Abstract
The mammalian inner ear consists of diverse cell types with important functions. Gene mutations in these diverse cell types have been found to underlie different forms of genetic hearing loss. Targeting these mutations for gene therapy development represents a future therapeutic strategy to treat hearing loss. Adeno-associated viral (AAV) vectors have become the vector of choice for gene delivery in animal models in vivo. To identify AAV vectors that target inner ear cell subtypes, we systemically screened 12 AAV vectors with different serotypes (AAV1, 2, 5, 6, 6.2, 7, 8, 9, rh.8, rh.10, rh.39, and rh.43) that carry a reporter gene GFP in neonatal and adult mice by microinjection in vivo. We found that most AAVs infect both neonatal and adult inner ear, with different specificities and expression levels. The inner ear cochlear sensory epithelial region, which includes auditory hair cells and supporting cells, is most frequently targeted for gene delivery. Expression of the transgene is sustained, and neonatal inner ear delivery does not adversely affect hearing. Adult inner ear injection of AAV has a similar infection pattern as the younger inner ear, with the exception that outer hair cell death caused by the injection procedure can lead to hearing loss. In the adult, more so than in the neonatal mice, cell types infected and efficiency of infection are correlated with the site of injection. Most infected cells survive in neonatal and adult inner ears. The study adds to the list of AAV vectors that transduce the mammalian inner ear efficiently, providing the tools that are important to study inner ear gene function and for the development of gene therapy to treat hearing loss.
Introduction
Genetic hearing loss is highly prevalent, occurring at a frequency of 1 in 500 newborns.1 Over 100 genetic hearing loss loci have been mapped, over 90 of which have been cloned in humans.2 Mouse models of hearing loss have been extensively studied because of a high degree of conservation between the structure and physiological roles of inner ears between humans and mice. New genes involved in genetic hearing loss in mice are being identified at a rapid rate, providing a growing list of candidate genes for human deafness. Despite the impressive progress in identification of deafness genes, no medical treatment is available for genetic hearing loss except for cochlear implantation. Development of new treatment that targets diverse types of genetic hearing loss is therefore a high priority.
One of the most promising approaches to develop treatment for genetic hearing loss is gene therapy. Gene therapy offers the possibility to supplement normal copies of genes whose functions are lost to correct gene defects. Adeno-associated virus (AAV) has been increasingly used as a primary choice of delivery vehicle for inner ear gene therapy because of its limited immunogenicity and toxicity, and its success in gene therapy programs for diverse genetic disorders such as retinitis pigmentosa, hemophilia A and B, and San Filippo A in humans.3–12 The elaborate structure and exquisite function of the inner ear require coordinated action of diverse inner ear cell types, including the sensory hair cells, supporting cells, neurons, and stria vascularis. Gene defects in any of these cell types result in genetic hearing loss.13–15 For instance, mutations in MYO7A, a gene affecting hair cell function, cause Usher syndrome type IB; GJB2 mutations in supporting cells and fibrocytes are responsible for the most common form of recessive deafness known as DFNB1, and mutations in NDP affecting the function of the stria vascularis and auditory neurons give rise to a form of syndromic deafness known as Norris disease.16–18 Targeting those cell types would likely require AAV with different tropisms. Further, other factors, including the effect on the delivery to young versus mature inner ears, the route of delivery, the control of expression of exogenous genes, and the impact of titer and long-term expression status, have yet to be determined.
AAV vectors have been studied for inner ear delivery. Most of the studies have focused on the serotypes commonly used. AAV3 was shown to specifically transduced cochlear inner hair cells (IHCs) in the basal and middle cochlear regions with high efficiency in vivo in the adult stage through the round window injection.4 By a nanoliter-level fluid delivery system, Kilpatrick et al. studied 5 AAV vectors (serotypes 1, 2, 5, 6, and 8) injected through the scala media in normal and deaf adult inner ear, and showed transduction in the sensory hair cells, supporting cells, the auditory nerve, and spiral ligament.9
To comprehensively survey AAV vectors for their transduction capacity into mouse inner ear at different stages and assess their impact on hearing, we systematically analyzed 12 AAV vectors with different serotypes. We identified AAV subtypes that infect major inner ear cell types in neonatal and adult mice. We showed that neonatal delivery does not affect hearing and maintains gene expression through development, establishing a significant step toward developing gene therapy to correct genetic deafness. Our study has implication in developing gene therapy that targets different inner ear cell types.
Materials and Methods
All the animals were used under protocols approved by the Massachusetts Eye & Ear Infirmary ALCUC committee.
Production of AAV
We used AAVs of different serotypes, including AAV1, 2, 5, 6, 6.2, 7, 8, 9, rh.8, rh.10, rh.39, and rh.43, with a GFP under the control of a chicken beta-actin promoter.20,21 All AAV vectors used in this study were produced at Horae Gene Therapy Center of UMass Medical School, Worcester, MA.
Microinjection of AAV to neonatal mouse inner ear
P1-2 CD1 mice were used for AAV-GFP (AAV1, 2, 5, 6, 6.2, 7, 8, 9, rh.8, rh.10, rh.39, rh.43) injection. The titer of AAV is 1–8 × 1012 genome copies (GCs)/ml. CD1 mice were from the Charles River Laboratory. Mice were anesthetized by lowering their temperature on ice. Cochleostomy was performed by making an incision behind the ear to expose the cochlear. Glass micropipettes (WPI, Sarasota, FL) held by a Nanoliter Microinjection System (WPI) were used to deliver the AAV into the scala media, which allows access to inner ear cells. The total delivery volume for each injection was ∼0.2 μl per cochlea and the release was controlled by a micromanipulator at the speed of 3 nl/sec. The mice were sacrificed at 2 weeks and 3 months after AAV injection.
Microinjection of AAV to adult mouse inner ear
Adult (6-week-old) male CBA/CaJ mice were used for AAV-GFP (AAV1, 2, 5, 6, 6.2, 7, 8, 9, rh.8, rh.10, rh.39, rh.43) injection. CBA/CAJ mice were from the Charles River Laboratory. Mice were anesthetized using an intraperitoneal injection of xylazine (20 mg/kg) and ketamine (100 mg/kg). Body temperature was maintained at 37°C using an electric heating pad. An incision was made from the right postauricular, and the tympanic bulla was exposed. The bulla was perforated with a surgical needle and the small hole was expanded to approach to the cochlea. The bony cochlear lateral wall of the scala media was thinned carefully by a dental drill but the membranous lateral wall was left intact. Glass micropipettes (WPI) were pulled and the tips were broken to a diameter of 15–20 mm. The Nanoliter Microinjection System (WPI) was used to deliver a total of ∼300 nl fluid into the inner ear at the speed of 2 nl/sec according to the manufacturer's protocol. The glass micropipette was left in place for 5 min after the injection. After cochleostomy, the opening in the tympanic bulla was sealed with dental cement. Muscle and skin were sutured with 4/0 suture. The mice were allowed to awaken from anesthesia, and their pain was controlled with 0.15 mg/kg buprenorphine hydrochloride for 3 days.
Immunohistochemistry and quantification
Two weeks and 3 months after injection, mice were sacrificed and cochlea were harvested by standard protocols.1 For immunohistochemistry, antibodies against markers for hair cells (Myo7a), supporting cells (Sox2), and GFP were used as previously described.10 To quantify the number of GFP-positive cells after AAV injection, we counted the total number of hair cells and supporting cells outside of IHCs in a region spanning 200 μm in the apex, middle, and base turn of the cochlea. The entire cochlear along the basilar membrane was divided into three pieces of equal length, designated basal, middle, and apical turns.
Hearing test after AAV injection in neonatal mice
Auditory brainstem response (ABR) was used to measure hearing, one month after infection in neonatal cochlea, with the uninjected inner ears as controls, using the method as described.22
Results
Transduction of 12 AAV serotypes in the cochlea in neonatal mice
The auditory system of the inner ear, also known as the cochlea, harbors diverse cell types, including the sensory hair cells that detect sound and balance, supporting cells, stria vascularis, and ganglion neurons. Mouse cochlea has 1½ turns that can be roughly divided into subregions, base, middle, and apex turns (Fig. 1A). To study how different serotypes of AAV infect these diverse cell types, we first performed cochleostomy in neonatal mice of P1–P2 age and injected AAV-GFP into the scala media in the base turn of the cochlea (Fig. 1B, C). By fluid movement and through intercellular junctions, AAV was transported to other regions (turns) of the cochlea, establishing the possibility of infecting cells distant from the injection site.
Figure 1.
AAV transduces diverse inner ear cell types in neonatal mice. (A) An confocal image of an whole-mount fluorescent immunolabeling P1 cochlea to illustrate 1½ turns from the base to the apex. Myo7a labeled hair cells. (B) A schematic diagram of a cross section of cochlea, showing injection into the scala media (SM) by cochleostomy. (C) Enlarged inset from (B) showing cochlear cell types. Cldc, Claudius cell; DC, Deiters cell; HeC, Hensen's cell; IHC, inner hair cells; IPC, inner pillar cell; OHC, outer hair cell; OPC, outer pillar cell. (D) Three months after injection, whole-mount fluorescent immunolabeling showed AAV infection in major cell types of the sensory epithelium in the middle-turn that included OHC and IHC, and different supporting cells (E, Sox2+). (E) was from the same field as in (D), with the focus on the Sox2-labeled supporting cells that were beneath the hair cells. (F) AAV2 infected inner and outer hair cells and the cells medial to the outer hair cell region. (G) AAV5 showed relatively weak GFP expression in the inner hair cells but absence in the outer hair cells, with higher GFP level in the cells medial to the outer hair cell region. (H) AAV6.2-transduced inner hair cells with strong GFP expression, with relatively weak GFP level in the limbus region (Lims). (I) AAV7 showed extensive infection in the limbus regions, as well as in the outer hair cell region. (J) Control cochlea without AAV injection showed no infection by the lack of GFP signals. Scale bars: (A) 50 μm; (D–J) 10 μm.
We examined GFP expression by immunohistochemistry with an antibody against the GFP protein, and compared the number and the type of cochlear cells that became GFP positive two weeks and three months after injections. Among 12 different AAVs tested, only AAVs 6 and rh.39 did not produce any discernible GFP-positive cells, whereas the remaining 10 AAVs resulted in GFP-positive cells in various cochlear cell subtypes. AAV infection did not induce serious toxicity as shown by the presence of all the major cell types at different points of development (Figs. 1 and 2).
Figure 2.
AAV transduces diverse inner ear cell types in neonatal mice. (A) AAVrh.8 injection produced strongly GFP-labeled inner border cells (IBC) and Deiters cells (DC), as well as cells in the limbus region (Lims). (B) AAV8 infection resulted in relatively weak GFP signal in broad cell types in the sensory epithelium, including auditory hair cells and all major cochlear-supporting cell types. (C) AAV9-transduced cells were mainly IHCs and inner pillar cells (IPC). (D) Robust GFP signal was detected in IHCs and OHCs, and the modiolus region after AAVrh.10 infection. (E) After AAVrh.43 infection, GFP was detected prominently in IBC, DC, and the Hensen cells (HeC). (F) AAV8 infected the lateral wall with GFP signal in discrete cells. (G) AAVrh.43 showed infection in the lateral wall with GFP signal that was broadly detected. Scale bars: 10 μm.
For inner ear auditory hair cells, GFP was detected in the outer hair cells (OHCs) when injections were performed using AAV1, 2, and 8 and in the IHCs by AAV1, 2, 5, 7, 8, 9, and rh.10 infection, 3 months after the initial injection (Figs. 1 and 2). Importantly, the types of cells transduced and the efficiency of transduction (percentage of GFP-positive cells) by AAV injection were similar at both two weeks and three months, establishing its sustainability. Two weeks after AAV2 infection, the number of GFP-positive OHCs in the base, middle, and apex turns were 39%, 33.3%, and 3.1%, respectively (Fig. 3B). At 3 months after AAV2 injection, the number of OHCs that were GFP positive was similar to two weeks in the base, middle, and apex turns with 39.5%, 28%, and 4.1% GFP positivity, respectively (Fig. 3C). Similarly, the number of GFP-positive IHCs 2 weeks or 3 months after infection were similar in the base, middle, and apex turns (19.3% vs. 22.6%, 11.4% vs. 15%, and 4.6% vs. 11.8%, respectively). Two weeks after AAV2 injection, GFP signals were detected in many supporting cell subtypes, with prominent expression in the pillar cells and the Claudius cells, and weaker in the Deiters cells (Fig. 3B), whereas after three months, GFP was similarly detected in the supporting cells, with the exception that GFP was absent in the Claudius cells (Fig. 3C). The number of GFP-positive supporting cells two weeks or three months after initial injections was similar (7.1% vs. 4.2% in the base; 2.4% vs. 3.5% in the middle turn). In controls, which did not receive injections, no cells were found to be GFP positive (Fig. 1J). In addition, we quantified the GFP-positive cells present in AAV1-infected cochleas, which showed consistent labeling between two weeks and three months after infection (Table 1).
Figure 3.
Inner ear cell subtypes infected by AAV. (A) Three months after infection of AAV1 in neonatal cochlea, confocal image of cross section of mid-turn cochlea showed that OHCs and Claudius cells (Cldc) expressed high level of GFP, whereas IHCs and inner and outer pillar cells (IPC, OPC) showed weak GFP signals. (B) Two weeks after AAV2 infection, prominent GFP signals were detected in the IHCs, OHCs, IPC, OPC, Deiters cells (DC), and Cldc. (C) Three months after AAV2 infection, the overall expression patterns of cell types infected were similar to 2 weeks postinfection (B), with the exception that GFP signal was below the level of detection in the Cldc. (D) Three months after AAV8 infection, prominent GFP signals were detected in the OHCs and Cldc, with weak expression in the IHCs. (E) AAV6.2 infection transduced IPC and Cldc with sustained GFP three months later. (F) AAVrh.43 infection produced strong GFP signals in the IBC and HeC, and weak GFP in the DC. Scale bars: 10 μm.
Table 1.
Transduction efficiency of AAV serotypes 1 and 2 in neonatal mice 2 weeks and 3 months postinfection
| 2 weeks | 3 months | ||||||
|---|---|---|---|---|---|---|---|
| Serotype | Cell type | Base (%) | Middle (%) | Apex (%) | Base (%) | Middle (%) | Apex (%) |
| AAV1 | OHCs | 15.5 ± 1.9 | 13.4 ± 1.83 | 3.2 ± 2.1 | 18.3 ± 6.5 | 15 ± 6.3 | 6.3 ± 1.1 |
| IHCs | 16.5 ± 2.64 | 13.6 ± 1.2 | 5.6 ± 2.1 | 14.9 ± 2.6 | 9.6 ± 4.1 | 2.6 ± 0.6 | |
| SCs | 2.5 ± 2 | 1.2 ± 1 | 5.1 ± 3.7 | 3 ± 1.5 | |||
| AAV2 | OHCs | 39 ± 5.4 | 33.3 ± 6.8 | 3.1 ± 1.5 | 39.5 ± 8.5 | 28 ± 4.5 | 4.1 ± 1.1 |
| IHCs | 19.3 ± 7.3 | 11.4 ± 2.1 | 4.6 ± 1.4 | 22.6 ± 4.8 | 15 ± 2.9 | 11.8 ± 2.1 | |
| SCs | 7.1 ± 3.6 | 2.4 ± 1.1 | 4.2 ± 2.6 | 3.5 ± 1.2 | |||
IHCs, inner hair cells; OHCs, outer hair cells; SCs, supporting cells.
Supporting cell subtypes were infected by AAVs with varying specificities. Pillar cells were infected by AAV1, 2, 8, and 6.2; the Deiters cell were infected by AAV1, 2, and rh.43; whereas the Claudius cells were infected by AAV1, 2, 5, 7, 8, 6.2, rh.8, rh.10, and rh.43. Furthermore, the cells in the limbus region were infected by AAV7, rh.8, and rh.10 (Figs. 1I and 2A, D). Finally, AAVrh.43 was demonstrated to infect the inner border cells and Hensen cells (Fig. 3F).
Infection efficiency and the level of GFP expression (visual observation of GFP intensity) by AAV inner ear injection were generally correlated with the site of the initial injection. With cochleostomy, AAV was injected into the scala media in the base turn (Fig. 1B). As a result the base turn had more cells with stronger GFP expression compared with other regions. We quantified the number of infected cells throughout different regions of cochlea and found similar trends with the highest number of GFP-positive cells in the base, and decreased GFP expression in the middle and apex, for all the AAVs that showed positive transduction in the inner ear (data summarized in Tables 1 and 2).
Table 2.
Transduction efficiency of AAV serotypes 5, 6.2, 7, 8, 9, rh.10, and rh.43 in neonatal mice 3 months postinfection
| Serotype | Cell type | Base (%) | Middle (%) | Apex (%) |
|---|---|---|---|---|
| AAV5 | OHCs | |||
| IHCs | 28.1 ± 3.4 | 11.2 ± 2.9 | ||
| SCs | 2.2 ± 1 | |||
| AAV6.2 | OHCs | |||
| IHCs | ||||
| SCs | 11.4 ± 2 | |||
| AAV7 | OHCs | |||
| IHCs | 20.5 ± 2.5 | 16.2 ± 2.6 | 3.1 ± 0.8 | |
| SCs | 3.6 ± 0.8 | 2.6 ± 1.1 | ||
| AAV8 | OHCs | 15 ± 3.1 | 14.2 ± 2.1 | 4.2 ± 0.9 |
| IHCs | 21 ± 2.5 | 18.7 ± 1.7 | 6.1 ± 1.3 | |
| SCs | 6.8 ± 1.5 | 4.1 ± 2 | ||
| AAV9 | OHCs | |||
| IHCs | 21 ± 3.1 | 16.2 ± 2.3 | 4.2 ± 0.9 | |
| SCs | 6.1 ± 1.5 | 3.2 ± 11 | ||
| AAVrh.10 | OHCs | |||
| IHCs | 34 ± 5.7 | 24 ± 4.7 | 8.1 ± 2 | |
| SCs | 5.2 ± 1.5 | 3.2 ± 0.8 | ||
| AAVrh.43 | OHCs | |||
| IHCs | 5.3 ± 2.1 | 3 ± 1.1 | ||
| SCs | 12.1 ± 3.9 | 8.9 ± 2.8 | 4.5 ± 1.7 |
Among the AAVs tested, AAV8 and rh.43 also infected the lateral walls. For AAV8, there was a discrete set of cells infected prominently (Fig. 2F), whereas weak GFP expression was detected in many cells that were likely to be the fibrocytes infected by AAVrh.43 (Fig. 2G). The identity of the GFP-positive cells is unknown. The number and the type of cochlear cells infected two weeks or three months after AAV transduction is summarized (Tables 1 and 2).
AAV infection in young ears does not impair hearing
How AAV infection affects normal hearing will determine its utility for future inner ear studies and potential therapies. We performed ABR to measure hearing one month after infection in neonatal cochlea and compared the findings with the uninfected control inner ears. Two weeks and 3 months after injection in neonatal mice, the structure of the inner ear was found to remain intact. We performed the ABR study in mice injected with six AAV serotypes and demonstrated efficient infection. Despite infection specificities associated with each AAV, the ABR tests were comparable between the injected and uninjected control inner ears, demonstrating preservation of hearing. With the exception of AAV1 and AAV9, where at the high frequency of 32 kHz the differences in ABR were 10 dB, the hearing tests in the inner ears transduced by different serotype AAVs showed preservation of hearing with ABR differences less than 5 dB across all frequencies (Fig. 4). Thus, neonatal injection of the AAV subtypes into the inner ear does not impair normal hearing significantly.
Figure 4.
Normal hearing was not affected by AAV transduction. Auditory brainstem response (ABR) tests showed relatively normal threshold profiles in the injected inner ears compared with the uninjected control inner ears, one month postinfection in neonatal mice. For most of AAV vectors tested, slight elevations of ABR threshold of less than 5 dB were observed across all frequencies, which were not significantly different from the control ears. Difference of 10 dB was observed only at 32 kHz for AAV1 and 22.63 and 32 kHz for AAV9. Five mice were tested for each AAV serotype. N = 3 in each group.
AAV transduction in adult mice
AAV transduction specificity and the efficiency may change as mice age. It is known that the inner ear cell types continue to mature in postnatal mice with the onset of hearing around P12.23 To evaluate how AAVs infect adult mouse inner ear, we performed cochleostomy in 6-week-old mice that have established normal hearing profiles with the inner ear cells terminally differentiated. The inner ears were analyzed three months after injection. In adult, the procedure of cochleostomy invariably damages most OHCs with the exception of the apex cells, leading to profound hearing loss, which is consistent with previous studies.9 We therefore focused on transfection of AAV on other inner ear cell types.
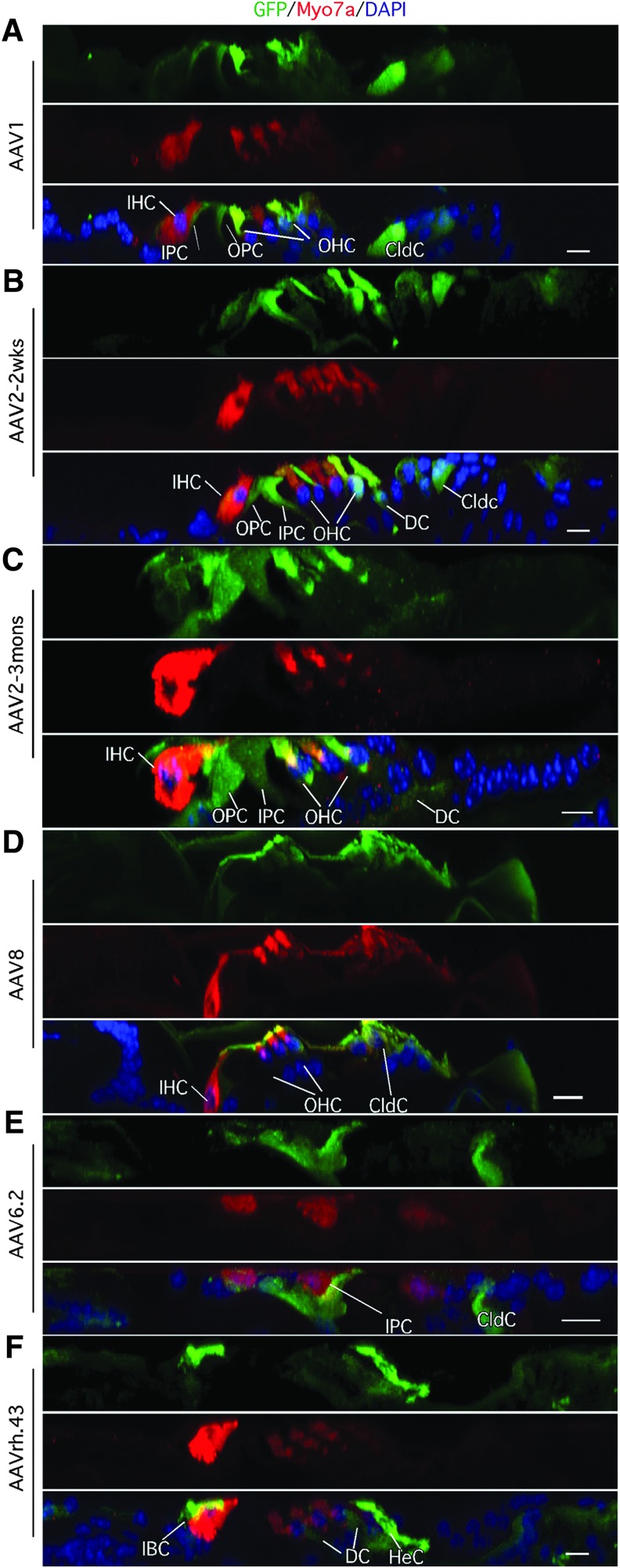
AAV1, 2, 6.2, 7, 8, 9, rh.39, and rh.43 demonstrated transduction in the adult mouse inner ear infecting IHCs with different efficiency (Figs. 5 and 6). Overall, AAV1, 2, 6.2, and 8 showed relatively strong GFP signals, whereas AAV7, 9, rh.39, and rh.43 exhibited moderate GFP signals. AAV1, 2, 8, and rh.43 efficiently transduced supporting cells with different specificities. AAV1 and 8 efficiently infected most supporting cell subtypes, including the pillar cells, Deiters cells, Claudius cells, and inner phalangeal cells (Fig. 5A, E), whereas AAV2, 6.2, and rh.43 infected these supporting cell subtypes with moderate efficiency (Figs. 5B, C and 6C). AAV7 did not infect supporting cells, making it a useful vector to specifically target adult IHCs (Fig. 5D). No infection by any vectors was observed for the cells in the limbus region, in contrast to neonatal infection, where AAV7, rh.8, and rh.10 had efficient transduction.
Figure 5.
AAV infection in adult inner ear three months after injection. (A) In a cross section of confocal image of mid-turn adult cochlea transduced by AAV1, prominent GFP signals were detected in IHCs, IPC, DC, and Cldc. OHCs died after cochleostomy. (B) A surface-mount confocal image of adult cochlea after AAV2 infection. Distinct GFP signals were detected in the IHCs, IPC, and Cldc. (C) In the AAV6.2-infected adult cochlea, GFP was mainly detected in the IHCs, and in the cells underneath the basilar membrane. Little expression was detected in any other cochlear cell types. (D) Moderate GFP signal was detected only in the IHCs after AAV7 infection. (E) A surface view of confocal image of adult cochlea mid-turn transduced by AAV8 showed GFP signals mainly in the IHCs, DC, and Cldc. (F) Image of cross section of adult cochlea three months after AAV8 infection showed high level of GFP in all cochlear cell types in the base turn, whereas in the apex prominent expression was detected in IHCs and Cldc and moderately in DC. Scale bars: 10 μm.
Figure 6.
Adult cochlea transduced by AAV. (A) AAV9-transduced adult IHCs and Cldc with moderate GFP expression three months postinfection. (B) AAVrh.39-transduced IHCs showed weak GFP expression. (C) In adult cochlea transduced with AAVrh.43, strong GFP was detected in the Cldc, whereas moderate GFP was seen in the IHCs and weak GFP in the DCs. (D) In control adult inner ear, no GFP was detected. Scale bars: 10 μm.
We found that the cell types infected and the intensity of GFP expression were correlated with the site of injection. The surgical procedure targeted primarily the base turn. As a result the cochlear cells near the base turn were preferentially infected with a higher GFP expression level. For instance, comparing the apex and the base turns of the same ear infected with AAV8, virtually all cochlear cells were strongly labeled with GFP in the base turn, including all supporting cells, some cells in the limbus, and the cells in the spiral prominence, whereas in the apex the infected cells included most supporting cells with significantly reduced GFP expression, and no GFP expression was detected in the spiral prominence (Fig. 5F, base vs. apex). Thus, in adults, local concentration is likely important in determining the level of transgene expression and the type of cells infected, with the cells in the base turn most efficiently transduced.
In adults, except for OHC loss, most cochlear cell types infected survived three months after injection, indicating a method that could be used to study these cochlear cell types. However, without OHCs, hearing is lost. Thus, to study hearing, alternative delivery strategies will be needed. The number of adult cochlear cell types infected with some AAV is summarized (Table 3). Comparison among all AAVs tested in neonatal and adult mice identified three AAVs that most efficiently infected OHCs, IHCs, and SCs, respectively (Table 4).
Table 3.
Transduction efficiency of AAV vectors in adult mice 3 months postinfection
| Serotype | Cell type | Base (%) | Middle (%) | Apex (%) |
|---|---|---|---|---|
| AAV2 | IHCs | 35.2 ± 6.3 | 27.2 ± 4.5 | 13.2 ± 2.1 |
| SCs | 13.6 ± 4.5 | 6 ± 1.4 | 1.6 ± 0.7 | |
| AAV8 | IHCs | 51.2 ± 7.5 | 22.2 ± 3.9 | 18.5 ± 1.8 |
| SCs | 10.1 ± 3.9 | 5.8 ± 2.1 | 3.1 ± 0.9 | |
| AAV1 | IHCs | 45.8 ± 7.3 | 24.1 ± 6.2 | 12.2 ± 2.3 |
| SCs | 8.2 ± 2.8 | 4.1 ± 1.4 | 2 ± 1.2 | |
| AAV6.2 | IHCs | 28 ± 4.8 | 18 ± 2.1 | 10.5 ± 1.5 |
| SCs | 3.5 ± 1.3 | 1.9 ± 1.1 | ||
| AAV9 | IHCs | 61.6 ± 8 | 35.1 ± 3.2 | 9.1 ± 1.4 |
| SCs | 6.2 ± 3.1 | 4.1 ± 2.1 | 2.1 ± 0.9 | |
| AAVrh.39 | IHCs | 20 ± 4.7 | 15 ± 2.3 | 5.6 ± 1.6 |
| SCs | 7 ± 2.9 | 3.1 ± 1 | 1.2 ± 1.1 |
Table 4.
Summary of three AAV serotypes with highest transduction in inner ear cell types
| Cell type | Neonatal mice | Adult mice |
|---|---|---|
| IHCs | AAV2, 5, rh.10 | AAV1, 8, 9 |
| OHCs | AAV1, 2, 8 | |
| SCs | AAV2, 6.2, rh.43 | AAV1, 2, 8 |
Discussion
It is increasingly recognized that AAV is an important vehicle for inner ear gene delivery, making it feasible to study gene function, and ultimately may represent a treatment for hearing loss. Previous studies have evaluated AAV vectors, including AAV1, 2/1, 2, 5, 6, and 8, in the inner ear.9,24–26 The present study evaluated additional AAV serotypes for their infectability in young and adult mouse inner ears. The study identified AAV viral vectors that can be used for targeted delivery into specific inner ear cell subtypes in neonatal and adult animals, with long-term expression patterns.
Gene mutations in diverse cell types of the inner ear have been found to underlie different forms of genetic hearing loss.13,14 Identification of AAV vectors with specificities for these cell types will allow for the specific evaluation of gene functions associated with these cell types and provide a mode of delivery of normal copies of genes as a potential therapy to treat genetic hearing loss. Among the 12 AAV serotypes studied, 10 infected neonatal inner ears and 8 infected adult inner ears. The sensory epithelial cell region that harbors hair cells and supporting cells has higher affinities for AAV infection, in both young and adult inner ears.
Because of the intricate structure and small size of inner ear, delivery of AAV presents a challenge especially in adult mice. Through the surgical procedure of cochleostomy, AAV is injected directly into the space of the scala media. The apical surface of the cochlea is bathed in the endolymph of scala media, which may facilitate the entrance of AAV into hair cells and supporting cells. In neonatal mice, the cochlear tissue is soft and it can be sealed automatically after glass-micropipette-mediated injection, with little endolymph leakage. Further, most of the cells are still in development, which makes them more resistant to damage induced during injection. Relatively normal hearing after AAV injection indicates (Fig. 4) that the AAV itself is likely benign to the survival and function of cells infected. The expression patterns of the transgene are maintained three months after injection in neonatal and adult mice. Thus, AAV is suitable for long-term expression of delivered genes.
There are similarities between the patterns of infection by AAV in both neonatal and adult inner ears. AAV1, 2, 6.2, 7, 8, 9, and rh.43 infect both, whereas AAVrh.8 and rh.10 mainly infect neonatal, and AAVrh.39 infects only the adult inner ear. In the adult inner ear, most OHCs near the injection site die, because of the trauma of the surgical cochleostomy procedure itself. The intensity of GFP expression in the adult inner ear is generally lower, which may reflect a reduced efficiency of AAV to infect terminally differentiated mature cochlear cells, or may be because of the rigid inner ear structure that prevents the diffusion of AAV to effectively infect other cells. This is illustrated by our data that, in the vicinity of the injection site, cells had stronger GFP expression compared with other cells at more distant areas. The procedure of cochleostomy severely damages OHCs and causes profound hearing loss especially at high frequencies.9 It is therefore important to identify a new route for AAV delivery into the adult inner ear, which allows the survival of OHCs and preserves hearing. Round window membrane injection has been experimented in adult mice with limited success. However, there is a reduced infection efficiency, and hearing in the injected inner ear is still affected adversely. One recent development is injection through the posterior canal, which may promote OHC survival and improve efficiency in diverse cochlear cell types. In adult studies, GFP expression appears to be maintained over time, similar to neonatal studies, an indication that AAV is suitable for long-term inner ear expression.
AAV vectors have been successfully used to design effective therapies for a variety of diseases, including blindness, and offer promise for other disorders.27,28 AAV1 and 2 have been used successfully to rescue hearing in a transgenic genetic hearing loss model,29–31 which open the door for future intervention in human patients. For AAV-based gene therapy to be applied successfully to treat patients with genetic hearing loss, however, a few hurdles still remain. Since the human inner ear develops during early embryonic stages, it is unknown whether the treatment at later stages of development would be effective for genetic mutations that cause defects in early development, such as GJB2 mutations. In progressive hearing loss, many of the mutations found are dominant. For those patients, suppression of dominant mutations by siRNA or antisense oligonucleotide or abolishing mutations by CRISPR/Cas9-mediated genome editing may be an effective approach.32–35 Further complicating the use of AAV for therapy development, the vectors used can accommodate only an insert size of less than ∼4 kb, which may constrain its use as many deafness genes are very large.
In this study we have provided a broader understanding of the cell types targeted by different AAVs. This represents an important step toward developing effective gene therapy strategies to treat different types of hearing loss. Further studies are needed to demonstrate the effectiveness of the AAV infection system for delivery in the inner ear of larger animal models and nonhuman primates. Establishing that AAV-mediated gene therapy can effectively restore hearing in adult animal models is the next step toward its application as a potential therapy in human adult hearing loss.
Acknowledgments
Z.-Y.C. was supported by U.S. National Institutes of Health (R01 DC006908). Y.S., Y.T., and Y.T. were supported by the Frederick and Ines Yeatts Hair Cell Regeneration Grant and Y.S. by the National Nature Science Foundation of China (NSFC81300824) and Science and Technology Commission of Shanghai Municipality (15pj1401000).
Author Disclosure
G.G. is a founder of Voyager Therapeutics and holds equity in the company. G.G. is an inventor on patents with potential royalties licensed to Voyager Therapeutics and other biopharmaceutical companies.
References
- 1.Morton CC, Nance WE. Newborn hearing screening—a silent revolution. N Engl J Med 2006;354:2151–2164 [DOI] [PubMed] [Google Scholar]
- 2.Hereditary Hearing Loss Homepage. Introduction. http://hereditaryhearingloss.org/
- 3.Lalwani AK, Walsh BJ, Reilly PG, et al. Development of in vivo gene therapy for hearing disorders: Introduction of adeno-associated virus into the cochlea of the guinea pig. Gene Ther 1996;3:588–592 [PubMed] [Google Scholar]
- 4.Liu Y, Okada T, Sheykholeslami K, et al. Specific and efficient transduction of cochlear inner hair cells with recombinant adeno-associated virus type 3 vector. Mol Ther 2005;12:725–733 [DOI] [PubMed] [Google Scholar]
- 5.Bedrosian JC, Gratton MA, Brigande JV, et al. In vivo delivery of recombinant viruses to the fetal murine cochlea: Transduction characteristics and long-term effects on auditory function. Mol Ther 2006;14:328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iizuka T, Kanzaki S, Mochizuki H, et al. Noninvasive in vivo delivery of transgene via adeno-associated virus into supporting cells of the neonatal mouse cochlea. Hum Gene Ther 2008;19:384–390 [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Okada T, Nomoto T, et al. Promoter effects of adeno-associated viral vector for transgene expression in the cochlea in vivo. Exp Mol Med 2007;39:170–175 [DOI] [PubMed] [Google Scholar]
- 8.Shibata SB, Di Pasquale G, Cortez SR, et al. Gene transfer using bovine adeno-associated virus in the guinea pig cochlea. Gene Ther 2009;16:990–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilpatrick LA, Li Q, Yang J, et al. Adeno-associated virus-mediated gene delivery into the scala media of the normal and deafened adult mouse ear. Gene Ther 2011;18:569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedele AO. Sanfilippo syndrome: Causes, consequences, and treatments. Appl Clin Genet 2015;8:269–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward P, Walsh CE. Current and future prospects for hemophilia gene therapy. Expert Rev Hematol 2016;9:649–659 [DOI] [PubMed] [Google Scholar]
- 12.Sahel J-A, Marazova K, Audo I. Clinical characteristics and current therapies for inherited retinal degenerations. Cold Spring Harb Perspect Med 2015;5:a017111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morton CC. Genetics, genomics and gene discovery in the auditory system. Hum Mol Genet 2002;11:1229–1240 [DOI] [PubMed] [Google Scholar]
- 14.Müller U, Barr-Gillespie PG. New treatment options for hearing loss. Nat Rev Drug Discov 2015;14:346–365 [DOI] [PubMed] [Google Scholar]
- 15.Géléoc GSG, Holt JR. Sound strategies for hearing restoration. Science 2014;344:1241062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weil D, Blanchard S, Kaplan J, et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature 1995;374:60–61 [DOI] [PubMed] [Google Scholar]
- 17.Kelsell DP, Dunlop J, Stevens HP, et al. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 1997;387:80–83 [DOI] [PubMed] [Google Scholar]
- 18.Chen ZY, Hendriks RW, Jobling MA, et al. Isolation and characterization of a candidate gene for Norrie disease. Nat Genet 1992;1:204–298 [DOI] [PubMed] [Google Scholar]
- 19.Laine H, Sulg M, Kirjavainen A, Pirvola U. Cell cycle regulation in the inner ear sensory epithelia: Role of cyclin D1 and cyclin-dependent kinase inhibitors. Dev Biol 2010;337:134–146 [DOI] [PubMed] [Google Scholar]
- 20.Yang B, Li S, Wang H, et al. Global CNS transduction of adult mice by intravenously delivered rAAVrh.8 and rAAVrh.10 and nonhuman primates by rAAVrh.10. Mol Ther 2014;22:1299–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao G, Alvira MR, Somanathan S, et al. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci U S A 2003;100:6081–6086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang M, Kantardzhieva A, Scheffer D, et al. Hair cell overexpression of islet1 reduces age-related and noise-induced hearing loss. J Neurosci 2013;33:15086–15094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus). J Am Audiol Soc 1976;1:179–184 [PubMed] [Google Scholar]
- 24.Praetorius M, Brough DE, Hsu C, et al. Adenoviral vectors for improved gene delivery to the inner ear. Hear Res 2009;248:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Q, Wang Y, Chang Q, et al. Virally expressed connexin26 restores gap junction function in the cochlea of conditional Gjb2 knockout mice. Gene Ther 2014;21:71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chien WW, Isgrig K, Roy S, et al. Gene therapy restores hair cell stereocilia morphology in inner ears of deaf whirler mice. Mol Ther 2016;24:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrs-Silva H, Linden R. Advances in gene therapy technologies to treat retinitis pigmentosa. Clin Ophthalmol 2014;8:127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierce EA, Bennett J. The status of RPE65 gene therapy trials: Safety and efficacy. Cold Spring Harb Perspect Med 2015;5:a017285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akil O, Seal RP, Burke K, et al. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 2012;75:283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang Q, Wang J, Li Q, et al. Virally mediated Kcnq1 gene replacement therapy in the immature scala media restores hearing in a mouse model of human Jervell and Lange-Nielsen deafness syndrome. EMBO Mol Med 2015;7:1077–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Askew C, Rochat C, Pan B, et al. Tmc gene therapy restores auditory function in deaf mice. Sci Transl Med 2015;7:295ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maeda Y, Fukushima K, Nishizaki K, Smith RJH. In vitro and in vivo suppression of GJB2 expression by RNA interference. Hum Mol Genet 2005;14:1641–1650 [DOI] [PubMed] [Google Scholar]
- 33.Lentz JJ, Jodelka FM, Hinrich AJ, et al. Rescue of hearing and vestibular function by antisense oligonucleotides in a mouse model of human deafness. Nat Med 2013;19:345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuris JA, Thompson DB, Shu Y, et al. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat Biotechnol 2015;33:73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou B, Mittal R, Grati M, et al. The application of genome editing in studying hearing loss. Hear Res 2015;327:102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]