU that are determinant of the assembly. We generated a series of N-terminal and C-terminal MCU deletions (Fig. 6B). Remarkably, deletions on both sides of the MCU CC domain disrupted human MCU multimer formation (Fig. 6B). We next examined the effect of N-terminal domain MRAP mutations on full-length MCU complex formation. Our immunopurification revealed that the D131R mutant completely abrogated human MCU complex formation (Fig. 6D), consistent with the greatest suppression of MCU channel activity among all mutants in ectopically expressing HeLa cells (Fig. 3B). Finally, we tested whether Mg2+ cations could affect the oligomerization pattern of full-length human MCU, observing a marked MgCl2 concentration-dependent decrease in MCU oligomer formation (Fig. 6E). Taken together, these data reveal that higher order oligomerization of human MCU requires both N- and C-terminal regions, and Mg2+ strikingly weakens MCU multimerization. A recent report suggested that the C. elegans MCU protein with an N-terminal domain deletion also forms a pentameric structure (Oxenoid et al., 2016). Our data show that the N-terminal domain contributes to human MCU oligomer formation and disruption of MRAP by mutation or divalent cations weakens this assembly.
Discussion
The β-grasp family of proteins exhibit a large structural diversity that can include as many as 10 or as few as 3 β-strands and up to 3 α-helices with the central α-helix crossing in either direction (Burroughs et al., 2012). Here, we have found that the β-grasp-like fold of MCU72-189 not only plays a role as a protein-interaction domain through a tendency for self-association, but also has an intrinsic ability to bind metals in the form of Ca2+ and Mg2+. Interestingly, this region was also shown to associate with MCUR1 (Lee et al., 2015). Our MCU72-189 structure shows a dynamic C-terminal tail (i.e. residues 168-189) that cannot be modeled in the electron density. A recent study found that the position of this C-terminus (i.e. residues 166-185) can be stabilized by a lipid-like molecule, ultimately placing a third α-helix close to α2 (Lee et al., 2015) (Fig. S7A). Considering the mobile nature of the C-terminal tail and the close proximity to MRAP, it is tempting to speculate that the 168-189 region could act as a dynamic cap, regulating the accessibility of cations and/or other larger molecules. The same study demonstrated that mutation of the proposed S92 phosphorylation site suppresses MCU activity, but showed an unfavourable distance for ionic interactions between R93 and E95 (Lee et al., 2015) (Fig. S7B). The formation of the R93:E95 salt bridge in our structure suggests this ionic interaction is also dynamic and, considering the close proximity to S92, may play a role in regulating the phosphorylation event that modulates MCU function.
We have found that the MCU72-189 matrix region binds Ca2+ and Mg2+ with ~mM affinity in a distinct electronegative patch. The coordination of Mg2+ involves oxygen atoms derived from the D147 side chain and five water molecules. Interestingly, the cytosolic domain of the T. maritima Mg2+ transporter similarly coordinates Mg2+ with one Asp and five water molecules; however, when assembled into a pentamer one water molecule is replaced with an Asp oxygen from an adjacent subunit (Lunin et al., 2006). Thus, additional high resolution structural data on assembled MCU72-189 multimers are needed to fully appreciate the precise roles of the MRAP residues in coordinating divalent cations in multiple shells and regulating self-association. Protein self-association is known to enhance stability, and thus, the destabilization of MCU72-189 due to monomerization caused by cation binding is fully consistent with this innate feature of myriad proteins.
Next to K+, Mg2+ is the second most abundant cation in the cell with cytosolic and nuclear concentrations maintained in the ~mM range (Romani et al., 1993; Szanda et al., 2009). Mg2+ enters mitochondria via the Mrs2p channel in an electrogenic manner; hence, changes in ΔΨm can modulate Mg2+ uptake (Kolisek et al., 2003; Schindl et al., 2007). The free Mg2+ concentration in the mitochondrial matrix is ~0.5-1.5 mM and can change depending on the abundance of PO43−, ADP and ATP (Jung et al., 1990; Rutter et al., 1990). The weak binding affinity estimated for Mg2+ is well-suited to the high Mg2+ levels of the mitochondrial matrix as proteins that are structurally sensitive to cation binding have evolved affinities close to the concentration range of the cation in the compartment in which they function. Thus, while a fraction of MCU molecules could be loaded with Mg2+ under physiological conditions, the level of saturation and MCU inhibition may change dependent on the metabolic state of the cell. With the known role of MICU1 as a gatekeeper of MCU in the resting state (Csordas et al., 2013; Mallilankaraman et al., 2012b; Tsai et al., 2016), it is conceivable that both MICU proteins and divalent cations modulate MCU activity. Indeed, it has long been known that mitochondrial Ca2+ uptake can be inhibited by Mg2+ (Favaron and Bernardi, 1985; Prentki et al., 1983), and inhibition can occur after physiologically-relevant elevations in cytosolic Mg2+ (Szanda et al., 2009).
Conversely, global free Ca2+ levels in the matrix would likely never approach mM levels; nevertheless, based on a ΔΨm of −180 mV, the Nernst equation shows that Ca2+ levels in the mitochondria could reach ~71 mM at cytosolic levels of ~0.1 μM if permitted to freely equilibrate between compartments. Thus, the driving force exists in the highly negative ΔΨm to establish mM Ca2+ levels within the matrix. Additionally, diffusion theory predicts Ca2+ levels as high as ~0.1 mM at a distance of ~10 nm from the exiting region of Ca2+ channel pores (Tadross et al., 2013) and ~mM at the immediate base in nanodomains (Bauer, 2001; Chad and Eckert, 1984). Therefore, the ability of MCU72-189 to bind Ca2+ will depend on the proximity to the open pore. Indeed, sustained cytosolic Ca2+ inactivates the mitochondrial Ca2+ uptake pathway (Moreau et al., 2006), and our observation that elevation of matrix Ca2+ inhibits the MCU-dependent Ca2+ uptake under normal ΔΨm maintenance (Fig. 4) is consistent with this earlier work; moreover, Ca2+-binding induced destabilization of MCU72-189 may be an important contributor to this inhibition. We believe that Ca2+-binding induced inactivation of MCU may be a protective buffering mechanism for mitochondrial Ca2+ overload-linked cell death. A recent study found that the MCU channel can be active in the absence of the N-terminal domain (Oxenoid et al., 2016), despite our compelling studies here and previous work (Lee et al., 2015) showing a vital functional role for the N-terminal domain. Thus, deletion of the N-terminal domain may relieve all the regulatory constraints placed on the channel region similar to the effect of deletions observed with other proteins.
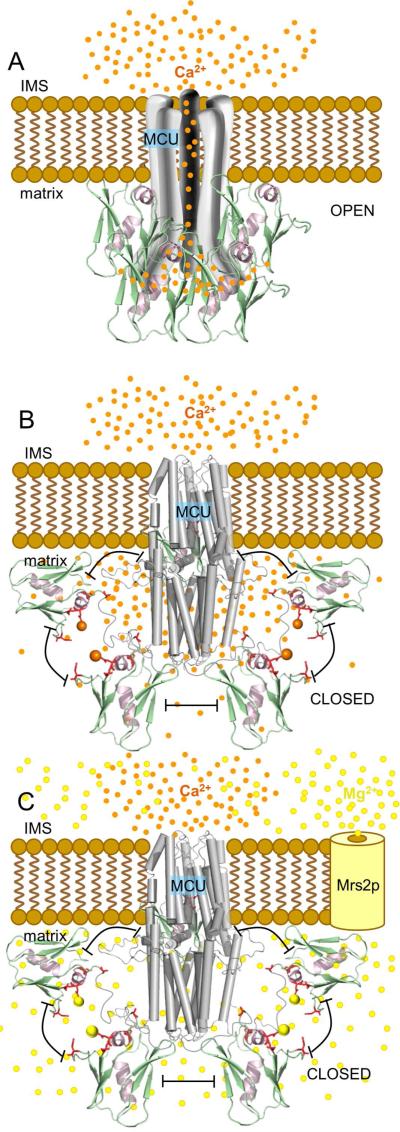
Taken together, our in vitro and live cell observations reveal a mode for MCU autoregulation mediated by Mg2+ and Ca2+ levels in the mitochondria. We demonstrate that Ca2+ and Mg2+ binding to MCU72-189 destabilize the protein and shift the self-association equilibrium toward monomer. Binding of these divalent cations occurs on a negatively charged region that we have termed MRAP. Disrupting MRAP monomerizes and destabilizes MCU72-189 similar to cation binding, decreases the sensitivity of the domain to Ca2+ and Mg2+, perturbs full length human MCU oligomerization and attenuates MCU uptake in live cells in a dominant negative manner. Blocking the Ca2+ extrusion path from the matrix decreases the rate of mitochondrial Ca2+ uptake without significantly altering ΔΨm; further, extramitochondrial Mg2+ similarly suppresses the rate of MCU-dependent mitochondrial Ca2+ uptake. Based on these observations, we propose a model of autoregulation where Mg2+ and/or Ca2+ binding to the MCU soluble matrix domain inhibits MCU activity via a destabilization that is concomitant with a suppression in the tendency for self-association (Fig. 7). MCU activity would be fine-tuned by the metabolic state of the cell and the abundance of ATP, ADP and PO43− as well as Mrs2p uptake, where increases in free Mg2+ would heighten MCU inhibition. Additionally, local increases in Ca2+ levels within nanodomains close to MCU72-189 established by an open MCU pore would also inhibit MCU activity in a negative feedback mechanism. It is tempting to speculate that the ~21 % fast inactivation of the peak inward currents observed during voltage step experiments of patched mitoplasts (Kirichok et al., 2004) is due to the Ca2+-dependent inhibition phenomenon we have described herein.
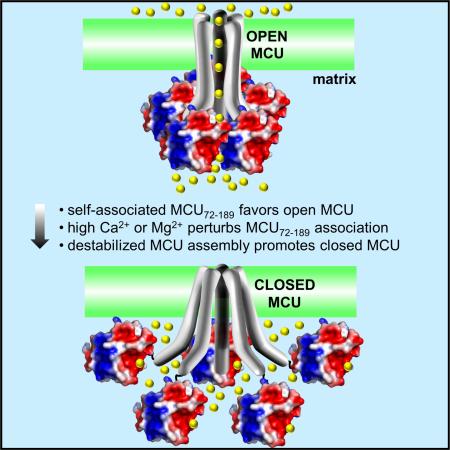
Fig. 7. Model of MCU autoregulation by Mg2+ and Ca2+.
(A) Activated MCU channel (open) due to increased IMS Ca2+ levels. Multimerized MCU72-189 is shown on the matrix side.
(B) Feedback inhibited MCU channel (closed) due to the disruption of self-association by high Ca2+ levels in nanodomains within ~1-6 nm of the channel pore. Monomerized MCU72-189 is shown relative to the closed C-terminal domain C. elegans MCU model (grey cylinders) (5id3).
(C) Inhibited MCU channel (closed) due to increased free Mg2+ levels entering the matrix through Mrs2p. In (A) – (C), Mg2+ and Ca2+ are depicted as yellow and orange spheres, respectively. The pentameric stoichiometry is based on our full-length human data (Fig. 6) and the recent C. elegans structural work (Oxenoid et al., 2016). Because our data shows that strong interactions under denaturing conditions are compromised and previous work under native conditions suggests the overall assembly between MCU subunits may be preserved after N-terminal deletion (Lee et al., 2015; Oxenoid et al., 2016), cation binding to MCU72-189 is depicted in (B) and (C) to disrupt the open architecture and not the stoichiometry of MCU. Additional structural and dynamical information is required to reveal the precise stoichiometry of the MCU complex in the presence of regulators and the relative location of the MCU72-189 region in an open and closed channel.
In summary, our structural and functional results reveal that MCU is autoregulated by prolonged high matrix Ca2+ and/or Mg2+ that binds to MRAP and destabilizes MCU. This regulatory model is consistent with a recent study demonstrating Mg2+-induced inhibition of MCU (Blomeyer et al., 2016) and work showing Ca2+-dependent inactivation of mitochondrial Ca2+ uptake (Moreau et al., 2006). Further studies are required to determine precisely how MRAP and MCU72-189 is involved in heteromeric protein-protein interactions known to mediate MCU complex assembly and activity. Nevertheless, these findings provide new mechanistic insights into MCU regulation and avenues to the development of MCU inhibitors that control cell death.
Significance
MCU is a Ca2+ selective inwardly rectifying inner mitochondrial membrane resident channel that mediates Ca2+ flux into the mitochondrial matrix and is essential for bioenergetics. However, the mechanisms of MCU regulation by cations are unclear. We show for the first time the structural basis for divalent cation interactions with an extensive electronegative surface patch termed MRAP located on the soluble MCU72-189 matrix domain. Further, we demonstrate that point mutations within MRAP can perturb the assembly of both the isolated MCU72-189 region and the full-length human MCU protein similar to interactions with cations, thereby leading to channel inhibition. Consistent with our biochemical approaches, we demonstrate that blockade of Ca2+ extrusion from the matrix or extramitochondrial Mg2+ incubation significantly suppresses MCU activity. Our work reveals a mechanism for divalent cation regulation of MCU activity and provides a framework for the development of small molecule modulators of MCU function by targeting MRAP within MCU72-189.
Experimental Procedures
Expression, purification and crystallization of MCU72-189
Native and mutant proteins used for the in vitro experiments were expressed in BL21 (DE3) codon plus Escherichia coli and purified as detailed in the Supplemental Experimental Procedures. All crystals were grown at 18 °C using the hanging-drop vapor diffusion method. Purified proteins in 20 mM HEPES, 150 mM KCl, 2 mM DTT, pH 7.5 were concentrated to ~4.5 mg/mL. Crystallization was performed by mixing 1 μL of protein with 1 μL of precipitant and structures were determined as detailed in the Supplemental Experimental Procedures. The wwPDB coordinate codes are 5KUE (I141M/L146M), 5KUG (lithium), 5KUI (calcium) and 5KUJ (magnesium).
Far-UV circular dichroism spectroscopy
CD spectra and thermal melts were acquired on a Jasco J-810 Spectropolarimeter equipped with a Peltier temperature control system using a quartz cell with a path length of 0.1 cm. Details of the acquisition settings are given in the Supplemental Experimental Procedures.
Analytical ultracentrifugation
Sedimentation equilibrium studies were carried out using a Beckman Optima XL-A Analytical Ultracentrifuge. An An60Ti rotor and six-channel cells with Epon-charcoal centerpieces were used for the data acquisition. Absorbance measurements at 280 nm were collected in 0.002 cm radial steps and averaged over 10 readings. Details of the data analyses are given in the Supplemental Experimental Procedures.
Size exclusion chromatography and multi-angle light scattering
SEC was performed using a Superdex 200 10/300 GL column (GE Healthcare) connected to an AKTA pure FPLC system (GE Healthcare) at 4°C. Three injections of 125 μL each were loaded onto the column and separated at 0.5 mL min−1. SEC-MALS was performed with a Superdex Increase S200 10/300 GL column in-line with a sixteen-angle Dawn Heleos II light-scattering instrument and Optilab TrEX differential refractometer (Wyatt Technologies). Analyses were performed as detailed in the Supplemental Experimental Procedures.
Ca2+/Mg2+ binding and chemical denaturation curves
Changes in intrinsic fluorescence as a function of CaCl2, MgCl2 or guanidine hydrochloride concentrations were taken as an estimate of binding affinity or conformational stability. Experiments were performed on a Cary Eclipse Spectrofluorimeter (Varian/Agilent). Details of these fluorescence measurements are given in the Supplemental Experimental Procedures.
Mitochondrial and cytosolic Ca2+ dynamics and confocal microscopy
HeLa cells were transiently transfected with genetically encoded mito-R-GECO1, mitochondrial targeted Ca2+ sensor or R-GECO1, cytosolic Ca2+ sensor along with Empty vector, MCU-WT-GFP, MCU D131A-GFP, MCU D131R-GFP, MCU D147A-GFP, and MCU D147R-GFP. After 48 hours, the change of mito-R-GECO1/R-GECO1 fluorescence was measured with 488 and 561-nm excitation on a Carl Zeiss META 510 confocal microscope equipped with a 40× oil objective. After 1 min of baseline recording, histamine (100 μM) was added, and the changes in mito-R-GECO1 and R-GECO1 fluorescence was recorded. Images were analyzed using ImageJ (NIH).
Measurement of resting mitochondrial Ca2+
Stable HEK293T cell lines were created using a pBSD plasmid which carries blasticidin resistance. HEK293T cells (~6 × 107) stably expressing Empty vector, MCU-WT-Flag, MCU D131A-Flag, MCU D131R-Flag, MCU D147A-Flag, and MCU D147R-Flag were resuspended and permeabilized with digitonin (40 μg/ml) in 1.5 ml of intracellular medium composed of 120 mM KCl, 10 mM NaCl, 1 mM KH2PO4, 20 mM HEPES-tris (pH 7.2), 5 mM succinate, bath Ca2+ indicator Fura-2FF (0.5 μM), MCU blocker Ru360 (1 μM) and NCLX blocker, CGP (1 μM), and 2 μM thapsigargin to block the SERCA pump. To assess the resting [Ca2+]m, after baseline recording of bath Ca2+ (i.e. [Ca2+]out), CCCP (2 μM) was added to release the mitochondrial Ca2+. Further details are described in the Supplemental Experimental Procedures.
Ca2+ and Mg2+ -dependent inactivation of mitochondrial Ca2+ uptake
HEK293T cells were washed in Ca2+-free PBS, pH 7.4. Approximately 7×106 cells were resuspended and permeabilized with 40 μg/ml digitonin in 1.5 ml of intracellular medium composed of 120 mM KCl, 10 mM NaCl, 1 mM KH2PO4, 20 mM HEPES-Tris, pH 7.2 and 2 μM thapsigargin to block the SERCA pump. All measurements were performed in the presence of 5 mM succinate. The simultaneous measurement of ΔΨm and extramitochondrial Ca2+ (i.e. [Ca2+]out) clearance was achieved by loading the permeabilized cells with JC-1 (800 nM) and Fura-2FF (1 μM), respectively, in the presence and absence of NCLX blocker CGP37157 (10 μM). A series of Ca2+ boluses and mitochondrial uncoupler, CCCP (2 μM), were added at the indicated time points. The Mg2+-inhibition experiments were performed in a similar manner as the Ca2+ inhibition experiments in the absence of the CGP37157, using a single bolus of 20 μM CaCl2 and varying concentrations of extramitochondrial MgCl2 from 0 – 10 mM. Additional details are described in the Supplemental Experimental Procedures.
Immunopurification and human MCU oligomerization
HEK293T cells were transiently transfected with the indicated constructs. Flag-tagged proteins were immunopurified and enriched using Flag-antibody (Sigma) and equal amounts of protein were separated on 4-12% Bis-Tris SDS-PAGE under reducing conditions, transferred to a PVDF membrane, and probed with α-Flag-antibody. Further details are described in the Supplemental Experimental Procedures.
Statistical Analysis
Significance was evaluated via Student's unpaired t-test or one-way and two-way ANOVAs.
Supplementary Material
Highlights.
The soluble MCU matrix domain contains an acidic patch on the β-grasp-like fold.
Cation binding or mutation in the acidic patch perturbs MCU assembly and activity.
Blockade of matrix Ca2+ extrusion or Mg2+ loading inhibits MCU uptake rates.
The N-terminal acidic patch confers a mode for MCU autoregulation.
Acknowledgements
This research was supported by Natural Sciences and Engineering Research Council of Canada (05239 to P.B.S.), Canadian Foundation for Innovation/Ontario Research Fund (to P.B.S.), Canadian Institutes of Health Research (MOP 89903 to M.S.J.) and National Institutes of Health (R01GM109882, R01HL086699, R01HL119306 and 1S10RR027327 to M.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceptualization, P.B.S. and M.M.; Methodology, P.B.S., M.M. and M.S.J.; Investigation, S.K.L, M.C.Y.M., M.S.J., P.B.S., S.S., Z.D., D.T., E.C., and S.R.; Visualization, P.B.S., S.K.L. and S.S.; Writing – Original Draft, P.B.S. and M.M.; Writing – Review & Editing, P.B.S., M.S.J., and M.M.; Supervision, P.B.S., M.S.J. and M.M.
References
- Bauer PJ. The local Ca concentration profile in the vicinity of a Ca channel. Cell Biochem Biophys. 2001;35:49–61. doi: 10.1385/CBB:35:1:49. [DOI] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Blomeyer CA, Bazil JN, Stowe DF, Dash RK, Camara AK. Mg differentially regulates two modes of mitochondrial Ca uptake in isolated cardiac mitochondria: implications for mitochondrial Ca sequestration. J Bioenerg Biomembr. 2016;48:175–188. doi: 10.1007/s10863-016-9644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burroughs AM, Iyer LM, Aravind L. Structure and evolution of ubiquitin and ubiquitin-related domains. Methods Mol Biol. 2012;832:15–63. doi: 10.1007/978-1-61779-474-2_2. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Historical review: mitochondria and calcium: ups and downs of an unusual relationship. Trends Biochem Sci. 2003;28:175–181. doi: 10.1016/S0968-0004(03)00053-7. [DOI] [PubMed] [Google Scholar]
- Chad JE, Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys J. 1984;45:993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Golenar T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, de la Fuente Perez S, Bogorad R, et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca(2)(+) uniporter. Cell Metab. 2013;17:976–987. doi: 10.1016/j.cmet.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaron M, Bernardi P. Tissue-specific modulation of the mitochondrial calcium uniporter by magnesium ions. FEBS Lett. 1985;183:260–264. doi: 10.1016/0014-5793(85)80789-4. [DOI] [PubMed] [Google Scholar]
- Hoffman NE, Chandramoorthy HC, Shamugapriya S, Zhang X, Rajan S, Mallilankaraman K, Gandhirajan RK, Vagnozzi RJ, Ferrer LM, Sreekrishnanilayam K, et al. MICU1 motifs define mitochondrial calcium uniporter binding and activity. Cell Rep. 2013;5:1576–1588. doi: 10.1016/j.celrep.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman NE, Chandramoorthy HC, Shanmughapriya S, Zhang XQ, Vallem S, Doonan PJ, Malliankaraman K, Guo S, Rajan S, Elrod JW, et al. SLC25A23 augments mitochondrial Ca(2)(+) uptake, interacts with MCU, and induces oxidative stress-mediated cell death. Mol Biol Cell. 2014;25:936–947. doi: 10.1091/mbc.E13-08-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, et al. CaMKII determines mitochondrial stress responses in heart. Nature. 2012;491:269–273. doi: 10.1038/nature11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung DW, Apel L, Brierley GP. Matrix free Mg2+ changes with metabolic state in isolated heart mitochondria. Biochemistry. 1990;29:4121–4128. doi: 10.1021/bi00469a015. [DOI] [PubMed] [Google Scholar]
- Kamer KJ, Mootha VK. The molecular era of the mitochondrial calcium uniporter. Nat Rev Mol Cell Biol. 2015;16:545–553. doi: 10.1038/nrm4039. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- Kolisek M, Zsurka G, Samaj J, Weghuber J, Schweyen RJ, Schweigel M. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. EMBO J. 2003;22:1235–1244. doi: 10.1093/emboj/cdg122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Min CK, Kim TG, Song HK, Lim Y, Kim D, Shin K, Kang M, Kang JY, Youn HS, et al. Structure and function of the N-terminal domain of the human mitochondrial calcium uniporter. EMBO Rep. 2015;16:1318–1333. doi: 10.15252/embr.201540436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunin VV, Dobrovetsky E, Khutoreskaya G, Zhang R, Joachimiak A, Doyle DA, Bochkarev A, Maguire ME, Edwards AM, Koth CM. Crystal structure of the CorA Mg2+ transporter. Nature. 2006;440:833–837. doi: 10.1038/nature04642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, Csordas G, Madireddi P, Yang J, Muller M, et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol. 2012a;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallilankaraman K, Doonan P, Cardenas C, Chandramoorthy HC, Muller M, Miller R, Hoffman NE, Gandhirajan RK, Molgo J, Birnbaum MJ, et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell. 2012b;151:630–644. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi S, Pinton P. The mitochondrial calcium uniporter complex: molecular components, structure and physiopathological implications. J Physiol. 2014;592:829–839. doi: 10.1113/jphysiol.2013.268235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G, Darling E, Eveleth J. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry. 1986;25:236–244. doi: 10.1021/bi00349a033. [DOI] [PubMed] [Google Scholar]
- Moreau B, Nelson C, Parekh AB. Biphasic regulation of mitochondrial Ca2+ uptake by cytosolic Ca2+ concentration. Curr Biol. 2006;16:1672–1677. doi: 10.1016/j.cub.2006.06.059. [DOI] [PubMed] [Google Scholar]
- Oxenoid K, Dong Y, Cao C, Cui T, Sancak Y, Markhard AL, Grabarek Z, Kong L, Liu Z, Ouyang B, et al. Architecture of the mitochondrial calcium uniporter. Nature. 2016;533:269–273. doi: 10.1038/nature17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plovanich M, Bogorad RL, Sancak Y, Kamer KJ, Strittmatter L, Li AA, Girgis HS, Kuchimanchi S, De Groot J, Speciner L, et al. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS One. 2013;8:e55785. doi: 10.1371/journal.pone.0055785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M, Janjic D, Wollheim CB. The regulation of extramitochondrial steady state free Ca2+ concentration by rat insulinoma mitochondria. J Biol Chem. 1983;258:7597–7602. [PubMed] [Google Scholar]
- Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, Checchetto V, Moro S, Szabo I, Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. Embo J. 2013;32:2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani A, Marfella C, Scarpa A. Cell magnesium transport and homeostasis: role of intracellular compartments. Miner Electrolyte Metab. 1993;19:282–289. [PubMed] [Google Scholar]
- Rutter GA, Osbaldeston NJ, McCormack JG, Denton RM. Measurement of matrix free Mg2+ concentration in rat heart mitochondria by using entrapped fluorescent probes. Biochem J. 1990;271:627–634. doi: 10.1042/bj2710627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Markhard AL, Kitami T, Kovacs-Bogdan E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindl R, Weghuber J, Romanin C, Schweyen RJ. Mrs2p forms a high conductance Mg2+ selective channel in mitochondria. Biophys J. 2007;93:3872–3883. doi: 10.1529/biophysj.107.112318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szanda G, Rajki A, Gallego-Sandin S, Garcia-Sancho J, Spat A. Effect of cytosolic Mg2+ on mitochondrial Ca2+ signaling. Pflugers Arch. 2009;457:941–954. doi: 10.1007/s00424-008-0551-0. [DOI] [PubMed] [Google Scholar]
- Tadross MR, Tsien RW, Yue DT. Ca2+ channel nanodomains boost local Ca2+ amplitude. Proc Natl Acad Sci U S A. 2013;110:15794–15799. doi: 10.1073/pnas.1313898110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar D, Dong Z, Shanmughapriya S, Koch DA, Thomas T, Hoffman NE, Timbalia SA, Goldman SJ, Breves SL, Corbally DP, et al. MCUR1 Is a Scaffold Factor for the MCU Complex Function and Promotes Mitochondrial Bioenergetics. Cell Rep. 2016;15:1673–1685. doi: 10.1016/j.celrep.2016.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MF, Phillips CB, Ranaghan M, Tsai CW, Wu Y, Willliams C, Miller C. Dual functions of a small regulatory subunit in the mitochondrial calcium uniporter complex. Elife. 2016;5:e15545. doi: 10.7554/eLife.15545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.