Abstract
Arsenic is a prevalent environmental toxin and a Group one human carcinogenic agent. Chronic arsenic exposure has been associated with many human diseases. The aim of this study is to evaluate zebrafish as an animal model to assess arsenic toxicity in elevated long-term arsenic exposure. With prolonged exposure (6 months) to various concentrations of arsenic from 50 ppb to 300 ppb, effects of arsenic accumulation in zebrafish tissues, and phenotypes were investigated. Results showed that there are no significant changes of arsenic retention in zebrafish tissues, and zebrafish did not exhibit any visible tumor formation under arsenic exposure conditions. However, the zebrafish demonstrate a dysfunction in their neurological system, which is reflected by a reduction of locomotive activity. Moreover, elevated levels of the superoxide dismutase (SOD2) protein were detected in the eye and liver, suggesting increased oxidative stress. In addition, the progenies of arsenic-treated parents displayed a smaller biomass (four-fold reduction in body weight) compared with those from their parental controls. This result indicates that arsenic may induce genetic or epigenetic changes that are then passed on to the next generation. Overall, this study demonstrates that zebrafish is a convenient vertebrate model with advantages in the evaluation of arsenic-associated neurological disorders as well as its influences on the offspring.
Introduction
Association of arsenic exposure with adverse human health
Arsenic is a prevalent environmental toxic substance which leads to various human disorders. Humans are exposed to arsenic mainly through dietary food and water. In some areas, drinking water is the major source of arsenic exposure. For example, in Bangladesh, epidemiological arsenic exposure through drinking water has been considered the largest poisoning of a human population in history.1 Clinical reports from cohort studies in epidemic arsenic-contaminated areas disclosed that long-term exposure of high arsenic in drinking water is closely associated with a range of disorders, including skin lesions, cancers, hypertension, neurological symptoms, and heart diseases.2–10 The Agency of Toxic Substances and Disease Registry (ATSDR) publically stated that arsenic is the causal agent for the following disorders during prenatal and childhood exposure: impaired fetal development, low birth weight, fetal malformations, fetal death, blood vessel damage, lower IQ, reduced nerve function, and a possible increase in mortality in young adults (ATSDR 2007).
Arsenic in tap water has been set at a limit of 10 ppb (10 parts per billion, or 10 μg/L) by WHO and EPA, which reflects a reduction from 50 ppb before 2004. This new standard has been enforced in developed countries, but in developing countries people have been consuming arsenic far above this limit.11–13 For example, arsenic levels in drinking water is over 100 ppb in many areas in Bangladesh.14 Currently, many epidemic studies have carried out investigation of diseases associated with arsenic exposure. Nevertheless, the treatment of arsenic-associated symptoms has remained challenging for the foreseeable future.
To investigate the relationship between human disorders and arsenic exposure, arsenic poisoning animal models such as rodent models have been constantly used.15 Currently, most studies using rodent models adopt a short-term exposure to mimic acute arsenic poisoning. However, the development of certain human disorders often requires years of arsenic exposure. Thus, a long-term exposure at sublethal arsenic concentrations is very useful for the assessment of chronic arsenic toxicity as well as the development of novel prevention and therapy.
Mechanisms of arsenic toxicity and biotransformation in vivo
There are two biologically important oxidation states of inorganic arsenic: As(V) (arsenate) and As(III) (arsenite). As(V) is reduced in the cellular environment to the more toxic trivalent form As(III). Many intracellular thiol-containing proteins are highly reactive to arsenic, therefore, arsenic can alter the function of these molecular targets involved in essential cellular processes. Some arsenic targets includes metallothioneins, transcriptional factors, and metabolic enzymes.16 The human liver is the primary organ of arsenic accumulation through aquaglyceroporins, where these inorganic arsenicals can be further metabolized into a profile of methylated organic arsenic, including MAs(V) and DMAs(V).17 Recent studies reveal that humans, rodents, and zebrafish share a high degree of evolutionary conservation in arsenic metabolism.18–20 These findings validated that zebrafish can be a convenient aquatic vertebrate model closer to human conditions in the studies of arsenic metabolism and toxic effects.
Long-term arsenic exposure in animal models
A good experimental animal model is necessary to be applied in the translation of arsenic toxicity-induced clinical manifestations. The effects of long-term arsenic exposure, induced pathogenicity, and carcinogenicity have been investigated in multiple rodent models.15 However, many experiments conducted with the rodent models have been jeopardized by several confounding factors. For instance, the metabolism of arsenic in rats is inconsistent with the human metabolism for the following reasons: (1) arsenic remains in the blood circulation of rats longer than that of humans; (2) arsenic is accumulated in erythrocytes of rats; (3) arsenic is methylated more extensively and excreted in the urine at a much slower rate in rats. As a result, rats are more susceptible to arsenic-induced cytotoxicity and tumorigenicity when compared with humans.21–23 On the other hand, other frequently used animal models, like mice and rabbits, are known to excrete double the amount of arsenic a human would. This difference in arsenic excretion indicates that the potential regulation of the intracellular arsenic metabolism may contribute to dissimilar arsenic-induced abnormalities and diverse variation in susceptibility to arsenic toxicity among these animal models.15,21,24 Therefore, an alternative model organism, like zebrafish, could potentially provide a better simulation of arsenic-induced human pathologies, and elucidate arsenic toxicity accurately.
Zebrafish as a new animal model to simulate arsenic-associated human diseases
Zebrafish (Danio rerio) has been used in scientific research for decades and its entire genome has been mapped and assembled. Zebrafish is a teleost vertebrate native to the fresh water springs of India, where arsenic concentration is high. Compared with other vertebrate models, zebrafish is relatively inexpensive to maintain in the laboratory environment for multiple generations.25,26The caveat of using zebrafish models for arsenic toxicology studies is that arsenic is water soluble, thereby, allowing it to simply dissolve in the tank water can generate a uniform and well-controlled arsenic-exposed environment. Our goal in this study was to establish zebrafish as an alternative aquatic model organism to evaluate arsenic toxicity, and simulate arsenic-induced human pathogenicity.
In this study, we examined the pathogenic response to chronic arsenic exposure and found that arsenic-treated zebrafish demonstrated altered cardiac and neurological function as well as adverse impacts on their progeny. In response to arsenic exposure, zebrafish displays increased antioxidative capacity against arsenic toxicity. In summary, zebrafish can be used as a convenient model in the elucidation of chronic arsenic toxicity in cardiovascular and neurological disorders, and process advantages in the studies of arsenic toxicity in reproduction and effects on offspring.
Materials and Methods
Establishment of adult zebrafish with chronic arsenic exposures
All fish studies were approved by IACUC of Oakland University.
Wild-type zebrafish were spawned in the morning, and their fertilized embryos were collected early that afternoon. The embryos were divided into separate but equal numbered groups, immediately cleaned, and cultured in static rearing buffer (15 mM NaCl, 0.5 mM KCl, 1 mM MgSO4, 1 mM CaCl2, 0.15 mM KH2PO4, 0.05 mM Na2HPO4, 0.7 mM NaHCO3, and 10%–5% Methylene Blue; pH 7.5) at 25°C–28.5°C with inorganic arsenite reflecting the desired concentration to be continuously applied. One group served as an untreated control and was cultivated under identical conditions to the arsenic-treated group(s), but was exempted from exposure. The rearing buffer was changed on a diurnal schedule until the embryos were mobile larva. At the larva developmental stage, the fish were fed three times per day with equal amounts of filtered paramecium. When the fish began to eat, the buffer was changed and replenished weekly. After 2 weeks on a paramecium-only diet, the juvenile fish were placed on a growth-encouraging dietary regimen of brine shrimp 2 times per day and the rearing buffer levels were increased to 1 L. All fish were allotted a 1-week period of time to acclimate to the increase in pressure ensuing from the increase in rearing buffer volume. At this point, the fish were 32 days postfertilization (dpf), and the buffer was replaced with tap water, which had been oxygenated and temperature regulated the previous night.
A static water facility was adopted for chronic toxicology studies and implemented a maximum adult zebrafish density of 5 per 1 L of liquid (water or rearing buffer). All treatment conditions were exposed to the same light–dark cycles, types of food, amount of food, and feeding times in the same heated water baths. The water columns and treatment conditions for each study (at least 1 untreated control and 1 arsenic-treated group) were recycled and replenished weekly on the same day, using the same method by the same researcher. Fish densities were regulated and equally sustained for each conditional group throughout the course of the studies, and observations were recorded. Chronic toxicology studies, including data processing were conducted for 6 months as the longest duration of continuous arsenic exposure on living zebrafish. The adult fish were then rinsed for 2 h in deionized water, euthanized with 0.3 mM Tricaine (Sigma), and specified tissues were harvested according to the specific study they were sacrificed for.
The effects of arsenic exposure on the cardiovascular system in zebrafish larvae
Wild-type embryos were collected promptly following fertilization and immediately exposed to the following concentrations of arsenic (as sodium arsenite, As(III)): 0, 50, 100, 300, and 500 ppb. The embryos were subjected to treatment conditions upon fertilization to ensure examination of arsenic exposure-induced effects on organogenesis, formation of the vascular system, the nervous system, and hatchling to larval development (about 72 h). Resting heart rate was visually monitored with a dissection microscope at 1-min intervals. In total, 10 times counting was performed and beats per minute was calculated by average of these 10 times counting. Experiments were performed at the same time of the day for each condition. The larvae were allocated 30 min to acclimate to the examination conditions before data collection. N = 3 was adopted for each group, and for each individual fish.
Arsenic retention in adult zebrafish tissues measured by ICP-MS
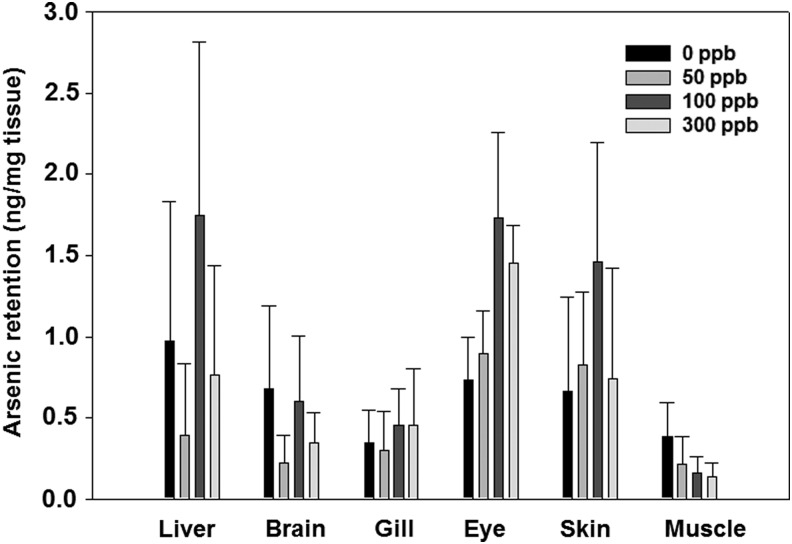
Tissue distribution as a result of arsenic exposure was evaluated following 6 months of treatment with arsenic in untreated control, 50, 100, and 300 ppb arsenic-treated groups, as described above. Each treatment group consists of five zebrafish, and the following tissues from those fish were harvested: the liver, brain, gill, eyes, skin, and muscle and individually digested in nitric acid (70%) for arsenic quantification. Instead of consolidating identical treatment tissues, testing every tissue individually ensued five data sets for each tissue for all conditions investigated and allowed us to derive standard errors. After digestion, samples were dissolved in HPLC water and quantified by ICP-MS (Inductively Coupled Plasma–Mass Spectrophotometer, PerkinElmer, NexION 300). Arsenic retention in each tissue is derived as ng arsenic/mg tissue.
The effect of arsenic exposure on adult zebrafish neurological system determined by locomotion assay
To evaluate the effects of chronic arsenic exposure on the neurological system of adult fish, a locomotion assay indicated by swimming behavior in zebrafish was adopted.27–30 Fish were exposed to arsenic treatment at 6 months of age and subjected to these treatment conditions throughout their lifespan. The three treatment conditions analyzed in this study consisted of the untreated control, 100 ppb, and 300 ppb arsenic exposure and each condition had two additional replicate groups, which were also evaluated (N = 10). Fish were housed in their typical 2-L beakers containing 1 L of water. During this 3-week study, the beakers (with the fish) were placed on a flat undisturbed surface, the fish groups were visually isolated from one another and their water was changed weekly, subsequent to recoding locomotion data pertaining to that week.
All the zebrafish treated with the three conditions were recorded by the same person to avoid human variation. A permanent line was placed on the mid section of the water level on each beaker. Each time a fish crossed the midsagittal plane of this water column, it was recorded. Data were collected for each treatment group and all replicate groups under identical conditions and at the same time of day, at 1 min intervals throughout a 10-min period, 3 days per week, and for 3 consecutive weeks. Data obtained from each condition and its replicate sets were pooled together and a standard error was calculated.
Evaluation of oxidative stress status in adult zebrafish with chronic arsenic exposure determined by western blotting
The status of oxidative stress (OS) in arsenic-treated fish was investigated indirectly by western blotting with an antibody (GeneTex; GTX124294) against superoxide dismutase, SOD2. SOD2 belongs to an antioxidant defense system located within the mitochondria. It destroys superoxide anion radicals by binding to the generated reactive oxygen species (ROS) and converting them into hydrogen peroxide and stable diatomic oxygen.31
The investigated conditions included an untreated control and a 300 ppb sodium arsenite-treated group. At 6 months of age, zebrafish from both treatment conditions were euthanized with 0.3 mM Tricaine, then eye, liver, skin, and muscle tissues were harvested (tissues from three fish were pooled as one sample). SDS-PAGE was used to resolve the proteins within the samples. The gel containing the separated proteins was then transferred onto a polyvinylidene difluoride membrane followed by western blotting.
Body weight of progeny from arsenic-treated and untreated parents
To determine the effect of arsenic on the growth of progeny, adult zebrafish, 6 months of age that had been raised in 300 ppb arsenite since fertilization, were interbred. The untreated control fish that served as the point of reference for the 6-month-old arsenic-exposed fish, and were also cultivated under identical conditions were also interbred. The fertilized embryos from the arsenic-exposed parents and those from the untreated control parents were collected the same day and raised under identical standards, and neither progenies were subjected to arsenic exposure. When the offspring had reached 3 months of age they were treated with anesthetic and individually weighed in grams. The body weight was derived for each progeny with or without parental arsenic exposure. N = 15 was adopted for each group.
Results
The tissue arsenic retention was not significantly altered after exposure
To monitor arsenic retention in zebrafish tissues after chronic arsenic exposure, various tissues were isolated from zebrafish exposed to 50, 100, and 300 ppb arsenite along with the untreated control group at 6 months. Tissues, including liver, brain, gill, eye, skin, and muscle, were individually harvested, processed, and analyzed by ICP-MS for quantifying arsenic levels. Arsenic content appeared relatively low or undetected in brain, gill, and muscle, whereas it was observed higher in the liver, eye, and skin (Fig. 1). Unlike rodents and humans, whose liver is the organ with the highest arsenic retention, zebrafish showed higher retention in not only the liver, but also eye and skin. The higher arsenic retention in eye and skin is likely due to absorption through direct contact with arsenic in water. Our results also showed that there is no further increase of arsenic retention in tissues at high arsenic concentration (>100 ppb). As zebrafish is native to rivers of India that naturally contains high levels of arsenic, it is possible that these animals have developed efflux mechanisms, which enable them to tolerate continuous arsenic poisoning. Large standard errors were observed in the experiments, which could be caused by smaller tissue size and lower arsenic reading.
FIG. 1.
Total arsenic retention in zebrafish tissue after chronic arsenic exposure to 50, 100, 300 ppb, and untreated control. Zebrafish were exposed after fertilization for continuous 6 months under concentrations indicated. Tissues were isolated at endpoint and processed for total arsenic quantification. N = 5 is used for statistics. SEM is calculated using SigmaPlot 10.0.
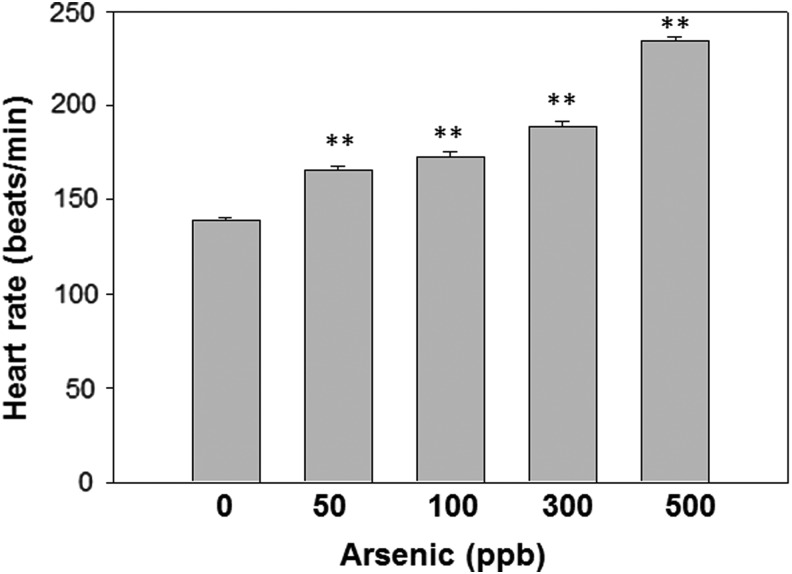
Arsenic exposure elevated resting heart rates in larva zebrafish
Arsenic exposure has been linked to cardiovascular disorders and heart diseases.32 To investigate the heart function in sublethal arsenic-exposed zebrafish larvae, promptly fertilized wild-type embryos exposed to varying concentrations of arsenite from conception were examined for heart rates. Arsenic-treated zebrafish showed increased resting heart rates during the larval developmental stage (juvenile) when compared with the untreated negative control group (Fig. 2). In addition, arsenic-induced resting heart rates increased in a concentration-dependent manner, and the highest arsenic concentration examined was 500 ppb. The larva heart rates from that group were increased by approximately 50% compared with those of untreated fish (Fig. 2).
FIG. 2.
Arsenic treatment of zebrafish larvae increased heart rate in a concentration-dependent manner. Zebrafish eggs were exposed to 50, 100, 300, 500 ppb sodium arsenite till hatched (3 days after fertilization) along with an untreated control. The rate of resting heart beats was recorded. N = 3 is used for statistics. **p < 0.01
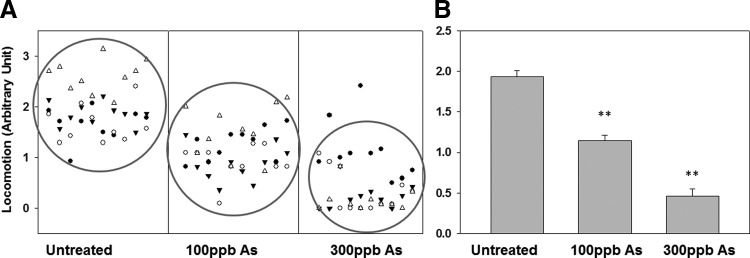
Chronic arsenic exposure disturbed neurological behavior in adult zebrafish
Previous studies have indicated that chronic arsenic poisoning is associated with neuropathies.33 To investigate the effects of chronic arsenite exposure (6-months) on neurological functions of zebrafish, embryos were collected and exposed to arsenic at 100 ppb and 300 ppb along with an untreated control cultivated under identical conditions. Neurological functions were determined by a well-adopted approach, which evaluates the locomotion of zebrafish. Locomotion was manually recorded as described. We used both a scatter plot and a bar graph to represent their standard deviations, as shown in Figure 3. Compared with the untreated control group, both the 100 ppb and 300 ppb arsenic-treated zebrafish displayed significantly reduced locomotion in a concentration-dependent manner, a sign of neurological pathology.
FIG. 3.
Locomotion of adult zebrafish with prolonged arsenic treatment at 100 ppb and 300 ppb conditions. (A) Locomotion of individual zebrafish was recorded. Each data point collected represents each time a fish crossed the midsagittal plane of the water column. (B) Statistical results that are corresponding to (A). N = 10 for each condition. **p < 0.01
Chronic arsenic exposure upregulated SOD2 expression in eye and liver
The toxicity of arsenicals to biological systems is contributed partly from enhanced OS.34 Antioxidative enzymes, such as superoxide dismutase (SOD), detoxify excess ROS to maintain intracellular redox homeostasis. SOD2 encodes the mitochondria superoxide dismutase. It is a natural ROS scavenger and supposed to be highly expressed in tissues where arsenic exposure increases OS. To determine whether chronic arsenic exposure induces a redox imbalance, the protein levels of SOD in zebrafish were examined. Zebrafish treated with 300 ppb arsenic, along with untreated control, were used to quantify SOD2 expression. The result shows that SOD2 was identified at 25 kDa, and expressed most extensively in the liver and eye tissues of the 300 ppb arsenic-treated group (Fig. 4). Two other tested tissues, muscle and skin, did not show any visible elevation in the arsenic-treated fish (data not shown). This proof-of-concept study establishes the upregulation of SOD2 expression in the liver, because it is the primary site for arsenic accumulation in humans, and it appears that zebrafish share this characteristic.
FIG. 4.
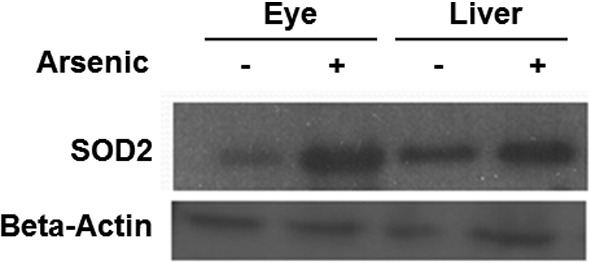
Expression of SOD2 in zebrafish eyes and liver with arsenic exposure to 300 ppb along with untreated controls. Western blotting is performed with antibody against zebrafish SOD2 (25 kDa). β-actin serves as a loading control expressed in tested tissues.
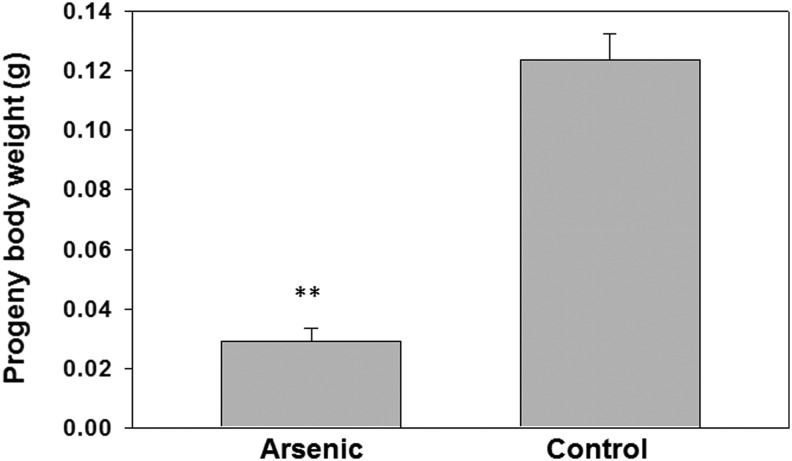
Parental arsenic exposure decreased the body mass of progeny
To determine whether the progeny from arsenic-treated parents were affected, we bred the fish from the arsenic-treated pool along with untreated controls. The progeny of each group were raised without treatment. Our results demonstrate that after a 3-month culture, the biomass of the progeny derived from the 300 ppb parental arsenic treatment group have about 75% less than those from the untreated control parents (Fig. 5).
FIG. 5.
Biomass of progeny derived from arsenic-treated parents were significantly lower than untreated control. Progenies are cultured from arsenic-treated parents along with untreated control. The 3-month-old offsprings were weighed and compared in body weight. N = 15 and **p < 0.01
Discussion
The scientific community has been aware of the dilemma regarding low-dose chronic arsenic exposure and its associations with induction of human pathogenicity and carcinogenicity for decades. Extensive research has been conducted with several animal models, including rodents, which have enlightened and demystified the field of arsenic toxicology.23 Although rodent models share analogous arsenic metabolism to humans, many reports are concerned that these models remain a certain degree of inconsistency with humans, and are costly. Mice for instance, excrete double the amount of arsenic that has been reported in humans.35 In rats, ingested arsenic remains in the blood significantly longer than in humans.15 Rats also methylate arsenic more extensively and generate methylated metabolites that are uncommon in human cohort studies.22,36,37
On the other hand, rodent models have limitations in the study of neurological disorders as well as its impact on their progenies. It has been known that behavioral studies using maze in mouse model have significant individual difference, and is also labor extensive. It is also a challenge to study arsenic impact on the next generation while maintaining the resemblance of arsenic administration to human arsenic exposure. When using these models, significant individual differences are frequently noticed. For instance, when mice are used to investigate chronic arsenic exposure, the arsenic is typically dissolved in their water supply, but it is nearly impossible to generate uniform exposure conditions among different mice subjects. Moreover, individual water consumption can vary significantly between mice of the same strain and the variation is more drastic between mice of two different strains.37 Another limit of using rodent model is the rising cost when a larger population of animals is desired, such as performing large-scale drug screening to seek for better treatment or prevention for countering arsenic toxicity.
A preponderance of experimental designs and confounding factors demand a new model organism to investigate chronic arsenic toxicity with precision and ease for simulation of arsenic-induced aberrations in human physiology. In this study, we propose to use zebrafish as a feasible and alternative animal model in the simulation of chronic arsenic exposure. Advantages in using the zebrafish model include its short lifespan, easiness of longer exposure, convenience in examination of neurological disorders through locomotion, and studies on the progeny.
Our past works support that the zebrafish model is useful for the investigation of chronic arsenic-induced abnormalities that are indicative of human response. It is evident that arsenic detoxification pathways in zebrafish include transport for inorganic arsenic through aquaporins and phosphate transporters, and arsenic methylation by methyltransferase. All these pathways share similar molecular mechanisms to humans. The tissue retention and arsenic metabolomic profiling are also consistent to that in humans.19,20
Significant human pathology that is associated with arsenic exposure includes multiple types of cancers. How arsenicals cause cancer has been postulated. One of the theories is that the initial biological response to arsenic toxicity is the significant increase of free radicals, such as ROS and reactive nitrogen species,38 which can modify intracellular macromolecules such as ribonucleic acids, proteins, and lipids. Consequently, these free radical-induced damages cause aberrant cellular processes. Natural defense against free radicals in biological systems are antioxidants and enzymes with antioxidant properties such as glutathione peroxidase, superoxide dismutase, and catalase.39,40 Thus, the status of these antioxidant proteins are often applied as surrogate markers in the assessment of OS, which could also serve as an indication of precancer condition.
In this study, no solid tumors have been identified in the arsenic-treated fish. The state of OS was altered in certain tissues, such as eye and liver, by using mitochondrial SOD2 as a biomarker. A multitude of laboratories have published evidence for an increase in ROS succeeding arsenic exposures, for example, low dose of arsenic exposure induce OS in the zebrafish brain.41 Therefore, it is logical to anticipate elevated SOD2 levels in the liver, where the majority of arsenic is accumulated. Eye is another organ where SOD2 is detected. There is no difference in SOD2 expression detected in skin and not much SOD2 is expressed in muscle (data not shown). The generation of arsenic-induced ROS have been actively involved in DNA damage, cell death, neurological disorders, diseases, and cancers in human.34,39,42 Thus, increased OS may be indicative of a precancerous condition in zebrafish with chronic arsenic exposure.
Our results showed that arsenic exposure increased zebrafish larvae heart rates in a concentration-dependent manner. This observation not only serves as a potential fast approach to monitor water quality, but it also indicates that arsenic exposure directly interferes with cardiovascular function. In a 2009 report, ATSDR stated that chronic arsenic exposure may cause adverse cardiovascular effects, including cardiovascular disease. A recent clinical study consisting of 10,519 men and 11,334 women reported that elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy populations.43 In 2003, Lee and associates published in vitro and in vivo findings obtained from the rat model that arsenic exposure inhibits acetylcholine's relaxation effects on blood vessels in a concentration-dependent manner. The authors' proposed reasoning was arsenic-induced impairment of vasomotor tone as a contributing factor in the onset of cardiovascular disease associated with arsenic exposure.44 It is possible that the treated larva also experienced an increase in resting heart rate as a result of arsenic-induced impairment of vasomotor tone.
Moreover, our results showed zebrafish exposed to arsenic for a prolonged term (one third of their lifespan) acquired neurological abnormalities. These findings are consistent with reports on humans that are chronically exposed to sublethal doses of arsenic. According to ATSDR, the sublethal dose of chronic arsenic exposure can cause neurological disturbances, including muscle cramps, muscle tenderness, numbness, paresthesia, encephalopathy, and the most common effect sensory-predominant peripheral neuropathy in a “stocking and glove” pattern (ATSDR 2009). Neurological disorders were also observed in rodents. For example, studies in rodent models have provided evidence to indicate that arsenic exposure influences several behaviors and systems related to aspects of memory, learning, motor function, and locomotion.45 Upon chronic exposure to arsenic, rats show significant memory impairment during the Morris water maze test46–48 as well as decreased learning ability on novel object exploration.49 On the other hand, mice exposed to chronic low-level arsenic exhibit gender-specific and dose-dependent alterations on spontaneous locomotor activity,50 while the mobility is reduced in rats.51,52 The neurological impact on early developmental stage attract attention also because arsenic has been detected in baby rice cereal, which form the major arsenic exposure source.
There are two different types of peripheral nerves, sensory and motor. Sensory nerves have a critical role in processing feelings such as pain, temperature, touch, and position. Motor nerves assist in the maintenance of muscle tone and are actively involved in physical movement. Findings, such as reduction of locomotive activity upon arsenic exposure, imply arsenic-induced irregularities within motor nerves in zebrafish. Compared with rodent model, locomotion studies in zebrafish possess advantages in easiness, low cost, and maintenance, and zebrafish can also be monitored in larger populations.
Zebrafish externally fertilize their oocytes, which provides a direct approach to study the impact on their progeny upon parental administration. In rodents, it was reported that arsenic has spermatotoxicity in animals,53,54 and also inhibits testicular steroidogenesis, ovarian steroidogenesis, and gonadotrophin secretion,55 and reduces the weight of the testes and accessory sex organs.56 In humans, several epidemiologic studies have reported that arsenic exposure in utero increased spontaneous abortion and stillbirth and decreased birth weight.57–59 The ATSDR toxicological profile for arsenic states that chronic exposure to arsenite through drinking water has been associated with excess incidence of miscarriages, stillbirths, preterm births, and infants with low birth weights (ATSDR 1998). Despite these studies to demonstrate the multitargeting properties of arsenic, the inherited defects from parents have not been reported. Our results showed that the offspring of parental zebrafish chronically exposed to arsenic have significant lower body mass. Thus, we provide the first evidence that zebrafish could be an ideal model in simulating those birth defects and inheritable damages to progeny due to chronic arsenic exposure, a field that has not drawn sufficient attention.
Zebrafish model might not be a perfect model to simulate all arsenic-associated human disorder, particularly cancers, but it shows promising properties in the assessment of human pathogenic responses to chronic arsenic exposure such as prenatal exposure, and neurological and cardiovascular disorders. It also possesses advantages in large-scale screening for treatments and preventions. Multiple studies will commence involving the offspring of the chronically exposed and untreated control zebrafish parents comprising: their overall intelligence, effects on specific organ systems, reproduction, and their entire developmental process. The carcinogenicity of arsenic can be done in p53 mutant fish with similar exposure studies. Further exploration of arsenic-induced pathologies will include its involvement as a cocarcinogen in combination with ultraviolet light exposure.
Acknowledgments
The authors thank Anoumid Vaziri (Oakland University) for critical editing of this article. This work was supported by NIEHS 022800 to Z.L.
Disclosure Statement
No competing financial interests exist.
References
- 1.Smith AH, Lingas EO, Rahman M. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. B World Health Organ 2000;78:1093–1103 [PMC free article] [PubMed] [Google Scholar]
- 2.Pierce BL, Argos M, Chen Y, Melkonian S, Parvez F, Islam T, et al. Arsenic Exposure, Dietary Patterns, and Skin Lesion Risk in Bangladesh: A Prospective Study. Am J Epidemiol 2010;171:S3–S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heck JE, Andrew AS, Onega T, Rigas JR, Jackson BP, Karagas MR, et al. Lung Cancer in a US Population with Low to Moderate Arsenic Exposure. Environ Health Persp 2009;117:1718–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argos M, Pierce BL, van Geen A, Graziano J, Ahsan H. Arsenic exposure from drinking water and mortality in Bangladesh Reply. Lancet 2010;376:1642. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Graziano JH, Parvez F, Liu ML, Slavkovich V, Kalra T, et al. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: prospective cohort study. Brit Med J 2011;342:d2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang CY, Chang CC, Chiu HF. Does Arsenic Exposure Increase the Risk for Prostate Cancer? J Toxicol Env Heal A 2008;71:1559–1563 [DOI] [PubMed] [Google Scholar]
- 7.Baastrup R, Sorensen M, Balstrom T, Frederiksen K, Larsen CL, Tjonneland A, et al. Arsenic in drinking-water and risk for cancer in Denmark. Environ Health Persp 2008;116:231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De BK, Majumdar D, Sen S, Guru S, Kundu S. Pulmonary involvement in chronic arsenic poisoning from drinking contaminated ground-water. J Assoc Physicians India 2004;52:395–400 [PubMed] [Google Scholar]
- 9.Chen Y, Wu F, Parvez F, Ahmed A, Eunus M, McClintock TR, et al. Arsenic Exposure from Drinking Water and QT-Interval Prolongation: Results from the Health Effects of Arsenic Longitudinal Study. Environ Health Persp 2013;121:427–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abhyankar LN, Jones MR, Guallar E, Navas-Acien A. Arsenic exposure and hypertension: a systematic review. Environ Health Persp 2012;120:494–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valenzuela OL, Borja-Aburto VH, Garcia-Vargas GG, Cruz-Gonzalez MB, Garcia-Montalvo EA, Calderon-Aranda ES, et al. Urinary trivalent methylated arsenic species in a population chronically exposed to inorganic arsenic. Environ Health Persp 2005;113:250–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall MN, Liu XH, Slavkovich V, Ilievski V, Mi ZY, Alam S, et al. Influence of Cobalamin on Arsenic Metabolism in Bangladesh. Environ Health Persp 2009;117:1724–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y, Wang Y, Zheng Q, Li X, Li B, Jin Y, et al. Association of oxidative stress with arsenic methylation in chronic arsenic-exposed children and adults. Toxicol Appl Pharmacol 2008;232:142–149 [DOI] [PubMed] [Google Scholar]
- 14.Jiang JQ, Ashekuzzaman SM, Jiang AL, Sharifuzzaman SM, Chowdhury SR. Arsenic Contaminated Groundwater and Its Treatment Options in Bangladesh. Int J Env Res Pub He 2013;10:18–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.States JC, Barchowsky A, Cartwright IL, Reichard JF, Futscher BW, Lantz RC. Arsenic toxicology: translating between experimental models and human pathology. Environ Health Persp 2011;119:1356–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson WH. Chapter Two–Molecular Mechanisms in Arsenic Toxicity. Adv Mol Toxicol 2015;9:3–37 [Google Scholar]
- 17.Yang HC, Fu HL, Lin YF, Rosen BP. Pathways of arsenic uptake and efflux. Curr Top Membr 2012;69:325–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamdi M, Sanchez MA, Beene LC, Liu QY, Landfear SM, Rosen BP, et al. Arsenic transport by zebrafish aquaglyceroporins. BMC Mol Biol 2009;10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamdi M, Yoshinaga M, Packianathan C, Qin J, Hallauer J, McDermott JR, et al. Identification of an S-adenosylmethionine (SAM) dependent arsenic methyltransferase in Danio rerio. Toxicol Appl Pharmacol 2012;262:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beene LC, Halluer J, Yoshinaga M, Hamdi M, Liu ZJ. Pentavalent Arsenate Transport by Zebrafish Phosphate Transporter NaPi-IIb1. ZEBRAFISH 2011;8:125–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen SM, Arnold LL, Eldan M, Lewis AS, Beck BD. Methylated arsenicals: the implications of metabolism and carcinogenicity studies in rodents to human risk assessment. Crit Rev Toxicol 2006;36:99–133 [DOI] [PubMed] [Google Scholar]
- 22.Vahter M. Methylation of inorganic arsenic in different mammalian species and population groups. Sci Prog 1999;82:69–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vahter M, Marafante E, Dencker L. Tissue distribution and retention of as-74-dimethylarsinic acid in mice and rats. Arch Environ Con Tox 1984;13:259–264 [DOI] [PubMed] [Google Scholar]
- 24.Liu B, Pan SG, Dong XS, Qiao HQ, Jiang HC, Krissansen GW, et al. Opposing effects of arsenic trioxide on hepatocellular carcinomas in mice. Cancer Sci 2006;97:675–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nayak AS, Lage CR, Kim CH. Effects of low concentrations of arsenic on the innate immune system of the zebrafish (Danio rerio). Toxicol Sci 2007;98:118–124 [DOI] [PubMed] [Google Scholar]
- 26.Delaney M, Follet C, Ryan N, Hanney N, Lusk-Yablick J, Gerlach G. Social interaction and distribution of female zebrafish (Danio rerio) in a large aquarium. Biol Bull 2002;203:240–241 [DOI] [PubMed] [Google Scholar]
- 27.Levin ED, Chen E. Nicotinic involvement in memory function in zebrafish. Neurotoxicol Teratol. 2004;26:731–735 [DOI] [PubMed] [Google Scholar]
- 28.Little EE, Finger SE. Swimming behavior as an indicator of sublethal toxicity in fish. Environ Toxicol Chem 1990;9:13–19 [Google Scholar]
- 29.He JH, Yang DR, Wang CY, Liu W, Liao JH, Xu T, et al. Chronic zebrafish low dose decabrominated diphenyl ether (BDE-209) exposure affected parental gonad development and locomotion in F1 offspring. Ecotoxicology 2011;20:1813–1822 [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Patino MA, Yu L, Cabral H, Zhdanova IV. Anxiogenic effects of cocaine withdrawal in zebrafish. Physiol Behav 2008;93:160–171 [DOI] [PubMed] [Google Scholar]
- 31.MacMillan-Crow LA, Thompson JA. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch Biochem Biophys 1999;366:82–88 [DOI] [PubMed] [Google Scholar]
- 32.Moon K, Guallar E, Navas-Acien A. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep 2012;14:542–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DN GM: Criteria for case definition of arsenicosis. In: Arsenic Exposure and Health Effects (V). Chappell WR, Abernathy CO, Calderon RL, and Thomas DJ. (eds), pp. 117–134, Elsevier Science Ltd, London, 2003 [Google Scholar]
- 34.Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, et al. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 2011;31:95–107 [DOI] [PubMed] [Google Scholar]
- 35.Marafante E, Vahter M, Norin H, Envall J, Sandstrom M, Christakopoulos A, et al. Biotransformation of Dimethylarsinic Acid in Mouse, Hamster and Man. J Appl Toxicol 1987;7:111–117 [DOI] [PubMed] [Google Scholar]
- 36.Yoshida K, Inoue Y, Kuroda K, Chen H, Wanibuchi H, Fukushima S, et al. Urinary excretion of arsenic metabolites after long-term oral administration of various arsenic compounds to rats. J Toxicol Environ Health Part A 1998;54:179–192 [DOI] [PubMed] [Google Scholar]
- 37.Ballatori N. Transport of toxic metals by molecular mimicry. Environ Health Persp 2002;110:689–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flora SJS. Arsenic-induced oxidative stress and its reversibility. Free Radic Bio Med 2011;51:257–281 [DOI] [PubMed] [Google Scholar]
- 39.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 2006;160:1–40 [DOI] [PubMed] [Google Scholar]
- 40.Chakraborty S, Ray M, Ray S. Toxicity of sodium arsenite in the gill of an economically important mollusc of India. Fish Shellfish Immun 2010;29:136–148 [DOI] [PubMed] [Google Scholar]
- 41.Sarkar S, Mukherjee S, Chattopadhyay A, Bhattacharya S. Low dose of arsenic trioxide triggers oxidative stress in zebrafish brain: expression of antioxidant genes. Ecotox Environ Safe 2014;107:1–8 [DOI] [PubMed] [Google Scholar]
- 42.Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res Rev Mutat 2004;567:1–61 [DOI] [PubMed] [Google Scholar]
- 43.Cooney MT, Vartiainen E, Laakitainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J 2010;159:612–619.e3 [DOI] [PubMed] [Google Scholar]
- 44.Lee MY, Jung BI, Chung SM, Bae ON, Lee JY, Park JD, et al. Arsenic-induced dysfunction in relaxation of blood vessels. Environ Health Persp 2003;111:513–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tyler CR, Allan AM. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr Environ Health Rep 2014;1:132–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo JH, Qiu ZQ, Shu WQ, Zhang YY, Zhang L, Chen JA. Effects of arsenic exposure from drinking water on spatial memory, ultra-structures and NMDAR gene expression of hippocampus in rats. Toxicol Lett 2009;184:121–125 [DOI] [PubMed] [Google Scholar]
- 47.Sharma B, Sharma PM. Arsenic toxicity induced endothelial dysfunction and dementia: pharmacological interdiction by histone deacetylase and inducible nitric oxide synthase inhibitors. Toxicol Appl Pharmacol 2013;273:180–188 [DOI] [PubMed] [Google Scholar]
- 48.Jing JF, Zheng G, Liu MC, Shen XF, Zhao F, Wang JY, et al. Changes in the synaptic structure of hippocampal neurons and impairment of spatial memory in a rat model caused by chronic arsenite exposure. Neurotoxicology 2012;33:1230–1238 [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Finley EJ, Ali AMS, Allan AM. Learning deficits in C57BL/6J mice following perinatal arsenic exposure: Consequence of lower corticosterone receptor levels? Pharmacol Biochem Be 2009;94:271–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bardullas U, Limon-Pacheco JH, Giordano M, Carrizales L, Mendoza-Trejo MS, Rodriguez VM. Chronic low-level arsenic exposure causes gender-specific alterations in locomotor activity, dopaminergic systems, and thioredoxin expression in mice. Toxicol Appl Pharmacol 2009;239:169–177 [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez VM, Limon-Pacheco JH, Carrizales L, Mendoza-Trejo MS, Giordano M. Chronic exposure to low levels of inorganic arsenic causes alterations in locomotor activity and in the expression of dopaminergic and antioxidant systems in the albino rat. Neurotoxicol Teratol 2010;32:640–647 [DOI] [PubMed] [Google Scholar]
- 52.Yadav RS, Sankhwar ML, Shukla RK, Chandra R, Pant AB, Islam F, et al. Attenuation of arsenic neurotoxicity by curcumin in rats. Toxicol Appl Pharmacol 2009;240:367–376 [DOI] [PubMed] [Google Scholar]
- 53.Waalkes MP. Cadmium carcinogenesis. Mutat Res Fund Mol M 2003;533:107–120 [DOI] [PubMed] [Google Scholar]
- 54.Pant N, Murthy RC, Srivastava SP. Male reproductive toxicity of sodium arsenite in mice. Hum Exp Toxicol 2004;23:399–403 [DOI] [PubMed] [Google Scholar]
- 55.Chattopadhyay S, Ghosh S, Chaki S, Debnath J, Ghosh D. Effect of sodium arsenite on plasma levels of gonadotrophins and ovarian steroidogenesis in mature albino rats: duration-dependent response. J Toxicol Sci 1999;24:425–431 [DOI] [PubMed] [Google Scholar]
- 56.Sarkar M, Chaudhuri GR, Chattopadhyay A, Biswas NM. Effect of sodium arsenite on spermatogenesis, plasma gonadotrophins and testosterone in rats. Asian J Androl 2003;5:27–31 [PubMed] [Google Scholar]
- 57.Ihrig MM, Shalat SLSL, Baynes C. A hospital-based case-control study of stillbirths and environmental exposure to arsenic using an atmospheric dispersion model linked to a geographical information system. Epidemiology 1998;9:290–294 [PubMed] [Google Scholar]
- 58.Ahmad SA, Sayed SU, Barua S, Khan MH, Jalil A, Hadi SA, et al. Arsenic in drinking water and pregnancy outcomes. Environ Health Persp 2001;109:629–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milton AH, Smith W, Rahman B, Hasan Z, Kulsum U, Dear K, et al. Chronic arsenic exposure and adverse pregnancy outcomes in Bangladesh. Epidemiology 2005;16:82–86 [DOI] [PubMed] [Google Scholar]