Abstract
Background
In a search for an effective ‘anti-alcohol pill’, three modern anti-craving agents have been studied in alcoholics of Army/ DSC, Air Force, Navy and Coast Guard.
Methods
129 patients of alcohol dependence syndrome were randomly assigned to three groups where topiramate, acamprosate and naltrexone were used as anti-craving agents in a year long prospective study. Of these 92 patients completed the study.
Result and Conclusion
Topiramate (76.3%) appears to be significantly more effective (p<0.01) in sustaining abstinence, though naltrexone (57.7%) and acamprosate (60.70%) offer moderate relapse-prevention efficacy. Side effects of all the three agents have been mild, transient and self-limiting. We recommend a trial of topiramate, before invaliding out of any alcoholic soldier.
Key Words: Topiramate, Naltrexone, Acamprosate, Alcohol dependence syndrome
Introduction
Alcohol dependence is one of the most common behavioural disorders in the Armed Forces. It is the cause of up to 15.53-20.90% of all psychiatric admissions and many medical, surgical and traumatic emergencies [1]. Unfortunately the treatment package (detoxification, counseling, educational group therapy) offered traditionally at the service psychiatric centres has been only partially successful, at best. Other methods tried, viz., electric aversion therapy, agnihotra [2], yoga therapy did not find favour with many psychiatrists. Pharmacotherapies with metronidazole, citrated calcium carbamide, disulfiram and selective serotonin reuptake inhibitors (SSRIs) have not stood the test of time.
The recent Army Orders (AO 03/2001 and 01/ 2004), allow only one year of observation period in low medical category. At any time during this period if there be relapse the individual is to be invalided out of service. This has accelerated the search for an effective ‘anti-alcohol pill’ by the service psychiatrists. The authors were inspired by the recent optimistic reports in the international journals about the efficacy as well as the safety of three such agents viz., Topiramate, Naltrexone and Acamprosate and took up a pragmatic study, besides offering a modern empirical benefit to the alcoholic-soldiers.
Material and Methods
A prospective study of one year duration to compare the relative efficacy of topiramate, naltrexone and acamprosate in promoting abstinence in soldiers diagnosed on the ICD-10 criteria was conducted from February 04 to March 06. 129 uniformed personnel from the Army/DSC, Air Force, Navy and the Coast Guard with alcohol dependence were taken up for the study after informed consent. Both new cases and the old relapsed cases were included. Cases with significant alcoholic liver disease and ischaemic heart disease were excluded. This was not a randomised control trial, by design, as ethical considerations did not permit to give only a placebo or an exclusive psychosocial therapy.
The protocol was designed with the routine soldierly duties in mind, necessitating a fair degree of daytime alertness. Unlike western studies where topiramate was used between 200- 300 mg/ day, we decided to limit it to 100- 125 mg / day, which was given in two divided doses. Naltrexone was used at the standard dose of 50 mg/ day as a single morning dose daily. Acamprosate was given as 333 mg tablets in 2-1-1 or 2-2-2 dosage depending on whether the body weight was below or above 60 kg. These were started after detoxification and return of liver function test to near-normal levels, usually 3-4 weeks after hospitalization. Apart from the medication, all cases were offered counseling sessions, Alcoholics Anonymous meetings, individual psychotherapy, cognitive behavioural therapy and occupational-recreational therapy.
A detailed advice to the patient and his unit authorities was provided for regular provision of medication, compliance, close surveillance, prompt referral in case of relapse and cessation of the issuance of liquor quota.
Those who were posted in and around Bangalore were reviewed fortnightly/ monthly in the Psychiatry OPD and those at distant units were advised similar reviews by the nearest Medical Specialist/ Authorised Medical Attendant (AMA) at MI Room. All of them were admitted and observed for at least seven days, when they reported for re-categorization after six months and then after one year. Minor lapses (occasional and brief drinking of less than three days) and Relapses (resumption of continuous drinking) were decided by the following parameters: historically by the self-report, the spouse/next of kin (NOK) report (if available), the AMA's report and the commanding officer's (CO) report on AFMSF-10; clinically by the presence of the symptoms and signs of intoxication/ withdrawal/ target organ damage and investigations to assess mean cell volume (MCV), liver function tests (LFT) including aspartate aminotransferase (AST), alanine aminotransferase (ALT) and abdominal ultrasonography (USG).
Results
Of the 129 patients selected 11 did not present for follow up at six months/one year intervals, probably after being posted out. Of the 118 who were followed up regularly for one year, 41 were on topiramate, 37 on naltrexone and 40 on acamprosate. Only 92 patients could complete the study and the rest discontinued for various reasons as shown in Table 1. The socio-demographic variables of the 92 patients who completed the study is shown in Table 2.
Table 1.
Non-compliance rates
| Group | Discontinuation due to non-availability | Discontinuation due to side effects |
|---|---|---|
| Topiramate (n=41) | 2 (4.9%) | 1 (2.4%) |
| Naltrexone (n=37) | 8 (21.6%) | 3 (8.1%) |
| Acamprosate (n=40) | 7 (17.5%) | 5 (12.5%) |
Table 2.
Socio-demographic profiles
| Socio-demographic variables | Topiramate group (n=38) | Naltrexone group (n=26) | Acamprosate group (n=28) | Total (n=92) |
|---|---|---|---|---|
| Gender | All male | All male | All male | All male |
| Age | 29-55 year | 27- 51 year | 30-54 year | 27-55 year |
| (mean 39.5 years) | (mean 37.3 years) | (mean 38 years) | (mean 38.4 years) | |
| Education | V Std – Post Graduate | V Std – Post Graduate | V Std – Post Graduate | V Std – Post Graduate |
| Marital status | ||||
| Married | 31 (81.57%) | 22 (84.61%) | 23 (82.14%) | 76 (82.60%) |
| Unmarried | 03 (07.89%) | 02 (07.69%) | 01 (03.57%) | 06 (06.52%) |
| Divorced/separated | 02 (05.26%) | 02 (07.69%) | 03 (10.71%) | 07 (08.69%) |
| Widowers | 02 (05.26%) | 00 | 01 (03.57%) | 03 (03.26%) |
| Rank | ||||
| Officers | 04 (10.52%) | 03 (11.53%) | 02 (07.14%) | 09 (09.78%) |
| JCOs | 09 (23.68%) | 10 (38.46%) | 08 (28.57%) | 27 (29.34%) |
| SNCOs | 25 (65.79%) | 14 (53.85%) | 17 (60.71%) | 56 (60.86%) |
| Organisation | ||||
| Army/ DSC | 24 (63.16%) | 15 (57.69%) | 17 (60.71%) | 56 (60.86%) |
| Air Force | 09 (23.68%) | 06 (23.08%) | 06 (21.42%) | 21 (22.82%) |
| Navy | 02 (05.26%) | 04 (15.38%) | 03 (10.71%) | 09 (09.78%) |
| Coast Guard | 03 (07.89%) | 01 (03.85%) | 02 (07.14%) | 06 (06.52%) |
| Service | 11-28 year | 9-26 year | 10-27 year | 9-28 year |
| (mean 17.3 year) | (mean 15.1 year) | (mean 16.0 year) | (mean 16.2 year) |
The final outcomes at the end of one year are shown in Table 3 and Fig. 1. Of 38 cases on topiramate, 29 (76.3%) maintained total abstinence for one year, two (5.3%) had minor lapses and seven (18.4%) had frank relapse. Of 26 cases in naltrexone group, 15 (57.7%) maintained complete abstinence, while three (11.5%) had minor lapses and eight (30.8%) had frank relapse. Of the 28 on acamprosate, 17 (60.70%) maintained total abstinence for one year, two (7.20%) had minor lapses, and nine (32.10%) had frank relapse.
Table 3.
Efficacy comparisons on the final outcome in one year
| Outcome | Topiramate group (n=38) | Naltrexone group (n=26) | Acamprosate group (n=28) |
|---|---|---|---|
| Abstinences | 29 (76.3%) | 15 (57.7%) | 17 (60.70%) |
| Minor lapses | 02 (05.3%) | 03 (11.5%) | 02 (07.20%) |
| Relapses | 07 (18.4%) | 08 (30.8%) | 09 (32.10%) |
Fig. 1.
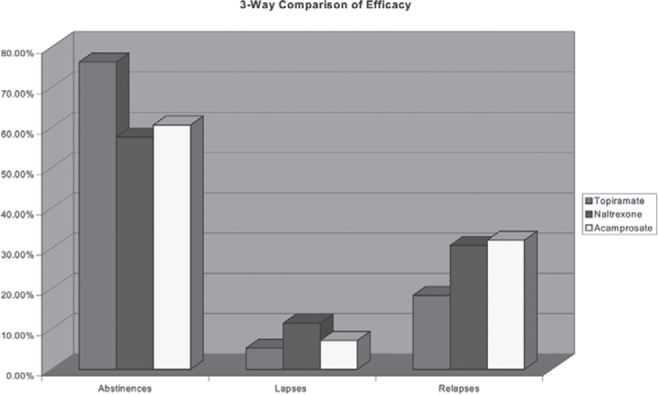
Efficacy comparisons of Topiramate, Naltrexone and Acamprosate
The profiles of side effect are shown in Table 4. Since the medications were started while the patients were still in hospital, the side effects were monitored closely and were noted to wear off quickly with education, reassurance, and general supportive measures. Topiramate group had fewer side effects, of mild intensity and in the initial days of therapy. None complained of impairment of memory/ concentration or word-naming difficulties.
Table 4.
Side effect profile
| Side effects | Topiramate group (n=38) | Naltrexone group (n=26) | Acamprosate group (n=28) |
|---|---|---|---|
| Nausea | - | 03 (11.5%) | 07 (25.0%) |
| Vomiting | - | - | - |
| Mild dizziness | 03 (07.9%) | 01 (03.8%) | - |
| Headache | - | 01 (03.8%) | 01 (03.57%) |
| Constipation | - | 02 (07.6%) | - |
| Fatigue | - | 03 (11.4%) | |
| Anxiety | - | 06 (23.07%) | |
| Nervousness | - | 06 (23.07%) | |
| Mild sedation | 05 (13.5%) | - | - |
| Insomnia | - | 04 (15.4%) | - |
| Tingling in skin | 01 (02.6%) | - | 02 (07.14%) |
| Psychomotor | 04 (10.5%) | - | - |
| slowing | |||
| Weight loss | 01 (02.6%) | - | - |
| Clinical signs of | - | - | - |
| organ dysfunction | |||
| Metabolic derangement | - | - | - |
| Diarrhoea | - | - | 06 (21.42%) |
| Pruritus | - | - | 02 (07.14%) |
| Confusion | - | - | - |
| Sexual dysfunction | - | - | 01 (03.57%) |
Naltrexone group mostly reported mild side effects which subsided quickly, but one side effect of concern was its unpleasant dysphoric side effect (anxiety, nervousness and sleep disturbances). None complained of vomiting.
Acamprosate group also reported mild side effects. Diarrhoea was most common which subsided with conservative measures. None complained of confusion.
No serious adverse events were noted in any of the three groups. In the abstinent and the only-occasionally-lapsed patients, clinical examinations and laboratory investigations did not reveal any metabolic or other organ dysfunction.
Discussion
Topiramate facilitates gamma amino butyric acid
(GABA) function and hence inhibits dopamine release in the midbrain. It can reduce the rewarding effect of pleasure and thus the craving in alcoholics by decreasing the mesocortico-limbic dopamine activity after alcohol intake. It can also antagonize the chronic changes induced by alcohol by decreasing the toxic glutamate activity at the kainate glutamate receptors.
Several studies have shown that topiramate is an effective anti-craving and abstinence-promoting agent in alcoholics [3, 4, 5, 6, 7, 8]. Johnson BA [9], showed that topiramate is a promising medication for treating the co-morbid alcohol and nicotine dependence due to its neuro-modulation of mesocortico-limbic dopamine function. Johnson et al [10] found that topiramate reduces the consequences of drinking and improves the quality of life. Myrick et al [11], noted that better understanding of the neuro-scientific underpinnings of addiction has led to the use of novel pharmacotherapeutic treatments for alcoholism, esp. naltrexone, acamprosate and topiramate. Kenna et al [12] felt that with the advent of more-efficacious medications like topiramate a transformation must occur in how alcoholism-treatment is viewed not only by the public but also by the clinicians. Addolarato et al [13] have recently reported that topiramate is not only an effective but also a safe GABAergic agent for treating alcoholism.
Naltrexone (an opiate antagonist), blocks the reward centres of the brain and thus blocks the pleasurable effects of alcohol. Several workers [14, 15, 16], have reported that naltrexone can lead to a reduction in alcohol consumption in alcoholics. Kiefer et al [17] found that naltrexone and acamprosate, especially in combination, considerably enhances relapse-prevention in alcoholics. However, a German multicentre study confirmed the safety but not the efficacy of naltrexone in the prevention of relapses in alcoholics [18].
Acamprosate, with a structure similar to GABA, normalizes the dysregulation of N-methyl-D-aspartate (NMDA)-mediated glutamatergic excitation that occurs in alcohol withdrawal and early abstinence [19]. It has also been approved for treatment of alcohol dependence in United States [20]. Acamprosate's effects on alcohol dependence have been examined in several European studies with over 4000 patients. In most of these studies, treatment with acamprosate was associated with higher rates of treatment completion, longer abstinence period to first drink and higher overall abstinence rates compared with placebo [21, 22].
Topiramate demonstrates significantly higher total abstinence, fewer minor lapses and decreased relapses, while naltrexone and acamprosate show moderate efficacy which is less than that of topiramate (Table 5, Table 6). Our study agrees with the encouraging reports by others [14, 15, 16] in the use of naltrexone when total abstinence is considered, but when degree-of-improvement or relapse rates are considered this study is more in agreement with the German multicentric study by Gastpar et al [18] in that naltrexone lacks efficacy in the prevention of relapses. With respect to acamprosate its efficacy is almost similar to naltrexone.
Table 5.
3 way efficacy comparison
| Relapses (%) | No relapses (%) | Total | |
|---|---|---|---|
| Topiramate | 18.40 | 81.60 | 100 |
| Naltrexone | 30.80 | 69.20 | 100 |
| Acamprosate | 39.30 | 60.70 | 100 |
| Total | 88.5 | 211.5 | 300 |
Degrees of freedom: 2; Chi-square = 10.623; p ≤ 0.01(S)
Table 6.
Splitting of comparison without Topiramate
| Relapses (%) | No relapses (%) | Total | |
|---|---|---|---|
| Naltrexone | 30.80 | 69.20 | 100 |
| Acamprosate | 39.30 | 60.70 | 100 |
| Total | 70.1 | 129.9 | 200 |
Degrees of freedom: 1; Chi-square = 1.586; p ≤(NS).
It is of interest to note that naltrexone and acamprosate had a much higher degree of drop-out rates than topiramate due to non-availability of the medicine. It means that in our country naltrexone and acamprosate could be difficult to procure, whereas topiramate is widely available due to its extensive use in epilepsies and pain syndromes. The side effect profiles noted for all the three medicines make all of them remarkably safe. No serious adverse events were reported in any group.
We feel that topiramate is worth trying in majority of alcoholics, not only for its reckonable anti-craving effects but also for its abstinence-promoting, thymostatic (anxiety, depression, restlessness and excitability are commonly associated with alcoholism) and mild sleep-promoting (insomnia is common in alcoholics) properties. Topiramate is excreted by kidneys which makes it a preferable agent in alcoholics, where liver function may be deranged.
This study had some limitations because of the inherent demographics of the serving soldier population, where only young, middle-aged male alcoholics could be studied. No placebo for comparison was used, because some medication of promise had to be offered to all the soldier-alcoholics to maximize the chances of abstinence. No randomisation or double-blinding was employed, as explicit instructions were to be given to the unit authorities to procure the particular medicine and supply it to a particular patient after discharge. The occupation as a variable, which could influence the outcome could not be controlled in this study.
In the outcome, the contribution of counseling, individual psychotherapy, alcholic annoymous meetings, cognitive – behavioural therapy, other psychosocial therapies, and of the administrative corrective measures taken at the unit level cannot be delineated. The study period was limited to one year, as that was the maximum period of observation permissible by the Army Order.
The findings of this study need further validation by larger multi-centre studies over longer periods.
Conflicts of Interest
None identified
Intellectual Contribution of Author
Study Concept : Wg Cdr PL Narayana
Drafting & Manuscript Revision : Wg Cdr PL Narayana, Sqn Ldr AK Gupta
Statistical Analysis : Wg Cdr PL Narayana
Study Supervision : Wg Cdr PL Narayana, Sqn Ldr AK Gupta, Wg Cdr PK Sharma
References
- 1.Raju MSVK, Valdiya PS, Tampi UR. Alcoholism in the Armed Forces. MJAFI. 2002;58:149–151. [Google Scholar]
- 2.Golechha GR, Sethi IC, Deshpande PL, Agnihotra U. The treatment of alcoholism. Indian J Psychiatry. 1991;33:44–47. [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K. Oral Topiramate for treatment of alcohol dependence: A randomised controlled trial. Lancet. 2003;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- 4.Swift RM. Topiramate for the treatment of alcohol dependence: initiating abstinence. Lancet. 2003;361:1666–1667. doi: 10.1016/S0140-6736(03)13378-8. [DOI] [PubMed] [Google Scholar]
- 5.Komanduri R. Two cases of alcohol craving curbed by Topiramate. J Clin Psychiatry. 2003;64:612. doi: 10.4088/jcp.v64n0518d. [DOI] [PubMed] [Google Scholar]
- 6.Anderson N, Oliver MN. Oral Topiramate effective for alcoholism. J Fam Pract. 2003;52:682–687. [PubMed] [Google Scholar]
- 7.Rubio G, Ponce G, Jimenez-Arriero MA, Palomo T, Manzanares J, Ferre F. Effects of topiramate in the treatment of alcohol dependence. Pharmacopsychiatry. 2004;37:37–40. doi: 10.1055/s-2004-815473. [DOI] [PubMed] [Google Scholar]
- 8.Johnson BA. Progress in the development of topiramate for treating alcohol dependence: from a hypothesis to a proof-ofconcept study. Alcohol Clin Exp Res. 2004;28:1137–1144. doi: 10.1097/01.alc.0000134533.96915.08. [DOI] [PubMed] [Google Scholar]
- 9.Johnson BA. An overview of the development of medications including novel anticonvulsants for the treatment of alcohol dependence. Expert Opin Pharmacother. 2004;5:1943–1955. doi: 10.1517/14656566.5.9.1943. [DOI] [PubMed] [Google Scholar]
- 10.Johnson BA, Akhtar FZ, Ait-Daoud N, Ma JZ. Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol-dependent individuals: a randomized controlled trial. Arch Gen Psychiatry. 2004;61:905–912. doi: 10.1001/archpsyc.61.9.905. [DOI] [PubMed] [Google Scholar]
- 11.Myrick H, Anton R. Recent advances in the pharmacotherapy of alcoholism. Curr Psychiatry Rep. 2004;6:332–338. doi: 10.1007/s11920-004-0019-7. [DOI] [PubMed] [Google Scholar]
- 12.Kenna GA, McGeary JE, Swift RM. Pharmacotherapy, pharmacogenomics, and the future of alcohol dependence treatment. Am J Health Syst Pharm. 2004;61:2272–2279. doi: 10.1093/ajhp/61.21.2272. [DOI] [PubMed] [Google Scholar]
- 13.Addolorato G, Johnson BA, Swift RM, Ciraulo DA, Myrick H. Safety and efficacy of GABAergic medications for treating alcoholism. Alcohol Clin Exp Res. 2005;29:248–254. doi: 10.1097/01.alc.0000153542.10188.b0. [DOI] [PubMed] [Google Scholar]
- 14.Rubio G, Manzanares J, Lopez-Munoz F. Naltrexone improves outcome of a controlled drinking program. J Subst Abuse Treat. 2002;23:361–366. doi: 10.1016/s0740-5472(02)00296-9. [DOI] [PubMed] [Google Scholar]
- 15.Drobes DJ, Anton RF, Thomas SE, Voronin K. A clinical laboratory paradigm for evaluating medication effects on alcohol consumption: Naltrexone and Nalmefene. Neuro Psychopharmacology. 2003;28:755–764. doi: 10.1038/sj.npp.1300101. [DOI] [PubMed] [Google Scholar]
- 16.O'Malley SS, Rounsaville BJ, Farren C, Namkoong K, Wu R, Robinson J, O'Connor PG. Initial and maintenance Naltrexone treatment for alcohol dependence using primary care vs specialty care: a nested sequence of 3 randomized trials. Arch Intern Med. 2003;163:1695–1704. doi: 10.1001/archinte.163.14.1695. [DOI] [PubMed] [Google Scholar]
- 17.Kiefer F, Jahn H, Tarnaske T, Helwig H, Briken P, Holzbach R. Comparing and combining Naltrexone and Acamprosate in relapse prevention of alcoholism: a double-blind, placebocontrolled study. Arch Gen Psychiatry. 2003;60:92–99. doi: 10.1001/archpsyc.60.1.92. [DOI] [PubMed] [Google Scholar]
- 18.Gastpar M, Bonnet U, Boning J, Mann K, Schmidt LG, Soyka M. Lack of efficacy of Naltrexone in the prevention of alcohol relapse: results from a German multicenter study. J Clin Psychopharmacol. 2002;22:592–598. doi: 10.1097/00004714-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Jung YC, Namkoong K. Pharmacotherapy for alcohol dependence: anticraving medications for relapse prevention. Yonsei Med J. 2006;47:167–178. doi: 10.3349/ymj.2006.47.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chawla PS, Kochar MS. What's new in clinical pharmacology and therapeutics. Wis Med J. 2006;105:24–29. [PubMed] [Google Scholar]
- 21.Mason BJ. Acamprosate and Naltrexone treatment for alcohol dependence: an evidence-based risk-benefits assessment. Eur Neuropsychopharmacol. 2003;13:469–475. doi: 10.1016/j.euroneuro.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Mason BJ. Treatment of alcohol-dependent out patients with Acamprosate: a clinical review. J Clin Psychiatry. 2001;62(Suppl 20):42–48. [PubMed] [Google Scholar]


