Abstract
We report the main characteristics of “Lachnoclostridium touaregense” strain Marseille-P2415T (= CSUR P2415 = DSM 102219), a new bacterial species isolated from the gut microbiota of a healthy young girl from Niger.
Keywords: Culturomics, gut microbiota, “Lachnoclostridium touaregense”, niger, taxonogenomics
Using the culturomics approach [1], [2], strain Marseille-P2415T was isolated in January 2016 from the stool sample of a 44-month-old healthy girl from Niger. Her weight-for-height z score was −0.65. This study was validated by the ethics committee of the Institut Fédératif de Recherche IFR48, and oral consent was obtained from the parents. We isolated strain Marseille-P2415T through 30-day preincubation in anaerobic Colombia-like broth supplemented with sheep's blood and seeding on 5% sheep's blood–enriched Colombia agar (bioMérieux, Marcy L'Etoile, France) in anaerobic atmosphere. Strain Marseille-P2415T forms on this medium a translucent biofilm formed by Gram-positive rods.
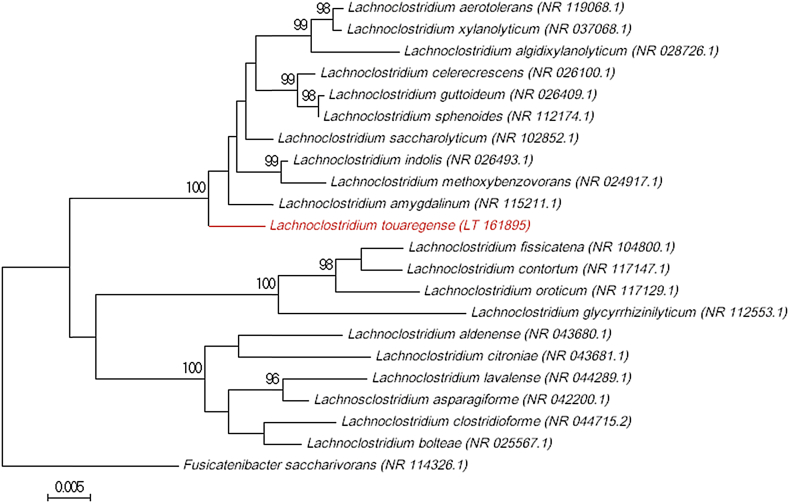
Cells have a mean diameter of 0.54 μm and a mean length of 3.35 μm. Oxidase and catalase activities were absent. Protein spectra were obtained for strain Marseille-P2415T using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) on a Microflex spectrometer (Bruker Daltonics, Brenen, Germany) [3], [4]. Because these spectra did not match any species in our database, the 16S rRNA gene was sequenced with fD1-rP2 primers as previously described [5] using a 3130-XL sequencer (Applied Biosciences, Saint Aubin, France). The sequence obtained had a 97.9% similarity with the 16S rRNA gene of Lachnoclostridium saccharolyticum strain WM1 (GenBank accession no. NR_102852.1), the closest species with a validly published name (Fig. 1). According to the 16S rRNA gene sequence similarity for species delineation of prokaryotes [6], [7], we propose that strain AT5T is representative of a new species within the recently described Lachnoclostridium genus [8], for which we propose the name Lachnoclostridium touaregense (twa.reg'ense, N.L. touaregense, “of Touareg,” because the stool sample was isolated from a young Touareg girl from Niger).
Fig. 1.
Phylogenetic tree showing position of Lachnoclostridium touaregense sp. nov. strain Marseille-P2415T relative to other phylogenetically close neighbors. Sequences were aligned using CLUSTALW, and phylogenetic inferences obtained using maximum-likelihood method within MEGA software. Numbers at nodes are percentages of bootstrap values (>95%) obtained by repeating analysis 500 times to generate majority consensus tree. Fusicatenibacter saccharivorans was used as outgroup. Scale bar indicates 5% nucleotide sequence divergence.
MALDI-TOF MS spectrum
The MALDI-TOF MS spectrum of “Lachnoclostridium touaregense” is available at http://www.mediterranee-infection.com/article.php?laref=256&titre=urms-database.
Nucleotide sequence accession number
The 16S rRNA gene sequence was deposited in GenBank under accession number LT161895.
Deposit in a culture collection
Strain Marseille-P2415T was deposited in the Collection de Souches de l’Unité des Rickettsies (CSUR, WDCM 875) under number P2415 and in the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ) under number DSM 102219.
Acknowledgement
This study was funded by the Fondation Méditerranée Infection.
Conflict of Interest
None declared.
References
- 1.Lagier J.C., Armougom F., Million M., Hugon P., Pagnier I., Robert C. Microbial culturomics: paradigm shift in the human gut microbiome study. Clin Microbiol Infect. 2012;18:1185–1193. doi: 10.1111/1469-0691.12023. [DOI] [PubMed] [Google Scholar]
- 2.Lagier J.C., Hugon P., Khelaifia S., Fournier P.E., La Scola B., Raoult D. The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clin Microbiol Rev. 2015;28:237–264. doi: 10.1128/CMR.00014-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seng P., Drancourt M., Gouriet F., La Scola B., Fournier P.E., Rolain J.M. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis. 2009;49:543–551. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- 4.Seng P., Abat C., Rolain J.M., Colson P., Lagier J.C., Gouriet F. Identification of rare pathogenic bacteria in a clinical microbiology laboratory: impact of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2013;51:2182–2194. doi: 10.1128/JCM.00492-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drancourt M., Bollet C., Carlioz A., Martelin R., Gayral J.P., Raoult D. 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J Clin Microbiol. 2000;38:3623–3630. doi: 10.1128/jcm.38.10.3623-3630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stackebrandt E., Ebers J. Taxonomic parameters revisited: tarnished gold standards. Microbiol Today. 2006;33:152. [Google Scholar]
- 7.Kim M., Oh H.S., Park S.C., Chun J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol. 2014;64:346–351. doi: 10.1099/ijs.0.059774-0. [DOI] [PubMed] [Google Scholar]
- 8.Yutin N., Galperin M.Y. A genomic update on clostridial phylogeny: gram-negative spore formers and other misplaced clostridia. Environ Microbiol. 2013;15:2631–2641. doi: 10.1111/1462-2920.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]