Abstract
Onychomycosis is usually caused by dermatophytes, although also other filamentous and yeast-like fungi are associated with nail invasion. Chaetomium is an environmental genus of ascomycetes exhibiting a certain degree of extremotolerance. We report the first case of onychomycosis in a 46-year-old woman in China caused by Chaetomium globosum. The patient showed yellowish black discoloration with periungual inflammation on the left first toenail. We confirmed the causative agent, C. globosum, by KOH mount, culture, micromorphology and DNA sequence analysis
Keywords: Chaetomium globsum, Onychomycosis, China
1. Introduction
The large genus Chaeomium, belonging to Ascomycota – Sordariomycetes comprises melanized, ascosporulating fungi that inhabit soil, dung and plant debris as a saprobe. The fungi are commonly encountered in indoor environments and show a certain degree of extremotolerance [1]. Although Chaetomium species are widely distributed, human and animal infections are uncommon. The prevalent species in clinical settings is C. globosum, a rare agent of subcutaneous infections, invasive pulmonary mycosis and systemic infections in immunocompromised patients, as well as onychomycosis in healthy subjects [2], [3].
Only a few cases of onychomycosis induced by C. globosum have been reported so far. Here, we report the first case of onychomycosis caused by C. globosum with periungual inflammation that was confirmed by clinical findings, repeated fungal isolation, light microscopy and sequencing analysis of the rDNA internal transcribed spacer (ITS) region.
2. Case
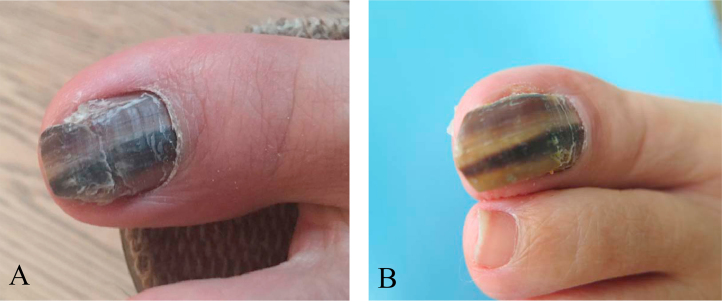
A 46-year-old Chinese woman visited our department in February 2016 (at day 0) with black and yellow discoloration and hypertrophy of the left first toenail with a somewhat painful periungual erythema (Fig. 1). Three years ago (at day – 3 years), the patient first noticed an infection in the first toenail. Physical examination showed that infection destroyed of the total nail plate, and the affected nails were discolored, yellow to dark brown to black, with subungual hyperkeratosis. She reported to have a history of doing nail fashion. Physical examination found no other abnormalities. She did not recall a history of long-term administration of steroids or any other drugs. No history of any underlying disease was reported.
Fig. 1.
A, the left thumb toenail was discolored, yellow brown to black with subungual hyperkeratosis and periungual inflammation at first examination. B, one month after initiation treatment. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Laboratory findings were within normal limits. Serological tests for hepatitis B virus, hepatitis C virus, HIV antibody and anti-nuclear antibodies were negative. Chest radiograph showed unremarkable findings.
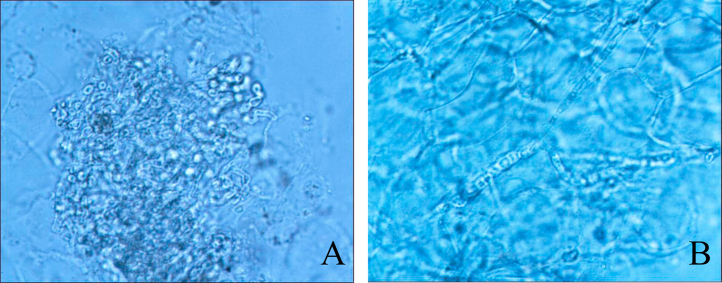
At day 0, direct microscopic examination of a toenail sample in 20% KOH revealed fungal infection. Light brown-colored hyphae with septa and spores were observed (Fig. 2).
Fig. 2.
Direct microscopic examination of the toenail sample in 20% KOH revealed fungal infection. Light brown-colored hyphae with septate were observed. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
At day 0, nail clippings and subungual debris, preserved in sterile saline, were aseptically homogenized and inoculated onto slopes of Sabouraud's glucose agar (SGA) containing chloramphenicol with and without cycloheximide (0.5 mg/ml), and OA, respectively, at 26 °C. Physiological tests included the growth of the fungus on SGA with cycloheximide (0.5 g/l) at 26 °C and 37 °C, and on OA at 26 °C, 37 °C, 40 °C and 45 °C.
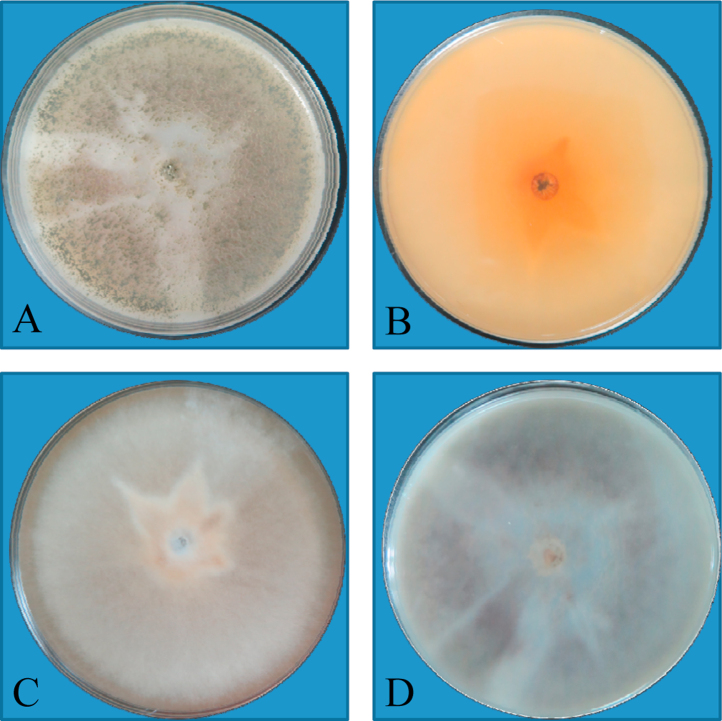
Colonies (SGA and OA without cycloheximide) expanded and grew rapidly, initially velvety, white then turning dark gray to brown, with a reddish diffusible pigment (Fig. 3, Fig. 4). Growth was evident at both 26 °C and 37 °C; no visible growth was observed at 40 °C and 45 °C at day +10. Repeated examination of the toenail sample after a week revealed hyaline, septate hyphae on direct microscopy, and a duplicate culturing on SGA yielded growth of C. globosum. No other fungi were isolated.
Fig. 3.
A, rapid growing, dark gray to brown colony with aerial mycelium on Sabouraud's dextrose agar plate after incubation at 25 °C for 1 week. B, Reverse surface of Sabouraud's dextrose agar plate after incubation at 25 °C for 1 week. C, Rapid growing, dark gray to brown colony with aerial mycelium on Sabouraud's dextrose agar plate after incubation at 25 °C for 1 week. D, Reverse surface of Sabouraud's dextrose agar plate after incubation at 25 °C for 1 week. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
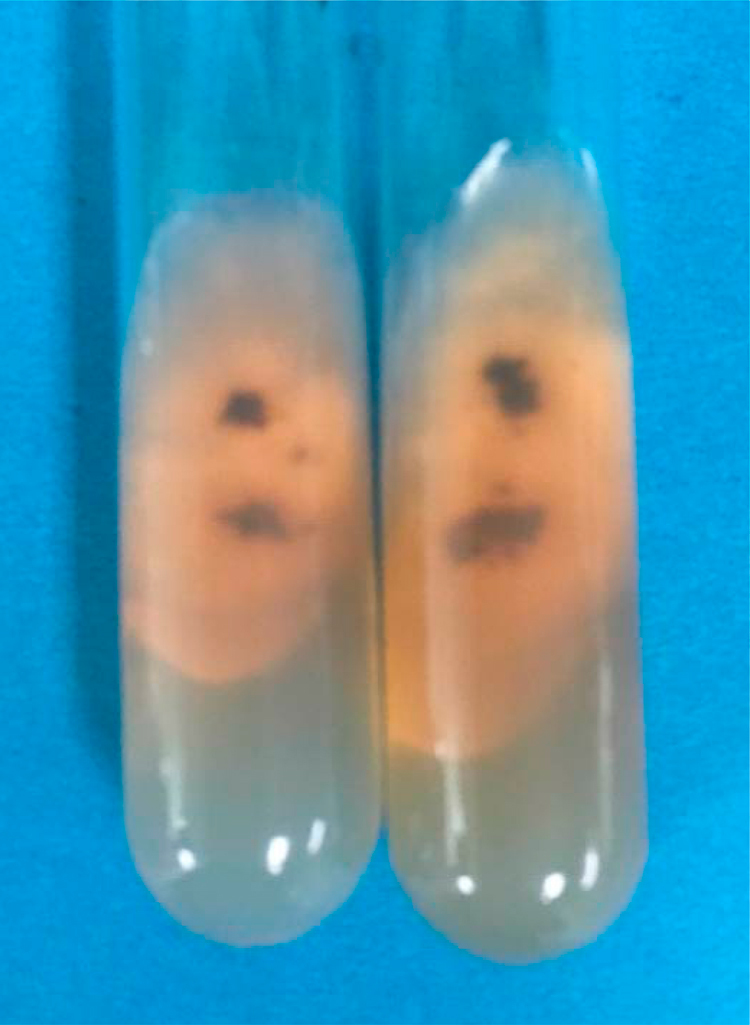
Colonies expanded and grew rapidly, which were initially velvety white then turned to dark gray to brown with reddish diffusible pigment. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
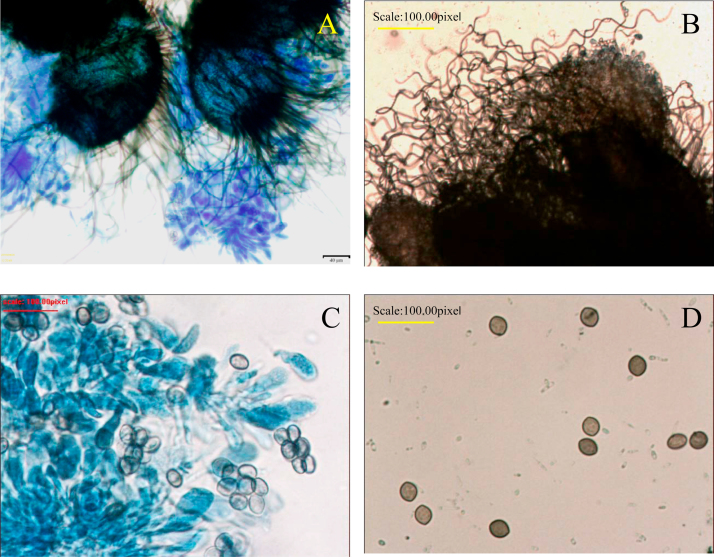
Microscopically, direct microscopy of the cultured fungi revealed many brown-colored, globose to subglobose perithecia. Ascomal hairs were numerous, usually unbranched, flexuose, undulate or coiled, septate, brownish. Ascospores limoniform in face view, bilaterally flattened, usually brownish with an apical germ pore (Fig. 5).
Fig. 5.
A, Close-up view of perithecium which demonstrates ostioles and contains asci and single-celled ascospores (Lactophenol cotton blue stain, ×200). B, C, Large, dark brown to black, flask shaped perithecia with hair-like filamentous appendages (Lactophenol cotton blue stain, ×400). D, Brown-colored septated hyphae and lemon-shaped ascospores (Lactophenol cotton blue stain, ×400). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The identity of the isolated agent was reconfirmed by sequencing of the D1/D2 region of rDNA and compared with reference sequences deposited in Genbank at day +14. The primers sequences as follows: (forward, GCATATCAATAAGCGGAGGAAAAG; reverse, GGTCCGTGTTTCAAGACGG). D1/D2 (608 bp) had 100% homology with C. globosum (KC425279.2). The isolated strain showed not only the typical morphological features of C. globosum but also a perfect homology to the D1/D2 sequence of C. globosum in an as yet unpublished database held at CBS (X. Wang, pers. comm.). The isolated agent has been added to the collection of Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College in Nanjing, Jiangsu, under accession number CMCCF 2160006.
The in vitro susceptibility of the strain was determined using the microdilution method in accord with the guidelines of the Clinical and Laboratory Standards Institute (CLSI) M38A at day +14. The minimum inhibitory concentrations (MICs) were defined as the lowest concentration at which no growth occurred which led to the following results: itraconazole, 0.125 μg/ml; ketoconazole, 0.25 μg/ml; fluconazole 32 μg/ml; econazole, 0.06 μg/ml; nystatin 4 μg/ml; nafitifine,>4 μg/ml; bifonazole, 0.5 μg/ml and terbinafine 0.5 μg/ml. According to the results of antifungal susceptibility testing, the patient was treated with oral administration of itraconazole 200 mg daily for one week associated with terbinafine cream twice per day. The patient responded well to this combination treatment. After 1 month (day +1 month) of oral therapy with itraconazole, the periungual lesion healed completely (Fig. 1B). Subsequently itraconazole therapy was maintained at a dose of 200 mg per day until day +3 month. The patient has remained available for follow up.
3. Discussion
Chaetomium is a large genus of Ascomycota. Since 1817, when Kunze defined the genus, more than 105 species have been described. The genus comprised melanized fungi found in soil, on dung, and on plant debris. Several species are regularly encountered in indoor environments [4], which may be due to an extremotolerant character of a number of species [1]. Chaetomium globosum, C. atrobrunneum, C. strumarium, C. perlucidum, and C. funicolum have been confirmed to cause infections in the human host. Most cases were related to systemic mycosis in immunocompromised patients [5], [6], [7].
Chaetomium globosum is the most frequently isolated species from patients with superficial mycoses such as cutaneous phaeohyphomycosis and systemic infection in immunocompetetent or immunocomprised patients [3], [8], [9]. To date, six cases of onychomycosis by C. globosum have been published [10], [11], [12], [13], [14], [15]. Our report of a toenail infection in an immunocompetent patient deviates clinically from published cases. A comparison with literature data showed that all cases occurred in adults and were chronic, lasting from 2 months to 4 years (Table 1). All six patients showed yellow to brownish discoloration without periungual inflammation. In our case, the infected nail showed yellow to black discoloration.
Table 1.
Comparison of reported cases with onychomycosis due to Chaetomium globosum.
| Author | Age/sex | Geography | Duration | Site | Nail sign | inflammation | Trauma history | Treatment/outcome |
|---|---|---|---|---|---|---|---|---|
| Naidu et al. [10] | 26/Male | India | 1 yr | Index finger of left hand, thumb third and fourth | Dark brown to sooty black, eroded, dystrophic | None | None | None |
| Stiller et al. [11] | 83/Female | India | 2 yr | All 10 toenails | Thickening and brown-black discoloration | None | None | None |
| Hattori et al. [12] | 57/Male | Japan | 2 month | Lt. 2nd, Rt. 2nd fingernail &, Lt. 1st toenail | Yellow brown to white discoloration | None | None | Oral itraconazole |
| Aspiroz et al. [13] | 23/Male | Spain | 4 yr | Lt. 1st toenail | Yellow-brown discoloration | None | Yes | Oral terbinanifin 12 week Complete recovery |
| Latha et al. [14] | 25/Male | India | 4 month | All Rt. fingernails | Yellow brown discoloration | None | Yes | Oral itraconozole 100 mg for 6 mo |
| Kim et al. [15] | 35/Male | South Korea | 2 yr | Rt. 1st, 5th. Lt. 1st, 4th toenail | Brownish yellow | None | None | Oral terbinafine daily and topical amorolfine 5% nail lacquer. |
| This case (2016) | 46/Female | China | 4 yr | Lt. 1st toenail | Yellow brown to black discoloration | Periungual inflammation | Yes | Oral itraconazole+laquer |
Lt: left, Rt: right.
Predisposing factors in cases of onychomycosis and cutaneous infection mostly involve local trauma [16]. Any process that breaks down the integrity of the horny layer of the nail will facilitate fungal penetration including species that are considered mild opportunists such as Chaetomium species. All patients infected with C. globosum were immunocompetent, and two had a recalled history of trauma. Our case lasted 4 years and occurred only in a single toenail. Our patient had a periungual inflammation, and had a history of nail fashion, which might cause minor nail barrier damage facilitating penetration by C. globosum. Chaetomium species are emerging as a cause of non-dermatophyte onychomycosis, especially in traumatized nails. The spectrum of fungi responsible for nail infections should be expanded to include C. globosum.
The identification of C. globosum was based on micromorphology revealing peritheca with hairy filamentous appendages, and ostioles liberating asci and ascospores. Ascospores are lemon-shaped olive-brown single cells. Sequence analysis of the ITS region of ribosomal DNA is considered necessary for definite identification among the genus Chaetomium [3]. Although the molecular diversity in Chaetomiaceae is unexpectedly large, and taxonomic rearrangements are necessary, our strain was confirmed to be C. globosum (X. Wang. pers. comm.). Our strain showed not only the typical morphological features of C. globosum but also a perfect homology to the ITS sequence of C. globosum in an as yet unpublished database held at CBS (X. Wang, pers. comm.).
Although in some cases, onychomycoses caused by non-dermatophyte molds does not respond well to common treatment, some cases respond well to common treatment. Administration of oral terbinafine to a patient with C. globosum onychomycosis for 3 months completely cured the disease [13]. Other studies with terbinafine and itraconazole appeared to be effective [12]. Likewise, our patient was treated with oral itraconazol 200 mg daily in addition to local application of amorolfine 5% nail lacquer for 1 month, and the patient responded well to the treatment, the periungual inflammation disappeared.
In conclusion, we note that onychomycosis caused by C. globosum is a recurrent feature. If C. globosum is isolated from onychomycosis patients, these should not be disregard as contaminants but as a potential causative agent requiring further mycological studies.
Conflict of interest
There are none.
Acknowledgements
This work was supported in part by grants from the Natural Science Foundation of Shandong Province, China (NM. ZR2015HL127), the National Natural Science Foundation of China (NM. 81401653) and the National Key Basic Research Program of China (NM. 2013CB531605) and funded by Jiangsu Provincial Special Program of Medical Science (BL2012003).
References
- 1.Ahmed Sarah A., Khan Ziauddin, Wang Xue-wei, Moussa Tarek A.A., Al-Zahrani Hassan S., Almaghrabi Omar A., Sutton Deanna A., Ahmad S., Groenewald Johannes Z., Alastruey-Izquierdo A., Diepeningen Anne, Menken S.B.J., Najafzadeh M.J., Crous Pedro W., Cornely Oliver, Hamprecht Axel, Vehreschild Maria J.G.T., Kindo A.J., de Hoog G. Sybren. Chaetomium-like fungi causing opportunistic infections in humans: a possible role for extremotolerance. Fungal Divers. 2016;76(1):11–26. [Google Scholar]
- 2.Capoor M.R., Agarwal P., Goel M. Invasive pulmonary mycosis due to Chaetomium globosum with false-positive galactomannan test: a case report and literature review. Mycoses. 2016;59(3):186–193. doi: 10.1111/myc.12446. [DOI] [PubMed] [Google Scholar]
- 3.Yu J., Yang S., Zhao Y., Li R. A case of subcutaneous phaeohyphomycosis caused by Chaetomium globosum and the sequences analysis of C. globosum. Med. Mycol. 2006;44(6):541–545. doi: 10.1080/13693780500525235. [DOI] [PubMed] [Google Scholar]
- 4.Flannigan B., Samson R.A., Miller J.D., editors. Diversity, Health Impacts, investigation and Control. 2nd ed. CRC Press; Boca Raton: 2011. Microorganisms in home and indoor work environments. [Google Scholar]
- 5.Segal R., Kusne S. Cerebral fungal infections in the immunocompromised host: a literature review and a new pathogen--Chaetomium atrobrunneum: case report. Neurosurgery. 1999;45(1):200. doi: 10.1097/00006123-199907000-00056. [DOI] [PubMed] [Google Scholar]
- 6.Aribandi M., Bazan I.C., Rinaldi M.G. Magnetic resonance imaging findings in fatal primary cerebral infection due to Chaetomium strumarium. Australas. Radiol. 2005;49(2):166–169. doi: 10.1111/j.1440-1673.2005.01367.x. [DOI] [PubMed] [Google Scholar]
- 7.Abbott S.P., Sigler L., McAleer R., McGough D.A., Rinaldi M.G., Mizell G. Fatal cerebral mycoses caused by the ascomycete Chaetomium strumarium. J. Clin. Microbiol. 1995;33(10):2692–2698. doi: 10.1128/jcm.33.10.2692-2698.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anandi V., John T.J., Walter A. Cerebral phaeohyphomycosis caused by Chaetomium globosum in a renal transplant recipient. J. Clin. Microbiol. 1989;27(10):2226–2229. doi: 10.1128/jcm.27.10.2226-2229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teixeira A.B., Trabasso P., Moretti-Branchini M.L. Phaeohyphomycosis caused by Chaetomium globosum in an allogeneic bone marrow transplant recipient. Mycopathologia. 2003;156(4):309–312. doi: 10.1023/b:myco.0000003563.29320.95. [DOI] [PubMed] [Google Scholar]
- 10.Naidu J., Singh S.M., Pouranik M. Onychomycosis caused by Chaetomium globosum Kunze. Mycopathologia. 1991;113(1):31–34. doi: 10.1007/BF00436384. [DOI] [PubMed] [Google Scholar]
- 11.Stiller M.J., Rosenthal S., Summerbell R.C., Pollack J., Chan A. Onychomycosis of the toenails caused by Chaetomium globosum. J. Am. Acad. Dermatol. 1992;26(5 Pt 1):775–776. doi: 10.1016/s0190-9622(08)80558-0. [DOI] [PubMed] [Google Scholar]
- 12.Hattori N., Adachi M., Kaneko T., Shimozuma M., Ichinohe M., Iozumi K. Case report. Onychomycosis due to Chaetomium globosum successfully treated with itraconazole. Mycoses. 2000;43(1–2):89–92. doi: 10.1046/j.1439-0507.2000.00523.x. [DOI] [PubMed] [Google Scholar]
- 13.Aspiroz C., Gené J., Rezusta A., Charlez L., Summerbell R.C. First Spanish case of onychomycosis caused by Chaetomium globosum. Med. Mycol. 2007;45(3):279–282. doi: 10.1080/13693780601164280. [DOI] [PubMed] [Google Scholar]
- 14.Latha R., Sasikala R., Muruganandam N., Shiva P.M.R. Onychomycosis due to ascomycete Chaetomium globosum: a case report. Indian J. Pathol. Microbiol. 2010;53(3):566–567. doi: 10.4103/0377-4929.68279. [DOI] [PubMed] [Google Scholar]
- 15.Kim D.M., Lee M.H., Suh M.K., Ha G.Y., Kim H., Choi J.S. Onychomycosis Caused by Chaetomium globosum. Ann. Dermatol. 2013;25(2):232–236. doi: 10.5021/ad.2013.25.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubka V., Mencl K., Skorepova M., Lyskova P., Zalabska E. Phaeohyphomycosis and onychomycosis due to Chaetomium spp., including the first report of Chaetomium brasiliense infection. Med. Mycol. 2011;49(7):724–733. doi: 10.3109/13693786.2011.572299. [DOI] [PubMed] [Google Scholar]