Abstract
Internet gaming disorder (IGD) is characterized by high levels of craving for online gaming and related cues. Since addiction-related cues can evoke increased activation in brain areas involved in motivational and reward processing and may engender gaming behaviors or trigger relapse, ameliorating cue-induced craving may be a promising target for interventions for IGD. This study compared neural activation between 40 IGD and 19 healthy control (HC) subjects during an Internet-gaming cue-reactivity task and found that IGD subjects showed stronger activation in multiple brain areas, including the dorsal striatum, brainstem, substantia nigra, and anterior cingulate cortex, but lower activation in the posterior insula. Furthermore, twenty-three IGD subjects (CBI + group) participated in a craving behavioral intervention (CBI) group therapy, whereas the remaining 17 IGD subjects (CBI − group) did not receive any intervention, and all IGD subjects were scanned during similar time intervals. The CBI + group showed decreased IGD severity and cue-induced craving, enhanced activation in the anterior insula and decreased insular connectivity with the lingual gyrus and precuneus after receiving CBI. These findings suggest that CBI is effective in reducing craving and severity in IGD, and it may exert its effects by altering insula activation and its connectivity with regions involved in visual processing and attention bias.
Keywords: Internet gaming disorder, fMRI, Cue reactivity, Craving, Intervention
Highlights
-
•
IGD subjects showed altered cue-induced neural activation in reward-related areas.
-
•
IGD subjects alleviated IGD symptoms after CBI.
-
•
IGD subjects showed higher insular activation after CBI.
-
•
IGD subjects showed lower insula-lingual gyrus/precuneus connectivity after CBI.
1. Introduction
Internet gaming disorder (IGD) constitutes a serious mental health issue worldwide, requiring additional investigation, as exemplified by its inclusion in section 3 of the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) as a topic deserving more research (American Psychiatric Association, 2013, Potenza, 2015). Craving is a hallmark feature of addictive disorders (Courtney et al., 2016, Engelmann et al., 2012), including IGD (Han et al., 2010a, Ko et al., 2009a). Similar to addictive drugs (e.g., stimulants), gaming may induce dopamine release, particularly in mesocorticolimbic pathways (Han et al., 2007, Kim et al., 2011, Koepp et al., 1998, Tian et al., 2014). Exposure to gaming-related cues may increase the salience of gaming-related cues and promote craving, which in turn may promote the development of IGD and exacerbate its symptoms (Ko et al., 2009a, Ko et al., 2013a). Considering the rewarding and motivational attributions of cue-induced craving, it has been hypothesized to be a promising target for interventions for IGD (Dong and Potenza, 2014, King and Delfabbro, 2014).
Cue-reactivity tasks represent valid and reliable measures to evaluate craving (Wilson et al., 2004) and provide important insight into motivational and reward dysfunctions in addictions (Courtney et al., 2016). Several studies have used cue-reactivity tasks to examine cue-induced craving in IGD and have shown that gaming pictures activate brain regions responsible for reward and motivational processing, such as the striatum and insula, in IGD subjects (IGDs) compared with healthy control subjects (HCs) (Han et al., 2010a, Ko et al., 2009a, Ko et al., 2013b). These findings are largely consistent with observations in substance dependence and pathological gambling (Engelmann et al., 2012, Goudriaan et al., 2013) and suggest there may be shared neural substrates between IGD and other addictions (Kuss and Griffiths, 2012). Moreover, although direct evidence in the field of IGD is still lacking, studies in substances-use disorders link craving with efficacies of interventions, with responsiveness being a strong predictor of relapse, even years after completing interventions (Courtney et al., 2016, Killen et al., 1992). These findings suggest that ameliorating cue-induced craving and altering responsivity of the underlying neural substrates may achieve promising treatment outcomes.
Cue-reactivity tasks provide a reliable way to investigate the neural mechanisms by which interventions may operate; however, to the best of our knowledge, only two studies have examined how interventions exert effects on cue-induced brain activation in IGD. Specifically, one study showed that 6-weeks of treatment with bupropion decreased cue-induced craving and activation in the left superior frontal gyrus in IGDs (Han et al., 2010a), whereas another study found that family therapy increased family cohesion and decreased gaming-cue-induced brain activation in frontal and occipital regions (Han et al., 2012). However, no existing fMRI study has investigated how an integrated behavioral intervention specifically targeting craving operates on neural levels. Behavioral rather than pharmacological interventions predominate in IGD studies, although this field is still nascent and more evidence is needed (King and Delfabbro, 2014, Winkler et al., 2013, Young, 2011, Young, 2013). Furthermore, behavioral interventions integrating multiple strategies (e.g., mindfulness, cognitive remediation) may reduce craving more efficiently than any of these strategies alone (Potenza et al., 2011, Young, 2011). For this reason, studies that evaluate neural effects of an integrated behavioral intervention targeting craving are necessary in the field of IGD since they may promote improved understanding of underlying mechanisms of IGD and provide insight into possible ways to enhance treatment efficacy.
In the current study, the main aim was to examine the effects of a craving behavioral intervention (CBI), which was developed to reduce craving for gaming, on cue-induced craving and neural activation in regions involved in reward and motivational processing. Furthermore, we aimed to investigate the functional connectivity of the regions altered by CBI with other regions to explore the neural networks through which CBI may operate. Based on previous findings, we hypothesized that, compared with HCs, IGDs would exhibit stronger brain activation in reward-related areas (e.g., ventral striatum, dorsal striatum, insula, anterior cingulate cortex, posterior cingulate cortex, substantia nigra) that have been implicated in cue-induced craving (Engelmann et al., 2012, Jasinska et al., 2014, Meng et al., 2014). We also hypothesized that CBI may exert its effects by decreasing brain activation in regions involved in reward processing and enhancing brain activation in regions involved in cognitive control (e.g., dorsolateral prefrontal cortex) (Konova et al., 2013, Yalachkov et al., 2010).
2. Materials and methods
2.1. Ethics statement
This study complied with the Declaration of Helsinki. All participants provided written informed consent and were financially compensated for their time. The protocol was approved by the Institutional Review Board of the State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University.
2.2. Participants
This study was part of a larger study of developing and evaluating an effective psychobehavioral intervention for IGD. Participants were recruited by means of online advertisements and word of mouth and were selected through an online questionnaire and in-person semi-structured screening. A total of 44 IGDs and 22 HCs participated in the fMRI cue-reactivity task based on their willingness and suitability for fMRI, and all participants were right-handed males. Because 4 IGDs and 3 HCs were excluded due to excessive head motion; thus, data from 40 IGDs and 19 HCs were included in final analyses.
Participants were selected according to their weekly Internet gaming time and scores on the Chen Internet addiction scale (CIAS; Chen et al., 2003). The CIAS consists of 26 items on a 4-point Likert scale (range: 26–104). Inclusion criteria for IGDs were the same as in previous studies (Liu et al., 2016, Yao et al., 2015, Zhang et al., 2016a, Zhang et al., 2016b) and included: 1) a score of 67 or higher on the Chen Internet Addiction Scale (CIAS) (Chen et al., 2003, Ko et al., 2009b); 2) engagement in Internet gaming for over 14 h per week for a minimum of one year; and, 3) reporting of one of the most popular Internet games as their primary online activity (Cross Fire: 4, Defense of the Ancient version 1: 11, Defense of the Ancient version 2: 2, League of Legends: 21, World of Warcraft: 2).
The inclusion criteria for HCs were: 1) a score of 60 or lower on the CIAS; and 2) never or occasional engagement (< 2 h per week) in Internet gaming. Ko et al. (2009b) suggest CIAS scores of 63 or lower identify HCs. We used a more conservative CIAS threshold (60 or lower) and a time limit for weekly gaming to ensure that HCs were free from IGD (Yao et al., 2015, Zhang et al., 2016a, Zhang et al., 2016b).
Participants who reported current or history of use of illegal substances and any gambling experience (including online gambling) were excluded given the illegality of gambling in China. Additional exclusion criteria were assessed through a semi-structured personal interview, consistent with previous studies in IGD (Yao et al., 2015, Zhang et al., 2016a). Exclusion criteria included: (1) any self-reported history of any psychiatric or neurological illness; and, (2) current use of any psychotropic medication.
Twenty-three IGDs (CBI + group) were willing to participate in a 6-week group CBI and were scanned before and after the CBI. The remaining 17 IGDs (CBI − group) did not receive any intervention and were scanned twice, with similar intervals between scans as for the CBI + group.
2.3. Craving behavioral intervention (CBI)
The integrated CBI was developed on the basis of behavioral intervention theories (Dong and Potenza, 2014), the craving framework of boundary conditions (McCarthy et al., 2010), and the fulfillment of psychological needs for Internet use (Suler, 1999). Since craving may impact significantly the development and maintenance of IGD, methods that help subjects to cope and reduce craving may improve therapeutic outcomes and prevent relapse (Brand et al., 2014, Dong and Potenza, 2014). CBI was conducted weekly with 8 to 9 IGD subjects in each group. The topic for each session was: 1) perceiving subjective craving; 2) recognizing and testing irrational beliefs regarding craving; 3) detecting craving and relieving craving-related negative emotions; 4) training in coping with cravings and altering participants' fulfillment of psychological needs; 5) learning time management and skills training for coping with craving; 6) reviewing, practicing, and implementing skills. In addition, mindfulness training was included in each session.
2.4. Questionnaires
Current status of depression and anxiety was assessed using the Beck Depression Inventory (Beck et al., 1961) and the Beck Anxiety Inventory (Beck et al., 1988), respectively. Cigarette and alcohol use was recorded, and the Fagerstrom Test for Nicotine Dependence (Fagerstrom, 1978) and alcohol consumption questions from the Alcohol Use Disorders Identification Test (Bush et al., 1998) were used to assess nicotine dependence and hazardous alcohol use, respectively.
2.5. fMRI cue-reactivity task
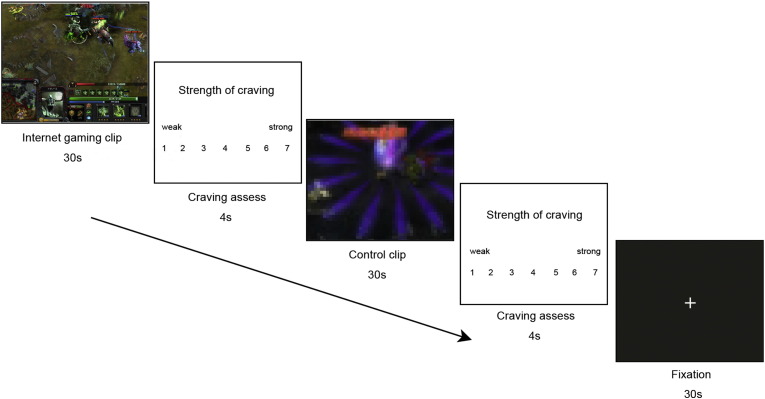
The block-design cue-reactivity task was adopted from previous studies (Han et al., 2010a, Han et al., 2010b). Participants were asked to passively watch three kinds of videos and rate their craving immediately after each video clip using 7-point visual analog scales. Six 30-second gaming video clips (G) were screen shots selected from official websites or gaming forums by 10 additional Internet gaming players (2 players for each of 5 following popular Internet games: Cross Fire, Defense of the Ancient version 1, Defense of the Ancient version 2, League of Legends, World of Warcraft) who did not participate subsequently in the fMRI and intervention study. The type of the gaming clips was individualized for IGDs' primary game and randomly assigned to HCs who did not play Internet games.
Matched control video (C) clips were selected from an unpopular online game which was not known to or played by any participants in the study. These clips were further obscured (as shown in Fig. 1) so that participants were unable to recognize the contents and details of these clips. We performed such manipulations to control for the possible effects of movement and color in gaming clips. In addition, six 30-second white-cross/black-background (Fixation, F) images were used as baseline. The order of the clips was fixed: G-F-C G-C-F C-F-G C-G-F F-C-G F-G-C. Each clip was followed by a 4-second rating screen. This task was presented by E-Prime 2.0 and lasted for 620 s. The graphical design of the task is shown in Fig. 1.
Fig. 1.
Schematic illustration of 2 blocks of the fMRI Internet-gaming cue-reactivity task.
2.6. Imaging acquisition and preprocessing
Data were acquired using a 3.0 T SIEMENS Trio scanner in the Imaging Center for Brain Research, Beijing Normal University. A gradient-echo echo-planar imaging (EPI) sequence was obtained (TR = 2000 ms; TE = 25 ms; flip angle = 90°; matrix = 64 × 64; resolution = 3 × 3 mm2; slices = 41). The slices were tilted 30° clockwise from the AC-PC plane to obtain better signals in frontal regions. A T1-weighted sagittal scan was acquired for anatomical reference with EPI data (TR = 2530 ms, TE = 3.39 ms, TI = 1100 ms, FA = 7°, FOV = 256 × 256 mm2, voxel size = 1 × 1 × 1.3 mm3, slice = 144).
Imaging data were pre-processed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8). Functional data were realigned, coregistered with the structural images, segmented for normalization to the standard MNI space, and smoothed with a 5-mm Gaussian kernel at full width at half maximum (FWHM). Subjects with head motion > 3 mm or 3° were excluded from further analysis (4 IGDs and 3 HCs were excluded).
2.7. Behavioral data analysis
Behavioral data were analyzed using SPSS version 20.0. Differences in baseline demographic, Internet-gaming characteristics (CIAS scores, durations of weekly gaming), and cue-induced craving between IGDs and HCs were analyzed using independent t-tests. Effects of CBI on cue-induced craving and Internet-gaming characteristics were analyzed using analyses of variance (ANOVAs) with repeated measures with group (CBI + and CBI −) as a between-subject factor and session (baseline and second tests) as a within-subject factor. The significance level was P < 0.05.
2.8. fMRI data analysis
Imaging data were analyzed using SPM8. Three regressors were distinguished: gaming and control videos and craving ratings. Regressors were constructed by convolving the onsets of these stimuli with a canonical hemodynamic response function. Six realignment parameters were also included as regressors of no interest. A high-pass filter (128 Hz) was applied to remove low-frequency signal drift. In the first-level fixed-effects analysis, a contrast image of gaming > control videos was built to examine cue-induced brain activation. To compare cue-induced activation between IGDs and HCs at baseline, contrast images were entered into a second-level random-effects analysis using a two-sample t-test. To examine for a group (CBI + and CBI −) by session (baseline and second tests) interaction on cue-induce activation, contrast images were entered into a second-level random-effects analysis using a flexible factorial design. To assess functional connectivity between regions associated with cue reactivity (gaming > control clips) that changed between the two sessions, we conducted a psychophysiological interaction (PPI) analysis in the CBI + and the CBI − groups using a flexible factorial design. At the group level, whole-brain analysis was performed to compare baseline cue-induced brain activation between IGDs and HCs and was corrected by means of Gaussian Random Field Theory (GRFT) with voxel-level P < 0.001 and cluster-level P < 0.05 to result in a family-wise error rate of 5%. For exploratory purposes, the group-by-session interaction on cue-induced activation and functional connectivity were corrected by a more liberal criterion (voxel level P < 0.005 and cluster-level P < 0.05). The results were visualized using BrainNet Viewer (Xia et al., 2013) and DPABI (http://rfmri.org/dpabi).
3. Results
3.1. Demographics and Internet gaming characteristics analyses
As shown in Table 1, IGDs and HCs did not differ on age, education, or use of alcohol and cigarettes. Consistent with the inclusion criteria, IGDs scored higher on the CIAS and reported higher craving for both gaming and control clips and fixation compared with HCs. In addition, IGDs showed higher levels of anxiety and depression.
Table 1.
Demographics and Internet gaming characteristics of IGDs and HCs at baseline.
| IGDs (n = 40) |
HCs (n = 19) |
t/χ2 value | P | Effect sizea | |
|---|---|---|---|---|---|
| Mean ± S.D. | Mean ± S.D. | ||||
| Age, years | 21.95 ± 1.84 | 22.89 ± 2.23 | − 1.72 | 0.091 | − 0.47 |
| Years of education | 15.75 ± 1.90 | 16.58 ± 1.98 | − 1.54 | 0.13 | − 0.43 |
| CIAS score | 79.88 ± 8.67 | 42.11 ± 8.27 | 15.86 | < 0.001 | 4.42 |
| Durations of weekly gaming, hours | 27.26 ± 10.58 | 1.67 ± 0.58b | 15.00 | < 0.001 | 8.98 |
| Craving for gaming clips | 5.36 ± 1.18 | 2.06 ± 1.57 | 8.99 | < 0.001 | 2.51 |
| Craving for control clips | 3.61 ± 1.36 | 1.75 ± 1.15 | 5.13 | < 0.001 | 1.43 |
| Craving for fixation | 3.75 ± 1.24 | 1.52 ± 0.61 | 9.24 | < 0.001 | 2.57 |
| Craving differences (gaming – control) | 1.75 ± 1.21 | 0.31 ± 0.59 | 6.14 | < 0.001 | 1.71 |
| BAI score | 5.35 ± 5.82 | 2.00 ± 3.18 | 2.85 | 0.006 | 0.79 |
| BDI score | 9.13 ± 5.35 | 2.79 ± 4.21 | 4.53 | < 0.001 | 1.26 |
| Alcohol use | 30/40 | 13/19 | 0.28 | 0.60 | 0.07 |
| AUDIT-C score | 3.20 ± 1.90c | 2.23 ± 1.17d | 1.70 | 0.10 | 0.56 |
| Tobacco use | 4/40 | 0/19 | – | – | – |
| FTND score | 3.25 ± 0.50e | – | – | – | – |
IGDs = Internet gaming disorder subjects; HCs = healthy control subjects; S.D. = standard deviation; CIAS = Chen Internet addition scale; AUDIT-C = alcohol consumption questions from the Alcohol Use Disorders Identification Test; FTND = Fagerstrom test for nicotine dependence; BAI = Beck Anxiety Inventory; BDI = Beck Depression Inventory.
Cohen's d value for t-tests and Cramer's V value for χ2 test.
n = 3.
n = 30.
n = 13.
n = 4.
3.2. Effects of CBI on behavioral measures
The CBI + and CBI − groups were matched well in age, education, and anxiety and depression symptoms at baseline (Table 2). ANOVAs with repeated measures on CIAS scores, (main effect of session: F(1,38) = 77.83, P < 0.001, partial η2 = 0.67; main effect of group: F(1,38) = 1.15, P = 0.29, partial η2 = 0.03; interaction effect: F(1,38) = 22.65, P < 0.001, partial η2 = 0.37), durations of weekly gaming (main effect of session: F(1,38) = 12.57, P = 0.001, partial η2 = 0.25; main effect of group: F(1,38) = 5.58, P = 0.02, partial η2 = 0.13; interaction effect: F(1,38) = 4.34, P = 0.04, partial η2 = 0.10), and gaming-related craving (main effect of session: F(1,38) = 25.77, P < 0.001, partial η2 = 0.40; main effect of group: F(1,38) = 4.40, P = 0.04, partial η2 = 0.10; interaction effect: F(1,38) = 5.73, P = 0.02, partial η2 = 0.13) showed similar results.
Table 2.
Demographics and Internet gaming characteristics of CBI + and CBI − groups.
| CBI + (n = 23) |
CBI − (n = 17) |
t value | P | Cohen's d value | |
|---|---|---|---|---|---|
| Mean ± S.D. | Mean ± S.D. | ||||
| Age | 21.91 ± 1.83 | 22.00 ± 1.90 | t (38) = − 0.15 | 0.89 | − 0.05 |
| Years of education | 16.09 ± 1.86 | 15.29 ± 1.93 | t (38) = 1.31 | 0.20 | 0.43 |
| BAI score | 3.78 ± 3.61 | 7.63 ± 7.73 | t (38) = − 1.85 | 0.08 | − 0.60 |
| BDI score | 8.83 ± 5.73 | 9.56 ± 5.09 | t (38) = − 0.41 | 0.46 | − 0.13 |
| CIAS score: baseline | 82.09 ± 8.75 | 76.88 ± 7.85 | t (38) = 1.94 | 0.06 | 0.63 |
| CIAS score: second test | 60.26 ± 9.83 | 70.35 ± 7.80 | t (38) = − 3.49 | 0.001 | − 1.13 |
| Durations of weekly gaming, hours: baseline | 27.20 ± 10.42 | 27.35 ± 11.13 | t (38) = − 0.05 | 0.96 | − 0.02 |
| Durations of weekly gaming, hours: second test | 11.36 ± 8.07 | 23.24 ± 17.51 | t (38) = − 2.88 | 0.007 | − 0.93 |
| Craving for gaming clips: baseline | 5.30 ± 1.21 | 5.43 ± 1.17 | t (38) = − 0.33 | 0.74 | − 0.11 |
| Craving for gaming clips: second test | 3.42 ± 1.50 | 4.75 ± 1.44 | t (38) = − 2.82 | 0.008 | − 0.91 |
CBI + = subjects with Internet gaming disorder who received craving behavioral intervention; CBI − = subjects with Internet gaming disorder who did not receive craving behavioral intervention; S.D. = standard deviation; CIAS = Chen Internet addition scale; BAI = Beck Anxiety Inventory; BDI = Beck Depression Inventory.
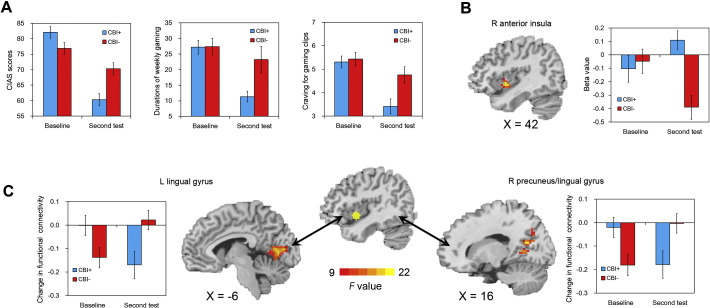
As shown in Table 2, single comparisons for session indicated that the CBI + and CBI − groups did not differ significantly on baseline CIAS scores, durations of weekly gaming, and gaming-related craving, but the CBI + group compared to the CBI − group showed significant reductions on these measures at the second test. Moreover, single comparisons for group indicated that the CBI + group showed significant reductions on CIAS scores (t(22) = 9.49, P < 0.001, d = 2.34), durations of weekly gaming (t(22) = 6.88, P < 0.001, d = 1.69), and gaming-related craving (t(22) = 5.21, P < 0.001, d = 1.38), but the CBI − group only showed significant reduction on CIAS scores with a smaller effect size (t(16) = 3.16, P < 0.001, d = 0.84) at the second test compared with baseline (Fig. 3).
Fig. 3.
Panel A: CIAS scores, durations of weekly gaming, and craving for gaming clips across groups and sessions. Panel B: Internet-gaming cue-induced activation in the right anterior insula across groups and sessions. Panel C: Functional connectivity (gaming versus control clips) between the right anterior insula and left lingual gyrus (right) and right precuneus/lingual gyrus (left) across groups and sessions. CIAS = Chen Internet addition scale; R = right; L = left.
3.3. fMRI results
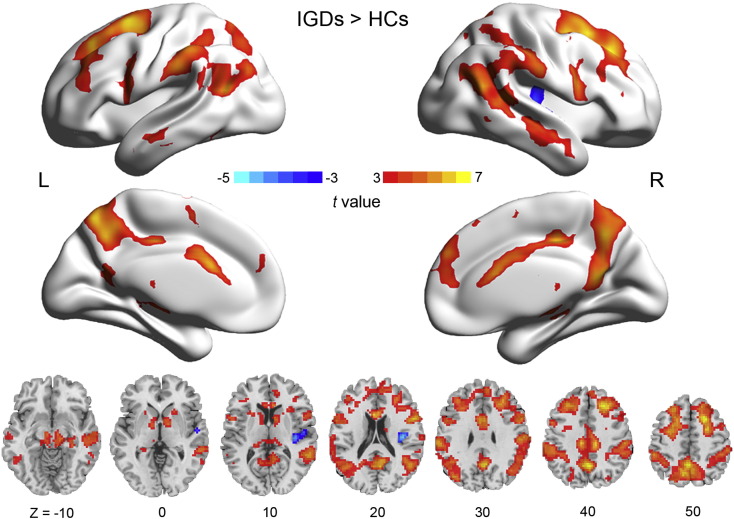
First, we conducted a two-sample t-test between the two IGD subgroups (CBI + and CBI −) at baseline. Since no significant differences between the CBI + and CBI − groups were identified, we combined them into an IGD group for subsequent baseline analyses. When comparing gaming-cue-induced brain activation between IGDs and HCs at baseline using a two-sample t-test, IGDs as compared with HCs showed greater activation in multiple brain regions including the dorsal striatum (caudate), brainstem, substantia nigra, anterior cingulate cortex, and posterior cingulate cortex; lower activation was observed in a relatively posterior portion of the right insula (Table 3 and Fig. 2). We further conducted correlational analyses between the mean beta value of these clusters and differences in craving intensities for gaming versus control clips and found a significant positive association in the MTG (r = 0.34, P = 0.035).
Table 3.
fMRI analysis results.
| Brain region | Side | BA | Cluster size | MNI coordinate |
Peak t/F values | Effect sizea | |||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||||
| Baseline: IGDs > HCs | Brainstem/caudate | L | 62 | − 6 | − 15 | − 9 | 4.57 | 1.21 | |
| Brainstem/SN | R/L | 92 | 0 | − 24 | − 24 | 5.01 | 1.33 | ||
| Precuneus/PCC/ACC | R/L | 7/24/31 | 1478 | 3 | − 57 | 45 | 6.84 | 1.81 | |
| MFG/ACC | R | 9/10 | 104 | 6 | 51 | 33 | 4.96 | 1.31 | |
| IPL/MTG | L | 40 | 649 | − 48 | − 60 | 15 | 5.68 | 1.50 | |
| IPL/STG | R | 39/40 | 740 | 51 | − 30 | 45 | 5.95 | 1.58 | |
| IFG | R | 9/44 | 188 | 57 | 9 | 21 | 5.72 | 1.52 | |
| IFG | L | 9/44 | 147 | − 54 | 9 | 33 | 4.81 | 1.27 | |
| MFG | R | 6/8/9 | 924 | 24 | 30 | 42 | 7.04 | 1.86 | |
| MFG/SFG | L | 6/8/9 | 855 | − 24 | 6 | 63 | 6.97 | 1.85 | |
| MTG | R | 21 | 138 | 63 | − 3 | − 18 | 4.31 | 1.14 | |
| Cerebellum posterior lobe | L | 131 | − 48 | − 48 | − 15 | 4.94 | 1.31 | ||
| Baseline: HCs > IGDs | Insula | R | 13 | 50 | 36 | − 18 | 21 | 4.94 | 1.31 |
| Group and session interaction | Insula | R | 13 | 29 | 42 | 3 | − 6 | 14.97 | 0.28 |
| PPI: R insula seed, group and session interaction | Lingual gyrus | L | 18/30 | 215 | − 6 | − 72 | 3 | 21.95 | 0.40 |
| Precuneus/lingual gyrus | R | 18/31 | 170 | 15 | − 60 | 18 | 17.22 | 0.31 | |
PGRFT < 0.05 for whole-brain analysis.
IGDs = Internet gaming disorder subjects; HCs = healthy control subjects; PPI = psychophysiological interaction; BA = Brodmann area; MNI = Montreal Neurological Institute; SN = substantia nigra; PCC = posterior cingulate cortex; ACC = anterior cingulate cortex; IPL = inferior parietal lobule; MTG = middle temporal gyrus; STG = superior temporal gyrus; IFG = inferior frontal gyrus; MFG = middle frontal gyrus.
Cohen's d value for t-tests and partial η2 value for F tests.
Fig. 2.
Whole-brain group comparison between IGDs and HCs in gaming-cue-induced brain activation. The 3D activation map is overlaid on an inflated surface using BrainNet Viewer, whereas the 2D activation maps are overlaid on a T1 image using DPABI.
In the assessment of effects of CBI on cue-induced brain activation, a significant interaction between group (CBI + and CBI −) and session (baseline and second tests) was observed in a relatively anterior portion of the right insula. Single-group comparisons indicated that the CBI + group showed a significant enhancement in the activation of the right anterior insula (t(22) = − 2.20, P = 0.04, d = − 0.47), whereas for the CBI − group, the opposite pattern was observed (t(16) = 3.01, P = 0.008, d = 1.08) (Fig. 3). Additionally, we conducted a correlational analysis to examine the association between changes in the intensities of craving for gaming clips and changes in activation of the anterior insula in the CBI + group; however, no significant relationship was observed (r = − 0.10, P = 0.66).
We further conducted a PPI analysis with the right insula as a seed region (identified in the previous analysis) to assess its functional connectivity with other brain regions that that were identified in the contrast of gaming versus control clips. We found a significant interaction between group and session implicating the bilateral lingual gyrus and right precuneus. Single-group comparisons indicated that the CBI + group showed deceased functional connectivity of the right insula and these two clusters (t(22) = 3.89, P = 0.001, d = 0.66, and t(22) = 3.05, P = 0.006, d = 0.57), whereas the CBI − group showed the opposite pattern (t(16) = − 3.24, P = 0.005, d = − 0.90, and t(16) = − 2.83, P = 0.01, d = − 0.87) (Table 2 and Fig. 3).
4. Discussion
To the best of our knowledge, this study is the first evaluating effects of CBI on gaming cue-induced brain activation in IGD. We found that, compared with HCs, IGDs exhibited generally higher gaming-cue-induced brain activation in multiple brain regions including reward-related areas, with the exception of lower activation in the posterior insula. Additionally, the CBI + group showed a significantly increased activation in the right anterior insula after completing CBI, whereas the CBI − group showed the opposite pattern. Moreover, the CBI + group, as compared with the CBI − group, showed reduced functional connectivity between the right anterior insula and the bilateral lingural gyrus and right precuneus. These results suggest that CBI may exert its effects through altering the anterior insula activity and its connectivity with brain regions previously implicated in visual processing and spatial attention.
Consistent with our hypothesis, IGDs in this study exhibited stronger gaming-cue-induced craving and brain activation in critical regions located in the mesocorticolimbic (e.g., anterior cingulate cortex) and nigrostriatal (e.g., caudate, substantia nigra) pathways in comparison with HCs. The mesocorticolimbic and nigrostriatal pathways are two major sources of dopaminergic release and contribute to the reinforcing effects of addiction-related cues (Jasinska et al., 2014, Koob and Volkow, 2010, Robinson and Berridge, 1993). Additionally, IGDs showed greater activation of the parietal cortex (e.g., precuneus) that has been implicated in attentional bias and episodic memory retrieval (Cavanna and Trimble, 2006). Together, these findings both largely replicate results of previous studies in IGD (Han et al., 2010a, Ko et al., 2009a, Liu et al., 2016) and other addictions (Engelmann et al., 2012, Goudriaan et al., 2013, Jasinska et al., 2014) and suggest that IGDs may be hypersensitive to gaming-related cues which may elicit greater neural activation in brain regions involved in reward and attention.
Inconsistent with our original hypothesis, IGDs exhibited hypoactivation of the right posterior insula compared with HCs. However, this finding largely parallels the results of previous studies of IGD using gaming screenshots as gaming-related cues and general Internet use screenshots unrelated to gaming (e.g., screenshot of online chatting) as control cues (Liu et al., 2016). This finding also resonates with those from a meta-analysis of cue-induced brain activation in obesity (Brooks et al., 2013). In addition, a negative association between self-reported craving and cortical thickness of the right insula has been reported in smokers (Morales et al., 2014). However, seemingly contradictory evidence exists and indicates that cue-induced activation in the insula is stronger in addicted individuals relative to HCs (Ko et al., 2009a, Luijten et al., 2011). The mixed results may relate to differences in methodology (e.g., different control stimuli) or differences in the status of the studied individuals (e.g., with respect to treatment-seeking). Moreover, as the insula is a multimodal structure in which the anterior part may be mainly involved in salience detection and cognitive control, whereas the posterior part may be predominately engaged in interoceptive and exteroreceptive processing and integrating the information from both processes (Cauda et al., 2011, Paulus and Stewart, 2014, Zhang et al., 2016b), differences in findings across studies may relate to regions of the insula implicated. Hypoactivation of the posterior insula found in the present study may reflect hyposensitivity to satiety by merely watching gaming clips (rather than playing games) in IGDs.
With regard to the effects of CBI, the CBI + group, compared with the CBI − group, showed enhanced neural activation in the right anterior insula and decreased insular connectivity with the bilateral lingual gyrus and right precuneus after receiving CBI. Since behavioral interventions (e.g., mindfulness meditation, also a critical component of CBI) were found to increase the gray-matter volume intensity of the right anterior insula (Hölzel et al., 2008) and improve cognitive control performance (Tang et al., 2015), it is possible that CBI may exert its effects by impacting the activity of the anterior insula to enhance cognitive control through a top-down mechanism. Furthermore, the lingual gyrus and precuneus contribute importantly to visual and attentional processing (Cavanna and Trimble, 2006, Hopfinger et al., 2000) and have been found to be activated by visual addiction-related cues (Hanlon et al., 2014). Decreased interactions between the right anterior insula and these regions may be related to deceased salience detection and attribution of the visual stimuli (Naqvi et al., 2014, Paulus and Stewart, 2014), although this possibility requires direct investigation. These findings suggest that CBI may exert its effects to reduce gaming-cue-induced craving not only through altering recruitment of specific brain regions but also by reducing connectivity within specific neural circuits.
Our study showed that CBI effectively decreased cue-induced craving and IGD severity at a behavioral level. At a neural level, however, it did not normalize abnormal cue-induced brain activation identified from the baseline comparison, but rather targeted another region (the anterior insula) that did not demonstrate differences at baseline in IGDs and HCs, suggesting that CBI may mainly modulated brain regions involved in relatively higher ordered cognitive function instead of directly altering those involved in reinforcement. Albeit speculative, our findings suggest that the insula (and perhaps both its anterior and posterior portions) may represent a critical target for intervention, and targeting different parts of the insula may achieve different therapeutic effects. However, it may be surprising that CBI showed no significant effects in other critical regions within the reward system (e.g., ventral striatum), and we propose that future interventions combining CBI and pharmacological interventions (Potenza et al., 2011), non-invasive procedures such as transcranial magnetic stimulation (Hayashi et al., 2013), or invasive procedures such as deep-brain stimulation (Luigjes et al., 2012) that directly manipulate the ventral striatum or other regions that may be involved in cue-reactivity may be explored in order to achieve optimal outcomes.
The findings of the present study are largely consistent with theoretical models (Brand et al., 2014, Dong and Potenza, 2014, Ko et al., 2014) that propose a central role for craving for gaming or related cues in the maintenance of and recovery from IGD, and brain regions involved in reward processing (e.g., striatum, PCC), executive control (e.g., DLPFC), or both processes (e.g., insula, ACC) interacting with each other as well as the sensory cortex contributing to craving for gaming in IGD (Brand et al., 2014, Dong and Potenza, 2014, Meng et al., 2014), paralleling with findings in other types of addiction or hypothetically related conditions (e.g., obesity) (Brooks et al., 2013, Engelmann et al., 2012, Hanlon et al., 2014, Jasinska et al., 2014). Furthermore, these findings suggest that the insula and its functional connectivity with visual and parietal cortices contribute importantly to gaming-cue-induced craving and may serve as a potential intervention target, consistent with therapeutic theories that psychological interventions may improve top-down control over bottom-up processes that promote craving (Konova et al., 2013, Potenza et al., 2011). Of note, our findings may not be limited to IGD and may generalize to other types of behavioral addictions, such as problematic Internet pornography use, since these constructs may share similar behavioral and neural mechanisms relating to cue-induced craving (Brand et al., 2016). Future studies could investigate directly whether intervention altering insula activity may decrease cue-induced craving in IGD and possibly other behavioral addictions.
Our findings should be viewed in the light of some limitations. First, the CBI + and CBI − groups were not randomly assigned but based on the willingness of the IGDs to participate in CBI, and the CBI − group did not participate in an alternative activity. For this reason, we cannot exclude possible confounding factors such as willingness to receive an intervention or effects of different amounts of work across the groups, and the current findings should be confirmed in studies employing randomized placebo-controlled trials. Second, different familiarity for game and control clips may influence participants' neural activity toward different kinds of stimuli, particularly for IGDs. Future studies may split gaming-related stimuli from the same game into high and low craving categories to deal with this issue. Third, the interval (4 s) between gaming and control clips is relatively short. Although studies with similar or shorter intervals exist when investigating IGD (Han et al., 2010a, Ko et al., 2009a, Liu et al., 2016, Sun et al., 2012), and the 6 fixation blocks used in this study could be regarded as 30-second intervals, future studies are recommended using intervals with longer durations to minimize possible contamination between conditions. Finally, the present study only evaluated immediate effects of CBI. Considering high relapse rates in IGD, long-term effects of interventions should be examined and could provide significant information with respect to optimizing the efficacies of interventions (King and Delfabbro, 2014).
In summary, this study provides new insights into the neural effects of CBI on cue-induced craving in IGD. These results suggest that IGDs exhibited aberrant gaming-cue-induced activation in brain regions involved in reward processing and higher-order cognitive functions, and CBI may exert its effects by enhancing cognitive control and reducing the salience of the gaming-related cues through altering the activity of the anterior insula and its functional connectivity with brain region involved in visual processing. Such findings advance our understanding of the underlying mechanisms of CBI and may help refine interventions for IGD.
Conflict of interest
JTZ, YWY, CCX, JL, LL, LJW, BL, SSM, and XYF declare that they have no conflict of interest.
MNP has consulted for and advised Lundbeck, Ironwood, Shire, INSYS, River Mend Health, Opiant/Lakelight Therapeutics, Jazz Pharmaceuticals and Pfizer; has received research support from the National Institutes of Health, Mohegan Sun Casino, the National Center for Responsible Gaming, and Pfizer pharmaceuticals; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has given academic lectures in grand rounds, CME events and other clinical or scientific venues.
Role of the funding source
This study was supported by the National Natural Science Foundation of China (No. 31170990 and No. 81100992), MOE (Ministry of Education in China) Project of Humanities and Social Sciences (No.15YJA190010), and Fundamental Research Funds for the Central Universities of China (No.2015KJJCA13). MNP's involvement was supported by National Institutes of Health (R01 DA035058, P50 DA09241), the National Center on Addictions and Substance Abuse, and the National Center for Responsible Gaming. The views in the manuscript reflect those of the authors and not necessarily those of the funding agencies.
References
- American Psychiatric Association . 5th ed. American Psychiatric Association; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Epstein N., Brown G., Steer R.A. An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Brand M., Young K.S., Laier C. Prefrontal control and Internet addiction: a theoretical model and review of neuropsychological and neuroimaging findings. Front. Hum. Neurosci. 2014;8:375. doi: 10.3389/fnhum.2014.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M., Snagowski J., Laier C., Maderwald S. Ventral striatum activity when watching preferred pornographic pictures is correlated with symptoms of Internet pornography addiction. NeuroImage. 2016;129:224–232. doi: 10.1016/j.neuroimage.2016.01.033. [DOI] [PubMed] [Google Scholar]
- Brooks S.J., Cedernaes J., Schiöth H.B. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS One. 2013;8:e60393. doi: 10.1371/journal.pone.0060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K., Kivlahan D.R., McDonell M.B., Fihn S.D., Bradley K.A. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch. Intern. Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Cauda F., D'Agata F., Sacco K., Duca S., Geminiani G., Vercelli A. Functional connectivity of the insula in the resting brain. NeuroImage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chen S., Weng L., Su Y., Wu H., Yang P. Development of a Chinese Internet addiction scale and its psychometric study. Chin. J. Psychol. 2003;45:279–294. [Google Scholar]
- Courtney K.E., Schacht J.P., Hutchison K., Roche D.J., Ray L.A. Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict. Biol. 2016;21:3–22. doi: 10.1111/adb.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G., Potenza M.N. A cognitive-behavioral model of Internet gaming disorder_theoretical underpinnings and clinical implications. J. Psychiatr. Res. 2014;58:7–11. doi: 10.1016/j.jpsychires.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann J.M., Versace F., Robinson J.D., Minnix J.A., Lam C.Y., Cui Y., Brown V.L., Cinciripini P.M. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. NeuroImage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom K.O. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict. Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Goudriaan A.E., Veltman D.J., van den Brink W., Dom G., Schmaal L. Neurophysiological effects of modafinil on cue-exposure in cocaine dependence: a randomized placebo-controlled cross-over study using pharmacological fMRI. Addict. Behav. 2013;38:1509–1517. doi: 10.1016/j.addbeh.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Han D.H., Lee Y.S., Yang K.C., Kim E.Y., Lyoo I.K., Renshaw P.F. Dopamine genes and reward dependence in adolescents with excessive internet video game play. J. Addict. Med. 2007;1:133–138. doi: 10.1097/ADM.0b013e31811f465f. [DOI] [PubMed] [Google Scholar]
- Han D.H., Hwang J.W., Renshaw P.F. Bupropion sustained release treatment decreases craving for video games and cue-induced brain activity in patients with Internet video game addiction. Exp. Clin. Psychopharmacol. 2010;18:297. doi: 10.1037/a0020023. [DOI] [PubMed] [Google Scholar]
- Han D.H., Kim Y.S., Lee Y.S., Min K.J., Renshaw P.F. Changes in cue-induced, prefrontal cortex activity with video-game play. Cyberpsychol. Behav. Soc. Netw. 2010;13:655–661. doi: 10.1089/cyber.2009.0327. [DOI] [PubMed] [Google Scholar]
- Han D.H., Kim S.M., Lee Y.S., Renshaw P.F. The effect of family therapy on the changes in the severity of on-line game play and brain activity in adolescents with on-line game addiction. Psychiatry Res. 2012;202:126–131. doi: 10.1016/j.pscychresns.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon C.A., Dowdle L.T., Naselaris T., Canterberry M., Cortese B.M. Visual cortex activation to drug cues: a meta-analysis of functional neuroimaging papers in addiction and substance abuse literature. Drug Alcohol Depend. 2014;143:206–212. doi: 10.1016/j.drugalcdep.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Ko J.H., Strafella A.P., Dagher A. Dorsolateral prefrontal and orbitofrontal cortex interactions during self-control of cigarette craving. Proc. Natl. Acad. Sci. U. S. A. 2013;110:4422–4427. doi: 10.1073/pnas.1212185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel B.K., Ott U., Gard T., Hempel H., Weygandt M., Morgen K., Vaitl D. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Soc. Cogn. Affect. Neurosci. 2008;3:55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger J.B., Buonocore M.H., Mangun G.R. The neural mechanisms of top-down attentional control. Nat. Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Jasinska A.J., Stein E.A., Kaiser J., Naumer M.J., Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci. Biobehav. Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen J.D., Fortmann S.P., Kraemer H.C., Varady A., Newman B. Who will relapse? Symptoms of nicotine dependence predict long-term relapse after smoking cessation. J. Consult. Clin. Psychol. 1992;60:797–801. doi: 10.1037//0022-006x.60.5.797. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Baik S.H., Park C.S., Kim S.J., Choi S.W., Kim S.E. Reduced striatal dopamine D2 receptors in people with Internet addiction. Neuroreport. 2011;22:407–411. doi: 10.1097/WNR.0b013e328346e16e. [DOI] [PubMed] [Google Scholar]
- King D.L., Delfabbro P.H. Internet gaming disorder treatment: a review of definitions of diagnosis and treatment outcome. J. Clin. Psychol. 2014;70:942–955. doi: 10.1002/jclp.22097. [DOI] [PubMed] [Google Scholar]
- Ko C.-H., Liu G.-C., Hsiao S., Yen J.-Y., Yang M.-J., Lin W.-C., Yen C.-F., Chen C.-S. Brain activities associated with gaming urge of online gaming addiction. J. Psychiatr. Res. 2009;43:739–747. doi: 10.1016/j.jpsychires.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Ko C.-H., Yen J.-Y., Chen S.-H., Yang M.-J., Lin H.-C., Yen C.-F. Proposed diagnostic criteria and the screening and diagnosing tool of Internet addiction in college students. Compr. Psychiatry. 2009;50:378–384. doi: 10.1016/j.comppsych.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Ko C.-H., Liu G.-C., Yen J.-Y., Yen C.-F., Chen C.-S., Lin W.-C. The brain activations for both cue-induced gaming urge and smoking craving among subjects comorbid with Internet gaming addiction and nicotine dependence. J. Psychiatr. Res. 2013;47:486–493. doi: 10.1016/j.jpsychires.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Ko C.H., Liu G.C., Yen J.Y., Chen C.Y., Yen C.F., Chen C.S. Brain correlates of craving for online gaming under cue exposure in subjects with Internet gaming addiction and in remitted subjects. Addict. Biol. 2013;18:559–569. doi: 10.1111/j.1369-1600.2011.00405.x. [DOI] [PubMed] [Google Scholar]
- Ko C.-H., Yen J.-Y., Chen S.-H., Wang P.-W., Chen C.-S., Yen C.-F. Evaluation of the diagnostic criteria of Internet gaming disorder in the DSM-5 among young adults in Taiwan. J. Psychiatr. Res. 2014;53:103–110. doi: 10.1016/j.jpsychires.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Koepp M.J., Gunn R.N., Lawrence A.D., Cunningham V.J., Dagher A., Jones T., Brooks D.J., Bench C.J., Grasby P.M. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–268. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- Konova A.B., Moeller S.J., Goldstein R.Z. Common and distinct neural targets of treatment: changing brain function in substance addiction. Neurosci. Biobehav. Rev. 2013;37:2806–2817. doi: 10.1016/j.neubiorev.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss D.J., Griffiths M.D. Internet gaming addiction: a systematic review of empirical research. Int. J. Ment. Health Addict. 2012;10:278–296. [Google Scholar]
- Liu L., Yip S.W., Zhang J.T., Wang L.J., Shen Z.J., Liu B., Ma S.S., Yao Y.W., Fang X.Y. Activation of the ventral and dorsal striatum during cue reactivity in Internet gaming disorder. Addict. Biol. 2016 doi: 10.1111/adb.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luigjes J., Van Den Brink W., Feenstra M., Van den Munckhof P., Schuurman P., Schippers R., Mazaheri A., De Vries T., Denys D. Deep brain stimulation in addiction: a review of potential brain targets. Mol. Psychiatry. 2012;17:572–583. doi: 10.1038/mp.2011.114. [DOI] [PubMed] [Google Scholar]
- Luijten M., Veltman D.J., van den Brink W., Hester R., Field M., Smits M., Franken I.H. Neurobiological substrate of smoking-related attentional bias. NeuroImage. 2011;54:2374–2381. doi: 10.1016/j.neuroimage.2010.09.064. [DOI] [PubMed] [Google Scholar]
- McCarthy D.E., Curtin J.J., Piper M.E., Baker T.B. Negative reinforcement: possible clinical implications of an integrative model. In: Kassel J.D., editor. Substance Abuse and Emotion. American Psychological Association; Washington, DC: 2010. [Google Scholar]
- Meng Y., Deng W., Wang H., Guo W., Li T. The prefrontal dysfunction in individuals with Internet gaming disorder: a meta-analysis of functional magnetic resonance imaging studies. Addict. Biol. 2014;20:799–808. doi: 10.1111/adb.12154. [DOI] [PubMed] [Google Scholar]
- Morales A.M., Ghahremani D., Kohno M., Hellemann G.S., London E.D. Cigarette exposure, dependence, and craving are related to insula thickness in young adult smokers. Neuropsychopharmacology. 2014;39:1816–1822. doi: 10.1038/npp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi N.H., Gaznick N., Tranel D., Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann. N. Y. Acad. Sci. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus M.P., Stewart J.L. Interoception and drug addiction. Neuropharmacology. 2014;76:342–350. doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza M. Behavioural addictions matter. Nature. 2015;522:S62. doi: 10.1038/522S62a. [DOI] [PubMed] [Google Scholar]
- Potenza M.N., Sofuoglu M., Carroll K.M., Rounsaville B.J. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69:695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T.E., Berridge K.C. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Suler J.R. To get what you need: healthy and pathological Internet use. Cyberpsychol. Behav. 1999;2:385–393. doi: 10.1089/cpb.1999.2.385. [DOI] [PubMed] [Google Scholar]
- Sun Y., Ying H., Seetohul R.M., Xuemei W., Ya Z., Qian L., Guoqing X., Ye S. Brain fMRI study of crave induced by cue pictures in online game addicts (male adolescents) Behav. Brain Res. 2012;233:563–576. doi: 10.1016/j.bbr.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Tang Y.-Y., Posner M.I., Rothbart M.K., Volkow N.D. Circuitry of self-control and its role in reducing addiction. Trends Cogn. Sci. 2015;19:439–444. doi: 10.1016/j.tics.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Tian M., Chen Q., Zhang Y., Du F., Hou H., Chao F., Zhang H. PET imaging reveals brain functional changes in internet gaming disorder. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:1388–1397. doi: 10.1007/s00259-014-2708-8. [DOI] [PubMed] [Google Scholar]
- Wilson S.J., Sayette M.A., Fiez J.A. Prefrontal responses to drug cues: a neurocognitive analysis. Nat. Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A., Dorsing B., Rief W., Shen Y., Glombiewski J.A. Treatment of internet addiction: a meta-analysis. Clin. Psychol. Rev. 2013;33:317–329. doi: 10.1016/j.cpr.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8:e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalachkov Y., Kaiser J., Naumer M.J. Sensory and motor aspects of addiction. Behav. Brain Res. 2010;207:215–222. doi: 10.1016/j.bbr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Yao Y.-W., Wang L.-J., Yip S.W., Chen P.-R., Li S., Xu J., Zhang J.-T., Deng L.-Y., Liu Q.-X., Fang X.-Y. Impaired decision-making under risk is associated with gaming-specific inhibition deficits among college students with Internet gaming disorder. Psychiatry Res. 2015;229:302–309. doi: 10.1016/j.psychres.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Young K.S. CBT-IA: the first treatment model for internet addiction. J. Cogn. Psychother. 2011;25:304–312. [Google Scholar]
- Young K.S. Treatment outcomes using CBT-IA with Internet-addicted patients. J. Behav. Addict. 2013;2:209–215. doi: 10.1556/JBA.2.2013.4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-T., Yao Y.-W., Potenza M.N., Xia C.-C., Lan J., Liu L., Wang L.-J., Liu B., Ma S.-S., Fang X.-Y. Altered resting-state neural activity and changes following a craving behavioral intervention for Internet gaming disorder. Sci. Rep. 2016;6:28109. doi: 10.1038/srep28109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-T., Yao Y.-W., Li C.S.R., Zang Y.-F., Shen Z.-J., Liu L., Wang L.-J., Liu B., Fang X.-Y. Altered resting-state functional connectivity of the insula in young adults with Internet gaming disorder. Addict. Biol. 2016;21:743–751. doi: 10.1111/adb.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]