Abstract
Background
Hypertension is increasingly prevalent among children. We sought to review provider adherence to the National High Blood Pressure Education Program (NHBPEP) recommendations at a single academic medical center.
Methods
We identified children 3–18 years of age with hypertension based on outpatient-visit International Classification of Diseases, Ninth Edition, Clinical Modification codes from 2006–2012. We calculated the odds of individual tests administration for 10 recommended tests, adjusting for demographic characteristics.
Results
We identified 3588/216,855 (1.7%) children diagnosed with hypertension at a median age of 14 years (25th, 75th percentile 10, 16). No child received all 10 recommended tests. The median number of tests administered was 2 (1, 4), but varied significantly by race and age. Urine drug screen (<1%) and renin levels (1%) were the least common, while serum creatinine (49%) and echocardiogram (40%) were the most common tests. Male children were more likely to receive an echocardiogram (odds ratio 1.43; 95% confidence interval 1.24–1.64), and black children and those ≥11 years old were less likely to have their serum creatinine checked. Adherence to the guidelines did not improve over time (p=0.24).
Conclusions
Children evaluated for hypertension in the outpatient setting infrequently receive the diagnostic tests recommended in the NHBPEP’s report. Test administration frequency varies by patient demographics, but has not improved significantly over time.
Keywords: pediatric hypertension, practice guidelines, children, adolescents
Introduction
The prevalence of hypertension in children and adolescents is rising, with estimated incidence of 1–4.5%.1 Both primary and secondary hypertension occur in children, but the rising incidence in children is primarily related to an increase in essential hypertension. Hypertension is also frequently clustered with other cardiovascular risk factors such as obesity, elevated cholesterol, and diabetes.2 Identifying associated cardiovascular risk factors and secondary causes of hypertension is essential to optimize treatment and improve outcomes.
To assist health care professionals in treating children with hypertension, the National Heart, Lung, and Blood Institute convened the National High Blood Pressure Education Program (NHBPEP) Working Group on Children and Adolescents. The working group’s most recent recommendations are summarized in the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents, released in 2004.3 The report recommends several diagnostic tests to evaluate the causes and comorbidities of hypertension, as well as the potential end organ damage that may result from it. Despite this extensive, well-structured, and well-written report, it is unclear whether clinicians follow the working group’s recommendations.
The purpose of this study was to retrospectively review adherence to the NHBPEP report recommendations by providers treating a cohort of children for hypertension at a single academic medical center. We hypothesized that overall adherence to the recommendations would be low, but variable across types of diagnostic tests and patient demographics.
Patients and Methods
Patient Cohort
We included all children age 3 to 18 years with an outpatient visit record that included an International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) code for hypertension (400.X, 402.X, 403.X, 404.X, 405.X, 405.91, 405.99) who were treated at a single academic medical center from 2006–2012. The study period was chosen to begin 2 years after the release of the NHBPEP report to allow for dissemination of the report’s findings. We included patients regardless of payer type. We excluded patients with a diagnosis of hypertension associated with pregnancy. Patients and data were identified using an electronic data warehouse containing information from all operational systems serving the medical center’s hospitals and clinics. This included data captured across multiple visits. To evaluate the accuracy of hypertension diagnosis by ICD coding, we performed a retrospective chart review in a random sample of 25% of our final cohort (900 children). This chart review found that all 900 children had documentation of at least 3 separate blood pressure measurements above the 95th percentile, confirming the diagnosis of hypertension. The Duke University Institutional Review Board approved the study with a waiver of informed consent because the data were de-identified.
Study Variables
We collected demographic variables including gender, race, ethnicity, and age at the time of hypertension diagnosis. Age at diagnosis was categorized as <11 years versus ≥11 years. We searched the data warehouse for 10 tests recommended by the NHBPEP report: serum creatinine, complete blood cell (CBC) count, serum total cholesterol level, serum catecholamine levels, serum renin level, urinalysis, urine drug screen, renal ultrasound, echocardiogram, and sleep study. Test completion dates and results were recorded. We considered a test as performed if it was completed within 1 year of the first outpatient visit record with an ICD-9-CM code for hypertension. If a patient had the same test repeated several times, we retained only the initial test results.
Statistical Analysis
We used summary statistics including counts and percentages and medians with 25th and 75th percentiles to describe categorical and continuous study variables. Odds ratios were calculated for the administration of each test and combination of tests for several demographic categories. We used the Cochran-Armitage test to evaluate trends in the administration of each test over time. We described the association between the total number of tests administered and patient age at the time of hypertension diagnosis using Spearman’s rank correlation coefficient. For all analyses, we used STATA 13.1 (College Station, TX), and considered a p-value < 0.05 statistically significant.
Results
Of 216,855 children ages 3-18 years seen at the outpatient clinic over the study period, 3588 were diagnosed with hypertension, for an estimated prevalence of 1.7%. The median (25th, 75th percentile) age at diagnosis was 14 years (10, 16), and nearly two-thirds of the study cohort were male (Table 1).
Table 1.
Demographics of Children Included in Study
| All Patients N=3588 (%) |
|
|---|---|
| Male gender | 2176 (61) |
| Ethnicity/race | |
| Black | 1642 (46) |
| Hispanic | 160 (4) |
| White | 1471 (41) |
| Other | 315 (9) |
| Age at diagnosis (years) | |
| <11 | 930 (26) |
| ≥11 | 2658 (74) |
| Diagnosis year | |
| 2006 | 679 (19) |
| 2007 | 543 (15) |
| 2008 | 465 (13) |
| 2009 | 477 (13) |
| 2010 | 491 (14) |
| 2011 | 426 (12) |
| 2012 | 507 (14) |
The median number of tests administered per child was 2 (1, 4), but 572/3588 (16%) of children had no tests administered, and none of the children had all 10 tests administered. Black children and White children were more likely to have at least one test administered (85% and 84%, respectively) compared to Hispanic children and those of other race/ethnicity (82% and 77%, respectively). Children <11 years old had fewer tests administered compared to children ≥11 years (median 2 [1, 3] vs. 3 [1, 4], p<0.001). The correlation between total number of tests administered and child’s age at diagnosis was weak, though it reached statistical significance (rho = −0.1, p<0.001).
The most commonly administered tests were serum creatinine in 49% of children and echocardiogram in 40% (Table 2). The least commonly administered tests were urine drug screen in <1% and serum renin levels in 1% of children. Serum creatinine, CBC count, and renal ultrasound were more likely to be administered in children <11 years old, while serum total cholesterol and urinalysis were more likely to be administered in children age 11 or older (Table 3). Male children were less likely to have a CBC count compared with female children, but more likely to have an echocardiogram.
Table 2.
Studies Performed
| All Patients N=3588 (%) |
Race/Ethnicity | Gender | Age at Diagnosis | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Black (n=1642) |
Hispanic (n=160) |
White (n=1471) |
Other (n=315) |
Male (n=2176) |
Female (n=1412) |
≥11 Years (n=2658) |
<11 Years (n=930) |
||
| Serum creatinine | 1751 (49) | 763 ( 47) | 88 (55) | 756 (51) | 144 (46) | 4057 (49) | 694 (49) | 1263 (48) | 488 (52) |
| CBC count | 1071 (30) | 438 (27) | 44 (28) | 508 (35) | 81 (26) | 605 (28) | 466 (33) | 712 (27) | 359 (39) |
| Serum total cholesterol |
749 (21) | 388 (24) | 31 (19) | 274 (19) | 56 (18) | 453 (21) | 296 (21) | 618 (23) | 131 (14) |
| Serum catecholamine | 58 (2) | 20 (1) | 0 (0) | 27 (2) | 11 (3) | 39 (2) | 19 (1) | 44 (2) | 14 (2) |
| Serum renin | 49 (1) | 18 (1) | 0 (0) | 21 (1) | 10 (3) | 32 (2) | 17 (1) | 37 (1) | 12 (1) |
| Urinalysis | 481 (13) | 206 (13) | 11 (7) | 219 (15) | 45 (14) | 273 (13) | 208 (15) | 377 (14) | 104 (11) |
| Urine drug screen | 9 (<1) | 8 (1) | 0 (0) | 1 (<1) | 0 (0) | 6 (<1) | 3 (<1) | 9 (<1) | 0 (0) |
| Renal ultrasound | 506 (14) | 208 (13) | 34 (21) | 216 (15) | 48 (15) | 304 (14) | 202 (14) | 307 (12) | 199 (21) |
| Echocardiogram | 1423 (40) | 624 (38) | 72 (45) | 598 (41) | 129 (41) | 935 (43) | 488 (35) | 1052 (40) | 371 (40) |
| Sleep study | 91 (3) | 53 (3) | 4 (3) | 31 (2) | 3 (1) | 52 (2) | 39 (3) | 60 (2) | 31 (3) |
CBC indicates complete blood cell.
Table 3.
Odds Ratios (95% Confidence Intervals) for Study Performance
| Race/Ethnicity | Gender | Age at diagnosis | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Black n=1642 |
Hispanic n=160 |
Other n=315 | White n=1471 |
Male n=2176 |
Female n=1412 |
≥11 years n=2658 |
<11 years n=930 |
|
| Serum creatinine | 0.82a (0.71–0.95) |
1.16 (0.83–1.60) |
0.80 (0.62–1.02) |
REF | 0.98 (0.85–1.12) |
REF | 0.82a (0.71–0.95) |
REF |
| CBC count | 0.69a (0.59–0.80) |
0.72 (0.50–1.03) |
0.66a (0.50–0.86) |
REF | 0.78a (0.68–0.90) |
REF | 0.58a (0.50–0.68) |
REF |
| Serum total cholesterol |
1.35a (1.14–1.61) |
1.05 (0.69–1.59) |
0.94 (0.69–1.30) |
REF | 0.99 (0.84–1.17) |
REF | 1.85a (1.50–2.27) |
REF |
| Serum catecholamine |
0.66 (0.37–1.18) |
N/A | 1.93 (0.95–3.94) |
REF | 1.33 (0.77–2.32) |
REF | 1.10 (0.60–2.02) |
REF |
| Serum renin | 0.77 (0.41–1.44) |
N/A | 2.26a (1.06–4.86) |
REF | 1.22 (0.68–2.21) |
REF | 1.08 (0.56–2.08) |
REF |
| Urinalysis | 0.82 (0.67–1.01) |
0.42a (0.23–0.79) |
0.95 (0.67–1.35) |
REF | 0.83 (0.68–1.01) |
REF | 1.31a (1.04–1.65) |
REF |
| Urine drug screen | 7.20 (0.90–57.6) |
N/A | N/A | REF | 1.30 (0.32–5.20) |
REF | N/A | REF |
| Renal ultrasound | 0.84 (0.69–1.03) |
1.57a (1.05–2.35) |
1.04 (0.74–1.47) |
REF | 0.97 (0.80–1.18) |
REF | 0.48a (0.39–0.58) |
REF |
| Echocardiogram | 0.89 (0.77–1.04) |
1.19 (0.86–1.66) |
1.01 (0.79–1.30) |
REF | 1.43a (1.24–1.64) |
REF | 0.99 (0.85–1.15) |
REF |
| Sleep study | 1.55 (0.99–2.43) |
1.19 (0.42–3.42) |
0.45 (0.14–1.47) |
REF | 0.86 (0.57–1.31) |
REF | 0.67 (0.43–1.04) |
REF |
CBC indicates complete blood cell; N/A, odds ratio could not be calculated due to homogeneous outcome in group.
Significant odds ratio.
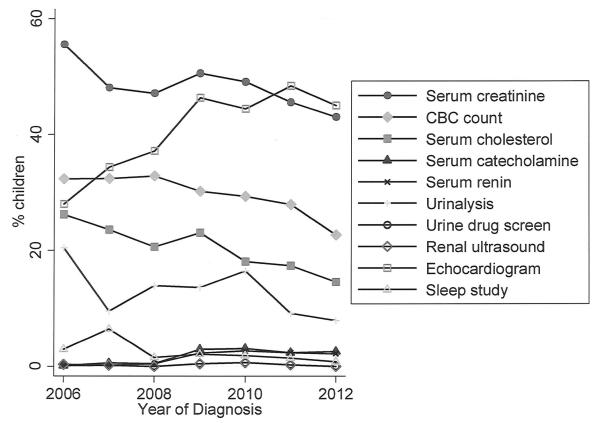
There was no significant trend over time in the proportion of children with at least one test administered (p=0.24). Analyzed individually, renal ultrasound, urine drug screen, serum catecholamine, and sleep study did not show any significant trends in administration over time. Serum creatinine, serum cholesterol, serum renin, urinalysis, and echocardiogram were administered less frequently over time, while CBC count was administered more frequently (all p-values <0.001) (Figure 1).
Figure 1.
Test administration over time.
Discussion
To the best of our knowledge, this is the first study evaluating the adherence by medical providers to the NHBPEP’s Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. We found that providers infrequently administered the tests recommended by the report and that adherence did not improve over time. The proportion of children receiving tests varied by demographic characteristics including race and age. Our findings serve as baseline data to evaluate interventions aimed at improving guideline adherence in the future.
Nearly one in six children diagnosed with hypertension received none of the NHBPEP-recommended tests, and 48% of children who received at least one test received no more than two. This profound lack of adherence to the NHBPEP guidelines, while disappointing, is not surprising. Several studies in adults have reported poor adherence to a variety of guidelines for diseases including hypertension, arrhythmias, and rheumatologic disorders.4-7 In the pediatric population, a longitudinal analysis of 951 adolescents with hypertension, identified through the state of Michigan’s Medicaid claims data, found that only 24% of adolescents received an echocardiogram and 22% a renal ultrasound, while 50% had an electrocardiogram performed.8 Other diagnostic tests were not evaluated in this study, which found that younger children were more likely to have both a renal ultrasound and an echocardiogram. These findings are in part consistent with our study. Compared to the Michigan findings, our cohort had a higher proportion of children receiving an echocardiogram (40%) and a lower proportion receiving a renal ultrasound (14%). There are several potential explanations for the observed differences. First, the Michigan study analyzed claims data from 2003 to 2008, a study period that spanned the release of the NHBPEP guidelines. Second, our study included children age 3 to 18 years, as opposed to the adolescents-only population analyzed in the Michigan study. Providers may be more likely to refer younger patients to specialists for imaging studies such as renal ultrasounds and echocardiograms to diagnose secondary causes of hypertension compared with adolescent patients, who may be treated more like their adult counterparts, and guidelines in the adult population differ significantly. Indeed, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure in adults, published in 2003, focuses primarily on the detection and treatment of hypertension.9 Its recommendation for diagnostic work-up includes electrocardiograms, laboratory studies, and other ancillary procedures, but not echocardiography, while renal ultrasounds are only recommended if renal disease is suspected based on other assessments. Finally, our study included patients of all payer types treated at a large academic medical center, as opposed to Medicaid patients treated at any medical facility in the Michigan study. Although our data source did not allow us to analyze the differences in guideline adherence and types of diagnostic studies performed by payer type, we can speculate that non-Medicaid patients may be more likely to receive certain tests including ultrasounds and echocardiograms.
The lack of adherence to the NHBPEP guidelines is disappointing because adherence to guidelines has been shown to improve the quality of care provided for a variety of conditions including hypertension in adults and several pediatric diseases.10 Most pediatric providers would agree that the variability and overall low proportion of tests received by the patients in our cohort indicates suboptimal management of hypertension in children. Lack of awareness and familiarity often is cited as a reason for poor adherence to guidelines and may have played a role in our study.11 The rising incidence of the so-called metabolic syndrome in children has resulted in a higher prevalence of primary hypertension in this population.2 The higher prevalence of primary hypertension shifts the burden of care for pediatric hypertension away from the subspecialist toward primary care pediatricians. Unfortunately, primary care pediatricians may be less familiar with hypertension diagnosis and management algorithms, and may not know about the existence of the NHBPEP guidelines. Even when aware of the guidelines, primary care pediatricians may struggle to follow them. The evaluation recommended in the current guidelines is extensive, requiring blood work, imaging, and likely referrals to specialists. Performing all these tasks may be particularly challenging in a high-volume primary care pediatric office. This is true not only for the pediatrician but also for the patients and their families, who may be required to make several visits to different medical facilities if they are to receive all the recommended studies. To ease the burden on both their practice and their patients, pediatricians may choose to limit the number of diagnostic studies ordered. Also, some providers may be familiar with the adult hypertension guidelines, which have been in circulation longer and are more widely disseminated, and may follow these guidelines when managing pediatric patients with hypertension, particularly adolescents. Unfortunately, there are significant differences between the adult and pediatric guidelines, and knowledge of the adult guidelines does not necessarily translate into adherence to the pediatric version.12
Another potential explanation for poor guideline adherence is provider disagreement with the recommendations.11 Again, this may have played a role in our study, and it is worth noting that the NHBPEP guidelines are based on expert consensus more so than on objective results from clinical studies. The finding that the number and type of tests obtained varied with patient age suggests that providers did not always feel that each test was indicated for a specific patient. Renal ultrasounds, for example, were ordered more frequently for younger children (<11 years old). Renal ultrasounds are recommended based on their utility for screening for hypertension secondary to structural renal disease, which is a much less common etiology of hypertension as children get older.2,13 Obtaining renal ultrasounds on patients who are hypertensive as part of the metabolic syndrome likely would have little utility. Providers may also choose to forgo certain tests based on cost containment principles, or because of the severity of the hypertension diagnosis. It is worth noting that echocardiograms were the second most common test ordered after serum creatinine, and the most commonly ordered imaging study. Echocardiograms may be frequently used because they can be justified as both a diagnostic test and a risk-stratifying tool. Although they are not recommended in the NHBPEP report for screening purposes, echocardiograms can be used to evaluate for coarctation of the aorta as a cause of hypertension.14 Hypertension secondary to coarctation is potentially reversible following surgical or catheter-based intervention, so an echocardiogram may be viewed as reasonable before starting long-term antihypertensive medication, although it is important to recall that coarctation can also be strongly suspected based on physical examination findings such as differential blood pressure and altered femoral pulses.14,15 The NHBPEP report does recommend echocardiograms to assess left ventricular mass as a surrogate for end organ damage, and several echocardiograms in our study were likely ordered for this indication. Left ventricular hypertrophy has prognostic significance as it is strongly associated with coronary artery disease, stroke, and sudden death in adults.15,16 However, it is unknown whether the same is true in children. Even so, a baseline echocardiogram can aid in the selection and timing of medications. It is also possible that providers chose to follow a different set of guidelines, such as in the treatment of hypertensive children with renal disease, for which different guidelines exist that vary substantially both in terms of diagnostic procedures and treatment recommendations.17
Our study has a number of strengths. First, our cohort is reflective of the target population of the NHBPEP report: We included all patients 3 to 18 years old and did not exclude any patients based on payer status, etiology of their hypertension, or whether they were being treated for hypertension. Second, our cohort was larger than any previous study evaluating the diagnostic work-up of children with hypertension. Third, we analyzed data over a 7-year period starting 2 years after the publication of the NHBPEP guidelines. This study period allowed sufficient time for at least early dissemination of the guidelines and provided us the opportunity to evaluate changes in adherence over time. Despite these strengths, our study suffers from several limitations. As a retrospective chart review, our study data have not undergone the scrutiny normally accorded a prospectively developed clinical study database. As such, certain important data elements were not available for review and analysis. Most importantly, we did not have access to actual blood pressure recordings, to categorize all children by stages of hypertension. We were able to address this potential limitation, however, by performing a detailed chart review of a subset of 900 children (25% of our cohort). In all cases, we were able to identify at least 3 blood pressure recordings above the 95th percentile, confirming a true diagnosis of hypertension. We also were not able to reliably assess if children were obese at the time of their evaluation. These limitations are particularly important given the fact that diagnostic study recommendations in the NHBPEP guidelines are stratified by severity of hypertension and diagnosis of obesity. Similarly, we did not have information about physical examination results, which may have pointed physicians toward a particular etiology including coarctation of the aorta. We chose a more conservative analysis approaching erring on the side of underreporting guideline adherence, by stipulating that all 10 analyzed tests were recommended for all children. However, the guidelines clearly do not recommend all tests in all cases, including those cases where an etiology is confirmed by an earlier test (e.g., coarctation of the aorta confirmed by echocardiography). Further, some of the recommended studies were not captured in our data source, such as ambulatory blood pressure monitoring and retinal examinations; however, we were able to reliably identify 10 of the recommended tests. Our data were limited to studies performed within a single academic medical center, which may have led us to underestimate the proportion of patients receiving a certain test if it was performed at other institutions or those performed prior to the documentation of a diagnostic code for hypertension. Our study also did not address the issue of spontaneous resolution of hypertension, which may have occurred in a subset of patients. Finally, we were unable to determine the exact indication for each diagnostic test, which may have caused us to overestimate the adherence to the guidelines when studies were done for indications other than hypertension, and any management changes that occurred following interpretation of the test results. On the other hand, our ability to record the exact date of each study allowed us to limit our analysis to studies completed within 1 year of the first outpatient visit for hypertension, likely limiting the chance that a study was ordered for another indication diagnosed later.
Conclusion
In summary, we found that children evaluated for hypertension in the outpatient setting infrequently receive the diagnostic tests recommended in the NHBPEP’s recent reports. Certain demographic characteristics are associated with higher rates of tests being administered. Unfortunately, adherence to the guidelines did not improve over time. Our findings serve as important baseline data to evaluate the performance of future interventions to improve adherence.
Acknowledgments
Funding
Dr Hornik receives salary support for research from the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR001117). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Conflicting Interests
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Lande MB, Carson NL, Roy J, Meagher CC. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension. 2006;48:40–44. doi: 10.1161/01.HYP.0000227029.10536.e8. [DOI] [PubMed] [Google Scholar]
- 2.Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA. 2007;298:874–879. doi: 10.1001/jama.298.8.874. [DOI] [PubMed] [Google Scholar]
- 3.National High Blood Pressure Education Program Working Group on Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 4.Allen LaPointe NM, Lokhnygina Y, Sanders GD, et al. Adherence to guideline recommendations for antiarrhythmic drugs in atrial fibrillation. Am Heart J. 2013;166:871–878. doi: 10.1016/j.ahj.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman MA, Fischer MA, Shlipak MG, et al. Clinician awareness of adherence to hypertension guidelines. Am J Med. 2004;117:747–754. doi: 10.1016/j.amjmed.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 6.Farah Z, Reddy V, Matthews W, Giles I. Poor adherence to guidelines on early management of acute hot swollen joint(s): an evaluation of clinical practice and implications for training. Int J Clin Pract. 2015;69:618–622. doi: 10.1111/ijcp.12580. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin DK, Jr, Hudak ML, Duara S, et al. Effect of fluconazole prophylaxis on candidiasis and mortality in premature infants: a randomized clinical trial. JAMA. 2014;311:1742–1749. doi: 10.1001/jama.2014.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon EY, Cohn L, Rocchini A, et al. Use of diagnostic tests in adolescents with essential hypertension. Arch Pediatr Adolesc Med. 2012;166:857–862. doi: 10.1001/archpediatrics.2012.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 10.Brownbridge G, Evans A, Fitter M, Platts M. An interactive computerized protocol for the management of hypertension: effects on the general practitioner's clinical behaviour. J R Coll Gen Pract. 1986;36:198–202. [PMC free article] [PubMed] [Google Scholar]
- 11.Arroll B. Why are guidelines not used and what can be done to change that? N Z Fam Physician. 2003;30:324–326. [Google Scholar]
- 12.Gooding HC, Rodday AM, Wong JB, et al. Application of pediatric and adult guidelines for treatment of lipid levels among US adolescents transitioning to young adulthood. JAMA Pediatr. 2015;169:569–574. doi: 10.1001/jamapediatrics.2015.0168. [DOI] [PubMed] [Google Scholar]
- 13.Gavrilovici C, Boiculese LV, Brumariu O, Dimitriu AG. [Etiology and blood pressure patterns in secondary hypertension in children] Rev Med Chir Soc Med Nat Iasi. 2007;111:70–81. [PubMed] [Google Scholar]
- 14.Sechtem U. Imaging of aortic coarctation—difficult choices. Eur Heart J. 1995;16:1315–1316. doi: 10.1093/oxfordjournals.eurheartj.a060737. [DOI] [PubMed] [Google Scholar]
- 15.O’Sullivan JJ, Derrick G, Darnell R. Prevalence of hypertension in children after early repair of coarctation of the aorta: a cohort study using casual and 24 hour blood pressure measurement. Heart. 2002;88:163–166. doi: 10.1136/heart.88.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniels SR, Arnett DK, Eckel RH, et al. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111:1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- 17.Dionne JM. Evidence-based guidelines for the management of hypertension in children with chronic kidney disease. Pediatr Nephrol. 2015;30:1919–1927. doi: 10.1007/s00467-015-3077-7. [DOI] [PubMed] [Google Scholar]