Abstract
Fetal alcohol spectrum disorders comprise the spectrum of cognitive, behavioral, and neurological impairments caused by prenatal alcohol exposure (PAE). Diffusion tensor imaging (DTI) was performed on 54 children (age 10.1 ± 1.0 years) from the Cape Town Longitudinal Cohort, for whom detailed drinking histories obtained during pregnancy are available: 26 with full fetal alcohol syndrome (FAS) or partial FAS (PFAS), 15 nonsyndromal heavily exposed (HE), and 13 controls. Using voxelwise analyses, children with FAS/PFAS showed significantly lower fractional anisotropy (FA) in four white matter (WM) regions and higher mean diffusivity (MD) in seven; three regions of FA and MD differences (left inferior longitudinal fasciculus (ILF), splenium, and isthmus) overlapped, and the fourth FA cluster was located in the same WM bundle (right ILF) as an MD cluster. HE children showed lower FA and higher MD in a subset of these regions. Significant correlations were observed between three continuous alcohol measures and DTI values at cluster peaks, indicating that WM damage in several regions is dose dependent. Lower FA in the regions of interest was attributable primarily to increased radial diffusivity rather than decreased axonal diffusivity, suggesting poorer axon packing density and/or myelination. Multiple regression models indicated that this cortical WM impairment partially mediated adverse effects of PAE on information processing speed and eyeblink conditioning. Hum Brain Mapp 37:2943–2958, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: prenatal alcohol exposure, fetal alcohol spectrum disorders, diffusion tensor imaging, IQ, eyeblink conditioning
INTRODUCTION
Prenatal alcohol exposure (PAE) has numerous adverse consequences on fetal development, which are encompassed under an umbrella term, fetal alcohol spectrum disorders (FASD). These include life‐long cognitive and behavioral impairments, as well as structural damage in the brain [Burden et al., 2005; Kalberg et al., 2006; Mukherjee et al., 2006; Spadoni et al., 2007; Streissguth, 2007; Vaurio et al., 2010]. The Centers for Disease Control and Prevention [2012] reported that, in a sample of 13,880 pregnant women in the US between 2006 and 2010, 7.6% admitted to consuming alcohol during the prior 30 days and 1.4% to binge drinking. While PAE does not always result in FASD, there are no medical guidelines for safe levels of alcohol consumption during pregnancy [Elliott and Bower, 2008; Jacobson and Jacobson, 2013; U.S. Department of Health and Human Services, 2005]. The prevalence of FASD in the US is estimated to be as high as 2–5% of the total population [May et al., 2009] and has been identified in all racial and ethnic groups [Abel, 1995]. In the Cape Coloured community in the Western Cape Province of South Africa, incidence rates 18 to 141 times higher than in the US have been reported [May et al., 2000]. The Cape Coloured population is a mixed ancestry group deriving from Africa, Europe, and Malaysia. Heavy maternal drinking is very prevalent in this community [Croxford and Viljoen, 1999] due, in part, to both poor psychosocial circumstances and the traditional dop system by which farm laborers were paid in part with wine, making this population ideally suited to studies of the effects of PAE.
Fetal alcohol syndrome (FAS) is the most severe of the FASD [Hoyme et al., 2005; Stratton et al., 1996]. It is characterized by central nervous system dysfunction, pre‐ and/or post‐natal growth deficiencies, and the presence of certain craniofacial features (specifically, short palpebral fissures, an indistinct philtrum, and a thin vermillion). Partial FAS (PFAS) is used to describe PAE in which at least two of the three key alcohol‐related facial anomalies and at least one of the following are present: small head circumference, growth retardation, or neurobehavioral impairment. Exposed individuals who do not meet these criteria but exhibit neurobehavioral deficits are clinically diagnosed as having alcohol‐related neurodevelopmental disorder.
Brain structure abnormalities have been reported in autopsies of children with FASD, including agenesis of the corpus callosum (CC), microcephaly, ventriculomegaly, a small cerebellum, and a variety of other malformations caused by neuronal and glial migration errors [Clarren, 1981; Clarren et al., 1978; Coulter et al., 1993; Jones and Smith, 1973; Kinney et al., 1980; Peiffer et al., 1979; Wisniewski et al., 1983]. Fifty‐two structural MRI studies have been published on individuals with PAE, typically using a T1‐weighted (T1w) protocol, including 22 studies on total brain volume [e.g., Chen et al., 2012; De Guio et al., 2014; Meintes et al., 2014; Rajaprakash et al., 2014], 5 studies on cerebral volume [Archibald et al., 2001; Mattson et al., 1992, 1994, 1996; de Zeeuw et al., 2012], and 11 studies on cerebellar volume [e.g., Astley et al., 2009; Spottiswoode et al., 2011; de Zeeuw et al., 2012]. Consistent with the autopsy studies, MRI has shown decreased brain volumes in FASD subjects compared to healthy controls.
A growing body of evidence suggests that white matter (WM) tissue is a specific target of alcohol teratogenesis. Reductions in the total WM volume have been found in individuals with FASD [Archibald et al., 2001; Bjorkquist et al., 2010; De Guio et al., 2014; Lebel et al., 2008; Nardelli et al., 2011; Roussotte et al., 2012; Sowell et al., 2001a; Treit et al., 2013; Yang et al., 2012; de Zeeuw et al., 2012]. Smaller WM volumes have also been reported in the frontal lobe [Astley et al., 2009; Sowell et al., 2002], parietal lobe [Archibald et al., 2001; Sowell et al., 2002], temporal lobe [Sowell et al., 2002], and cerebellum [Spottiswoode et al., 2011], though many of these differences were not significant after correcting for whole‐brain volume. Further investigation of regional WM alterations in individuals with FASD is necessary to better understand the effects of PAE on WM integrity and the complex relations between behavior, cognition, and brain structure.
Diffusion tensor imaging (DTI) has been shown to provide useful quantitative information regarding brain anatomy and particularly WM fiber pathways [Basser et al., 1994]. This MRI‐based method measures water diffusion in brain tissue, which tends to be highly anisotropic (i.e., varying with orientation) in WM and strongly isotropic (i.e., homogenous in all orientations) in gray matter and cerebrospinal fluid. The diffusion tensor can be represented geometrically as an ellipsoid surface with semi‐axes whose lengths are described by the eigenvalues, L1, L2, and L3; L1 is often called parallel or axial diffusivity (AD), while the average of L2 and L3 is termed perpendicular or radial diffusivity (RD). The average of the three eigenvalues is the mean diffusivity (MD), which quantifies the average random motion within a voxel, and their normalized standard deviation is the fractional anisotropy (FA), which represents the degree to which water diffuses preferentially parallel to the axonal axis rather than perpendicular to it.
To date, 14 DTI studies have been performed on individuals with FASD, often focusing on the CC as a region of interest (ROI), since it is relatively easy to delineate and crucial for interhemispheric communication. These studies have employed a variety of techniques, including manual tracing [Ma et al., 2005; Wozniak et al., 2006, 2009], tractography [Colby et al., 2012; Green et al., 2013; Lebel et al., 2008; Taylor et al., 2015; Treit et al., 2013], tract‐based spatial statistics (TBSS) [Fryer et al., 2009; Li et al., 2009], and voxelwise analysis [Fan et al., 2015; Lebel et al., 2010; Sowell et al., 2008; Spottiswoode et al., 2011]. Microstructural abnormalities of the CC have been reported in FASD in the splenium [Lebel et al., 2008; Ma et al., 2005, Sowell et al., 2008; Wozniak et al., 2009], isthmus [Li et al., 2009; Wozniak et al., 2006, 2009], genu [Lebel et al., 2008; Ma et al., 2005; Treit et al., 2013] and body of the CC [Fryer et al., 2009; Wozniak et al., 2009]. WM deficits have also been reported in the inferior [Colby et al., 2012; Lebel et al., 2008; Sowell et al., 2008] and superior longitudinal fasciculi [Fryer et al., 2009; Lebel et al., 2008; Treit et al., 2013].
Eyeblink conditioning is a Pavlovian learning paradigm that involves contingent temporal pairing of a conditioned stimulus (tone) with an unconditioned stimulus (air puff to the eye that elicits a reflexive blink). Typically developing children learn to blink in anticipation of the air puff after hearing the tone. In our Cape Town longitudinal FASD cohort, at 5 years, we found that not a single child with full FAS met criterion for conditioning, compared with about one‐third of the PFAS and HE groups and 75% of controls [Jacobson et al., 2008]. We found a similar pattern of eyeblink conditioning deficits in a separate group of Cape Town school‐age children with FASD [Jacobson et al., 2011]; poorer delay conditioning was also reported in a U.S. school‐age sample of alcohol exposed children [Coffin et al., 2005]. The cerebellar peduncles are large bundles of myelinated nerve fibers that connect the cerebellum to the brainstem and constitute the principal WM element of the EBC circuit. Using path analytic models, DTI studies of the two Cape Town samples found that the effect of PAE on EBC was partially mediated by lower FA and higher MD in the left middle cerebellar peduncle [Fan et al., 2015; Spottiswoode et al., 2011] and lower FA in the superior peduncles [Fan et al., 2015]. We found increased RD in these regions, suggesting that these effects are due to poorer myelination and/or axon packing density. Thus these WM deficits may account, in part, for the heightened risk for impairment in EBC performance in children with PAE.
Our finding that the adverse effects of PAE on EBC are partially attributable to microstructural deficits in the cerebellar peduncles led us to explore the degree to which WM deficits in cerebral cortex might mediate effects on IQ and other aspects of cognitive function. Whole‐brain DTI was acquired from each study participant. One strength of this study is that, because the participating mothers and children are part of a longitudinal cohort recruited during pregnancy, detailed prospectively obtained information is available about maternal drinking during pregnancy [Jacobson et al., 2008]. This study thus provided a unique opportunity to study cerebral WM abnormalities in relation to degree of alcohol exposure in a well‐characterized cohort of children. We hypothesized that (1) PAE will adversely impact cortical WM and that effects will vary with clinical diagnosis, (2) the extent of WM alterations (in terms of volume and numbers of regions) will be related to the degree of alcohol exposure (i.e., the amount or frequency of drinking), (3) PAE‐related deficits in cortical WM integrity will be related to poorer child performance on IQ, learning and memory, and EBC, and (4) these effects will be mediated primarily by increased RD, suggesting poorer myelination.
MATERIALS AND METHODS
Participants
Pregnant women were recruited into the Cape Town Longitudinal Cohort from an antenatal clinic of a midwife obstetrical unit in a predominantly Cape Coloured neighborhood in the Western Cape, South Africa [Jacobson et al., 2008]. The Cape Coloured community is comprised primarily of descendants of white European settlers, Malaysian slaves, Khoi‐San aboriginals, and black African ancestors. The incidence of FASD in this population is exceptionally high due to poor socioeconomic circumstances and historical practices of compensating farm laborers with wine, which have contributed to a tradition of heavy recreational weekend binge drinking [May et al., 2007]. Exclusionary criteria included: age <18 years, diabetes, epilepsy, or cardiac problems requiring treatment.
Timeline follow‐back interviews were conducted to determine incidence and amount of alcohol consumed on a day‐by‐day basis during a typical 2‐week period, both at time of conception and time of recruitment [Jacobson et al., 2002]. The timeline follow‐back interview was repeated in mid‐pregnancy and again at 1 month postpartum to obtain information on drinking during the third trimester. Drinking was quantified in ounces (oz) of absolute alcohol (AA; 1 oz AA ≈ 2 standard drinks), and maternal consumption was recorded using three measures: oz AA consumed per day across pregnancy (AA/day), oz AA consumed per occasion (AA/occasion), and number of drinking days per week. Two groups of women were invited to participate in the study: (1) any woman reporting a minimum of 14 drinks per week (1.0 oz AA/day) or at least two incidents of binge drinking (≥5 drinks per occasion) during the first trimester of pregnancy and (2) controls who abstained from drinking or drank no more than minimally during pregnancy. Smoking during pregnancy was reported in terms of cigarettes smoked per day; drug use, days/month. The mother's age at delivery and years of education were also recorded.
Procedures
DTI and behavioral data were obtained from 54 right‐handed children from the cohort. Each child was examined for growth and FAS dysmorphology by two dysmorphologists (HE Hoyme, MD, LK Robinson, MD) using the Revised Institute of Medicine criteria [Hoyme et al., 2005] at an FAS diagnostic clinic held in 2005; one child, who could not attend the clinic was later examined at our University of Cape Town (UCT) laboratory by a third dysmorphologist (N Khaole, MD) using the same criteria [see Jacobson et al., 2008]. There was substantial interexaminer agreement on the assessment of the principal fetal alcohol‐related dysmorphic features among the three expert dysmorphologists. Following a case conference, each of the alcohol‐exposed children was assigned to one of the three diagnostic groups: FAS, PFAS, or nonsyndromal heavily exposed (HE). It should be noted that the children in the HE group did not meet diagnostic criteria for syndromal FAS or PFAS but were born to mothers recruited prospectively based on their heavy alcohol consumption during pregnancy. The sample for this study comprised 7 children with FAS, 19 with PFAS, 15 HE, and 13 controls.
For behavioral assessments, both mother and child were transported to our UCT Child Development Research Laboratory. The Wechsler Intelligence Scale for Children‐IV (WISC‐IV), which generates full scale IQ and four indices (verbal comprehension, perceptual reasoning, working memory, and processing speed), was administered at mean age 9.4 ± 0.5 years. The WISC‐IV scores obtained for this sample were strongly correlated (r = 0.76, p < 0.001) with IQ scores obtained at 5 years on the Junior South African Individual Scale [Madge et al., 1981], which is normed for South African children. The children were also administered the California Verbal Learning Test‐Children's Version (CVLT‐C) [Lewis et al., 2015] and delay EBC, measured as at least 40% conditioned responses within 3 EBC sessions (50 trials each; Jacobson et al., 2008]. Postnatal lead exposure was obtained from a venous blood sample at mean age 5.1 ± 0.2 years because lead levels in this population are within the range in which effects on cognitive function have been documented [Chiodo et al., 2004; Lanphear et al., 2000].
Approval for human research was obtained from the Wayne State University and UCT ethics committees. Informed consent was obtained from mothers at recruitment and at the child assessment visits; assent was obtained from the child. Children received a small gift, and mothers received a photo of their child and compensation consistent with guidelines from the UCT ethics committee.
Neuroimaging Protocol
Each child was transported to the Cape Universities Brain Imaging Centre (CUBIC) together with his/her mother at mean age 10.1 ± 1.0 years. After familiarization with the scanning procedures on a mock scanner, data were acquired using two DTI acquisitions with alternating phase encoding directions (i.e., anterior–posterior and posterior–anterior (AP‐PA)) on a 3 T Allegra MRI (Siemens, Erlangen, Germany). For each acquisition, the following parameters were used: 4 reference images with b = 0 s/mm2 and 30 diffusion‐weighted images (DWIs) with b = 1000 s/mm2; 72 slices; field of view (FOV) = 230 × 230 × 130 mm3; slice thickness 1.8 mm; 1.8 × 1.8 mm2 in‐plane resolution; TR 10,000 ms; TE 88 ms. A 3D echo planar imaging (EPI) navigated [Tisdall et al., 2009] multiecho magnetization prepared rapid gradient echo (MEMPRAGE) [van der Kouwe et al., 2008] structural image (resolution 1.3 × 1.3 × 1.0 mm3, FOV = 256 × 256 × 167 mm3, 128 slices, TR 2530 ms, TI 1100ms, TEs 1.53/3.21/4.89/6.57 ms, flip angle 7°) was also acquired for each subject.
Preprocessing
Preprocessing included motion correction using FSL‐flirt [Smith et al., 2004] and susceptibility correction in Matlab applied to the AP‐PA acquisitions [Andersson et al., 2003]. The DTI data were initially inspected visually for the presence of dropout slices. Any subjects with dropout slices in any of their DTI acquisitions were excluded from all further analyses. After exclusions, for each volume we computed the resultant displacement relative to the first unweighted (B0) volume using the three translation parameters from mcflirt in FSL. The resultant displacements for all volumes were below 2.0 mm for all subjects included in the analyses, maximum resultant displacement did not differ between diagnostic groups. Rotations in any direction were <1.3° in all subjects. To compute z‐scores, we calculated the mean and standard deviation based only on values between the 25 and 75 percentile limits and generated z‐score maps for each acquisition; any data points more than 3 standard deviations (SDs) beyond the mean of the z‐score map were discarded. The diffusion tensors (DTs) were estimated, and the relevant maps of DTI scalar parameters (FA, MD, etc.) were generated.
A mean standard space and WM mask for this study were created as follows: for each subject, the B0 volumes were co‐registered to his/her own T1w structural image using nonlinear algorithms in FSL. T1w images of controls were coregistered to a single control image and then averaged to create a mean T1w image. Each subject's T1w and DTI parameter images were transformed to this mean T1w space. As a final step, coregistered FA maps of all subjects were averaged, after which individual DTI parameter maps were coregistered to the mean FA image. The cerebrum was extracted using an MNI 1 × 1 × 1 mm3 template and thresholded at an average FA > 0.2 to ensure that only WM was included in the analysis [Mori and van Zijl, 2002]. For presentation and reporting of cluster locations, final clusters were mapped to a 1 mm3 MNI pediatric standard image [Fonov et al., 2011].
Statistical Analyses
Differences in DTI parameters were investigated between the control group and each of two FASD groups: (1) a combined FAS and PFAS group (FAS/PFAS) and (2) an HE group, composed of heavily exposed children who did not meet criteria for a clinical diagnosis of FAS or PFAS. In each group comparison voxelwise, two sample (unpaired) t‐tests were performed using permutation tests (with FSL‐randomise) to identify clusters showing significant differences between groups. To control for Type I error, Monte Carlo simulations [Forman et al., 1995] were performed using AFNI‐AlphaSim [Cox, 1996], which indicated that activation clusters of at least 168 mm3 in the mean template space were significant at p < 0.01.
To examine dose dependence in regions where diagnostic groups revealed WM differences, we extracted each subject's average FA (or MD), AD, and RD in a 2 × 2 × 2 mm3 region‐of‐interest (ROI) around the peak coordinates of each cluster. We computed group means and examined in each cluster the association of DTI measures with the three PAE parameters (AA/day, AA/occasion, and days/week). These statistical analyses were performed using SPSS (version 21). The AA/day values were log transformed to correct for skewness (using the natural log of one plus the value). Alcohol days/week, maternal smoking during pregnancy, and postnatal lead exposure each had a single outlier (>3 SD above the mean) that was recoded to 1 point higher than the next highest observed value [Winer et al., 1971]. Missing demographic data were estimated as the median for their diagnostic group (as noted in Table 1); in each case, correlations were also examined while excluding subjects with missing data, without any significant alteration of results.
Table 1.
Sample characteristics (N = 54)
| FAS/PFAS | HE | Ctl | F or χ 2 | |
|---|---|---|---|---|
| N | 26 | 15 | 13 | |
| Maternal characteristics | ||||
| Maternal age at delivery | 28.8 (7.5) | 25.2 (5.0) | 26.0 (3.3) | 2.04 |
| Education (years)a | 7.8 (2.2) | 9.5 (2.2) | 10.3 (1.4) | 7.46*** |
| Absolute alcohol consumed per day across pregnancy (oz)b | 1.3 (1.4) | 0.5 (0.5) | 0.0 (0.0) | 7.26** |
| Absolute alcohol consumed per occasion across pregnancy (oz)b | 4.1 (1.8) | 2.9 (1.4) | 0.1 (0.3) | 31.75*** |
| Drinking days per week across pregnancyb, c | 2.1 (1.3) | 1.1 (0.8) | 0.0 (0.0) | 17.75*** |
| Cigarettes smoked per day during pregnancyd | 8.2 (5.7) | 8.0 (7.2) | 4.2 (10.9) | 1.32 |
| Child characteristics | ||||
| Sex (% male) | 50 | 53 | 54 | 0.03 |
| Age at cognitive assessments (years) | 9.3 (0.3) | 9.6 (0.5) | 9.4 (0.4) | 2.04 |
| Age at DTI scan (years) | 10.4 (0.5) | 10.5 (0.3) | 10.4 (0.4) | 0.63 |
| Maximum resultant displacement during scan (mm) | 1.0 (0.5) | 1.1 (0.5) | 0.9 (0.5) | 0.39 |
| Lead exposurec, e | 11.9 (5.5) | 9.7 (3.7) | 8.1 (3.3) | 3.24* |
| WISC‐IV full‐scale IQ | 64.3 (9.7) | 73.1 (8.0) | 74.9 (9.0) | 7.70*** |
Values are mean (SD); *p < 0.05, **p < 0.01, ***p < 0.001.
FAS/PFAS = combined fetal alcohol syndrome (FAS) and partial FAS (PFAS) group; HE = nonsyndromal heavily exposed group; Ctl = control group; WISC‐IV = Wechsler Intelligence Scale for Children, 4th Edition.
Missing values estimated at group median for 2 children with FAS/PFAS.
Missing values estimated at group median for 2 children with FAS/PFAS, 1 HE.
One outlier in the FAS/PFAS group was winsorized (value >3 SD above the mean recoded to 1 point higher than highest observed nonoutlier) [Winer et al., 1971].
One outlier in the Ctl group was winsorized [Winer et al., 1971].
Missing values estimated at group median for 2 children with FAS/PFAS, 1 HE.
Seven control variables were considered as potential confounders: four child characteristics (sex, age at scan, postnatal lead exposure, and maximum resultant displacement during DTI), and three maternal characteristics (age at delivery, years of education, and mean number of cigarettes smoked/day during pregnancy). Model selection of potential confounders was determined as follows: each potential control variable that was even weakly related (at p < 0.10) to the average FA or MD in the cluster was included in the final model. Analysis of covariance was used to determine whether group differences persisted after controlling for potential confounders; linear regression, to examine whether associations with the alcohol exposure measures remained significant after control for confounders.
The mothers of three children (1 with FAS, 2 with PFAS) reported using marijuana during pregnancy, and the mother of one child with PFAS reported using cocaine. Because prenatal exposure to these drugs was too rare for statistical adjustment, associations with PAE were rerun omitting the children exposed to these drugs to see if the alcohol‐related effects persisted.
Pearson correlations were used to examine the relation of DTI measures in the ROIs related to PAE after control for confounders with behavioral measures from three domains known to be affected in FASD—WISC‐IV IQ (Full Scale and each of the four index scores), CVLT (short‐ and long‐delay free recall), and EBC. Given the extensive overlap in the ROIs in which group differences were seen in FA with those generated from the MD maps, these correlations were examined only for the MD values. The hypothesis that MD in each of these ROIs mediates the effect of PAE on these behavioral outcomes was tested using hierarchical multiple regression analysis. AA/day during pregnancy was entered in the first step of the regression; MD in the ROI was entered in the second step. Mediation was inferred if the addition of MD substantially reduced the magnitude of the regression coefficient for AA/day [Baron and Kenny, 1986]. The Difference in Coefficients Test [Clogg et al., 1992] was used to assess whether the reduction in the magnitude of the regression coefficient was statistically significant. The Clogg Test was selected because in a Monte Carlo study comparing 14 methods to test the statistical significance of mediation hypotheses, MacKinnon et al [2002] found that it was one of the two with the greatest power.
RESULTS
Sample Characteristics
The sample characteristics are summarized in Table 1. The children came from a socioeconomically disadvantaged population. As in previous studies [Jacobson et al., 2004; May and Gossage, 2011], mothers of more severely affected children were older than those of the other exposed children and had completed fewer years of education. As expected, there were significant differences in alcohol use across groups, with the highest average values in the FAS/PFAS group. Mothers of exposed children concentrated their drinking, resulting in consumption of an average of 5.8–8.2 standard drinks/occasion on 1–2 days/week. Twelve of the 13 mothers in the control group (92.3%) abstained from alcohol use during pregnancy, while one consumed 2 drinks on 3 occasions. Drug use other than alcohol and smoking during pregnancy was rare: three women (4.8%) reported using marijuana (1–3 days/month), one used cocaine, and none used methaqualone (“mandrax”). There were no significant between‐group differences in child sex, age at scan, maximum displacement during DTI, maternal age at delivery, or smoking during pregnancy. Lead exposure was significantly higher in the FAS/PFAS group compared to controls (p < 0.05). As expected, children with FAS/PFAS had lower IQs than either of the other groups (ps < 0.001).
FA Comparisons
Voxelwise group comparisons revealed significant differences in FA between children with FAS/PFAS and controls in four (suprathresholded) clusters, located in the left and right inferior longitudinal fasciculi (L‐ and R‐ILF, respectively) and the splenium and isthmus of the corpus callosum (SCC and ICC, respectively) (Fig. 1A–C). Table 2 (top) shows the cluster sizes, peak coordinates, and group averages of the mean FA extracted for each child in ROIs defined around the peak coordinates of each cluster. A separate pairwise voxelwise analysis revealed three clusters where HE children had lower FA than the controls—one in the L‐ILF and two in the SCC. Each of these clusters overlapped with the clusters derived from the FAS/PFAS versus control comparison, and they are, therefore, not reported separately in Table 2 and were not used to examine dose dependence. Columns on the right in Table 2 show the correlations of mean FA, AD, and RD extracted for each child in ROIs around peak coordinates of regions showing differences between FAS/PFAS and control groups with the three continuous measures of alcohol exposure. Examples of the correlations of DTI and continuous alcohol measures are shown in Figure 2. In each of the ROIs, increasing alcohol exposure was associated with lower FA, effects that appear to be attributable to greater RD. In addition, increased alcohol exposure was associated with decreased AD in the ICC.
Figure 1.
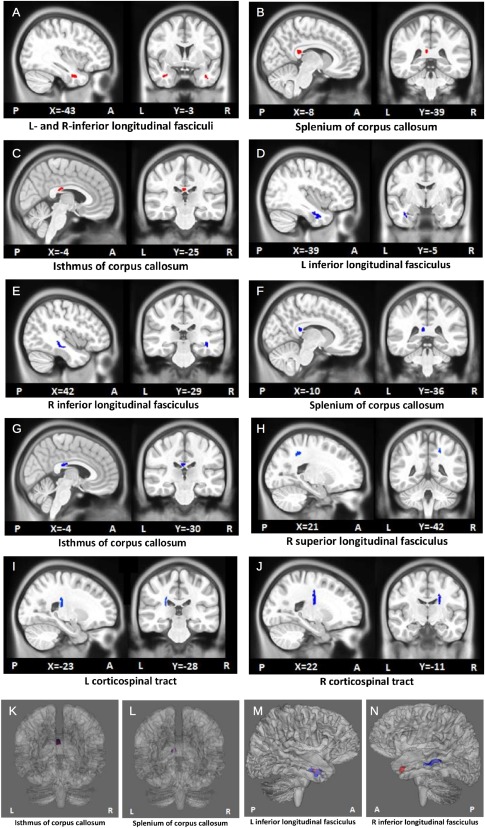
Clusters in which the mean FA is lower (red; A–C) or the mean MD is higher (blue; D–J) in children with FAS/PFAS compared to controls. Volumes are shown in MNI pediatric standard space. See Table 2 for associated cluster information. The locations of the FA‐ and MD‐derived clusters which directly overlapped are also highlighted (K,L), as well as one set which was found within the same WM structure (M). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Size and peak coordinates (in MNI pediatric standard space) of regions where lower FA (top) or higher MD (bottom) were seen in children with FAS/PFAS compared to controls. Average FA (or MD), AD, and RD were extracted for each child in 8mm3 ROIs around the peak coordinates of each region. We report group averages of FA or MD as well as relations of continuous alcohol exposure measures to DTI measures in the ROIs.
| Region MNI coordinates (mm) | Size (mm3) | Mean FA or MD in ROIs | Correlations with DTI measures in ROIs | |||||
|---|---|---|---|---|---|---|---|---|
| FAS/PFAS (26) | HE (15) | Ctl (13) | AA/day | AA/occasion | days/week | |||
| FA | ||||||||
| L inferior longitudinal fasciculus a | ||||||||
| −43, −3, −30 | 282 | 0.30 (0.04) | 0.30 (0.03) | 0.35 (0.04) |
FA AD RD |
−0.39** −0.10 0.33* |
−0.35** −0.02 0.33* |
−0.35** −0.12 0.28* |
| R inferior longitudinal fasciculus | ||||||||
| 37, 2, −30 | 198 | 0.30 (0.04) | 0.32 (0.04) | 0.35 (0.04) |
FA AD RD |
−0.35** 0.07 0.48*** |
−0.32* 0.03 0.40** |
−0.39** 0.01 0.45*** |
| Splenium of corpus callosum b | ||||||||
| −8, −39, 18 | 217 | 0.39 (0.05) | 0.42 (0.08) | 0.46 (0.05) |
FA AD RD |
−0.35** −0.08 0.45*** |
−0.33* −0.16 0.40** |
−0.36** −0.11 0.43** |
| Isthmus of corpus callosum | ||||||||
| −4, −25, 21 | 208 | 0.33 (0.05) | 0.35 (0.04) | 0.40 (0.04) |
FA AD RD |
−0.49*** −0.36** 0.30* |
−0.35** −0.24 0.23 |
−0.48*** −0.33* 0.30* |
| MD | ||||||||
| L inferior longitudinal fasciculus c | ||||||||
| −39, −5, −25 | 1057 | 0.80 (0.03) | 0.78 (0.04) | 0.76 (0.03) |
MD AD RD |
0.44*** 0.29* 0.37** |
0.58*** 0.46*** 0.41** |
0.37** 0.25 0.30* |
| R inferior longitudinal fasciculus | ||||||||
| 42, −29, −12 | 434 | 0.83 (0.04) | 0.81 (0.02) | 0.77 (0.04) |
MD AD RD |
0.39** 0.31* 0.20 |
0.37** 0.35** 0.14 |
0.39** 0.27* 0.23 |
| Splenium of corpus callosum d, e | ||||||||
| −10, −36, 18 | 222 | 0.82 (0.04) | 0.81 (0.05) | 0.76 (0.04) |
MD AD RD |
0.41** 0.34* 0.22 |
0.31* 0.17 0.24 |
0.41** 0.35** 0.21 |
| Isthmus of corpus callosum f | ||||||||
| −4, −30, 18 | 307 | 0.85 (0.06) | 0.85 (0.06) | 0.79 (0.04) |
MD AD RD |
0.53*** 0.42** 0.39** |
0.36** 0.25 0.30* |
0.50*** 0.40** 0.37** |
| R superior longitudinal fasciculus | ||||||||
| 21, −42, 45 | 234 | 0.76 (0.02) | 0.75 (0.03) | 0.73 (0.02) |
MD AD RD |
0.35** 0.20 0.13 |
0.34** 0.28* 0.04 |
0.35** 0.19 0.14 |
| L corticospinal tract | ||||||||
| −23, −28, 20 | 207 | 0.76 (0.02) | 0.78 (0.04) | 0.74 (0.03) |
MD AD RD |
0.34* 0.25 0.26 |
0.35** 0.21 0.31* |
0.31* 0.23 0.23 |
| R corticospinal tract g | ||||||||
| 22, −11, 26 | 526 | 0.70 (0.02) | 0.70 (0.02) | 0.68 (0.02) |
MD AD RD |
0.29* 0.14 0.18 |
0.41** 0.28* 0.18 |
0.27* 0.09 0.18 |
Values are mean (SD); L = left, R = right; *p < 0.05, **p < 0.01, ***p < 0.001.
FAS/PFAS = combined fetal alcohol syndrome (FAS) and partial FAS (PFAS) group; HE = nonsyndromal heavily exposed group; Ctl = control group.
FA = fractional anisotropy; MD = mean diffusivity (10−3 mm2/s); AD = axial diffusivity (10−3 mm2/s); RD = radial diffusivity (10−3 mm2/s).
AA/day = ounces absolute alcohol consumed per day across pregnancy; AA/occasion = ounces absolute alcohol consumed per occasion; days/week = drinking days per week across pregnancy.
HE children had lower FA compared to controls in an overlapping region (peak coordinates: −39, −10, −19; size 342 mm3).
HE children had lower FA compared to controls in two clusters that overlap with this region (peak coordinates: −18, −42, 9 and −14, −33, 23; sizes 247 and 243 mm3).
HE children had greater MD than controls in an overlapping region (peak coordinates: −43, −13, −18; size: 250 mm3).
Another smaller region (197 mm3) in the same tract with peak coordinates (−6, −33, 21) survived in the FAS/PFAS > Ctl group comparison.
A similar region (288 mm3) with peak coordinates (−13, −36, 11) survived in the HE > Ctl group comparison.
Two similar regions (329 and 203 mm3) with peak coordinates (−11, −32, 24) and (−6, −32, 20) survived in the HE > Ctl group comparison.
A similar region (291 mm3) with peak coordinates (22, −8, 25) survived in the HE > Ctl group comparison.
Figure 2.
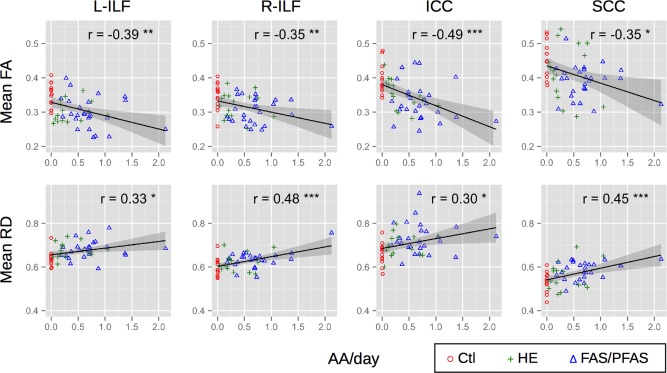
Relations of mean FA (top row) and RD (×10−3 mm2/s; bottom row) with the continuous alcohol measure AA/day (absolute alcohol consumed per day across pregnancy, log transformed) for each FA‐derived ROI in Table 2. L‐ and R‐ILF = left and right inferior longitudinal fasciculus; SCC = splenium of corpus callosum; ICC = isthmus of the corpus callosum. FAS/PFAS = combined fetal alcohol syndrome (FAS) and partial FAS (PFAS) group; HE = nonsyndromal heavily exposed group; Ctl = control group. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
MD Comparisons
Voxelwise group comparisons of MD maps produced seven regions (Fig. 1D–J), where children with FAS/PFAS had significantly higher MD than controls (Table 2). Three of these clusters overlapped with those derived in the voxelwise FA analysis described above; namely, the L‐ILF, SCC, and ICC (Fig. 1K–M). However, in the R‐ILF, the MD‐derived cluster (MNI peak coordinate: 42, −29, −12) was found at a different location than the FA‐derived one (MNI peak coordinate: 37, 2, −30) (Fig. 1N). Greater MD was also observed in the FAS/PFAS group compared to controls in the right superior longitudinal fasciculus (R‐SLF) and in the left and right corticospinal tracts (L‐ and R‐CSTs). All the regions are well‐removed from the ventricles, excepting the latter two, which may be affected by partial voluming. Comparing the HE group with controls separately, higher MD was found in the HE children in 5 clusters within 4 WM pathways, all of which overlapped with MD clusters derived from the FAS/PFAS versus controls comparisons. Two clusters were located in the ICC; a single cluster, in each of the following: L‐ILF, SCC, and R‐CST. Since the HE versus control comparison did not yield any additional clusters, these regions are not reportedly separately in Table 2 and were not used to examine dose dependence. Group averages of mean MD in ROIs around the peak coordinates of regions showing differences between the FAS/PFAS group and controls are presented in Table 2, as well as associations with extent of PAE. In each of the seven clusters, the mean MD values in the peak ROIs were positively associated with each of the 3 continuous measures of alcohol exposure. Increased AD was associated with higher PAE in the L‐ and R‐IFL, SCC, and ICC; increased RD, with higher PAE in L‐ILF and ICC.
After controlling for confounders, group differences remained significant in all regions (all ps ≤ 0.05). AA/day remained significantly associated with FA in L‐ILF, SCC, and ICC and with MD in L‐ and R‐ILF, SCC, and ICC (ps < 0.05) (Table 3). The FA and MD findings were essentially unchanged when the analyses were rerun omitting the three children whose mothers used marijuana and the one who reported using cocaine.
Table 3.
Associations of AA/day with FA and MD in the regions of interest after controlling for potential confounders. AA/day values were log transformed to correct for skewness.
| Potential confounders | AA/day | ||||
|---|---|---|---|---|---|
| With all subjects | Omitting 4 subjectsa | ||||
| r | β | r | β | ||
| FA | |||||
| L inferior longitudinal fasciculus | None | −0.39** | −0.39** | −0.37** | −0.37** |
| R inferior longitudinal fasciculus | Maternal education, lead exposure | −0.35** | −0.22 | −0.34* | −0.22 |
| Splenium of corpus callosum | None | −0.35** | −0.35** | −0.34* | −0.34* |
| Isthmus of corpus callosum | Maternal education, smoking, lead exposure | −0.49*** | −0.39** | −0.53*** | −0.40** |
| MD | |||||
| L inferior longitudinal fasciculus | Maternal age, maternal education, smoking, lead exposure | 0.44*** | 0.27* | 0.43** | 0.28* |
| R inferior longitudinal fasciculus | Maternal education, smoking, lead exposure | 0.39** | 0.29* | 0.37** | 0.33* |
| Splenium of corpus callosum | Smoking | 0.41** | 0.40** | 0.41** | 0.41** |
| Isthmus of corpus callosum | Smoking | 0.53*** | 0.47*** | 0.53*** | 0.47*** |
| R superior longitudinal fasciculus | Maternal education, smoking | 0.35** | 0.23† | 0.33** | 0.24† |
| L corticospinal tract | Smoking | 0.34* | 0.14 | 0.35* | 0.14 |
| R corticospinal tract | Maternal age, smoking | 0.29* | 0.07 | 0.30* | 0.10 |
Omitting the three children whose mothers used marijuana and the one who reported using cocaine.
β = correlation of DTI measures and AA/day after controlling for potential confounders.
L = left, R = right; † p < 0.10, *p < 0.05, **p < 0.01, ***p < 0.001.
FA = fractional anisotropy; MD = mean diffusivity.
AA/day = ounces absolute alcohol consumed per day across pregnancy.
Mediation of Effects of PAE on Behavioral Outcomes
The effect of AA/day on MD in each of the four principal ROIs associated with PAE after control for confounders was examined in relation to three sets of PAE‐related behavioral outcomes (Table 4). Higher MD in the L‐ILF was associated with poorer performance on all five IQ measures and to short‐delay free recall on the CVLT. Higher MD in the SCC and ICC was associated with lower Full Scale IQ, slower processing speed, and poorer EBC. Higher MD in the SCC was also associated with poorer WISC‐IV Verbal Comprehension and CVLT short‐delay free recall. Mediation of the effect of AA/day on each of the behavioral measures by MD was tested statistically using multiple regression analysis. As shown in Table 5, the standardized regression coefficient for AA/day in relation to WISC‐IV Processing Speed decreased from −0.29 to −0.08 when MD in the SCC was entered into the regression, a decrease that was statistically significant. This mediation of the effect of AA/day on WISC‐IV Processing Speed by higher MD in the SCC is illustrated in Figure 3. The regression coefficients for AA/day in relation to EBC also decreased significantly when MD in the splenium and the isthmus were added to their respective regression analyses (Table 5).
Table 4.
Correlation of behavioral measurements with MD in the four FA‐derived ROIs described in Table 2
| L‐ILF | R‐ILF | SCC | ICC | |
|---|---|---|---|---|
| WISC‐IV Full‐scale IQ | −0.39** | −0.19 | −0.38** | −0.29* |
| WISC‐IV Verbal Comprehension Index a | −0.27* | −0.06 | −0.32* | −0.16 |
| WISC‐IV Perceptual Reasoning Index | −0.35** | −0.22 | −0.22 | −0.19 |
| WISC‐IV Working Memory Index b | −0.32* | −0.14 | −0.24 | −0.20 |
| WISC‐IV Processing Speed Index c | −0.35** | −0.08 | −0.43** | −0.30* |
| CVLT short‐delay recall d | −0.17 | −0.20 | −0.29* | −0.25 |
| CVLT long‐delay recall d | −0.32** | −0.17 | −0.20 | −0.18 |
| Eyeblink conditioning e | −0.16 | −0.22 | −0.39** | −0.42** |
L = left, R = right; *p < 0.05, **p < 0.01, ***p < 0.001.
ILF = inferior longitudinal fasciculus; SCC = splenium of corpus callosum; ICC = isthmus of corpus callosum.
CVLT = California Verbal Learning Test‐Children's Version.
Missing values for 1 child with FAS/PFAS.
Missing values for 1 child with HE.
Missing values for 2 children with FAS/PFAS, 1 HE.
Missing values for 3 children with FAS/PFAS, 3HE, 1 Ctl.
Missing values for 2 children with FAS/PFAS, 2 HE, 2 Ctl.
Table 5.
Mediation of the effect of prenatal alcohol exposure on WISC‐IV processing speed and eyeblink conditioning by MD in three ROIs
| Behavioral outcomes and ROIs | β 1 | β 2 | p a |
|---|---|---|---|
| WISC‐IV Processing Speed Index | |||
| L inferior longitudinal fasciculus | −0.29* | −0.17 | −1.76† |
| Splenium of corpus callosum | −0.29* | −0.08 | −2.49* |
| Eyeblink conditioning | |||
| Splenium of corpus callosum | −0.32* | −0.18 | −1.99* |
| Isthmus of corpus callosum | −0.32* | −0.15 | −2.22* |
L = left; † p < 0.10, *p < 0.05.
β 1 = Standardized correlation coefficient for AA/day before controlling for the mediating variable; β 2 = Standardized correlation coefficient for AA/day after controlling for MD in the ROI listed in the first column.
Significance of the reduction in β following the addition of the mediator to the model, based on the difference in coefficients method [Clogg et al., 1992].
Figure 3.

Path model illustrating mediation of the effect of AA/day by MD in the splenium of corpus callosum on WISC‐IV Processing Speed.
DISCUSSION
This voxel‐based DTI study of effects of PAE on cortical WM identified microstructural deficits in several brain regions reported to be affected in previous studies of FASD. This study extended these findings by examining DTI effects in both syndromal and nonsyndromal heavily exposed children and by assessing the degree to which these deficits are also seen in relation to continuous PAE measures derived from detailed maternal drinking interviews conducted prospectively during pregnancy. This study also examined these deficits in relation to a range of cognitive outcomes and is the first to test statistically the degree to which they mediate the well‐documented associations between PAE and these outcomes.
Voxelwise group comparisons showed decreased FA in four WM regions and increased MD in seven regions in children with FAS/PFAS compared to non‐ or minimally exposed controls. Three regions derived from the FA group differences (L‐ILF, SCC, and ICC) overlapped with the MD‐derived clusters, and the fourth FA‐derived cluster was located in a different location of the same fiber bundle (R‐ILF) as an MD cluster. The additional MD clusters were in the R‐SLF and bilaterally in the CSTs. The nonsyndromal alcohol‐exposed children (HE) showed lower FA only in the L‐ILF and SCC, as well as higher MD in L‐ILF, SCC, ICC, and R‐CST. For each DTI parameter, the HE‐derived clusters formed a subset of those derived from the comparison with the more severely affected FAS/PFAS group.
Table 6 provides the summary of previous studies that found WM alterations in regions similar to the FA and MD clusters reported here. Abnormalities in the corpus callosum in individuals with FASD have been observed previously by autopsy, structural MRI, and DTI [e.g., Clarren and Smith, 1978; Coulter et al., 1993; Jones and Smith, 1973; Taylor et al., 2015]. The splenium appears to be particularly vulnerable to PAE [Autti‐Rämö et al., 2002; Riley et al., 1995; Sowell et al., 2001b], and the locations of peak DTI differences (FA [−8, −39, 18] and MD [−10, −36, 18]) between the FAS/PFAS and control groups in the SCC in this study were similar to those seen in the Sowell et al [2008] study (MNI coordinates: [−14, −50, 15] and [−4, −43, 18]). Decreased FA in the SCC in FASD has also been reported in one semiautomated DTI tractography study [Lebel et al., 2008] and two manually defined DTI‐tractography studies [Ma et al., 2005; Wozniak et al., 2009], one of which also found increased MD in this region [Ma et al., 2005]. Our findings of PAE‐related abnormalities in the ICC are consistent with reports from previous studies using manually defined tractography [Wozniak et al., 2006, 2009] and voxel‐wise TBSS [Fryer et al., 2009; Li et al., 2009]. Although higher MD in the genu of the corpus callosum has been previously reported in individuals with FASD [Lebel et al., 2008; Ma et al., 2005; Treit et al., 2013], this region (peak MNI coordinates: [−11, 27, 6]) did not survive cluster size correction at p < 0.01 in our study.
Table 6.
Summary of previous DTI studies that have reported white matter alterations in FASD in regions similar to those found in this study
| FAS/PFAS | HE | Previous studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | FA | MD | FA | MD | References | Subjects' age (years) | Methods | Results | ||
| L‐ILF | ↓ | ↑ | ↓ | ↑ | Lebel et al., 2008 | 5–12 | Semiautomated tractography | FA↓ | ||
| R‐ILF | ↓ | ↑ | Lebel et al., 2008 | 5–12 | Semiautomated tractography | FA↓,MD↑ | ||||
| Sowell et al., 2008, a | 7–15 | Voxelwise | FA↓ | |||||||
| SCC | ↓ | ↑ | ↓ | ↑ | Sowell et al., 2008, b | 7–15 | Voxelwise | FA↓ | ||
| Lebel et al., 2008 | 5–12 | Semiautomated tractography | FA↓ | |||||||
| Ma et al., 2005 | 18–25 | Manually defined tractography | FA↓,MD↑ | |||||||
| Wozniak et al., 2009 | 10–17 | Manually defined tractography | FA↓ | |||||||
| ICC | ↓ | ↑ | ↑ | Wozniak et al., 2006 | 10–19 | Manually defined tractography | MD↑ | |||
| Wozniak et al., 2009 | 10–17 | Manually defined tractography | FA↓ | |||||||
| Li et al., 2009 | 8–18 | TBSS | FA↓ | |||||||
| Fryer et al., 2009 | 19–27 | TBSS | FA↓ | |||||||
| R‐SLF | ↑ | Lebel et al., 2008 | 5–12 | Semiautomated tractography | FA↓ | |||||
| Fryer et al., 2009 | 19–27 | TBSS | FA↓ | |||||||
| L‐CST | ↑ | Lebel et al., 2010 | 5–13 | Voxelwise | FA↓ | |||||
| R‐CST | ↑ | ↑ | Lebel et al., 2008 | 5–12 | Semiautomated tractography | MD↑ | ||||
L = left, R = right; FA = fractional anisotropy; MD = mean diffusivity.
FAS/PFAS = combined fetal alcohol syndrome (FAS) and partial FAS (PFAS) group; HE = nonsyndromal heavily exposed group.
ILF = inferior longitudinal fasciculus; SCC = splenium of corpus callosum; ICC = isthmus of corpus callosum; SLF = superior longitudinal fasciculus; CST = corticospinal tract.
The coordinate where the maximum MD difference (42, −29, −12) in the R‐ILF occurred in the FAS/PFAS vs Ctl comparison overlapped with the maximum FA difference (45, −22, −15) seen in the Sowell et al. [2008] study.
Coordinates where the peak FA (−8, −39, 18) and MD (−10, −36, 18) differences occurred in the SCC in the FAS/PFAS vs Ctl comparison were close to the SCC regions (MNI coordinates: (−14, −50, 15) and (−4, −43, 18)) seen in the Sowell et al. [2008] study.
Our findings of lower FA and higher MD bilaterally in the ILF in children with FASD are consistent with results from a semiautomated DTI tractography study by Lebel et al. [2008]. The coordinates where MD differences were maximal in the R‐ILF (MNI coordinates: [42, −29, −12]) in our study overlapped with the region where Sowell et al [2008] found lower FA in children with FASD (MNI coordinates: [45, −23, −15]). Lower FA has been reported in children with FASD in the SLF both on the right [Fryer et al., 2009] and bilaterally [Lebel et al., 2008], and higher MD in the R‐CST [Lebel et al., 2008].
Since detailed maternal drinking histories were collected during pregnancy, a novel aspect of the current study was the ability to quantitatively relate extent of alcohol exposure to WM alterations. All three continuous measures of PAE (AA/day, AA/occasion and days/week) were significantly associated with reduced FA in the L‐ and R‐ILF, SCC, and ICC and with increased MD in the L‐ and R‐ILF, SCC, ICC, R‐SLF, L‐ and R‐CST. The associations of the DTI parameters with continuous measures of alcohol exposure indicate that the degree of microstructural alterations in these regions is dose dependent. It is noteworthy that the majority of the mothers in the FAS/PFAS and HE groups engaged in weekend binge drinking, with the mothers of the more severely affected children with FAS or PFAS concentrating their drinking on roughly 2 days/week, and the mothers of the nonsyndromal HE children drinking on average only 1 day/week. After controlling for potential confounders, AA/day remained significantly correlated with both the FA and MD measures only in the L‐ILF, SCC, and ICC, as well as with MD in the R‐ILF. Associations of AA/day with MD were no longer significant in the R‐SLF and L‐ and R‐CST, where MD was related to maternal smoking during pregnancy (p < 0.10). In the L‐CST, smoking was significantly related (p < 0.05) to MD after controlling for alcohol, which may provide additional evidence of neurobehavioral deficits related to maternal smoking in children with PAE [Cornelius et al., 2001; Gusella and Fried, 1984; Martin et al., 1977].
Decreases in FA with increasing alcohol exposure were largely attributable to increased RD, rather than to decreased AD. Although the biological interpretation of the MD constituents RD and AD is not straightforward, this pattern of increased RD has previously been associated with myelination deficits or reduced axonal packing and density in animal studies [Beaulieu, 2002] and is consistent with results from our previous studies of effects on the cerebellar peduncles [Fan et al., 2015; Spottiswoode et al., 2011]. Other DTI studies of children within the same age range have shown similar patterns. One other DTI study of children with FASD, math achievement test scores were associated with higher RD in left anterior WM tracts in the middle cerebellar peduncles [Lebel et al., 2010]. Several studies have shown that alcohol exposure may result in the impairment of oligodendrocytes, which produce the myelin sheaths [Ozer et al., 1999; Phillips and Krueger, 1992].
Several previous DTI studies have reported correlations between DTI measures and cognitive performance. In this study, higher MD in three of the four ROIs in which significant WM deficits were detected—left ILF, splenium, and isthmus—showed significant associations with poorer performance in IQ, memory recall, and EBC. Moreover, when we tested directly whether mediation of the effect of PAE on behavior by MD was statistically significant, we found that the effect on processing speed was significantly mediated by MD in the SCC and the effect on EBC was significantly mediated by MD in the splenium and isthmus. It is perhaps not surprising that the mediation of behavioral deficits by WM impairment was most clearly evident in relation to effects on processing speed and EBC, outcomes that are particularly dependent on rapid transmission of neural signals for successful performance. These data are consistent with our previous finding that the effect of PAE on EBC is partially mediated by lower FA and higher MD in the left middle penduncle, a WM structure known to be critical to EBC performance [Fan et al., 2015; Spottiswoode et al., 2011]. They are also consistent with numerous behavioral studies linking PAE to slower information processing in infancy [Jacobson et al., 1993, 1994; Kable and Coles, 2004] and childhood [e.g., Burden et al., 2005; Ma et al., 2005; Streissguth et al., 1986, 1984, 1994; Willford et al., 2010].
One limitation of our study is that, using a voxel‐based approach, we found only a limited number of discrete clusters of impairment in the WM tracts. A tractography‐based approach might reveal WM deficits in more extensive regions along the tracts. Moreover, although care was taken to ensure good coregistration, any voxelwise analysis is limited by potential misregistration errors due to the low resolution of DTI acquisitions. To control for Type 1 error, Monte Carlo simulations [Forman et al., 1995] were applied to determine the minimum size of clusters for significance. In this study, clusters had to be 168 mm3 or larger to be deemed significant. This approach makes it difficult to identify small regions with WM abnormalities, which may explain why some regions reported in other studies were not found here. One approach to overcome this limitation in future studies may be to use regions where functional deficits have previously been detected as seed regions for tractography studies, thus limiting the numbers of voxels being compared to those within particular WM tracts. Although it is never possible in a correlational study to assess all extraneous variables that might provide alternative explanations for observed effects, we controlled for seven important variables, including biological (age, sex), environmental (lead exposure), sociological (maternal education, which is highly associated with socioeconomic status), and modality‐related (subject motion while scanning) factors. With respect to interpretation of the findings, it should be noted that it is difficult to translate DTI parameters to a single, direct biological phenomenon, and studies using additional methodologies would, therefore, be warranted to further investigate the effects observed here.
CONCLUSIONS
This study examined the effects of PAE on cortical WM in 10‐year‐old children using both FASD diagnosis and prospectively obtained continuous measures of extent of PAE. Voxelwise group comparisons revealed four regions with FA alterations and seven with MD alterations in children with FAS or PFAS, a subset of which were also found in HE children. The mean FA and MD values in the ROIs were significantly associated with all three continuous measures of alcohol exposure (AA/day, AA/occasion and days/week). After controlling for confounders, associations of PAE with FA and MD remained significant in the L‐ILF, SCC, and ICC, and with MD in the R‐ILF, suggesting dose‐dependent impairments in these regions. Lower FA in the ROIs was attributable primarily to increased RD rather than decreased AD, suggesting poorer axon packing density and/or myelination. Mediation of behavioral deficits by WM impairment was most clearly observed in effects of PAE on processing speed and eyeblink conditioning, which may be related to the fact that these outcomes are particularly dependent on rapid transmission of neural signals. This finding is consistent with numerous behavioral studies linking PAE to slower information processing in infancy and childhood. Further study examining both DTI and resting‐state fMRI to examine the relation between brain structural and functional connectivity in the same population may provide greater insight into mechanisms underlying PAE‐related neurocognitive deficits.
ACKNOWLEDGMENTS
The authors thank A. Alhamud and the CUBIC radiographers Marie‐Louise de Villiers and Nailah Maroof, and the UCT and WSU research staff Nicolette Hamman, Mariska Pienaar, Maggie September, Emma Makin, Catherine Lewis, and Renee Sun. They also thank the dysmorphologists H. Eugene Hoyme, Luther K. Robinson, and Nathaniel Khaole, who performed the dysmorphology examinations of the children in conjunction with the NIAAA Collaborative Initiative on Fetal Alcohol Spectrum Disorders. They greatly appreciate the contributions of the mothers and children who have participated in their Cape Town Longitudinal Study.
REFERENCES
- Abel EL (1995): An update on incidence of FAS: FAS is not an equal opportunity birth defect. Neurotoxicol Teratol 17:437–443. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Skare S, Ashburner J (2003): How to correct susceptibility distortions in spin‐echo echo‐planar images: Application to diffusion tensor imaging. Neuroimage 20:870–888. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Fennema‐Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL (2001): Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol 43:148–154. [PubMed] [Google Scholar]
- Astley SJ, Aylward EH, Carmichael Olson H, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B Jirikowic T, Kraegel P, Maravilla K, Richards T (2009): Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 33:1671–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autti‐Rämö I, Autti T, Korkman M, Kettunen S, Salonen O, Valanne L (2002): MRI findings in children with school problems who had been exposed prenatally to alcohol. Dev Med Child Neurol 44:98–106. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA (1986): The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Personality Soc Psychol 51:1173–1182. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, Le Bihan D (1994): MR diffusion tensor spectroscopy and imaging. Biophysical J 66:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C (2002): The basis of anisotropic water diffusion in the nervous system–a technical review. NMR Biomed 15:435–455. [DOI] [PubMed] [Google Scholar]
- Bjorkquist OA, Fryer SL, Reiss AL, Mattson SN, Riley EP (2010): Cingulate gyrus morphology in children and adolescents with fetal alcohol spectrum disorders. Psychiatr Res Neuroimaging 181:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Jacobson JL (2005): Relation of prenatal alcohol exposure to cognitive processing speed and efficiency in childhood. Alcohol Clin Exp Res 29:1473–1483. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2012): Alcohol use and binge drinking among women of childbearing age–United States, 2006–2010. Morb Mortal Wkly Rep 61(28):534–538. [PubMed] [Google Scholar]
- Chen X, Coles CD, Lynch ME, Hu X (2012): Understanding specific effects of prenatal alcohol exposure on brain structure in young adults. Hum Brain Mapp 33:1663–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo LM, Jacobson SW, Jacobson JL (2004): Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol Teratol 26:359–371. [DOI] [PubMed] [Google Scholar]
- Clarren SK (1981): Recognition of fetal alcohol syndrome. JAMA 245:2436–2439. [PubMed] [Google Scholar]
- Clarren SK, Smith DW (1978): The fetal alcohol syndrome. N Engl J Med 298:1063–1067. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Alvord EC, Sumi SM, Streissguth AP, Smith D (1978): Brain malformations related to prenatal exposure to ethanol. J Pediatr 92:64–67. [DOI] [PubMed] [Google Scholar]
- Clogg CC, Petkova E, Shihadeh ES (1992): Statistical methods for analyzing collapsibility in regression models. J Educ Behav Stat 17:51–74. [Google Scholar]
- Coffin JM, Baroody S, Schneider K, O'Neill J (2005): Impaired cerebellar learning in children with prenatal alcohol exposure: A comparative study of eyeblink conditioning in children with ADHD and dyslexia. Cortex 41:389–398. [DOI] [PubMed] [Google Scholar]
- Colby JB, Smith L, O'Connor MJ, Bookheimer SY, Van Horn JD, Sowell ER (2012): White matter microstructural alterations in children with prenatal methamphetamine/polydrug exposure. Psychiatr Res Neuroimaging 204:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Ryan CM, Day NL, Goldschmidt L, Willford JA (2001): Prenatal tobacco effects on neuropsychological outcomes among preadolescents. J Dev Behav Pediatr 22:217–225. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Leech RW, Schaefer GB, Scheithauer BW, Brumback RA (1993): Midline cerebral dysgenesis, dysfunction of the hypothalamic‐pituitary axis, and fetal alcohol effects. Arch Neurol 50:771–775. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comp Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Croxford J, Viljoen D (1999): Alcohol consumption by pregnant women in the Western Cape. S Afr Med J 89:962–965. [PubMed] [Google Scholar]
- De Guio F, Mangin JF, Riviere D, Perrot M, Molteno CD, Jacobson SW, Meintjes EM, Jacobson JL (2014): A study of cortical morphology in children with fetal alcohol spectrum disorders. Hum Brain Mapp 35:2285–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zeeuw P, Zwart F, Schrama R, Van Engeland H, Durston S (2012): Prenatal exposure to cigarette smoke or alcohol and cerebellum volume in attention‐deficit/hyperactivity disorder and typical development. Trans Psychiatr 2:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott EJ, Bower C (2008): Alcohol and pregnancy: The pivotal role of the obstetrician. Austral New Zeal J Obstetr Gynaecol 48:236–239. [DOI] [PubMed] [Google Scholar]
- Fan J, Meintjes EM, Molteno CD, Spottiswoode BS, Dodge NC, Alhamud AA, Stanton ME, Peterson BS, Jacobson JL, Jacobson SW (2015): White matter integrity of the cerebellar peduncles as a mediator of effects of prenatal alcohol exposure on eyeblink conditioning. Hum Brain Mapp 36:2470–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL, Group BDC (2011): Unbiased average age‐appropriate atlases for pediatric studies. Neuroimage 54:313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Res Med 33:636–647. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Schweinsburg BC, Bjorkquist OA, Frank LR, Mattson SN, Spadoni AD, Riley EP (2009): Characterization of white matter microstructure in fetal alcohol spectrum disorders. Alcohol Clin Exp Res 33:514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CR, Lebel C, Rasmussen C, Beaulieu C, Reynolds JN (2013): Diffusion tensor imaging correlates of saccadic reaction time in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res 37:1499–1507. [DOI] [PubMed] [Google Scholar]
- Gusella J, Fried P (1984): Effects of maternal social drinking and smoking on offspring at 13 months. Neurobehav Toxicol Teratol 6:13–17. [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS Khaole N, Viljoen DL, Jones KL, Robinson LK (2005): A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 institute of medicine criteria. Pediatrics 115:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL (2013): The risk of low‐to‐moderate prenatal alcohol exposure on child academic underachievement and behavior may be difficult to measures and should not be underestimated. Evidence Based Med 19:e7. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW (1993): Prenatal alcohol exposure and infant information processing ability. Child Dev 64:1706–1721. [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ (1994): Effects of fetal alcohol exposure on infant reaction time. Alcohol Clin Exp Res 18:1125–1132. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL (2002): Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics 109:815–825. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Chiodo LM, Corobana R (2004): Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5‐year intellectual function. Alcohol Clin Exp Res 28:1732–1745. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL (2008): Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res 32:365–372. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N (2011): Impaired delay and trace eyeblink conditioning in school‐age children with fetal alcohol syndrome. Alcohol Clin Exp Res 35:250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K, Smith D (1973): Recognition of the fetal alcohol syndrome in early infancy. Lancet 302:999–1001. [DOI] [PubMed] [Google Scholar]
- Kable JA, Coles CD (2004): The impact of prenatal alcohol exposure on neurophysiological encoding of environmental events at six months. Alcohol Clin Exp Res 28:489–496. [DOI] [PubMed] [Google Scholar]
- Kalberg WO, Provost B, Tollison SJ, Tabachnick BG, Robinson LK, Eugene Hoyme H, Trujillo PM, Buckley D, Aragon AS, May PA (2006): Comparison of motor delays in young children with fetal alcohol syndrome to those with prenatal alcohol exposure and with no prenatal alcohol exposure. Alcohol Clin Exp Res 30:2037–2045. [DOI] [PubMed] [Google Scholar]
- Kinney H, Faix R, Brazy J (1980): The fetal alcohol syndrome and neuroblastoma. Pediatrics 66:130–132. [PubMed] [Google Scholar]
- Lanphear BP, Dietrich K, Auinger P, Cox C (2000): Cognitive deficits associated with blood lead concentrations< 10 microg/dL in US children and adolescents. Pub Health Rep 115:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Walker L, Andrew G, Yager J, Beaulieu C (2008): Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res 32:1732–1740. [DOI] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Andrew G, Beaulieu C (2010): Brain microstructure is related to math ability in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res 34:354–363. [DOI] [PubMed] [Google Scholar]
- Lewis CE, Thomas KG, Dodge NC, Molteno CD, Meintjes EM, Jacobson JL, Jacobson SW (2015): Verbal learning and memory impairment in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 39:724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Coles CD, Lynch ME, Hu X (2009): Voxelwise and skeleton‐based region of interest analysis of fetal alcohol syndrome and fetal alcohol spectrum disorders in young adults. Hum Brain Mapp 30:3265–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Coles CD, Lynch ME, LaConte SM, Zurkiya O, Wang D, Hu X (2005): Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcohol Clin Exp Res 29:1214–1222. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V (2002): A comparison of methods to test mediation and other intervening variable effects. Psychological Meth 7:83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madge E, Van den Berg A, Robinson M, Landman J (1981): Junior South African Individual Scales. Pretoria: Human Sciences Research Council. [Google Scholar]
- Martin J, Martin DC, Lund CA, Streissguth AP (1977): Maternal alcohol ingestion and cigarette smoking and their effects on newborn conditioning. Alcohol Clin Exp Res 1:243–247. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Jernigan TL, Ehlers CL, Delis DC, Jones KL, Stern C, Johnson KA, Hesselink JR, Bellugi U (1992): Fetal alcohol syndrome: A case report of neuropsychological, MRI, and EEG assessment of two children. Alcohol Clin Exp Res 16:1001–1003. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Jernigan TL, Garcia A, Kaneko WM, Ehlers CL, Jones KL (1994): A decrease in the size of the basal ganglia following prenatal alcohol exposure: A preliminary report. Neurotoxicol Teratol 16:283–289. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL (1996): A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcohol Clin Exp Res 20:1088–1093. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP (2011): Maternal risk factors for fetal alcohol spectrum disorders: Not as simple as it might seem. Alcohol Res Health 34:15–26. [PMC free article] [PubMed] [Google Scholar]
- May PA, Brooke L, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson L, Viljoen D (2000): Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. Am J Pub Health 90:1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais A‐S, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NC, Snell C, Kalberg WO (2007): The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend 88:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE (2009): Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in‐school studies. Dev Disabil Res Rev 15:176–192. [DOI] [PubMed] [Google Scholar]
- Meintjes E, Narr K, van der Kouwe A, Molteno C, Pirnia T, Gutman B, Woods R, Thompson P, Jacobson J, Jacobson S (2014): A tensor‐based morphometry analysis of regional differences in brain volume in relation to prenatal alcohol exposure. NeuroImage Clin 5:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Van Zijl PC (2002): Fiber tracking: Principles and strategies‐a technical review. NMR Biomed 15:468–480. [DOI] [PubMed] [Google Scholar]
- Mukherjee RA, Hollins S, Turk J (2006): Fetal alcohol spectrum disorder: An overview. J Royal Soc Med 99:298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli A, Lebel C, Rasmussen C, Andrew G, Beaulieu C (2011): Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 35:1404–1417. [DOI] [PubMed] [Google Scholar]
- Ozer E, Sarioglu S, Güre A (1999): Effects of prenatal ethanol exposure on neuronal migration, neuronogenesis and brain myelination in the mice brain. Clin Neuropathol 19:21–25. [PubMed] [Google Scholar]
- Peiffer J, Majewski F, Fischbach H, Bierich J, Volk B (1979): Alcohol embryo‐ and fetopathy: Neuropathology of 3 children and 3 fetuses. J Neurologic Sci 41:125–137. [DOI] [PubMed] [Google Scholar]
- Phillips D, Krueger S (1992): Effects of combined pre‐and postnatal ethanol exposure (three trimester equivalency) on glial cell development in rat optic nerve. Int J Dev Neurosci 10:197–206. [DOI] [PubMed] [Google Scholar]
- Rajaprakash M, Chakravarty MM, Lerch JP, Rovet J (2014): Cortical morphology in children with alcohol‐related neurodevelopmental disorder. Brain Behav 4:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL (1995): Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res 19:1198–1202. [DOI] [PubMed] [Google Scholar]
- Roussotte FF, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O'Connor MJ, Narr KL, Sowell ER (2012): Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Hum Brain Mapp 33:920–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW (2001a): Voxel‐based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. Neuroreport 12:515–523. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson S, Thompson P, Jernigan T, Riley E, Toga A (2001b): Mapping callosal morphology and cognitive correlates: Effects of heavy prenatal alcohol exposure. Neurol 57:235–244. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW (2002): Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex 12:856–865. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Johnson A, Kan E, Lu LH, Van Horn JD, Toga AW, O'Connor MJ, Bookheimer SY (2008): Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. J Neurosci 28:1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni AD, McGee CL, Fryer SL, Riley EP (2007): Neuroimaging and fetal alcohol spectrum disorders. Neurosci Biobehav Rev 31:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spottiswoode BS, Meintjes EM, Anderson AW, Molteno CD, Stanton ME, Dodge NC, Gore JC, Peterson BS, Jacobson JL, Jacobson SW (2011): Diffusion tensor imaging of the cerebellum and eyeblink conditioning in fetal alcohol spectrum disorder. Alcohol Clin Exp Res 35:2174–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia FC (1996): Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment. Washington, DC: National Academies Press. [Google Scholar]
- Streissguth AP (2007): Offspring effects of prenatal alcohol exposure from birth to 25 years: The Seattle prospective longitudinal study. J Clin Psychol Med Settings 14:81–101. [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD, Parrish‐Johnson JC, Kirchner GL, Martin DC (1986): Attention, distraction and reaction time at age 7 years and prenatal alcohol exposure. Neurotoxicol Teratol 8:717–725. [PubMed] [Google Scholar]
- Streissguth AP, Martin DC, Barr HM, Sandman BM (1984): Intrauterine alcohol and nicotine exposure: Attention and reaction time in 4‐yearold children. Dev Psychol 20:533–541. [Google Scholar]
- Streissguth AP, Sampson PD, Olson HC, Bookstein FL, Barr HM, Scott M, Feldman J, Mirsky AF (1994): Maternal drinking during pregnancy: Attention and short‐term memory in 14‐year‐old offspring: A longitudinal prospective study. Alcohol Clin Exp Res 18:202–218. [DOI] [PubMed] [Google Scholar]
- Taylor PA, Jacobson SW, van der Kouwe A, Molteno CD, Chen G, Wintermark P, Alhamud A, Jacobson JL, Meintjes EM (2015): A DTI‐based tractography study of effects on brain structure associated with prenatal alcohol exposure in newborns. Hum Brain Mapp 36:170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdall M, Hess A, van der Kouwe A (2009): MPRAGE using EPI navigators for prospective motion correction. Proc Int Soc Magn Res Med 17:4656. [Google Scholar]
- Treit S, Lebel C, Baugh L, Rasmussen C, Andrew G, Beaulieu C (2013): Longitudinal MRI reveals altered trajectory of brain development during childhood and adolescence in fetal alcohol spectrum disorders. J Neurosci 33:10098–10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2005): News release. U.S. Surgeon General releases advisory on alcohol use in pregnancy. Available at https://wayback.archive-it.org/3926/20140421162517/http://www.surgeongeneral.gov/news/2005/02/sg0222(2005):html.
- van der Kouwe AJ, Benner T, Salat DH, Fischl B (2008): Brain morphometry with multiecho MPRAGE. Neuroimage 40:559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaurio L, Crocker N, Mattson S (2010): Fetal Alcohol Spectrum Disorders. The Handbook of Pediatric Neuropsychology. New York: Springer Publishing Company. [Google Scholar]
- Willford JA, Chandler LS, Goldschmidt L, Day NL (2010): Effects of prenatal tobacco, alcohol and marijuana exposure on processing speed, visual–motor coordination, and interhemispheric transfer. Neurotoxicol Teratol 32:580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ, Brown DR, Michels KM (1971): Statistical Principles in Experimental Design, Vol. 2 New York: McGraw‐Hill. [Google Scholar]
- Wisniewski K, Dambska M, Sher J, Qazi Q (1983): A clinical neuropathological study of the fetal alcohol syndrome. Neuropediatr 14:197–201. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Chang PN, Muetzel RL, Caros L, aLim KO (2006): Diffusion tensor imaging in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 30:1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Muetzel RL, Mueller BA, McGee CL, Freerks MA, Ward EE, Nelson ML, Chang PN, Lim KO (2009): Microstructural corpus callosum anomalies in children with prenatal alcohol exposure: An extension of previous diffusion tensor imaging findings. Alcohol Clin Exp Res 33:1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Phillips OR, Kan E, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O'Connor MJ (2012): Callosal thickness reductions relate to facial dysmorphology in fetal alcohol spectrum disorders. Alcohol Clin Exp Res 36:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]


