Abstract
Significance: Recent breakthroughs in mitochondrial research have advanced, reshaped, and revolutionized our view of the role of mitochondria in health and disease. These discoveries include the development of novel tools to probe mitochondrial biology, the molecular identification of mitochondrial functional proteins, and the emergence of new concepts and mechanisms in mitochondrial function regulation. The discovery of “mitochondrial flash” activity has provided unique insights not only into real-time visualization of individual mitochondrial redox and pH dynamics in live cells but has also advanced understanding of the excitability, autonomy, and integration of mitochondrial function in vivo. Recent Advances: The mitochondrial flash is a transient and stochastic event confined within an individual mitochondrion and is observed in a wide range of organisms from plants to Caenorhabditis elegans to mammals. As flash events involve multiple transient concurrent changes within the mitochondrion (e.g., superoxide, pH, and membrane potential), a number of different mitochondrial targeted fluorescent indicators can detect flash activity. Accumulating evidence indicates that flash events reflect integrated snapshots of an intermittent mitochondrial process arising from mitochondrial respiration chain activity associated with the transient opening of the mitochondrial permeability transition pore. Critical Issues: We review the history of flash discovery, summarize current understanding of flash biology, highlight controversies regarding the relative roles of superoxide and pH signals during a flash event, and bring forth the integration of both signals in flash genesis. Future Directions: Investigations using flash as a biomarker and establishing its role in cell signaling pathway will move the field forward. Antioxid. Redox Signal. 25, 534–549.
Introduction
The chemiosmotic theory established more than half a century ago has put mitochondria in the center of cellular energy metabolism (52). Mitochondria are often referred to as the “powerhouse” of eukaryotic cells. However, the “switch” of this powerhouse, that is, whether energy metabolism through mitochondrial respiration is deliberately controlled by a specific mechanism/regulator, is not clear. Given the fact that intracellular energy expenditure and workload fluctuate constantly while intracellular ATP levels remain largely stable, it is speculated that mitochondrial respiration is a spontaneous and passive process mainly controlled by changes in downstream energy demand or substrate availability rather than a specific regulatory mechanism or pathway (13). In addition, alternative functions of mitochondria in cell apoptosis, calcium (Ca2+) homeostasis, and reactive oxygen species (ROS) generation are now widely appreciated. These functions are coupled or closely associated with mitochondrial respiration. The central question then arises: How do mitochondria orchestrate these multiple functions/processes? For instance, in vitro studies have suggested that electron leak from the electron transport chain (ETC) complexes is responsible for mitochondrial ROS production. However, whether the same mechanism exists in in vivo or under physiologically relevant conditions (e.g., temperature, oxygen tension) is not clear. In this regard, the discovery of multidimensional mitochondrial flash activity was a breakthrough that provides some answers to this central question. The ETC-dependent and stochastic occurrence of discrete flash events in an individual mitochondrion suggests that respiration and other mitochondrial functions are orchestrated and controlled by specific mechanisms or regulators. Moreover, the frequency of mitochondrial flashes can be used as a biomarker for evaluating mitochondrial respiration status and related functions, since these events reflect a composite of overlapping signals, including mitochondrial ROS production, matrix alkalization, ETC activity, and membrane potential dissipation (23, 28, 76, 77). By monitoring mitochondrial flash activity, several exciting and important discoveries have been made that link mitochondrial function/dysfunction to a broad range of human diseases and aging.
Discovery and Characterization of Single Mitochondrial Flash Events
The serendipitous discovery of mitochondrial flash
The word “serendipity” accurately describes how our groups observed flash activity with mitochondria-targeted circularly permuted yellow fluorescent protein (mt-cpYFP). Our initial intention was to investigate mitochondria Ca2+ handling during skeletal muscle and cardiac excitation–contraction (EC) coupling. At the time, while the role of mitochondrial Ca2+ uptake in promoting energy metabolism and mitochondrial Ca2+ overload in cell death was well known, whether mitochondria sequester Ca2+ during EC coupling was under debate. A study reported transient Ca2+ uptake events in individual mitochondria, referred to as “Ca2+ marks” since they reflected Ca2+ spark-like behavior in single mitochondria (59). This intriguing finding prompted us to use a mitochondrial-targeted genetic fluorescent Ca2+ indicator, named ratiometric mt-pericam (56), to probe mitochondrial Ca2+ handling during EC coupling. However, initial testing was disappointing, since transient millisecond duration mitochondrial events were not detected. This could be due to the relatively slow Ca2+ binding kinetics of mt-pericam, in comparison to small molecule Ca2+ indicators (such as rhod-2) used previously to measure Ca2+ marks. Based on this speculation, we increased the scanning time and focused on 2D time-lapse scanning. Very unexpectedly, we found stochastic and transient increases in mt-pericam fluorescence from individual mitochondria under resting conditions in intact cells (76). These events exhibited much longer time-to-peak (∼3 s) and mean duration (10–20 s) compared to that of Ca2+ sparks (∼30 ms duration) and Ca2+ marks (∼170 ms duration) and were only observed at one of the two Ca2+-sensitive excitation wavelengths of mt-pericam (488 nm but not 405 nm). In addition, flash events in cardiac myocytes occurred at a very low frequency, more than 3 orders of magnitude lower compared with Ca2+ sparks in the same cells. Further characterization indicated that acute manipulation of extracellular Ca2+ concentrations or application caffeine/ryanodine to alter Ca2+ spark frequency did not significantly impact mitochondrial flash frequency. These initial observations suggested that the events detected with mt-pericam were unlikely due to mitochondrial Ca2+ signals similar to “Ca2+ marks” and, thus, were referred to as “flashes” to differentiate them from Ca2+ sparks/marks. Indeed, subsequent structure–function studies of the probe revealed that identical flash events were also observed even after removal of the Ca2+ sensing elements (calmodulin and M13) of mt-pericam (resulting in mt-cpYFP) (76).
Mitochondrial flash renamed to superoxide flash
We puzzled over the nature and mechanism of mitochondrial flash activity for nearly 2 years following its initial observation. First, we found that flash activity was observed in all cell types screened, including rodent cardiomyocytes, skeletal myotubes, primary neurons, and human cancer cell lines (76, 82). Although the frequency of flash events differed dramatically across the different cell types, likely due to the differences in mitochondrial number, morphology, and bioenergetics, the properties of individual events, including amplitude and kinetics, were essentially invariant. These findings strongly suggest that flash activity reflects a fundamental, well-conserved mitochondrial biological process or phenomenon. Since probe structure–function studies revealed that identical events were observed using a Ca2+-insensitive variant of the probe (mt-cpYFP), subsequent studies focused on the role of changes in other potential mitochondrial signals (e.g., redox, superoxide, pH, membrane potential, ATP, ADP, and NADH), during flash events (76).
After confirming that flash events were not the result of laser-induced phototoxicity, we monitored changes in mitochondrial membrane potential during flash activity. Indeed, the stochastic nature of flash activity in quiescent cells is reminiscent of previously described spontaneous transient depolarization in the mitochondrial membrane potential (termed “flickers”) that exhibits similar spatial–temporal properties (14, 22, 38, 58). Consistent with this, we found that every flash is accompanied with an abrupt reduction in mitochondrial membrane potential (76). However, since not all mitochondrial membrane potential flickers are coincident with a flash event (76, 79), mitochondrial flashes likely represent only a subset of these events. Since ETC activity is stimulated by mitochondrial depolarization, studies manipulating mitochondrial respiration revealed that flash activity requires an intact mitochondrial ETC. Besides using known ETC inhibitors, one piece of key evidence comes from the study on PC12 cells after long-term treatment of ethidium bromide, which gradually depletes mitochondrial DNA. To our surprise, flash frequency decreased proportionally with a decrease in expression of mitochondrial DNA encoded proteins. In a ρ0 cell line, in which mitochondrial DNA is totally depleted, no flash events were observed. Together, these observations provide strong evidence that flash events are coupled to ETC activity or mitochondrial respiration (76). Since mitochondrial respiration is the major process for the generation of mitochondrial ROS, the relationship between ROS and mitochondrial flash activity was tested. Indeed, flash frequency and amplitude were found to be decreased and increased by antioxidants and oxidants, respectively. Following in vitro studies of purified cpYFP (see The Superoxide/ROS Component in Mitochondrial Flash section) showing that the probe fluorescence is reversibly increased by superoxide ions, mitochondrial flashes were named “superoxide flashes” (76).
The superoxide/ROS component in mitochondrial flash
Substantial evidence supports the conclusion that superoxide or ROS represent the major signal detected by mt-cpYFP during each flash event. First, we conducted in vitro calibrations on purified cpYFP protein using a spectrofluorometer while strictly ensuring inert environmental conditions throughout the whole process (76). When purified, the cpYFP protein is fully oxidized and exhibits a maximal fluorescence at 488 nm excitation because the purification process is conducted under ambient oxygenated conditions. Following the detailed protocol described previously (30), we successfully reduced purified cpYFP using dithiothreitol (DTT) and, after removing the reducing agent, were able to reoxidize cpYFP back to the fully oxidized condition. Surprisingly, however, fully reduced cpYFP could only be oxidized by superoxide (from xanthine + xanthine oxidase) and O2, but not various other ROS (including hydrogen peroxide [H2O2], hydroxyl radical, peroxynitrite, nitric oxide) or changes in redox potential. More importantly, addition of superoxide dismutase (SOD) before or after the xanthine + xanthine oxidase reaction attenuated or blocked the increase in fluorescence (76). This inhibition by SOD provides strong evidence that superoxide underlies the increased cpYFP fluorescence. During these in vitro experiments, the solution pH was set at 8.0 to better mimic the physiological pH of the mitochondrial matrix. The superoxide sensitivity of cpYFP was further tested in live cells and animals, which showed acute increases in cpYFP fluorescence upon addition of multiple superoxide generators or oxidants, including menadione, aldrithiol, and paraquat (37, 76, 83).
The superoxide component of mitochondrial flash events in cells was supported by the inhibitory effect on flash frequency and amplitude of various general and mitochondria-targeted antioxidants (35, 37, 60, 76). Acute treatment of oxidants such as menadione and paraquat (which generate superoxide inside cells) or deletion of SOD2 (which scavenges superoxide in the mitochondrial matrix) dramatically increased flash activity (35, 37, 69, 76). The most physiologically relevant evidence for the contribution of superoxide to mitochondrial flash events comes from studies of ischemia–reperfusion, which is well known to first decrease (during ischemia) and then transiently increase (during early reperfusion) mitochondrial superoxide production. Indeed, ischemia drastically suppressed and anoxia abolished, while reperfusion caused a transient increase in flash frequency in intact cardiomyocytes (37, 76). Superoxide/ROS signal in flash events detected using mt-cpFYP was confirmed by several groups using various cell types, oxidants, and antioxidants (32, 35, 37, 60, 65, 86). Finally, other superoxide/ROS indicators, including MitoSOX, 2′,7′-dichlorodihydrofluorescein diacetate (DCF) and redox green fluorescent protein (roGFP), have been used to detect increases in superoxide during single mitochondrial flash events (Table 1) (5, 11, 60, 79, 87).
Table 1.
Properties of Mitochondrial “Flash-Like” Events Detected by Different Indicators
| Name of the event | Superoxide flash | pH transient/flash | Redox transientsa |
|---|---|---|---|
| Indicator | cpYFP, pericam, MitoSOX, DCF | SypHer, cpYFP | roGFP |
| Basic characteristics | |||
| Frequency (unit) | ∼4 in CM, ∼10 in SM, 30–60 in other cells (/1000 μm2/100 s) | 0.54 ± 0.04 in Hela (/min/cell) | 0.6 ± 0.1 (per hour) |
| Amplitude (dF/F0) | 0.41 ± 0.02 (CM), 0.93 ± 0.02 (SM) | 0.5–0.6 (estimated) | ∼0.2 |
| Rising time | 3.5 ± 0.1 s (CM) | 1.63 ± 0.08 s | ∼20 s |
| Decay time | Half time: 8.6 ± 0.2 s (CM) Total time ∼20 s (SM) | 8.6 ± 0.6 s | ∼200 s |
| Species | Caenorhabditis elegans, zebrafish, rodent, human | Plant, human cell lines | Mouse neurons, in vivo |
| Single mitochondria | Yes | Yes | Yes |
| Superoxide/ROS/redox | |||
| Antioxidants | Blocked | No effect | Blocked |
| Oxidants | Induced | Stimulated | Increased |
| SOD2 | Effective | N/A | N/A |
| ETC dependence | |||
| ETC substrates | Induce | Induce | N/A |
| ETC components | Complex I | N/A | N/A |
| ETC inhibitors | Inhibited | Inhibited | Inhibited |
| FCCP | Abolished | Abolished | N/A |
| pH dependence | |||
| pH change | 30% increase (0.1 U) | 100% increase (0.4 U) | Brief increase then decrease |
| Nigericin | Increase at low-dose block at high dose | Block at high dose | N/A |
| NH4Cl | Increased | Decreased | N/A |
| mPTP dependence | |||
| Δψ dissipation | Yes | Yes | Yes |
| Δψ recovery | Time course varies | Mirror | N/A |
| mPTP opener | Induced | Induced | N/A |
| mPTP blocker | Inhibited in CM, but not SM | No effect | No effect |
| Other features | |||
| Contraction | Estimated 1–2% | N/A | Coupled |
| Calcium | Stimulate | Stimulate | No effect |
| Other regulators | N/A | OPA1 dependent | N/A |
| References | 60, 76, 79, 82, 87 | 63, 65 | 11 |
Properties are mostly deduced from accompanying mitochondrial “contraction” events.
CM, cardiomyocytes; DCF, 2′,7′-dichlorodihydrofluorescein diacetate; ETC, electron transport chain; FCCP, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone; mPTP, mitochondrial permeability transition pore; OPA1, optic atrophy 1; roGFP, redox green fluorescent protein; ROS, reactive oxygen species; SM, skeletal muscles; SOD, superoxide dismutase; N/A, not available; Δψ, mitochondrial membrane potential.
The pH component in mitochondrial flash events
During the in vitro calibration of cpYFP, we confirmed that cpYFP is pH sensitive as its fluorescence emission increases with pH as is documented for other GFP-based biosensors (76). Given the high HEPES concentration (20 mM) used in the calibration studies, pH changes were not a concern for the in vitro determination of the superoxide response of purified cpYFP. However, since cpYFP has a pKa of ∼8.5, which approximates the pH of the mitochondrial matrix, changes in mitochondrial pH during a flash could contribute to the signal (64, 66) (see Transient pH Flash Events Detected with mt-cpYFP and mt-SypHer section for details). This possibility was initially ruled out, since a known pH-sensitive GFP derivative, enhanced yellow fluorescent protein (EYFP), when targeted to mitochondria, failed to detect transient flash-like signals (76). To further investigate whether pH contributes to mt-cpYFP-detected mitochondrial flashes, we conducted experiments to simultaneously monitor changes in fluorescence of cpYFP and a red-shifted commercial pH indicator, SNARF-1, in the same mitochondria. SNARF-1 is a small molecule with a pKa of 7.5 determined in vitro. By using an optimized protocol, we preferentially loaded SNARF-1 into the mitochondrial matrix. Intracellular calibration of SNARF-1 using two imaging protocols revealed a pKa of ∼8.3, which is close to that of cpYFP (∼8.5). The pH fluorescence response curves of cpYFP and SNARF-1 in cells were overlapping, suggesting the two indicators respond equally to changes in intramitochondrial pH. This approach enabled the use of SNARF-1 as an internal control to determine the relative contribution of changes in pH to mitochondrial flash events. Indeed, we found that SNARF-1 detected a small alkalization signal during each flash event, which was equal to ∼30% of the peak cpYFP signal increase during the flash (79). Based on in-cell calibrations, the pH increase during a flash was estimated to be ∼0.1 pH unit. Thus, each flash event represents a mixed signal with ∼30% due to a modest alkalization of the matrix and the rest from superoxide/ROS. In light of this fact, the events were renamed as “mitochondrial flashes” (or “mitoflashes” for short, which have been used in several reports) to acknowledge the composite nature of signal (21, 27, 31, 69, 84–86).
Mitochondrial Flash Integrates ROS and pH Signals in Single Mitochondria
Mitochondrial flash detected by other ROS/redox indicators
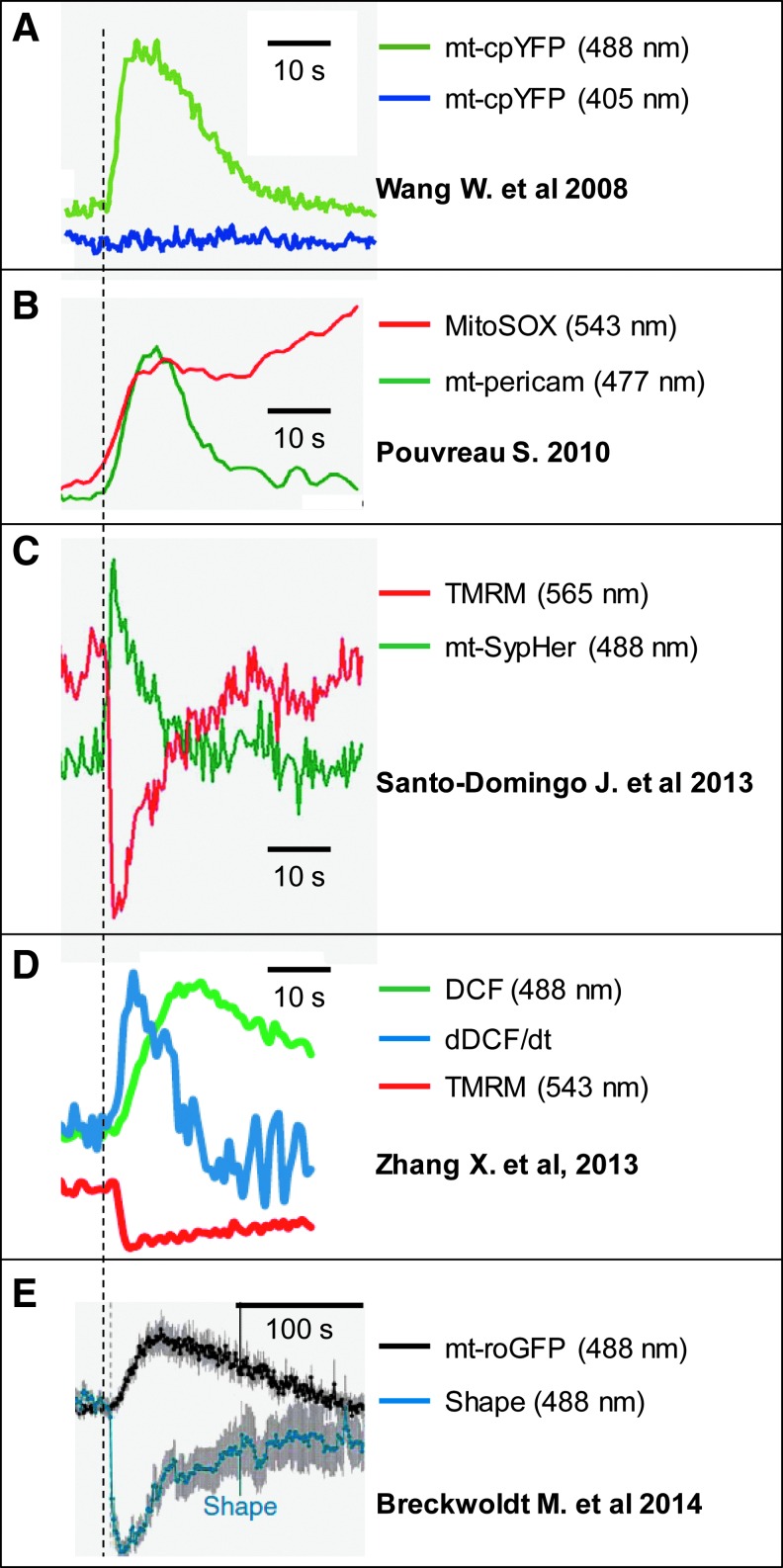
Shortly after the first report of mitochondrial flashes with mt-cpYFP, Pouvreau detected similar flash events in skeletal muscle cells using mt-pericam (60). The mt-pericam-detected flashes in skeletal muscles share many of the same features as the mitochondrial flashes detected by cpYFP in cardiomyocytes. In the skeletal muscle, flashes were transient with a time course of 10–20 s, were reduced by antioxidants, depended on ETC activity, and were accompanied by a drop in mitochondrial membrane potential (60). However, the skeletal muscle flash did not depend on transient opening of the mitochondrial permeability transition pore (mPTP). In addition, the author also determined the spectra of mt-pericam inside skeletal muscle cells and showed that when it binds to Ca2+, its fluorescence at 405 nm excitation dropped 90%, while its fluorescence at 488 nm excitation only increased by 25% (60). This is consistent with previous in vitro calibrations (56), but drastically differs from the fluorescence changes during mitochondrial flashes. During mitochondrial flashes, the increased fluorescence of mt-pericam and mt-cpYFP is solely at 488 nm excitation with no change detected at 405 nm excitation. This largely refutes the possibility of Ca2+-dependent signals in mt-pericam-detected mitochondrial flashes. Pouvreau was also the first to simultaneously monitor MitoSOX red with mt-pericam to independently confirm an increase in ROS production during each flash event (60). These findings critically support the superoxide contribution of mitochondrial flashes, since MitoSOX is an accepted and widely used mitochondrial superoxide indicator that is not sensitive to physiological pH changes (87).
Thanks, in part, to an ongoing debate over the relative pH and superoxide sensitivity of mt-cpYFP, we and others used other commercially available genetic and organic small molecule indicators (it should be noted that each indicator has its own limitations such as lack of specificity for single ROS and dependence on mitochondrial membrane potential) to detect superoxide/ROS/redox flash-like events in cells and live animals (Table 1). Several groups confirmed that MitoSOX red detects sudden steep and irreversible increases in superoxide production during mitochondrial flash events (5, 60, 79, 87). Since the MitoSOX signal is not reversible, a burst in superoxide results in an enhanced rate of fluorescence increase (i.e., an increase in slope) during the time-to-peak of a flash event. Thus, plotting the rate of change in MitoSOX fluorescence over time results in a trace that shares a similar time course as the changes in mt-cpYFP fluorescence during a flash (87). Similarly, the cytosolic ROS indicator, DCF, preferentially loaded into mitochondria also exhibits an increased rate of change in fluorescence during mitochondrial flash events (87). Finally, a recent study using mitochondrial targeted mt-roGFP, a redox indicator, observed “redox transients” that were coincident with changes in mitochondrial morphology (“contraction”) in neurons in vivo (11). Moreover, mt-roGFP redox transients were accompanied by an abrupt loss in membrane potential, confined within single mitochondria, and subjected to antioxidant regulation. These features of “redox transients” are remarkably similar to mitochondrial flash activity detected with mt-cpYFP and mt-pericam. However, the mt-roGFP redox events occurred at a much lower frequency and were longer in duration compared to that reported for mitochondrial flash events (Table 1). The longer time course of redox transient could be explained by the shorter superoxide lifetime compared to a general redox potential change, which requires the re-equilibration of the concentrations of electron donors and electron acceptors, and/or slower redox reaction between the indicator and underlying redox event. In summary, both genetic (mt-cpYFP, mt-pericam, and mt-roGFP) or small molecule (MitoSOX and DCF) indicators confirm that ROS production is an important component of single mitochondrial flash events in mammalian cells across multiple species and tissues (Table 1 and Fig. 1). Similarities in the basic characteristics of these flash events, such as frequency, amplitude, kinetics (Fig. 1), and sensitivity to antioxidants, indicate that the events likely reflect a common fundamental quantal event of mitochondrial function. To further elucidate their role and origin, more studies are warranted to more thoroughly characterize mitochondrial flash activity in single mitochondria using the different indicators in the same cell type and under the same conditions.
FIG. 1.
Single mitochondrial flash events detected with various indicators. Representative traces are from the indicated publications from different groups and aligned in a way to better show the coordinated change of the various signals. Note in (E), the mt-roGFP-detected redox transient is much longer than the other events and the scale bar is different from panels (A–D). roGFP, redox green fluorescent protein. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Transient pH flash events detected with mt-cpYFP and mt-SypHer
As mentioned earlier, the pH sensitivity of cpYFP was demonstrated in our first report on mitochondrial flash activity. Following introduction of mt-cpYFP into root epidermis cells of Arabidopsis, Schwarzlander et al. found single mitochondrial flashes in isolated mitochondria from these cells (64). Next, they manipulated mitochondrial respiration, ROS, and pH of the isolated mitochondria. However, instead of quantifying flash events from individual mitochondria, they used a plate reader to monitor changes in steady-state (basal) fluorescence of mt-cpYFP from a large population of isolated mitochondria. The results showed that energizing mitochondria increased basal cpYFP fluorescence, but further stimulation or inhibition of respiration did not change the mt-cpYFP fluorescence in the mitochondrial population (64). Antioxidants also failed to decrease mt-cpYFP fluorescence. Therefore, the authors concluded that mt-cpYFP is not sensitive to superoxide or ROS. In addition, the authors also monitored the pH response of mt-cpYFP and found that nigericin, a K+/H+ antiporter that dissipates the pH gradient across mitochondrial membrane, totally abolished flash events in isolated mitochondria. Taken together, the authors concluded that flash activity detected by mt-cpYFP was not related to superoxide production, but rather was due to changes in mitochondrial pH.
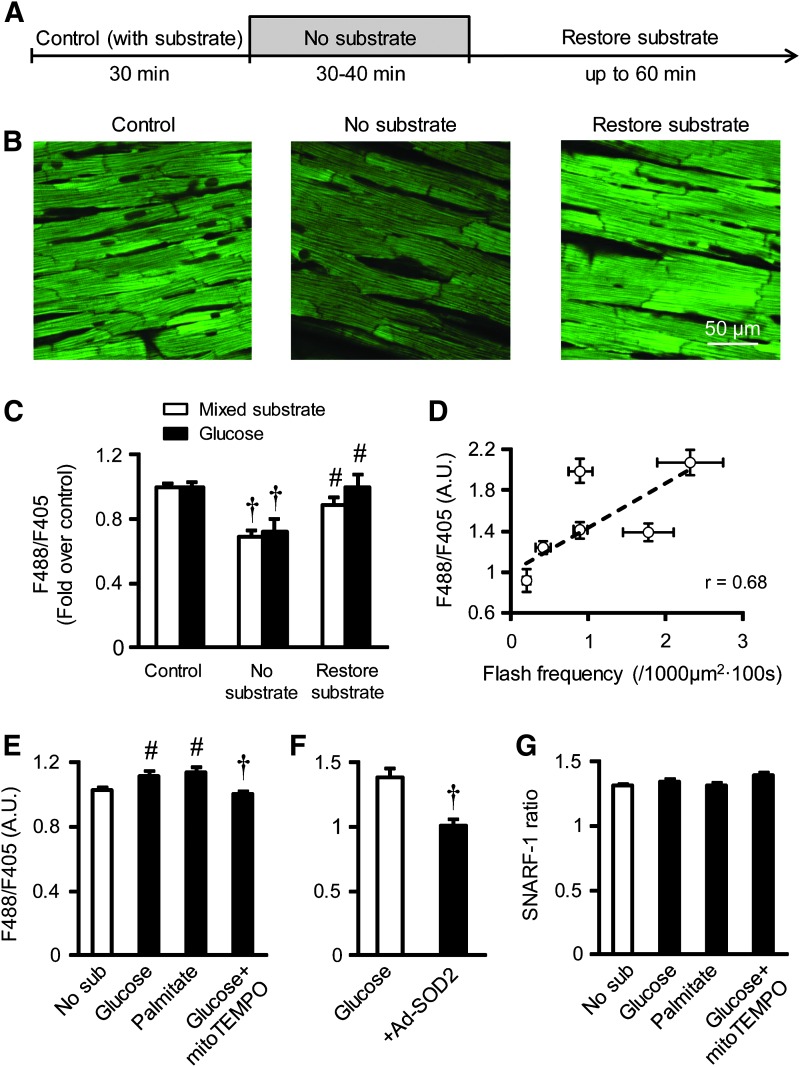
It should be noted, however, that monitoring basal mt-cpYFP fluorescence in a large population of mitochondria using a plate reader may not be sensitive enough to detect small transient stochastic changes in mt-cpYFP signal. This is because superoxide is short lived and mt-cpYFP fluorescence is fully reversible. Indeed, we recently monitored basal mt-cpYFP fluorescence by confocal imaging in the intact perfused heart. The results confirmed a significant change in basal mt-cpYFP fluorescence occurred during manipulation of mitochondrial substrate availability (Fig. 2A–C), the magnitude of which positively correlated with flash frequency in the same cells (Fig. 2D). In intact adult rat cardiomyocytes, stimulating mitochondrial respiration induced a mild, but significant, increase in basal mt-cpYFP fluorescence, which was sensitive to antioxidants (Fig. 2E, F). However, parallel imaging of SNARF-1 revealed no significant change in mitochondrial pH during manipulation of mitochondrial substrate availability (Fig. 2G).
FIG. 2.
Regulation of basal mt-cpYFP fluorescence by mitochondrial respiration substrates and antioxidants. (A) Experimental protocol for manipulating physiological substrates in the intact perfused mouse heart. (B) Representative images showing mt-cpYFP fluorescence in intact myocardium of mt-cpYFP transgenic mice during substrate manipulation. (C) Changes in whole cell mt-cpYFP fluorescence (ratio of 488 nm and 405 nm excitation, F488/F405) during substrate manipulation. Data are mean ± SEM. n = 22–34 images from three hearts for each group. †p < 0.001 versus Control (with substrate). #p < 0.01 versus No substrate. (D) Correlation between whole cell mt-cpYFP fluorescence (F488/F405) and mitochondrial flash frequency during substrate manipulation in the perfused heart. The data points are from Figure 2C and Figure 1D of Ref. (27). (E) Whole cell mt-cpYFP fluorescence (F488/F405) in freshly isolated adult cardiac myocytes from the mt-cpYFP mouse with no substrate (No sub), with glucose (10 mM), palmitate (0.5 mM), or with mitoTEMPO (1 μM) added after glucose (10 mM). Data are mean ± SEM. n = 43–104 cells from five mice for each group. #p < 0.01 versus No sub. †p < 0.001 versus Glucose. (F) Whole cell mt-cpYFP fluorescence in adult rat cardiac myocytes in the presence of glucose (10 mM) with or without SOD2 overexpression (+Ad-SOD2). Data are mean ± SEM. n = 27–35 cells from three rats for each group. †p < 0.001 versus glucose. (G) Mitochondrial pH monitored by SNARF-1 (SNARF-1 ratio) during the same treatments as in F. Data are mean ± SEM. n = 27–45 cells from three rats. Detailed methods for these experiments are described in Refs. (27, 79). mt-cpYFP, mitochondria-targeted circularly permuted yellow fluorescent protein; SOD, superoxide dismutase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In a follow-up study, the same group attributed mt-cpYFP flash events solely to matrix alkalization and showed that they were coupled to transient reductions in mitochondrial membrane potential (pulsing of membrane potential) in cells and isolated mitochondria from the root of Arabidopsis (Table 1) (65). Although oxidants increased the incidence of membrane potential pulsing events, the study did not assess whether oxidants also increased the frequency of mt-cpYFP flash events. A simultaneous increase in the matrix Ca2+ levels was detected during membrane potential pulsing, which was proposed to serve as the trigger for the membrane potential pulsing. However, blockers of the mitochondrial calcium uniporter (MCU) failed to inhibit the pulsing events. Nevertheless, the authors proposed a model in which Ca2+ influx into the matrix, through an unknown non-MCU channel, stimulates membrane potential pulsing to uncouple mitochondria, accelerate electron flux, and trigger transient matrix alkalization (detected by mt-cpYFP) (65). In addition, the authors proposed that opening of a nonspecific large pore (e.g., mPTP) was not responsible for the pulsing and the transient alkalization events, since the pulsing events were not blocked by cyclosporine A, a known mPTP inhibitor, and were accompanied by transient alkalization and increased matrix Ca2+ levels, two events that should decrease when the mPTP opens (65). Therefore, besides differences with regard to interpretations of the signal detected by mt-cpYFP (i.e., pH vs. superoxide), the trigger of membrane depolarization during flash events in plants was fundamentally different from that previously reported for mammalian cells (Ca2+ influx vs. opening of the mPTP). Despite these specific differences, the proposed model is in general agreement with previous hypotheses on flash activity (5, 66, 76), since it also suggests that individual mitochondria switch between quiescent and activated states, which are closely coupled to mitochondrial respiration.
Using a newly developed pH indicator, Santo-Domingo et al. reported pH flash events in Hela cells (Fig. 1C) (63). The pH indicator, SypHer, was derived from a previous H2O2 indicator named HyPer (8) by mutating a cysteine residue in the H2O2 sensing domain. It should be noted that HyPer or SypHer shares a very similar cpYFP domain as the one originally used to detect mitochondrial flashes. They differ only by a few amino acids (61, 62). mt-SypHer-detected pH flashes were dependent on mitochondrial respiration and an intact ETC, induced by oxidants, but not inhibited by mPTP blockers or antioxidants. The authors calibrated mt-SypHer in vitro and found that it senses pH, but not superoxide or other ROS. The pH flashes exhibited high amplitude, a peak increase of ∼0.4 U pH (63), which was significantly higher than ∼0.1 U determined using mt-cpYFP and SNARF-1 (79). Another feature of the pH flashes in this study was that they occurred together with a simultaneous loss of membrane potential that always mirrored the flash event time course. The authors further determined pH flash events in cells with altered mitochondrial morphology by genetic manipulations of fission and fusion proteins. Intriguingly, they found that pH flash events were dependent on optic atrophy 1 (OPA1), an inner membrane fusion protein (63). Since OPA1 regulates inner membrane cristae and mitochondrial respiration, this is consistent with the mitochondrial respiration dependence of pH flash activity. Azarias and Chatton also reported pH flash events using the same SypHer indicator. However, these studies also used MitoSOX to show that the pH flash events were accompanied by a burst in superoxide (5) and that the alkalization and ROS events coexisted in individual mitochondria (5). In the recent report that used roGFP to detect “redox transients,” the authors also monitored mitochondrial pH using mt-SypHer and found that brief matrix alkalization preceded the onset of a redox transient (Fig. 1E) (11). Interestingly, their results showed that during the redox transients, matrix pH actually decreased (became more acidic) rather than increased (11). Taken together, in these reports, pH flashes in single mitochondria share many similarities, but also exhibit important differences compared to mitochondrial flash activity detected using ROS indicators (e.g., mt-cpYFP, MitoSOX, DCF).
Mitochondrial flash events reflect an integration of ROS and pH signals
As discussed earlier, although being named differently due to the different indicators used (e.g., mt-cpYFP, mt-SypHer, mt-roGFP) and signals measured (e.g., ROS, pH, or redox), the underlying events exhibit remarkably similar features, including transient fluorescence changes, stochastic signals confined within single mitochondria, comparable quantal properties, including frequency, amplitude, and kinetics, coincidence with membrane potential depolarization, and dependence on mitochondrial respiration/metabolism (Table 1). Despite disagreement and debate over the pH and superoxide sensitivity of mt-cpYFP, the universal existence of “flash-like” events detected by mitochondrial pH and ROS indicators in intact cells and live animals represents a major breakthrough as characterization of their activity advances our understanding of real-time mitochondrial function/signals at the individual mitochondrion level (23, 28, 70, 77, 81). Moreover, the similar unitary features and regulatory mechanisms of “flash-like” events across species and cell types suggest that the events reflect different components/signals of the same underlying mitochondrial phenomenon (31, 79). Previous work has suggested that many mitochondrial processes/functions are integrated or interconnected and changes in one component directly or indirectly affect the others (2, 10, 12, 39, 55). Since flash events are coupled to mitochondrial respiration, it is very likely that the coincidence of increased ROS generation and matrix alkalization during a flash is due to transient bursts of electron flow, which are accompanied by parallel transient increases in electron slippage to oxygen and a burst of H+ efflux. Perhaps, it is fair to say that just like the story of “the blind men and an elephant” (where a group of blind men touch different parts of an elephant and conclude that they are in complete disagreement), the different indicators preferentially detect different components of the same phenomenon. In other words, mitochondrial flash activity reflects an integration of ROS and pH signals within the matrix of single mitochondria, which are difficult to separate. In addition, these integrated events also involve changes in other mitochondrial signals, including Na+, Mg2+, Ca2+, membrane potential, and more (5, 11). The relationship and dynamics between these different mitochondrial parameters (including energetics, pH, membrane potential, redox, and ROS) have been nicely integrated with computational models (43, 80).
Structural Insights into the Possible Dual Signal Sensing of cpYFP
The ongoing debate over the nature of signals detected by cpYFP (superoxide vs. pH) is a direct result of the known dual sensitivity of cpYFP (76). Although we have shown that purified cpYFP responds to superoxide while pH is strongly buffered, the mechanism for how the cpYFP fluorophore reacts with superoxide remains unclear (76). We previously reported that mutation of the only two cysteines in cpYFP to alanine or methionine residues significantly decreased basal cpYFP fluorescence, presumably due to impaired folding (76). However, the fact that cpYFP is not sensitive to redox, H2O2, and hydroxyl radicals suggests that the reaction between cpYFP and superoxide is unlikely mediated by a common redox reaction involving the thiol groups of these two cysteine residues in cpYFP. Definitive evidence for the structural basis for the interaction between superoxide and cpYFP must await the results of ongoing collaborative X-ray crystallography studies of cpYFP solved under reduced and oxidized conditions.
Schwarzlander et al. conducted in vitro studies of purified cpYFP and concluded that the probe did not respond to superoxide (67). These data were presented in a Brief Communication Arising letter to Nature, thus, precise details regarding the conditions in which the in vitro studies were conducted are not available. From the brief description provided in the methods section, a number of important differences from the original in vitro study of Wang et al (76). could potentially explain the lack of cpYFP responsiveness to superoxide in this communication (16). One critical difference involves the different Escherichia coli. expression systems used in the two studies. The strain used by Schwarzlander et al. lacks glutathione reductase needed to fully limit cysteine oxidation and, thus, may result in defects in proper cpYFP folding (see above). In addition, during purification under atmospheric conditions, cpYFP proteins are fully oxidized and, thus, no longer able to respond to superoxide. Although Schwarzlander et al. used DTT to reduce the probe, data were not provided to definitively show that the purified cpYFP was fully reduced under the conditions used. Confirmation that cpYFP is fully reduced before in vitro calibration of ROS sensitivity is required to unequivocally determine the probe's relative sensitivity to superoxide and other ROS (16).
Taken together, determination of the superoxide sensitivity of cpYFP is critical, but also challenging. Further studies applying high-resolution structural approaches are needed to definitively determine the mechanism by which superoxide alters the structure of cpYFP. As one speculative possibility, analogous to certain EGFP-based calcium sensors (72), superoxide could react with cpYFP to induce a reversible deprotonization of the chromophore that mimics an alkalinization-induced increase in probe fluorescence.
At present, available data from isolated mitochondria, single cells, and live animals support on the existence of quantal transient mitochondrial events, termed mitochondrial flashes, which reflect the integration of multiple concurrent signals (e.g., ROS, pH, membrane potential) intimately linked to aerobic respiration, and thus, the metabolic state of the cell (see Mitochondrial Flashes Are Coupled to Mitochondrial Respiration Through ETC Electron Flow section). In this regard, mt-cpYFP (and other probes) can be used as a reporter for mitochondrial flash activity. Through simultaneous or parallel use of multiple indicators, one can determine the relative contribution of ROS and pH signals during these flash events in different species/cell types and under specific conditions.
Mitochondrial Flash Activity as a Biomarker of Mitochondrial Function in Health and Disease
Mitochondrial flashes are coupled to mitochondrial respiration through ETC electron flow
As discussed earlier, mitochondrial flashes detected by different groups, using different indicators and in different species/cell types, share the common feature of a strong dependence on mitochondrial respiration through ETC electron flow (5, 11, 27, 63, 65, 76, 79). This finding is in agreement with the notion that mitochondrial ETC electron flow underlies both ROS production and proton pumping. Therefore, mitochondrial flash activity can be used as a biomarker for mitochondrial respiration (Fig. 3).
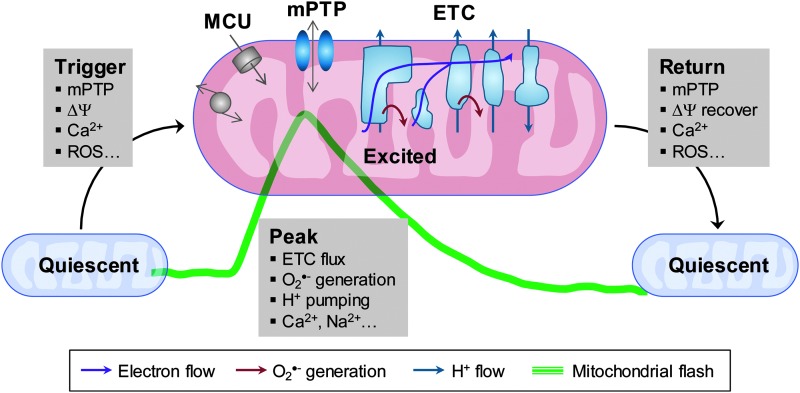
FIG. 3.
Mitochondrial flash events integrate the inseparable signals of ROS, pH, and Ca2+ in individual mitochondria. The diagram was generated based on the models presented in Refs. (5, 27, 65, 66, 76, 82). ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Several studies have reported that mitochondrial flashes are stimulated by respiration substrates, including oxygen, glucose, fatty acids, and specific substrates for ETC complexes (23, 27, 60, 63, 69, 76, 82). We demonstrated that stimulation of respiration with physiological substrates increased the frequency of mt-cpYFP-detected mitochondrial flash events in living cells, tissues, and animals (23, 27, 76, 82). In human cell lines, both mt-cpYFP- and mt-SypHer-detected mitochondrial flashes were absent when mitochondrial DNA was depleted (in ρ0 human osteosarcoma cells) (63, 76). Mitochondrial flash activity was also enhanced in permeabilized cells and isolated mitochondria under conditions promoting State 3 respiration using Complex I, II, or IV substrates (27). In addition, mitochondria under State 4 respiration also exhibit increased flash frequency, consistent with increased superoxide generation when electron flow through the ETC slows down (19, 63, 79). For mt-cpYFP-detected mitochondrial flashes in plant cells/mitochondria, indirect evidence also supports the idea that respiration is coupled to mitochondrial flash activity (65). For instance, mt-cpYFP flash activity in plant cells is tightly coupled with membrane potential pulsing events, which are increased in State 3 respiration and further increased in State 4 respiration (65). In addition, the pulsing events are coupled to transient increase in matrix Ca2+, a known activator of mitochondrial respiration.
The causal role of mitochondrial respiration through ETC electron flow in mitochondrial flash genesis is supported by the fact that all pharmacological inhibitors of the ETC reduce or abolish mitochondrial flash activity. Specifically, mt-cpYFP- and mt-SypHer-detected mitochondrial flash activity in mammalian cells is abolished by mitochondrial inhibitors, including rotenone (Complex I), antimycin A (Complex III), NaCN or azide (Complex IV), oligomycin A (Complex V), and carbonyl cyanide m-chlorophenylhydrazone (CCCP) or carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) (uncoupler) (63, 76). Overall, these results suggest that electron flow along the entire spectrum of ETC is required for mitochondrial flash generation (2, 43, 80). However, the effect of uncouplers is an exception. Although uncouplers stimulate maximal electron flow, they also dissipate the proton gradient across the inner membrane, which is a key component of the membrane potential and proton motive force. Therefore, uncouplers decrease electron leak, prevent ROS production, and cause acidosis of the matrix. In addition, previous reports have shown that antimycin A induces reverse electron flow and increases mitochondria ROS production (2, 53). However, antimycin A significantly reduced mitochondrial flash activity detected with either mt-cpYFP or mt-SypHer. This observation could be explained by the fact that antimycin A (like FCCP) collapses the proton motive force needed for ROS production by Complex I (47, 48), thus inducing ROS production from Complex III toward the intermembrane space rather than within the mitochondrial matrix where the probe is located (63, 76). The decreased pH gradient may further decrease mt-cpYFP fluorescence. In addition, the fluorescence of mt-cpYFP is reversible and depends on the balance between superoxide production and scavenging. In contrast, other ROS indicators, such as Amplex red, DCF, and MitoSOX red, detect the cumulative signal from stable ROS such as H2O2 likely from both inside and outside of mitochondrial matrix. Results obtained with oligomycin A are also interesting. Oligomycin A acutely induces mitochondrial flash production followed by an inhibition after 20–30 min of treatment (27). This is likely due to the fact that oligomycin A slows down rather than totally blocks electron flow, which, similar to State 4 respiration, promotes electron leak and temporarily induces ROS production (73).
Recent reports using genetic animal models have provided explicit evidence to demonstrate the ETC dependence of mitochondrial flash activity. In these studies, knock out (KO) of ETC components or related mitochondrial proteins resulted in decreased or abolished mitochondrial flash activity in live cells and animals. For example, using a Complex I-deficient mouse model, where the Ndufs4 subunit of Complex I is knocked out in the heart, mitochondrial flash activity was decreased proportionally with decreased Complex I activity. Importantly, Complex I substrates cannot stimulate flash activity in myocytes from Ndufs4 KO mice to the same level as that observed for myocytes from wild-type mice (27). The parallel reduction in respiration and mitochondrial flash activity is consistent with decreased ROS production and a more reduced state in cardiomyocytes/mitochondria from the Ndufs4 KO hearts under resting and stress conditions (42). In Caenorhabditis elegans, a number of strains with defective respiratory chain were generated, all of which exhibited dramatically decreased mitochondrial flash activity (69). In the Hela cells, mt-SypHer-detected mitochondrial flashes are abolished by depletion of OPA1 (63). However, the effects of OPA1 ablation of flash activity were not due to altered mitochondrial morphology and fusion since flash activity was unaltered by inhibiting fusion through KO of the outer membrane fusion protein mitofusin 1. Since OPA1 also plays an important role in inner membrane cristae organization and formation of ETC supercomplexes that augment respiration efficiency (17, 74), it is possible that OPA1 ablation abolished mitochondrial flash activity through suppression of respiration.
Mitochondrial flash and transient mPTP opening
An abrupt reduction in membrane potential accompanies each mitochondrial flash event detected by the different flash indicators (mt-cpYFP, mt-SypHer, and mt-roGFP) (5, 8, 60, 63, 65, 76, 79). This is another common feature of these events and suggests a drastic change in inner membrane permeability during mitochondrial flash activity. In addition, the loss of membrane potential is temporary and recovers during or after each event, and two or more consecutive events can be detected within the same mitochondrion. These observations suggest that changes in mitochondrial inner membrane permeability are brief and reversible. Thus, transient openings of a large pore such as mPTP have been proposed to underlie this temporary loss of membrane potential.
Three lines of evidence suggest that mt-cpYFP-detected mitochondrial flashes in mammalian cells are accompanied by transient mPTP openings. First, the mPTP pore is a nonspecific large pore that can conduct small molecules up to 1.5 kDa. Mitochondria loaded with small fluorescence molecules, such as rhod-2 and mag-rhod-2 (molecular weight less than 1 kDa), were monitored during mt-cpYFP flash activity in the same mitochondria in skeletal muscle and adult cardiomyocytes. The results demonstrate a clear temporal coupling between flash events and the loss of fluorescence from these small molecules (76, 78, 79). These findings are consistent with the opening of a large pore during flash activity that permits release of these small molecules from the matrix. Second, mitochondrial flash activity is altered by pharmacological and genetic manipulations of mPTP opening. While the precise molecular identity of mPTP is not fully resolved, cyclophilin D (CypD) regulates the Ca2+ sensitivity of mPTP and is also a regulator of the ATP synthase (Complex V), in which the c-ring of the Fo or the dimers of the ATP synthase are proposed to form the mPTP pore (1, 6, 9, 24). The most widely used pharmacological inhibitor of mPTP, cyclosporine A, which targets CypD, blocks cpYFP-detected mitochondrial flash activity in cardiomyocytes and neurons. Conversely, flash activity is increased by atractyloside, an mPTP opener that binds to adenine nucleotide translocator and locks it into opening conformation (33–35, 50, 76, 79). Knocking down or knocking out CypD, which suppresses but does not abolish mPTP openings, decreases flash frequency, while overexpressing CypD increases flash frequency (68, 76, 84). Similarly, mt-SypHer-detected mitochondrial flash activity is also induced by atractyloside, and the authors suggested that the pH flash events are triggered by the opening of a large pore during fusion (63). Finally, mPTP opening is regulated by a number of signals, such as ROS and Ca2+. The roles of oxidants and antioxidants on mitochondrial flash regulation are consistent with mPTP as a trigger, and mitochondrial Ca2+ uptake indeed enhances mitochondrial flash activity (26, 32, 41, 86). In plant cells, atractyloside and H2O2 induce mitochondrial flashes. However, based on the interpretation that mt-cpYFP flash activity reflects transient matrix alkalization, the authors excluded the possibility that opening of a large pore triggers the flash events and accompanying loss of membrane potential (pulsing events) (65). Mitochondrial redox events detected with mt-roGFP coincident with mitochondrial “contraction” are also accompanied by a loss of membrane potential and are regulated by oxidants/antioxidants. However, the role of mPTP activity in the contraction events was excluded due to the lack of an effect by cyclosporine A. Since mitochondrial flash frequency in skeletal muscle is not altered by either cyclosporine A or CypD KO, but is coincident with the opening of a large pore, it is likely that CypD-independent mPTP openings (possibly via ROS activation) are coupled to flash activity in skeletal muscle (60, 68, 79, 82). Taken together, the data suggest that transient openings of the mPTP likely accompany mitochondrial flash activity (Fig. 3).
While transient mPTP opening accompanies each mitochondrial flash, whether they are causally linked is a topic of current investigation. In cardiomyocytes and neurons, transient openings of mPTP play a causal role in triggering mitochondrial flashes. This conclusion is derived from the inhibitory effect of cyclosporine A and CypD KO or knockdown (27, 33–35, 68, 76). In skeletal muscles, even though a large pore opening also accompanies each flash event, mPTP inhibition does not alter flash frequency (60, 68, 82). Because CypD controls the Ca2+ sensitivity of mPTP, but not other triggers of the mPTP, including ROS (7), flash activity in skeletal muscle may be triggered by CypD-independent mPTP openings (81). This possibility is supported by the very low abundance of CypD in skeletal muscle compared to the heart (68). Furthermore, a “pedestal precursor” signal was found before some flashes in both cardiomyocytes and skeletal muscle cells, which could reflect small increases in ROS that trigger mPTP opening (49, 60). A detailed discussion of the relationship between mPTP openings and flash activity is provided in our previous review article (81). Finally, whether genetic manipulation of mPTP constituents modulates mt-cpYFP-detected flashes in plant cells or mt-roGFP-detected redox flashes in neurons awaits future studies.
Physiological functions of mitochondrial flash activity
Mitochondrial flash activity is an evolutionarily conserved process that crosses a broad range of species and tissues. Thus, mitochondrial flashes likely reflect a fundamental physiological mitochondrial phenomenon that has important implications in mitochondrial biology and cell biology. However, debate over the elusive nature of the different overlapping signals that occur during these events has hampered broader investigations into the biological importance of the phenomenon. Moreover, the relative low frequency and discrete/stochastic occurrence of flash activity have raised concerns as to whether these events exert a significant functional impact on whole cell energy metabolism, redox regulation, and/or ion homeostasis. Nevertheless, several studies have clearly shown that mitochondrial flash activity contributes to both local physiological signaling and pathological conditions in the heart, skeletal muscle, neurons, and stem cells (Fig. 4).
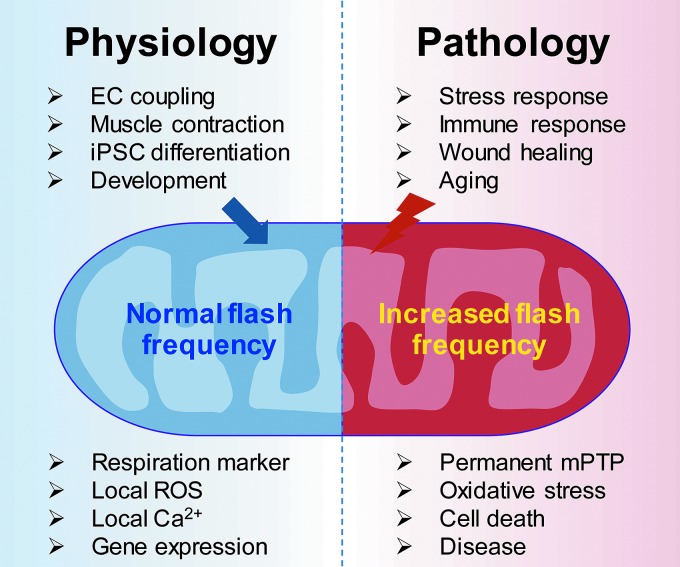
FIG. 4.
Mitochondrial flash activity as a biomarker for health (physiology) and disease (pathology). Various physiological and pathological triggers modulate mitochondrial flash frequency. Pathological stresses usually increase flash frequency, thus contributing to stress-induced mitochondrial and cell dysfunction, ultimately leading to the progression of disease. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
We have studied the role of mitochondrial flash activity in cardiac EC coupling, which provides the driving force for the continuous pumping function of the heart. The results revealed that a positive feedback loop exists in the cardiomyocytes, whereby mitochondria respond to an increased cytosolic energy demand by mitochondrial Ca2+ uptake boosting respiration. Furthermore, the increased mitochondrial respiration is coupled to increased generation of mitochondrial flashes, which provide local ROS signaling to further promote Ca2+ release from the sarcoplasmic reticulum. This feedback loop provides an important mechanism for the heart to continually match energy production with increased energy demand on a beat-to-beat basis (26).
The role of mitochondrial flash activity in skeletal muscle development was investigated in mt-cpYFP-expressing transgenic zebrafish. The results demonstrate striking multiphasic changes in mitochondrial flash frequency during muscle development, which are accompanied by mitochondrial morphological remodeling and functional maturation (85). However, whether alterations in mitochondrial flash activity play a causal role in mitochondrial maturation and muscle development is an unanswered question ripe for future study. The roles of mitochondrial flash activity in somatic cell reprogramming and neural progenitor differentiation have also been studied. Mitochondrial flash activity is increased during the early phase of somatic cell reprogramming, which induces DNA demethylation in the nucleus and transcriptional upregulation of Nanoga, a key gene in self-renewal and maintaining stemness of cells (84). In neural progenitor cells, mitochondrial flash activity negatively regulates cell proliferation and promotes cell differentiation (34, 35). These data indicate that mitochondrial flash activity regulates context-dependent stem cell differentiation and somatic cell dedifferentiation. With the demonstration that mitochondrial flash activity is coupled to both ETC-dependent respiration and mPTP opening, future investigations will undoubtedly uncover additional important cell type-specific physiological functions linked to mitochondrial flash activity.
Mitochondrial flash activity and disease
Given the importance of mitochondrial ROS production and mPTP in human disease, several studies have explored the role of mitochondrial flash activity under pathological conditions. Selenite-induced Hela cell death is mediated partially by increased mitochondrial ROS production through increased mitochondrial flash activity, and the antiapoptotic protein, Bcl-2, ameliorates this effect by reducing mitochondrial flash frequency (51). Proinflammatory cytokines stimulate both mitochondrial flash activity and mPTP openings in articular chondrocytes, which may contribute to cartilage inflammatory diseases (15). During heart failure, mitochondrial flash activity is decreased, which likely reflects compromised mitochondrial metabolism (26). During reperfusion following cardiac ischemia, mitochondrial flash activity mirrors the burst in mitochondrial ROS production and mPTP openings (54, 76). In the skeletal muscle, uncontrolled mitochondrial flash activity contributes to enhanced muscle oxidative stress in a murine model of malignant hyperthermia (82), a muscular dystrophy model in zebrafish (85), and in the ob/ob mouse model of obesity and insulin resistance (21).
Several groups have reported a role for altered mitochondrial flash activity in neuronal disease. Increased mitochondrial flash activity mediates the suppressive effect of amyloid beta 1–42 on neural progenitor cell proliferation (33), mitochondrial Ca2+-induced mitochondrial DNA damage in fibroblasts from Huntington disease patients (75), and glutamate toxicity in motor neurons (50). roGFP-detected redox flashes and accompanying mitochondrial “contraction” events are induced by stresses that promote neuron damage (11). Recent studies conducted on C. elegans revealed an important role of mitochondrial flash activity in aging and wound healing. In one study, mitochondrial flash frequency peaked 3 days after maturation, which was negatively correlated to the life span of the worm. In general, the higher the level of day 3 flash activity, the shorter the worm's life span, such that mitochondrial flash activity could be used to predict how long the organism will live (69). In a second study, physical damage of the worm skin (by laser or needle stab) induced a wave of mitochondrial ROS production featured by a dramatic increase in mitochondrial flash activity around the injured site. This ROS production was induced by a wave of intracellular Ca2+ right after the injury and was responsible for actin-based wound closure (83). In most of these studies, increased mitochondrial flash activity serves as a universal stress response signal downstream of changes in mitochondrial and/or cytosolic Ca2+ and upstream of either detrimental outcomes, including DNA damage, or compensatory outcomes, such as wound healing (Fig. 4).
Compatible with the significantly increased flash frequency under pathological conditions outlined earlier is the “ROS-induced ROS release” model. This model was developed based on the findings that laser-induced local ROS production triggers either mPTP or inner membrane anion channels (IMAC) to release ROS (2, 89). The released extramitochondrial ROS subsequently triggers neighboring mitochondria to undergo a similar process that leads to whole cell bursting of ROS oscillations. These whole cell mitochondrial ROS oscillations are coincident with loss of mitochondrial membrane potentials, which could lead to cellular dysfunctions such as cardiac arrhythmias (2–4, 18, 71). Recently, computational models have integrated the ROS signals with other mitochondrial functions during ROS oscillations (43–46, 80). Since synchronized flash activity is observed in a group of interconnected mitochondria in skeletal muscles (23, 25), individual mitochondrial flashes and whole cell ROS oscillations could be mechanistically linked and reflect the dynamic ROS regulation (and integrated mitochondrial functions) under physiological or pathological conditions, respectively. However, important characteristics of flash events that differentiate them from global ROS-induced ROS release events are that mitochondrial flashes occur spontaneously and reversibly in quiescent cells under physiological conditions, represent a stochastic rather than oscillatory process, and are confined to single mitochondria or a local interconnected mitochondrial network.
Mitochondrial Flash Activity Provides New Insights into Mitochondrial Biology
The autonomy and excitability of individual mitochondria
The discovery and characterization of mitochondrial flash activity provide new insights into mitochondrial biology. First, flash frequency provides an index of mitochondrial respiration at the individual mitochondrion level. Mitochondrial flash activity also represents a fundamental mechanism linking ATP utilization (energy demand) with ETC electron flow (energy production). Thus, mitochondrial flash activity may reflect a “switch” within each mitochondrion by which an increase in energy demand is sensed and translated into a transient burst or acceleration in aerobic respiration. In this regard, individual (or interconnected) mitochondria are regulated and work autonomously. Such regulatory signals could involve mitochondrial ROS and/or Ca2+, which both trigger transient mPTP opening and subsequent mitochondrial flash activity (Fig. 3). At the whole cell level, controlling the autonomy of individual mitochondria could enable precise and prompt manipulation of energy production to match fluctuations in energy demand. Under certain conditions, such as during whole cell ROS oscillations, individual mitochondrion could function in synchrony or form functional networks (2, 4, 25, 43, 45, 46, 80).
Moreover, mitochondria have been suggested to be excitable organelles in terms of Ca2+ signaling (39), and this concept now appears to extend to ROS and pH, which are concomitantly changed during mitochondrial respiration and thus are inseparable. We and others have proposed that mitochondrial excitability is reflected in integrated functions of mitochondria switching between quiescent and excited states (2, 5, 43, 49, 76, 80). In this regard, mitochondrial excitability could shift current paradigms that view mitochondria as passive energy producers to a more active participant and regulator of whole cell energy metabolism and signaling, which could broaden and deepen our understanding of the complex role of mitochondria in health and disease (Figs. 3 and 4).
Amplitude versus frequency mode of regulation
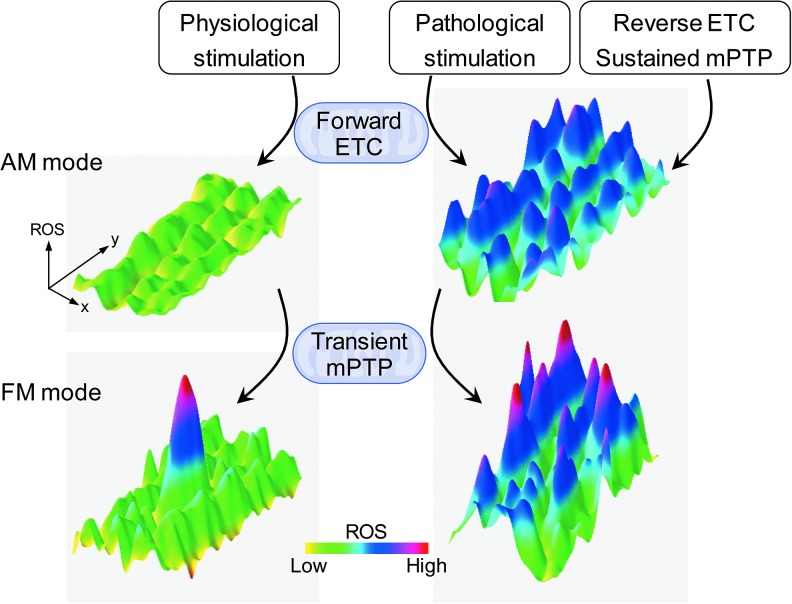
The concept of “switch” and “excitability” of mitochondria in bioenergetics discussed earlier brings forth a fundamental mechanism in encoding biological signals physiologically and effectively through stochastic rather than tonic signals. One example is how Ca2+ signals can be encoded by altering the frequency of Ca2+ oscillations. In an analogous manner, frequency-encoded ROS (and pH) signal via changes in mitochondrial flash activity represents a new mechanism for mitochondrial ROS signaling. As discussed earlier, mitochondrial flash activity is controlled by multiple physiological and pathological stresses, suggesting that the spatiotemporal regulation of mitochondrial flash frequency could represent a powerful mechanism of mitochondrial and cellular ROS regulation. We, therefore, refer to this “digitized” ROS production as a “frequency-modulatory (FM) mode” of ROS regulation. In comparison, sustained or steady-state mitochondrial ROS production (55) would represent an “amplitude-modulatory (AM) mode” of ROS regulation (Fig. 5). Similar distinct regulatory mechanisms have also been shown for controlling mitochondrial Ca2+, in which oscillatory and sustained Ca2+ changes play different roles in energy metabolism (29). Accumulating evidence suggests that the FM mode of ROS regulation is uniquely suitable to regulate local signals such as EC coupling and gene expression under physiological conditions (27, 84). Under extreme conditions or strong stresses, such as ischemia–reperfusion or cell damage, the FM mode activity becomes uncontrolled and significantly augments global or whole cell ROS signaling (2, 76, 84, 89). In contrast, the degree of AM mode activity may regulate the sensitivity of FM mode activity through triggering transient mPTP openings (Fig. 5) (32). Future studies are needed to provide more evidence for the existence, interaction, and significance of the two proposed modes of mitochondrial ROS regulation in cells. In addition, the composite nature of mitochondrial flashes indicates that other signals produced during these events (e.g., pH, Ca2+, membrane potential, and redox) could display similar dual-mode forms of regulation, one for local and compartmentalized regulation and another for global and steady-state regulation. In the face of dynamic nature of mitochondria that tend to preferentially localize in the hot spots where the energy demands are high as well as their critical role in the interorganelle (e.g., mitochondria–nucleus, mitochondria–endo/sarcoplasmic reticulum) communications, these oscillatory and localized mitochondrial signals will play a key role in cell regulation.
FIG. 5.
Proposed two modes of mitochondrial ETC-dependent superoxide production that coexist in vivo or under physiologically relevant conditions. The first mode, termed AM mode, reflects basal or constitutive ROS production. The second mode, termed FM mode, involves quantal events of mitochondrial flash activity that provide pulsatile transients of ROS production (plus other signals, including pH, Ca2+, redox, membrane potential). The two modes of ROS production may be linked through the effects of constitutive ROS production on transient mPTP activation. AM, amplitude-modulatory; ETC, electron transport chain; FM, frequency-modulatory; mPTP, mitochondrial permeability transition pore. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Conclusions and Perspectives
Perhaps the most important advance resulting from the discovery and characterization of mitochondrial flash activity is that intracellular metabolism can be controlled at the individual mitochondrion level. Specifically, within one mitochondrion, respiration, ROS production, proton pumping, membrane depolarization, and other concurrent processes are integrated and undergo intermittent bursting cycles analogous to that of action potentials used to encode electrical excitability and signaling. With more studies using mitochondrial flash activity as an in vivo biomarker for mitochondrial function, new features and regulatory mechanisms are likely to be discovered, which will further advance medicine by providing new therapeutic targets for aging and diseases related to alterations in mitochondrial and cellular metabolism. As the question of how mt-cpYFP senses superoxide in addition to pH does not have a straightforward answer due to limitations in current technology and the lack of structural information, future efforts are clearly warranted to fully resolve this issue. Nevertheless, ongoing debate over the sensitivity and selectivity of a specific indicator should not prevent investigations designed to further dissect the biology of mitochondrial flash activity. Moreover, ongoing debates should stimulate the design and development of new generations of indicators that are more specific for individually distinct mitochondrial signals. A few such indicators, including HKSox (for superoxide), ATeam (for ATP), and SoNar (for NAD+/NADH ratio), have been reported and need to be further tested and validated in different biological systems (20, 36, 40, 57, 88). Future studies designed to advance our understanding of the composite nature of the mitochondrial flash activity, their regulation by cell signaling pathways and posttranslational modifications (e.g., phosphorylation, acetylation, and nitrosylation), and significance in health and disease are all crucial for moving this field forward.
Abbreviations Used
- ADP
adenosine diphosphate
- AM
amplitude-modulatory
- ATP
adenosine triphosphate
- Ca2+
calcium
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- CM
cardiomyocytes
- CypD
cyclophilin D
- DCF
2′,7′-dichlorodihydrofluorescein diacetate
- DTT
dithiothreitol
- EC
excitation–contraction
- ETC
electron transport chain
- EYFP
yellow fluorescent protein
- FCCP
carbonyl cyanide-p-trifluoromethoxyphenylhydrazone
- FM
frequency-modulatory
- H2O2
hydrogen peroxide
- IMAC
inner membrane anion channels
- KO
knock out
- MCU
mitochondrial calcium uniporter
- Mg2+
magnesium
- mPTP
mitochondrial permeability transition pore
- mt-cpYFP
mitochondria-targeted circularly permuted yellow fluorescent protein
- Na+
sodium
- NAD+
nicotinamide adenine dinucleotide (oxidized)
- NADH
nicotinamide adenine dinucleotide (reduced)
- OPA1
optic atrophy 1
- roGFP
redox green fluorescent protein
- ROS
reactive oxygen species
- SM
skeletal muscles
- SOD
superoxide dismutase
Acknowledgments
This work is in part supported by grants from the National Institutes of Health (HL114760 to W.W., HL122124 and HL093671 to S.-S.S., AR059646 to R.T.D.), American Heart Association (10SDG3450009 to W.W.), National Key Basic Research Program of China (2013CB531200 to H.P.C.), and the National Science Foundation of China (31130067 to H.P.C.).
References
- 1.Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA, Jr., and Jonas EA. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A 111: 10580–10585, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aon MA, Cortassa S, Marban E, and O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem 278: 44735–44744, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Aon MA, Cortassa S, and O'Rourke B. The fundamental organization of cardiac mitochondria as a network of coupled oscillators. Biophys J 91: 4317–4327, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aon MA, Cortassa S, and O'Rourke B. Mitochondrial oscillations in physiology and pathophysiology. Adv Exp Med Biol 641: 98–117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azarias G. and Chatton JY. Selective ion changes during spontaneous mitochondrial transients in intact astrocytes. PLoS One 6: e28505, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, and Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, and Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem 280: 18558–18561, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, and Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 3: 281–286, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Bernardi P, Rasola A, Forte M, and Lippe G. The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol Rev 95: 1111–1155, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brand MD. and Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J 435: 297–312, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breckwoldt MO, Pfister FM, Bradley PM, Marinkovic P, Williams PR, Brill MS, Plomer B, Schmalz A, St Clair DK, Naumann R, Griesbeck O, Schwarzlander M, Godinho L, Bareyre FM, Dick TP, Kerschensteiner M, and Misgeld T. Multiparametric optical analysis of mitochondrial redox signals during neuronal physiology and pathology in vivo. Nat Med 20: 555–560, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Brookes PS, Yoon Y, Robotham JL, Anders MW, and Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287: C817–C833, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Brown GC. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem J 284: 1–13, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckman JF. and Reynolds IJ. Spontaneous changes in mitochondrial membrane potential in cultured neurons. J Neurosci 21: 5054–5065, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao Y, Zhang X, Shang W, Xu J, Wang X, Hu X, Ao Y, and Cheng H. Proinflammatory cytokines stimulate mitochondrial superoxide flashes in articular chondrocytes in vitro and in situ. PLoS One 8: e66444, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng H, Wang W, Wang X, Sheu SS, Dirksen RT, and Dong MQ. Cheng , et al. reply. Nature 514: E14–E15, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A, Gomes LC, Perales-Clemente E, Salviati L, Fernandez-Silva P, Enriquez JA, and Scorrano L. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155: 160–171, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortassa S, Aon MA, Winslow RL, and O'Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys J 87: 2060–2073, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortassa S, O'Rourke B, and Aon MA. Redox-optimized ROS balance and the relationship between mitochondrial respiration and ROS. Biochim Biophys Acta 1837: 287–295, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Michele R, Carimi F, and Frommer WB. Mitochondrial biosensors. Int J Biochem Cell Biol 48: 39–44, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Ding Y, Fang H, Shang W, Xiao Y, Sun T, Hou N, Pan L, Sun X, Ma Q, Zhou J, Wang X, Zhang X, and Cheng H. Mitoflash altered by metabolic stress in insulin-resistant skeletal muscle. J Mol Med (Berl) 93: 1119–1130, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duchen MR, Leyssens A, and Crompton M. Transient mitochondrial depolarizations reflect focal sarcoplasmic reticular calcium release in single rat cardiomyocytes. J Cell Biol 142: 975–988, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang H, Chen M, Ding Y, Shang W, Xu J, Zhang X, Zhang W, Li K, Xiao Y, Gao F, Shang S, Li JC, Tian XL, Wang SQ, Zhou J, Weisleder N, Ma J, Ouyang K, Chen J, Wang X, Zheng M, Wang W, and Cheng H. Imaging superoxide flash and metabolism-coupled mitochondrial permeability transition in living animals. Cell Res 21: 1295–1304, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, and Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A 110: 5887–5892, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glancy B, Hartnell LM, Malide D, Yu ZX, Combs CA, Connelly PS, Subramaniam S, and Balaban RS. Mitochondrial reticulum for cellular energy distribution in muscle. Nature 523: 617–620, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong G, Liu X, and Wang W. Regulation of metabolism in individual mitochondria during excitation-contraction coupling. J Mol Cell Cardiol 76: 235–246, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong G, Liu X, Zhang H, Sheu SS, and Wang W. Mitochondrial flash as a novel biomarker of mitochondrial respiration in the heart. Am J Physiol Heart Circ Physiol 309: H1166–H1177, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong G. and Wang W. Confocal imaging of single mitochondrial superoxide flashes in intact heart or in vivo. J Vis Exp 81: e50818, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajnoczky G, Robb-Gaspers LD, Seitz MB, and Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82: 415–424, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, and Remington SJ. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem 279: 13044–13053, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Hou T, Wang X, Ma Q, and Cheng H. Mitochondrial flashes: new insights into mitochondrial ROS signalling and beyond. J Physiol 592: 3703–3713, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou T, Zhang X, Xu J, Jian C, Huang Z, Ye T, Hu K, Zheng M, Gao F, Wang X, and Cheng H. Synergistic triggering of superoxide flashes by mitochondrial Ca2+ uniport and basal reactive oxygen species elevation. J Biol Chem 288: 4602–4612, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou Y, Ghosh P, Wan R, Ouyang X, Cheng H, Mattson MP, and Cheng A. Permeability transition pore-mediated mitochondrial superoxide flashes mediate an early inhibitory effect of amyloid beta1-42 on neural progenitor cell proliferation. Neurobiol Aging 35: 975–989, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou Y, Mattson MP, and Cheng A. Permeability transition pore-mediated mitochondrial superoxide flashes regulate cortical neural progenitor differentiation. PLoS One 8: e76721, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou Y, Ouyang X, Wan R, Cheng H, Mattson MP, and Cheng A. Mitochondrial superoxide production negatively regulates neural progenitor proliferation and cerebral cortical development. Stem Cells 30: 2535–2547, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu JJ, Wong NK, Ye S, Chen X, Lu MY, Zhao AQ, Guo Y, Ma AC, Leung AY, Shen J, and Yang D. Fluorescent Probe HKSOX-1 for Imaging and Detection of Endogenous Superoxide in Live Cells and In Vivo. J Am Chem Soc 137: 6837–6843, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Huang Z, Zhang W, Fang H, Zheng M, Wang X, Xu J, Cheng H, Gong G, Wang W, Dirksen RT, and Sheu SS. Response to “A critical evaluation of cpYFP as a probe for superoxide”. Free Radic Biol Med 51: 1937–1940, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Huser J, Rechenmacher CE, and Blatter LA. Imaging the permeability pore transition in single mitochondria. Biophys J 74: 2129–2137, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ichas F, Jouaville LS, and Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell 89: 1145–1153, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Imamura H, Nhat KP, Togawa H, Saito K, Iino R, Kato-Yamada Y, Nagai T, and Noji H. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci U S A 106: 15651–15656, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jian C, Hou T, Yin R, Cheng H, and Wang X. Regulation of superoxide flashes by transient and steady mitochondrial calcium elevations. Sci China Life Sci 57: 495–501, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC, Jr., Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, and Tian R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab 18: 239–250, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kembro JM, Aon MA, Winslow RL, O'Rourke B, and Cortassa S. Integrating mitochondrial energetics, redox and ROS metabolic networks: a two-compartment model. Biophys J 104: 332–343, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kembro JM, Cortassa S, and Aon MA. Complex oscillatory redox dynamics with signaling potential at the edge between normal and pathological mitochondrial function. Front Physiol 5: 257, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurz FT, Aon MA, O'Rourke B, and Armoundas AA. Spatio-temporal oscillations of individual mitochondria in cardiac myocytes reveal modulation of synchronized mitochondrial clusters. Proc Natl Acad Sci U S A 107: 14315–14320, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurz FT, Derungs T, Aon MA, O'Rourke B, and Armoundas AA. Mitochondrial networks in cardiac myocytes reveal dynamic coupling behavior. Biophys J 108: 1922–1933, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lambert AJ. and Brand MD. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J 382: 511–517, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lambert AJ, Buckingham JA, Boysen HM, and Brand MD. Low complex I content explains the low hydrogen peroxide production rate of heart mitochondria from the long-lived pigeon, Columba livia. Aging Cell 9: 78–91, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Li K, Zhang W, Fang H, Xie W, Liu J, Zheng M, Wang X, Wang W, Tan W, and Cheng H. Superoxide flashes reveal novel properties of mitochondrial reactive oxygen species excitability in cardiomyocytes. Biophys J 102: 1011–1021, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu X, Xu S, Wang P, and Wang W. Transient mitochondrial permeability transition mediates excitotoxicity in glutamate-sensitive NSC34D motor neuron-like cells. Exp Neurol 271: 122–130, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma Q, Fang H, Shang W, Liu L, Xu Z, Ye T, Wang X, Zheng M, Chen Q, and Cheng H. Superoxide flashes: early mitochondrial signals for oxidative stress-induced apoptosis. J Biol Chem 286: 27573–27581, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191: 144–148, 1961 [DOI] [PubMed] [Google Scholar]
- 53.Muller FL. A critical evaluation of cpYFP as a probe for superoxide. Free Radic Biol Med 47: 1779–1780, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Murphy E. and Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88: 581–609, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagai T, Sawano A, Park ES, and Miyawaki A. Circularly permuted green fluorescent proteins engineered to sense Ca2+. Proc Natl Acad Sci U S A 98: 3197–3202, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakano M, Imamura H, Nagai T, and Noji H. Ca(2)(+) regulation of mitochondrial ATP synthesis visualized at the single cell level. ACS Chem Biol 6: 709–715, 2011 [DOI] [PubMed] [Google Scholar]
- 58.O'Reilly CM, Fogarty KE, Drummond RM, Tuft RA, and Walsh JV., Jr. Quantitative analysis of spontaneous mitochondrial depolarizations. Biophys J 85: 3350–3357, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pacher P, Thomas AP, and Hajnoczky G. Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc Natl Acad Sci U S A 99: 2380–2385, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pouvreau S. Superoxide flashes in mouse skeletal muscle are produced by discrete arrays of active mitochondria operating coherently. PLoS One 5: pii:, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pouvreau S. Genetically encoded reactive oxygen species (ROS) and redox indicators. Biotechnol J 9: 282–293, 2014 [DOI] [PubMed] [Google Scholar]
- 62.Quatresous E, Legrand C, and Pouvreau S. Mitochondria-targeted cpYFP: pH or superoxide sensor? J Gen Physiol 140: 567–570, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santo-Domingo J, Giacomello M, Poburko D, Scorrano L, and Demaurex N. OPA1 promotes pH flashes that spread between contiguous mitochondria without matrix protein exchange. EMBO J 32: 1927–1940, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwarzlander M, Logan DC, Fricker MD, and Sweetlove LJ. The circularly permuted yellow fluorescent protein cpYFP that has been used as a superoxide probe is highly responsive to pH but not superoxide in mitochondria: implications for the existence of superoxide ‘flashes’. Biochem J 437: 381–387, 2011 [DOI] [PubMed] [Google Scholar]
- 65.Schwarzlander M, Logan DC, Johnston IG, Jones NS, Meyer AJ, Fricker MD, and Sweetlove LJ. Pulsing of membrane potential in individual mitochondria: a stress-induced mechanism to regulate respiratory bioenergetics in Arabidopsis. Plant Cell 24: 1188–1201, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwarzlander M, Murphy MP, Duchen MR, Logan DC, Fricker MD, Halestrap AP, Muller FL, Rizzuto R, Dick TP, Meyer AJ, and Sweetlove LJ. Mitochondrial ‘flashes’: a radical concept repHined. Trends Cell Biol 22: 503–508, 2012 [DOI] [PubMed] [Google Scholar]
- 67.Schwarzlander M, Wagner S, Ermakova YG, Belousov VV, Radi R, Beckman JS, Buettner GR, Demaurex N, Duchen MR, Forman HJ, Fricker MD, Gems D, Halestrap AP, Halliwell B, Jakob U, Johnston IG, Jones NS, Logan DC, Morgan B, Muller FL, Nicholls DG, Remington SJ, Schumacker PT, Winterbourn CC, Sweetlove LJ, Meyer AJ, Dick TP, and Murphy MP. The ‘mitoflash’ probe cpYFP does not respond to superoxide. Nature 514: E12–E14, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shang W, Gao H, Lu F, Ma Q, Fang H, Sun T, Xu J, Ding Y, Lin Y, Wang Y, Wang X, Cheng H, and Zheng M. Cyclophilin D regulates mitochondrial flashes and metabolism in cardiac myocytes. J Mol Cell Cardiol 91: 63–71, 2016 [DOI] [PubMed] [Google Scholar]
- 69.Shen EZ, Song CQ, Lin Y, Zhang WH, Su PF, Liu WY, Zhang P, Xu J, Lin N, Zhan C, Wang X, Shyr Y, Cheng H, and Dong MQ. Mitoflash frequency in early adulthood predicts lifespan in Caenorhabditis elegans. Nature 508: 128–132, 2014 [DOI] [PubMed] [Google Scholar]
- 70.Sheu SS, Wang W, Cheng H, and Dirksen RT. Superoxide flashes: illuminating new insights into cardiac ischemia/reperfusion injury. Future Cardiol 4: 551–554, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Slodzinski MK, Aon MA, and O'Rourke B. Glutathione oxidation as a trigger of mitochondrial depolarization and oscillation in intact hearts. J Mol Cell Cardiol 45: 650–660, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang S, Wong HC, Wang ZM, Huang Y, Zou J, Zhuo Y, Pennati A, Gadda G, Delbono O, and Yang JJ. Design and application of a class of sensors to monitor Ca2+ dynamics in high Ca2+ concentration cellular compartments. Proc Natl Acad Sci U S A 108: 16265–16270, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tocchetti CG, Caceres V, Stanley BA, Xie C, Shi S, Watson WH, O'Rourke B, Spadari-Bratfisch RC, Cortassa S, Akar FG, Paolocci N, and Aon MA. GSH or palmitate preserves mitochondrial energetic/redox balance, preventing mechanical dysfunction in metabolically challenged myocytes/hearts from type 2 diabetic mice. Diabetes 61: 3094–3105, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Varanita T, Soriano ME, Romanello V, Zaglia T, Quintana-Cabrera R, Semenzato M, Menabo R, Costa V, Civiletto G, Pesce P, Viscomi C, Zeviani M, Di Lisa F, Mongillo M, Sandri M, and Scorrano L. The opa1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab 21: 834–844, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang JQ, Chen Q, Wang X, Wang QC, Wang Y, Cheng HP, Guo C, Sun Q, and Tang TS. Dysregulation of mitochondrial calcium signaling and superoxide flashes cause mitochondrial genomic DNA damage in Huntington disease. J Biol Chem 288: 3070–3084, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, and Cheng H. Superoxide flashes in single mitochondria. Cell 134: 279–290, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X, Fang H, Huang Z, Shang W, Hou T, Cheng A, and Cheng H. Imaging ROS signaling in cells and animals. J Mol Med (Berl) 91: 917–927, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang X, Jian C, Zhang X, Huang Z, Xu J, Hou T, Shang W, Ding Y, Zhang W, Ouyang M, Wang Y, Yang Z, Zheng M, and Cheng H. Superoxide flashes: elemental events of mitochondrial ROS signaling in the heart. J Mol Cell Cardiol 52: 940–948, 2012 [DOI] [PubMed] [Google Scholar]
- 79.Wei-LaPierre L, Gong G, Gerstner BJ, Ducreux S, Yule DI, Pouvreau S, Wang X, Sheu SS, Cheng H, Dirksen RT, and Wang W. Respective contribution of mitochondrial superoxide and pH to mitochondria-targeted circularly permuted yellow fluorescent protein (mt-cpYFP) flash activity. J Biol Chem 288: 10567–10577, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei AC, Aon MA, O'Rourke B, Winslow RL, and Cortassa S. Mitochondrial energetics, pH regulation, and ion dynamics: a computational-experimental approach. Biophys J 100: 2894–2903, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei L. and Dirksen RT. Perspectives on: SGP symposium on mitochondrial physiology and medicine: mitochondrial superoxide flashes: from discovery to new controversies. J Gen Physiol 139: 425–434, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei L, Salahura G, Boncompagni S, Kasischke KA, Protasi F, Sheu SS, and Dirksen RT. Mitochondrial superoxide flashes: metabolic biomarkers of skeletal muscle activity and disease. FASEB J 25: 3068–3078, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu S. and Chisholm AD. C. elegans epidermal wounding induces a mitochondrial ROS burst that promotes wound repair. Dev Cell 31: 48–60, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ying Z, Chen K, Zheng L, Wu Y, Li L, Wang R, Long Q, Yang L, Guo J, Yao D, Li Y, Bao F, Xiang G, Liu J, Huang Q, Wu Z, Hutchins AP, Pei D, and Liu X. Transient activation of mitoflashes modulates nanog at the early phase of somatic cell reprogramming. Cell Metab 23: 220–226, 2016 [DOI] [PubMed] [Google Scholar]
- 85.Zhang M, Sun T, Jian C, Lei L, Han P, Lv Q, Yang R, Zhou X, Xu J, Hu Y, Men Y, Huang Y, Zhang C, Zhu X, Wang X, Cheng H, and Xiong JW. Remodeling of mitochondrial flashes in muscular development and dystrophy in zebrafish. PLoS One 10: e0132567, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang W, Li K, Zhu X, Wu D, Shang W, Yuan X, Huang Z, Zheng M, Wang X, Yang D, Liu J, and Cheng H. Subsarcolemmal mitochondrial flashes induced by hypochlorite stimulation in cardiac myocytes. Free Radic Res 48: 1085–1094, 2014 [DOI] [PubMed] [Google Scholar]
- 87.Zhang X, Huang Z, Hou T, Xu J, Wang Y, Shang W, Ye T, Cheng H, Gao F, and Wang X. Superoxide constitutes a major signal of mitochondrial superoxide flash. Life Sci 93: 178–186, 2013 [DOI] [PubMed] [Google Scholar]
- 88.Zhao Y, Hu Q, Cheng F, Su N, Wang A, Zou Y, Hu H, Chen X, Zhou HM, Huang X, Yang K, Zhu Q, Wang X, Yi J, Zhu L, Qian X, Chen L, Tang Y, Loscalzo J, and Yang Y. SoNar, a Highly responsive NAD+/NADH sensor, allows high-throughput metabolic screening of anti-tumor agents. Cell Metab 21: 777–789, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zorov DB, Filburn CR, Klotz LO, Zweier JL, and Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192: 1001–1014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]