Abstract
A sustainable economy can be achieved only by assessing processes finalized to optimize the use of resources. Waste can be a relevant source of energy thanks to energy-from-waste processes. Concerns regarding the toxic fly ashes can be solved by transforming them into resource as recycled materials. The commitment to recycle is driven by the need to conserve natural resources, reduce imports of raw materials, save landfill space and reduce pollution. A new method to stabilize fly ash from Municipal Solid Waste Incinerator (MSWI) at room temperature has been developed thanks to COSMOS-RICE LIFE+ project (www.cosmos-rice.csmt.eu). This process is based on a chemical reaction that occurs properly mixing three waste fly ashes with rice husk ash, an agricultural by-product. COSMOS inert can replace critical raw materials (i.e. silica, fluorspar, clays, bentonite, antimony and alumina) as filler. Moreover the materials employed in the stabilization procedure may be not available in all areas. This paper investigates the possibility of substituting silica fume with corresponding condensed silica fume and to substitute flue-gas desulfurization (FGD) residues with low-cost calcium hydroxide powder. The removal of coal fly ash was also considered. The results will be presented and a possible substitution of the materials to stabilize fly ash will be discussed.
Keywords: Engineering, Materials science, Environmental science
1. Introduction
Every year about 11 billion tons of solid waste are collected worldwide and the European Union alone generates about 3 billion tons of waste (http://ec.europa.eu/eurostat). Highly dependent on imported raw materials, Europe aims to reduce the amount of waste generated by improving its resource efficiency through recycling, avoiding waste and using unavoidable waste as a resource, when possible.
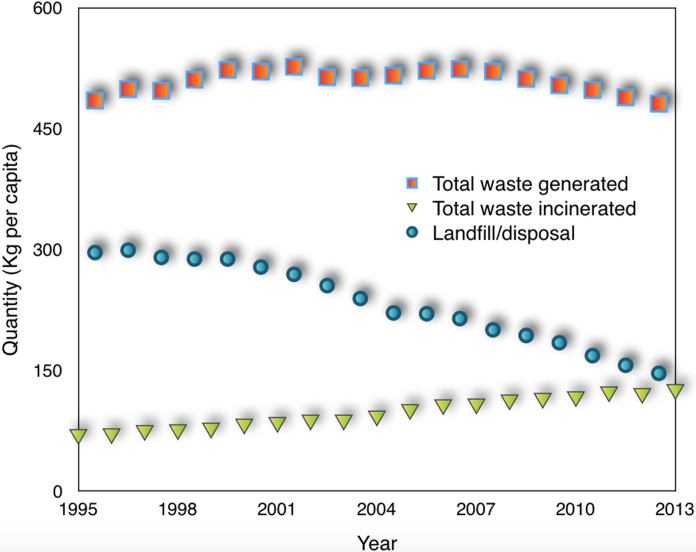
Fig. 1 shows the amount of waste generated per capita in EU27 from 1995 to 2013. The corresponding quantity of landfilled and incinerated wastes are also shown (http://ec.europa.eu/eurostat). Although the global amount of waste produced is almost constant, it is evident that the waste landfilling shows a negative trend, with a reduction at about one half of the waste designated to landfill in 2013, in comparison to data of 1995. The opposite trend can be highlighted for incineration. Waste incineration, that is acquiring a prominent role for domestic waste management in several countries in Europe, is now exceeding the amount of waste that is landfilled.
Fig. 1.
Amount of waste generated per capita in EU27 from 1995 to 2013. The corresponding quantity of landfilled and incinerated wastes are also shown (http://ec.europa.eu/Eurostat).
However, while modern waste incineration represents a consolidated technology for volume and mass waste reduction, combined with efficient energy recovery, increasing concern regarding the production of toxic waste exists in society. Indeed, fly ash from municipal solid waste incineration (MSWI) is hazardous so landfilling is still considered the most appropriate management strategy (Zacco et al., 2014).
Solidification and stabilization using cement as a binder is recognized worldwide as the pre-landfill treatment method most often applied (Polettini et al., 2001). However, it has several disadvantages as for example the large amount of cement requested for fly ash treatment (Van Gerven et al., 2005), the significant increase of the volume of the final obtained product, and the high amount of CO2 obtained for the cement production (Van Gerven et al., 2005). From an environmental point of view, also LCA analysis demonstrated that solidification and stabilization technologies without cement are preferred (Billen et al., 2014). For this reason, alternatives to cementitious binders with reduced cement content were proposed in the last years (Colangelo et al., 2015; Galiano et al., 2011).
With the Critical Raw Material initiative (http://ec.europa.eu/growth/sectors/raw-materials/index_en.htm), the European policy focus on the fundamental role of innovation to develop new materials, by optimizing the resource and minimizing the waste. The European need of raw materials substitution pushed the research policy towards development of innovative solution involving waste recovering.
In this frame, the COSMOS project, financed by European Commission, developed a new technology for MSWI fly ash stabilization and recovery. The stabilization method is based on the use of other fly ashes (such as coal fly ash and flue-gas desulfurization (FGD) residues) and colloidal silica.
By the use of zebrafish embryos test, it was recently shown that the MSWI fly ashes stabilization in classical concrete matrices allows to obtain a material, with some concerns about its safety (Guarienti et al., 2014). On the contrary, no significant mortality and developmental defects were observed in zebrafish embryos exposed to COSMOS solution. This makes it possible to deduce that COSMOS technology provides a biologically safe inert (Guarienti et al., 2016).
Because the cost of this process is high, being dependent on the colloidal silica quotation, recently the technology was improved by the substitution of commercial silica with rice husk ash (RHA), an agricultural by product of rice cultivation (Benassi et al., 2015a; Bosio et al., 2014a). This project, named COSMOS-RICE, has already shown the capability to produce new fillers, by using stabilized waste (Ponsot et al., 2015; Benassi et al., 2015b; Bosio et al., 2014b; Besco et al., 2014; Zacco et al., 2012; Besco et al., 2013), that were inserted in polypropylene, polyethylene, nylon, some resins and ceramics. It is possible to obtain attractive and sustainable products that can compete with the market of natural raw materials on the global stage.
The high sustainability of the new proposed technology is based on the fact that all the materials employed in the stabilization are wastes and by-products. Indeed, the materials recycling represents a large and significant contribution to protecting the environment (Brunner and Rechberger, 2015) and a way to reach the objectives established in the framework of COP21 meeting.
In the following we briefly analyze the required ingredients for the COSMOS process, that are necessary to obtain the MSWI fly ashes stabilization.
Amorphous silica source is necessary to promote heavy metals entrapment, as already reported (Bontempi et al., 2010). COSMOS-RICE project demonstrated the possibility to employ RHA as a source of silica, to substitute commercial colloidal silica use (Benassi et al., 2015a). However, also other by-products, containing amorphous reactive silica, can be employed instead of colloidal silica. Very recently we showed that silica fume can be considered a low-cost valid alternative silica source (Rodella et al., 2014).
Silica fume is a by-product of the silicon and ferro-silicon industry. It is formed by condensation from the vapor phase of SiO2. Typically, 85% to 95% of the silica fume is in the form of amorphous silica (Bonen and Khayat, 1995), with a sizes of the clusters ranging between about 0.3 μm and slightly over 1 μm (Diamond et al., 2004).
Silica fume was originally supplied for use in concrete. For a better handling of such material, and to decrease the impact of cost of its transport, the realization of a more compact silica fume form, i.e. densified silica fume, was made. Densified silica fume is generally produced by air floatation within silos. The end products of this process are agglomerates of various shapes and of sizes much larger than the original clusters. Agglomerates present in densified silica fumes range in size from about 10 μm to as much as several thousand microns (Diamond et al., 2004).
The densification process is carried out at temperatures very much lower than the melting point of silica; thus, it is expected that this does not induce any additional permanent bonding between individual spheres (Diamond et al., 2004).
Coal fly ash is a predominantly inorganic residue obtained from the flue gases of furnaces at pulverized coal power plants. The characteristics of this material differ depending on the coal source, the method of combustion of power plants, typologies of emission control devices, storage and handling (Zacco et al., 2014). Therefore, it shows a wide variation in its physicochemical and mineralogical properties. The main detected crystalline phases are mullite, quartz, magnetite, hematite and anhydrite (Helmuth, 1987). Glass is also found. This material was considered, in the COSMOS technology, to improve the mechanical characteristics of the obtained filler (Bontempi et al., 2010).
FGD residues are the by-product generated by the air pollution control equipment in coal-fired power plants, to reduce sulphur amount in air. Indeed, the coal burning can convert sulphur-bearing impurities to gaseous SO2, an atmospheric pollutant and a precursor to acid rain. As a consequence, power plants have to remove increasing amounts of SO2 from the flue gases before releasing them to the atmosphere (Zacco et al., 2014). This material is added as an inexpensive calcium hydroxide source. The aim is to promote the carbonation reaction, that takes place with atmospheric carbon dioxide. Indeed we have recently shown (Bosio et al., 2014a) that it also promote metal entrapment.
The COSMOS technology was optimized by using these ashes, that are available in northern Italy, but MSWI fly ash is present in all European countries and its quantity is continuously increasing (as shown in Fig. 1). As a consequence, it is fundamental to provide adequate substitute of some materials, used in the process, to guarantee the possibility to make COSMOS technology also in countries where the availability of the employed materials is too onerous.
As silica fume can be considered the cheapest amorphous silica source and is available in almost all European countries, the aim of this work is to evaluate the possibility of realizing COSMOS technology in those countries where FGD residue and coal fly ash availability is not necessarily guaranteed. For this reason in the present work, some samples were realized, testing the possibilities of coal fly ash removing and FGD residue substitution with calcium hydroxide powder.
Calcium hydroxide, Ca(OH)2, has been used for approximately 2000 years in building applications, as for example mortar, internal plaster, external render, foundations, flooring, decorative applications and so on (Carran et al., 2012). This material is not only abundant but is also low cost and naturally reacts with the carbon dioxide contained in the air, to produce calcium carbonate (carbonation reaction). Finally the possibility to substitute silica fume with condensed silica fume was also investigated. Indeed, the agglomeration of this material increases its density, making its transport less expensive, in respect to conventional silica fume.
2. Materials and methods
Silica fume was used to stabilize MSWI fly ashes from an Italian incinerator by following the protocols reported in (Rodella et al., 2014). Briefly, a mixture of MSWI fly ash (130 g), flue-gas desulfurization (FGD) residues (40 g), coal fly ash (30 g) and silica fume (20 g) was incorporated to 200 mL of milliQ water, and then stirred for 30 minutes in a laboratory mixer at room temperature. This sample is the reference sample (S0). Other samples were prepared by using calcium hydroxide powder (instead of FGD residues) and densified silica fume (instead of non densified silica fume) and removing coal fly ash, as reported in Table I (samples S1-4).
Table I.
Samples composition, considering all the materials employed to obtain stabilized MSWI fly ash.
| Sample | MSWI [g] |
FGD [g] |
Ca(OH)2 [g] |
Coal Ash [g] |
S.Fume [g] |
Dens.S.Fume [g] |
|---|---|---|---|---|---|---|
| S0 | 59 | 18 | 0 | 14 | 9 | 0 |
| S1 | 59 | 0 | 32 | 0 | 9 | 0 |
| S2 | 59 | 0 | 32 | 0 | 0 | 9 |
| S3 | 59 | 32 | 0 | 0 | 9 | 0 |
| S4 | 59 | 32 | 0 | 0 | 0 | 9 |
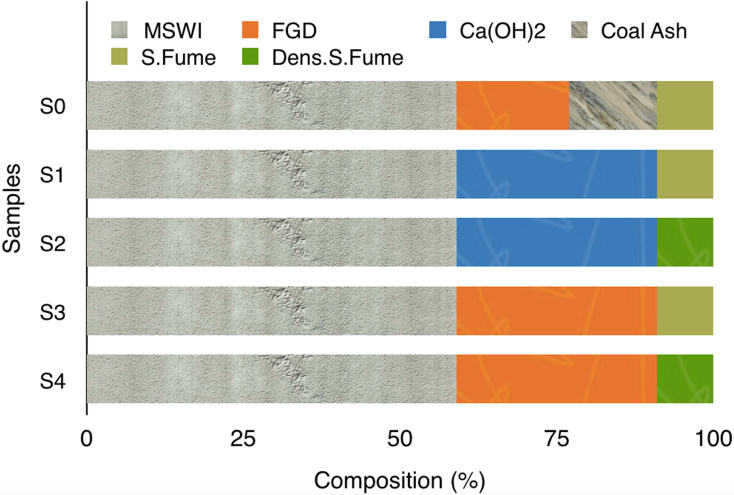
Fig. 2 reports the percent composition of all obtained samples (these percent values correspond to data reported in Table I). In this Figure it appears evident that MSWI fly ash and silica fume contents are always constant. However, in samples S2 and S4 conventional silica fume is substituted by condensed silica fume. Moreover, coal fly ash, originally contained in S0 sample, was completely substituted by FGD residues or calcium hydroxide powder, in all the other samples.
Fig. 2.
Percent composition of the materials employed to obtain all stabilized samples (the data correspond to those reported in Table I, but the values in this figure are reported in percent).
After stirring, the samples were thermally treated at 120 °C for 4 hours allowing the water to totally evaporate, the stabilization reactions to complete and the samples to solidify. The final material immobilized heavy metals in its matrix and it may be washed out with water in order to eliminate (and recover) soluble salts (Bontempi et al., 2010).
Leaching tests in water were performed according to the CEN EN 12457–2 normative.
The leaching tests were performed on samples immediately after their preparation, i.e. just after the thermal treatment.
TXRF analysis on the leaching solutions were performed by the Bruker TXRF system S2 Picofox (air cooled, Mo tube, Silicon-Drift Detector), with operating values of 50 kV and 750 μA and using an acquisition time of 600 seconds. A proper amount of gallium, used as an internal standard, was added. Full details on protocols are given in (Borgese et al., 2009).
X-ray Diffraction (XRD) measurements were performed with Panalytical X’Pert Pro diffractometer equipped with the X’Celerator detector and Cu anode. Operating values were 40 kV and 40 mA.
The Raman characterization of the samples was carried out by a high-resolution Raman micro-spectrometer (Labram HR-800, Horiba Jobin-Yvon). Exciting Source: He-Ne laser (λ = 632.8 nm). The spectra were acquired in backscattering mode, with a 100x (N.A.: 0.9) microscope objective. To perform the Raman analysis, compact pellets were prepared to limit the effects of florescence due to the powdery nature of the samples.
3. Results
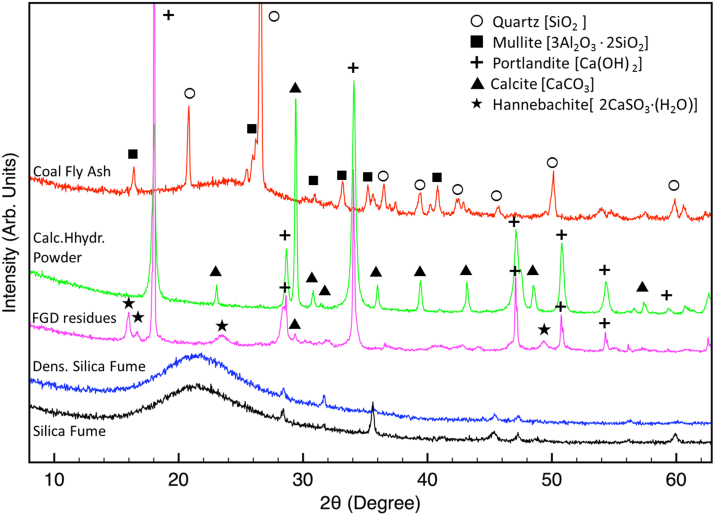
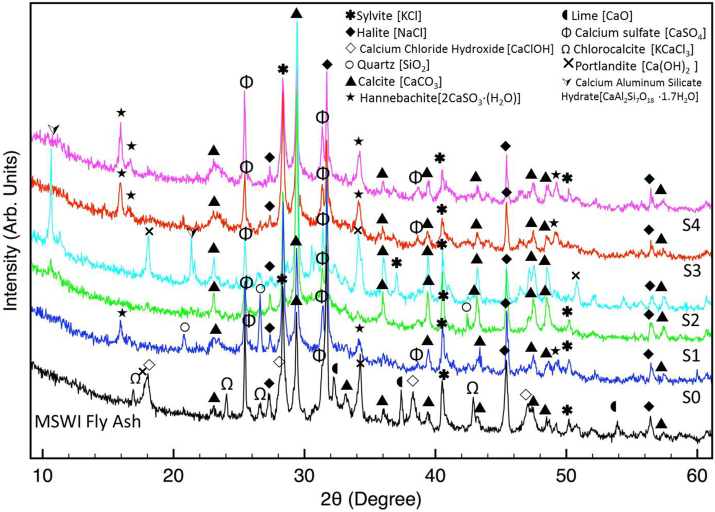
Fig. 3 reports the XRD patterns collected on the materials used for the stabilization procedure. All the crystalline phases found in these patterns are also reported.
Fig. 3.
XRD patterns collected on the materials used for the stabilization procedure. All the crystalline phases found in these patterns are also reported.
Analyzing the spectra of Fig. 3, it is possible to compare the XRD patterns of silica fume and corresponding condensed silica fume. It is clear that, as expected, silica results amorphous. Moreover, some peaks appear in both patterns. They can be attributed to the presence of few amounts of crystalline silica phases. Because of the procedure applied to obtain condensation of silica fume (see the introduction section), it is not plausible to suppose a change in the amount of crystalline silica in the two samples. Indeed this hypothesis was never reported in literature.
The mechanism of silica entrapment can be justified by the presence on the surface of silanol groups. The sorption of metal ions on silica may take place by the cation exchange reaction through the substitution of protons from silanol on the surface by the metal ions from the solution (Srivastava et al., 2006; Bosio et al., 2014a).
In accord with literature results, the main crystalline detected phases in coal fly ash are mullite, and quartz (Helmuth, 1987).
FGD residues contain portlandite and hannebachite. As already reported in literature (Ecke, 2003), the carbonation reaction due to the presence of calcium hydroxide, is found to promote heavy metals stabilization in MSWI fly ash. It was supposed that the active phase promoting metals stabilization is the portlandite, due to the following reaction:
Ca(OH)2 + CO2 —> CaCO3 + H2O
Then, to eventually substitute FGD residues, calcium hydroxide powder could be used. For this reason a low cost calcium hydroxide powder, generally employed for building application (to realize cement), was considered. The aim is to provide another sustainable material that can support the carbonation reaction. Although calcium hydroxide powder is not a waste or by-product material, its availability is very good and its cost is quite low. A great advantage of this material is the sulphate phases absence. Indeed, the COSMOS material, obtained after the stabilization procedure, realized using FGD residues and amorphous silica, generally contains calcite and sulphate phases. Hannebachite, that originate from FGD residues, is often found in the final material (Rodella et al., 2014). The presence of this phase can limit some possible applications of the final obtained stabilized material (for example it is difficult to propose it as a filler for cement, in large quantities).
XRD pattern (see Fig. 3) shows that the calcium hydroxide powder contains not only portlandite (calcium hydroxide), but also calcium carbonate, possibly due to natural carbonation of portlandite. Indeed, the provided calcium hydroxide powder is a low-cost material (then it may be expected to obtain a non pure material).
To distinguish the calcium hydroxide phase (portlandite) from the sample of commercial calcium hydroxide powder used for FGD residues substitution, which actually contains not only calcium hydroxide but also calcite, in the following the calcium hydroxide phase will be always called portlandite.
Fig. 3 shows that, as already discussed, also FGD residues contain not only portlandite, but at least another phase other than calcium hydroxide. As a consequence, to compare the effect of these two materials it was chosen to substitute FGD residues in the COSMOS technology (to prepare samples S1 and S2) with the same amount of the calcium hydroxide powder (see Table I).
Stabilized samples, obtained by MSWI fly ash treatments with different raw materials (i.e by-products of industrial applications) were realized, as reported in the experimental section. The aim is to investigate the change in the heavy metal entrapment efficiency.
The traditional COSMOS technology requires only the opportune mixing of wastes and by-products, with the addition of a proper amount of water. Generally the samples are then left to ripen at ambient conditions for 30 days allowing the water to totally evaporate, the stabilization reactions to complete and the samples to solidify. In the present work the COSMOS procedure was accelerated, by using a thermal annealing made at 120 °C for 4 hours, to promote a rapid water evaporation and to increase the reactions kinetics. This thermal treatment was realized to avoid the last step (samples ripening). Indeed, although natural water evaporation is a low-cost process, it is dependent on the meteorological conditions, therefore, it can then take up to several days.
In order to assess the environmental impact of a waste material and the efficacy of a stabilization procedure, it is important to determine the leachable metals contained in the starting waste and in the final stabilized material. Table II reports the elements concentration in the solutions obtained after the leaching tests on the final obtained materials. As a reference, the concentration of the elements found in the leaching solution of MSWI fly ash before the stabilization is reported as well.
Table II.
Elements concentration in the solutions, obtained after the leaching tests on the final obtained materials. As a reference, also the concentration of the elements found in the leaching solution of MSWI fly ash before the stabilization, is reported.
| Element | Fly ash [mg/L] |
S0 [mg/L] |
S1 [mg/L] |
S2 [mg/L] |
S3 [mg/L] |
S4 [mg/L] |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | 33 | ± | 22 | 204 | ± | 19 | 43 | ± | 12 | 111 | ± | 20 | 223 | ± | 19 | 228 | ± | 27 |
| Cl | 8991 | ± | 1671 | 3779 | ± | 1276 | 5667 | ± | 475 | 4255 | ± | 935 | 3900 | ± | 567 | 5505 | ± | 524 |
| K | 1935 | ± | 423 | 855 | ± | 350 | 1600 | ± | 141 | 914 | ± | 244 | 851 | ± | 155 | 1283 | ± | 128 |
| Ca | 5354 | ± | 1006 | 2206 | ± | 553 | 3538 | ± | 298 | 3195 | ± | 505 | 2210 | ± | 258 | 3344 | ± | 354 |
| Fe | 1.7 | ± | 0.4 | 0.66 | ± | 0.17 | 1.3 | ± | 0.2 | 0.85 | ± | 0.28 | 0.63 | ± | 0.07 | 1.1 | ± | 0.2 |
| Cu | 0.79 | ± | 0.2 | n.d | 0.26 | ± | 0.09 | n.d | n.d | n.d | ||||||||
| Zn | 7.98 | ± | 1.28 | 0.15 | ± | 0.03 | 0.32 | ± | 0.05 | 0.66 | ± | 0.08 | 0.24 | ± | 0.04 | 0.31 | ± | 0.03 |
| Br | 136 | ± | 22 | 65 | ± | 18 | 69 | ± | 6 | 58 | ± | 10 | 72 | ± | 8 | 73 | ± | 6 |
| Sr | 5.6 | ± | 0.9 | 5.7 | ± | 1.1 | 10.3 | ± | 0.8 | 9.4 | ± | 1.2 | 7.2 | ± | 0.6 | 6.9 | ± | 0.6 |
| Ba | 8.8 | ± | 1.7 | 2.4 | ± | 0.5 | 5.4 | ± | 0.5 | 3.5 | ± | 1.2 | 0.7 | ± | 0.5 | 2.1 | ± | 0.3 |
| Pb | 104 | ± | 17 | n.d | 0.35 | ± | 0.04 | 1.5 | ± | 0.2 | n.d | 0.033 | ± | 0.004 | ||||
From this Table it appears that the starting values of Zn and Pb (in the MSWI fly ash solution) exceed the regulatory limits of contaminant, to be considered toxic. The amount of these two metals, in the leaching solutions of all stabilized samples, always results reduced (in particular, Pb concentration results about 2 orders of magnitude lower than the corresponding one found in the solution of MSWI fly ash, for all samples).
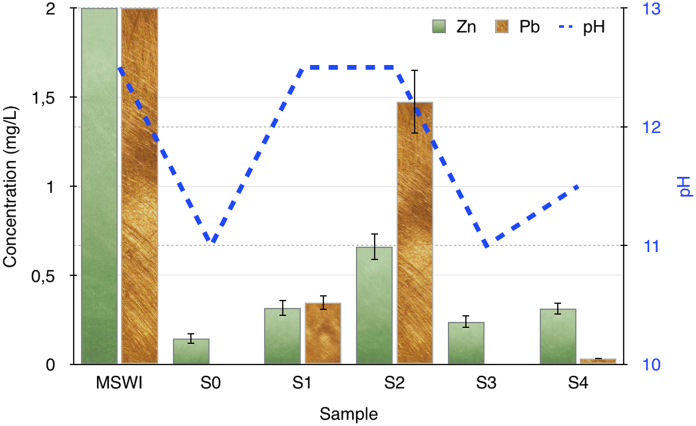
To highlight and discuss in more details the results about stabilization of heavy metals, made by using different starting materials, the concentration values of Pb and Zn in the leaching solution of all samples are shown in Fig. 4.
Fig. 4.
Concentration values of Pb and Zn in the leaching solution of all samples. The y-axis in this figure was cut at the concentration of 2 mg/L, to compare the residual low quantities of heavy metals in stabilized samples. As a consequence, the concentrations of Pb and Zn in the leaching solution of the MSWI fly ash result out of the scale. The corresponding pH of the solutions is also reported.
The y-axis in this Figure was cut at the concentration of 2 mg/L, to compare the residual low quantities of heavy metals in the stabilized samples. As a consequence, the concentrations of Pb and Zn in the leaching solution of the MSWI fly ash result out of the scale.
To discuss the data reported in Fig. 4, it is important to remember that the samples that have been better stabilized exhibit lower Pb and Zn concentrations in their leaching solutions (see also Table II). By analyzing these data it appears that sample S0 results to be better (Pb is undetectable and the Zn concentration is about 0,15 mg/L). As a consequence, it is confirmed that the already established COSMOS procedure (Rodella et al., 2014) for heavy metals stabilization, based on the use of amorphous silica source, with the addition of coal fly ash, FGD residues, and silica fume, results extremely effective in Pb and Zn stabilization. Moreover, also sample S3 shows comparable results; this sample was obtained by applying the COSMOS procedure, with only one difference: coal fly ash was substituted by FGD residues. This was done, as already reported in the introduction, because the coal fly ash can have a commercial value, that generally is considered higher in respect to FGD residues. The eventual substitution of coal fly ash with FGD residues can make the stabilization technology more economic, in respect to the traditional COSMOS.
Sample S4, that was realized with the same ingredients of sample S3, but by substituting the silica fume, with the corresponding condensed silica fume, appears to have a higher amount of Pb and Zn in the leaching solution, in respect to samples S0 and S3. This leads to the conclusion that the reactivity of condensed silica fume seems to be less than corresponding non-condensed silica. The decrease of silica fume reactivity, due to its condensation, was already observed and reported in literature, mainly related to its reactivity during the cement production. In particular, it was reported, that the densified silica may be not easily dispersed during the mixing procedure and the agglomerates may not be broken down completely (Mitchell et al., 1998). Indeed, despite the fact that sometimes the agglomeration process is considered “reversible”, most of the available scientific evidence suggests that this is generally not so (Diamond et al., 2004). However, despite the reduced reactivity of condensed silica fume, the stabilization procedure may be optimized, probably by increasing the quantity of silica in respect to the MSWI fly ash amount.
Samples S1 and S2 produced the leaching solutions with the higher quantities of Pb and Zn. These two samples were obtained by employing calcium hydroxide powder instead of FGD residues. Then it is possible to conclude that the efficacy of FGD residues in heavy metals stabilization seems higher than low-cost calcium hydroxide powder. Moreover, comparing samples S1 and S2, it appears that S2 contain a higher amount of Pb and Zn in the leaching solution. Indeed, this sample was realized also by using the condensed silica fume, that already showed lower reactivity in respect to corresponding non-condensed silica. Then this result is in accord with data obtained from samples S3 and S4.
However, even with the use of this powder, optimization of the stabilization procedure could be possible, for example by increasing the amount of the silica fume.
Fig. 5 shows XRD patterns of MSWI fly ash and stabilized samples obtained by using the different stabilizing agents, as reported in Table I.
Fig. 5.
XRD patterns of MSWI fly ash and stabilized samples obtained by using the different stabilizing agents, in accord to quantities reported in Table I. All the crystalline phases found in these patterns are also reported.
The MSWI fly ash crystalline composition is in accord with results already found and reported in literature (Bontempi et al., 2010; Abbas et al., 2003). In particular, it contains calcium sulphate, calcium carbonate (calcite), chlorides (KCl and NaCl, CaClOH, KCaCl3), lime (CaO), and portlandite. CaClOH is an intermediate phase formed during the absorption of HCl (Wang et al., 2010). Also CaClOH is available for carbonation reaction (Tian and Jiang, 2012) as:
2CaClOH + CO2 —> CaCl2 + CaCO3 + H2O
We have already discussed the changes in the XRD patterns of stabilized MSWI fly ash, in respect to the starting material (Bontempi et al., 2010). The disappearance of CaClOH in the XRD patterns of all stabilized samples and of portlandite, in the XRD patterns of samples S0, S1, S3 and S4, suggests that carbonation reactions have occurred, resulting in the formation of calcite, CaCO3 (Bontempi et al., 2010). The carbonation process reduces the alkalinity of the materials mixture since it involves CO2 dissolution. It is recognized that carbonation reduces metal leachability not only by changing metals solubility because of precipitation of their carbonates and/or by their sorption to the newly formed minerals (Ecke, 2003), but also by its effect on the pH.
The pH was measured for starting MSWI fly ash and for all stabilized samples. The pH of wetted MSWI fly ash is 12,5 which is very close to the pH of a Ca(OH)2 saturated solution. The pH of the leaching solution of MSWI fly ash (12,5) and of the leaching solution of the samples obtained after the stabilization reactions, is reported in Fig. 4.
Comparing the pH of leaching tests, it is evident that sample S0, S3, and S4 that exhibit the best results in terms of heavy metals stabilization, show a decrease of the pH. It is possible to deduce that the chemical reactions occurring during carbonation contribute to an increase in the efficiency of stabilization, by reducing the metals mobility. This was already found for samples stabilized by using amorphous silica contained in the rice husk ash (Bosio et al., 2014a), but this is the first time that a similar behavior can be highlighted also for samples stabilized with silica fume. It is extremely interesting to notice that samples S1 and S2, obtained by using calcium hydroxide powder, instead of FGD residues, are different from the others; the pH remains at the initial value of 12,5 with no reduction. On the contrary, S0 and S3, realized by using FGD residues and silica fume, are the samples with the higher pH reduction due to the stabilization reactions. Finally, sample S4, that was obtained by using FGD residues and condensed silica fume, shows an intermediate behavior: pH was reduced to 11,5.
XRD patterns of stabilized samples (see Fig. 5) show that samples S0, S3, and S4 contains also hannebachite, that is originally contained in the FGD residues. These data are in accord with the results reported in Table II. In particular, it appears that the amount of S found in leaching solutions of S1 and S2 is lower than the one found in corresponding solutions of S0, S3, and S4.
Sample S2 shows an XRD spectrum different from the other samples: the main difference concerns the presence of portlandite, added as calcium hydroxide powder to promote the carbonation reaction, but that is still present in the final material. It is possible to deduce that the total amount of portlandite does not completely react. It is extremely important also to notice that corresponding sample, realized with calcium hydroxide powder, but using non-condensed silica fume (S1) shows better results in terms of heavy metals stabilization. Indeed, the XRD pattern of S1 does not show the presence of un-reacted portlandite.
Another difference occurring in the XRD pattern of S2 sample concerns the presence of a zeolite phase (calcium aluminium silicate hydrate). The main peak of this phase (at about 10,5° in 2Theta) appears also in the XRD pattern of sample S1, but with very low intensity. Silica fume was already employed for the synthesis zeolite (Prud'homme et al., 2012). It was also shown that this zeolite phase can be formed by the reaction of portlandite with silica (Rodriguez-Navarro et al., 2013). The presence of this phase can be attributed to a so-called pozzolanic reaction, that occurs in building materials.
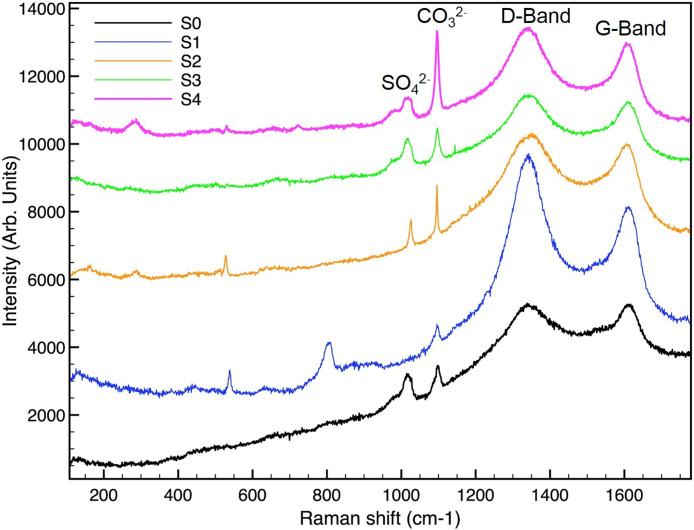
Fig. 6 shows the Raman spectra of all stabilized samples. From Fig. 6 it is possible to observe the presence of the same absorption signals in all samples, in particular the peaks at ∼ 1010 cm−1 and ∼ 1020 cm−1 are relative at the sulfate group (v1[SO4][2]2−) (Chang et al., 1999) the absorption signal at ∼ 1095 cm−1 can be attribute to the carbonate group (v1[CO3][2]2−) (Garg et al., 2013).
Fig. 6.
Raman spectra of all stabilized samples.
By a comparison with the XRD analysis, it is possible to conclude that the sulfate group is due to the calcium sulfate and the carbonate group is due to the presence of calcite. Moreover it was not possible to observe the portlandite (that results present in sample S2) absorption signal because the O-H stretching vibration is at 3640 cm−1, out of the instrument investigation range.
In all the Raman spectra collected on the samples two peaks are found in the region of 1200–1700 cm-1. They are due to the characteristic of C—C carbon bonds in elemental carbon: the peak at about 1600 cm-1 corresponds to ordered or graphitic structure (G) and the peak at about 1350 cm-1 corresponds to disordered structure (D). The disordered form is due to randomly orientated carbon (Keiluweit et al., 2010) while graphite is characterized by a crystalline structure with carbon atoms organized on specific planes (Bjurström et al., 2014). The ratio of the D-band area to the G-band area has long been indicative of the graphite-like order of carbon substrates. In particular, the extent of graphitization highly influence the adsorption properties of coal (Du and Miller, 2007; Han et al., 2003).
The presence of unburned carbon in MSWI fly ash is already reported in literature (Bjurström et al., 2014) and it was found that the ratio between graphitic and disordered form of elemental carbon corresponds to values found for commercial activate carbon. It is well-known that activated carbon, due to its strong sorption capacity, reduces the bioavailability of organic contaminants (Hilber and Bucheli, 2010). This is extremely interesting and may help also to understand the adsorption properties attributed to fly ash (Chen et al., 2003).
Indeed, the use as adsorbent of COSMOS material was already tested. It was shown (Bosio et al., 2014b) that the capability of this material to adsorb methylene blue is higher in comparison to fly ash (also coal and lignite fly ash).
4. Conclusions
One of the major obstacles to the use of MSWI fly ash as raw material is the leaching of potentially hazardous substances. In recent years a new sustainable technology for MSWI fly ash stabilization was developed, based on the use of waste silica source as heavy metals stabilizer. The final obtained material was employed as a filler in several matrices (plastics, bioplastic, resins, plasters, etc.), also in the frame of Raw Materials Initiative.
Moreover, the materials employed in the stabilization procedure may be not available in all areas.
This paper investigates the possibility of substituting silica fume with corresponding condensed silica fume and of substituting FGD residues with low-cost calcium hydroxide powder. The removal of coal fly ash was also considered. The results of leaching tests show that the substitution of these materials reduces the efficacy of the COSMOS stabilization technology. In particular, it was shown that the use of condensed silica fume with calcium hydroxide powder does not allow the occurrence of complete carbonation reaction. Then the replacement of both materials (silica fume and FGD residues) promoting the chemical reactions, with low cost substituting materials, is not desirable.
However, this may be made in areas where the availability of some materials employed in the COSMOS technology is not possible. In this case the stabilization procedure may be optimized, for example by the addition of a larger amount of silica, in respect to the traditional developed technology.
Declarations
Author contribution statement
Elza Bontempi: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Nicola Rodella, Niccolò Bontempi, Giuseppe Tomasoni: Performed the experiments.
Michela Pasquali: Analyzed and interpreted the data.
Annalisa Zacco: Contributed reagents, materials, analysis tools or data.
Laura Eleonora Depero: Wrote the paper.
Funding statement
This work was supported LIFE+, the financial instrument of the European Community to support environmental projects (LIFE+ 2011 project ENV/IT/000256) and by Italian Ministry of the Environment (RISANA project).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abbas Z., Moghaddam A.P., Steenari B.-M. Release of salts from municipal solid waste combustion residue. Waste Manage. 2003;23(4):291–305. doi: 10.1016/S0956-053X(02)00154-X. [DOI] [PubMed] [Google Scholar]
- Benassi L. Comparison between rice husk ash grown in different regions for stabilizing fly ash from a solid waste incinerator. J. Environ. Manage. 2015;159:128–134. doi: 10.1016/j.jenvman.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Benassi L. Rice Husk Ash to Stabilize Heavy Metals Contained in Municipal Solid Waste Incineration Fly Ash: First Results by Applying New Pre-treatment Technology. Materials. 2015;8(10):6868–6879. doi: 10.3390/ma8105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besco S. Processing and properties of polypropylene-based composites containing inertized fly ash from municipal solid waste incineration. J. Appl. Polym. Sci. 2013;130(6):4157–4164. [Google Scholar]
- Besco S. Structural and Mechanical Characterization of Sustainable Composites Based on Recycled and Stabilized Fly Ash. Materials. 2014;7:5920–5933. doi: 10.3390/ma7085920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billen P. Comparison of solidification/stabilization of fly ash and air pollution control residues from municipal solid waste incinerators with and without cement addition. J. Mater. Cycles Waste. 2014;17(2):229–236. [Google Scholar]
- Bjurström H., Lind B.B., Lagerkvist A. Unburned carbon in combustion residues from solid biofuels. Fuel. 2014;117:890–899. [Google Scholar]
- Bonen D., Khayat K.H. Characterization and pozzolanic properties of silica fume stored in an open pond. Cem. Concr. Res. 1995;25(2):395–407. [Google Scholar]
- Bontempi E. A new method for municipal solid waste incinerator (MSWI) fly ash inertization, based on colloidal silica. J. Environ. Monit. 2010;12(11):2093–2099. doi: 10.1039/c0em00168f. [DOI] [PubMed] [Google Scholar]
- Borgese L. Total reflection of x-ray fluorescence (TXRF): a mature technique for environmental chemical nanoscale metrology. Meas. Sci. Technol. 2009;20(8) [Google Scholar]
- Bosio A. A sustainable technology for Pb and Zn stabilization based on the use of only waste materials: A green chemistry approach to avoid chemicals and promote CO2 sequestration. Chem. Eng. J. 2014;253:377–384. [Google Scholar]
- Bosio A. Rice husk ash based composites, obtained by toxic fly ash inertization, and their applications as adsorbents. Chem. Eng. Trans. 2014;37:631–636. [Google Scholar]
- Brunner P.H., Rechberger H. Waste to energy–key element for sustainable waste management. Waste Manage. 2015;37:3–12. doi: 10.1016/j.wasman.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Carran D. A Short History of the Use of Lime as a Building Material Beyond Europe and North America. Int. J. Archit. Herit. 2012;6(2):117–146. [Google Scholar]
- Chang H., Jane Huang P., Hou S.C. Application of thermo-Raman spectroscopy to study dehydration of CaSO4·2H2O and CaSO4·0.5H2O. Mater. Chem. Phys. 1999;58(1):12–19. [Google Scholar]
- Chen X., Farber M., Gao Y., Kulaots I., Suuberg E.M., Hurt R.H. Mechanisms of surfactant adsorption on non-polar, air-oxidized and ozone-treated carbon surfaces. Carbon. 2003;41(8):1489–1500. [Google Scholar]
- Colangelo F., Messina F., Cioffi R. Recycling of MSWI fly ash by means of cementitious double step cold bonding pelletization: Technological assessment for the production of lightweight artificial aggregates. J. Hazard. Mater. 2015;299:181–191. doi: 10.1016/j.jhazmat.2015.06.018. [DOI] [PubMed] [Google Scholar]
- Diamond S., Sahu S., Thaulow N. Reaction products of densified silica fume agglomerates in concrete. Cem. Concr. Res. 2004;34(9):1625–1632. [Google Scholar]
- Du H., Miller J.D. Adsorption states of amphipatic solutes at the surface of naturally hydrophobic minerals: a molecular dynamics simulation study. Langmuir. 2007;23(23):11587–11596. doi: 10.1021/la701604u. [DOI] [PubMed] [Google Scholar]
- Ecke H. Sequestration of metals in carbonated municipal solid waste incineration (MSWI) fly ash. Waste Manage. 2003;23(7):631–640. doi: 10.1016/S0956-053X(03)00095-3. [DOI] [PubMed] [Google Scholar]
- Galiano Y.L., Pereira C.F., Vale J. Stabilization/solidification of a municipal solid waste incineration residue using fly ash-based geopolymers. J. Hazard. Mater. 2011;185(1):373–381. doi: 10.1016/j.jhazmat.2010.08.127. [DOI] [PubMed] [Google Scholar]
- Garg N., Wang K., Martin S.W. A Raman spectroscopic study of the evolution of sulfates and hydroxides in cement–fly ash pastes. Cem. Concr. Res. 2013;53:91–103. [Google Scholar]
- Van Gerven T. Management of incinerator residues in Flanders (Belgium) and in neighbouring countries A comparison. Waste Manage. 2005;25(1):75–87. doi: 10.1016/j.wasman.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Guarienti M. Biosafe inertization of municipal solid waste incinerator residues by COSMOS technology. J. Hazard. Mater. 2014;279:311–321. doi: 10.1016/j.jhazmat.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Guarienti M. COSMOS-rice technology abrogates the biotoxic effects of municipal solid waste incinerator residues. Environ. Pollut. 2016;3:713–721. doi: 10.1016/j.envpol.2016.04.053. [DOI] [PubMed] [Google Scholar]
- Han S. Simple Solid-Phase Synthesis of Hollow Graphitic Nanoparticles and their Application to Direct Methanol Fuel Cell Electrodes. Adv. Mat. Res. 2003;15(22):1922–1925. [Google Scholar]
- Helmuth R. Portland Cement Association; Skokie: 1987. Fly ash in cement and Concrete; pp. 31–61. [Google Scholar]
- Hilber I., Bucheli T. Activated carbon amendment to remediate contaminated sediments and soils: a review. Global Nest J. 2010;12(3):305–317. [Google Scholar]
- Keiluweit M. Dynamic molecular structure of plant biomass-derived black carbon (biochar) Environ. Sci. Technol. 2010;44(4):1247–1253. doi: 10.1021/es9031419. [DOI] [PubMed] [Google Scholar]
- Mitchell D.R.G., Hinczak I., Day R.A. Interaction of silica fume with calcium hydroxide solutions and hydrated cement pastes. Cem. Concr. Res. 1998;28(11):1571–1584. [Google Scholar]
- Polettini A. Properties of Portland cement–stabilised MSWI fly ashes. J. Hazard. Mater. 2001;88(1):123–138. doi: 10.1016/s0304-3894(01)00292-8. [DOI] [PubMed] [Google Scholar]
- Ponsot I. Recycling of pre-stabilized municipal waste incinerator fly ash and soda-lime glass into sintered glass-ceramics. J. Clean. Prod. 2015;89:224–230. [Google Scholar]
- Prud'homme E. Influence of raw materials and potassium and silicon concentrations on the formation of a zeolite phase in a geopolymer network during thermal treatment. J. Non-Cryst. Solids. 2012;358(16):1908–1916. [Google Scholar]
- Rodella N. Waste silica sources as heavy metal stabilizers for municipal solid waste incineration fly ash. Arab. J. Chem. 2014 [Google Scholar]
- Rodriguez-Navarro C., Suzuki A., Ruiz-Agudo E. Alcohol dispersions of calcium hydroxide nanoparticles for stone conservation. Langmuir. 2013;29(36):11457–11470. doi: 10.1021/la4017728. [DOI] [PubMed] [Google Scholar]
- Srivastava V.C., Mall I.D., Mishra I.M. Characterization of mesoporous rice husk ash (RHA) and adsorption kinetics of metal ions from aqueous solution onto RHA. J. Hazard. Mater. 2006;134(1-3):257–267. doi: 10.1016/j.jhazmat.2005.11.052. [DOI] [PubMed] [Google Scholar]
- Tian S., Jiang J. Sequestration of flue gas CO2 by direct gas-solid carbonation of air pollution control system residues. Environ. Sci. Technol. 2012;46(24):13545–13551. doi: 10.1021/es303713a. [DOI] [PubMed] [Google Scholar]
- Wang L., Jin Y., Nie Y. Investigation of accelerated and natural carbonation of MSWI fly ash with a high content of Ca. J. Hazard. Mater. 2010;174(1-3):334–343. doi: 10.1016/j.jhazmat.2009.09.055. [DOI] [PubMed] [Google Scholar]
- Zacco A. Review of fly ash inertisation treatments and recycling. Environ. Chem. Lett. 2014;12(1):153–175. [Google Scholar]
- Zacco A. Use of colloidal silica to obtain a new inert from municipal solid waste incinerator (MSWI) fly ash: First results about reuse. Clean Technol. Envir. 2012;14(2):291–297. [Google Scholar]