CASE
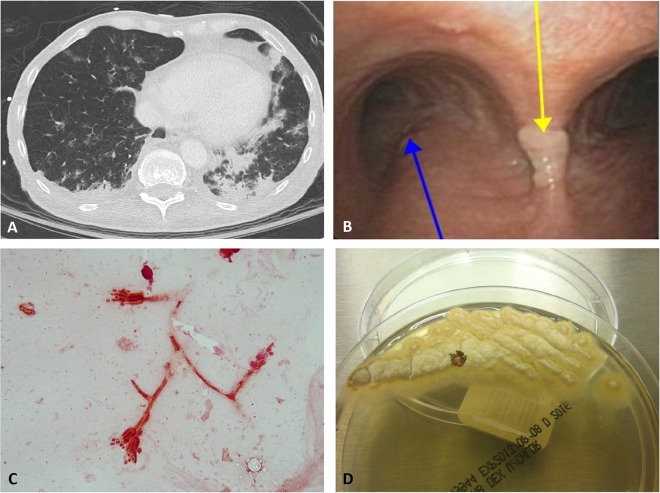
A 61-year-old male presented to a hospital in Missouri with several months of progressive shortness of breath and low-grade fevers. He had a past medical history of chronic obstructive pulmonary disease and diffuse large B-cell lymphoma, which was treated with a matched unrelated donor bone marrow transplant 10 months prior and was complicated by chronic graft-versus-host disease, requiring significant immunosuppression with prednisone, tacrolimus, and mycophenolate mofetil. This was further complicated by presumed Aspergillus species pneumonia (diagnosed by imaging and clinical syndrome). He was initially treated with voriconazole and subsequently liposomal amphotericin B, with gradual worsening. The patient lived in rural Missouri, where he worked as a farmer; he denied any recent travel. Computed tomography (CT) of the chest was performed and was concerning for worsening fungal pneumonia (Fig. 1A). When a bronchoscopy was performed to obtain a specimen, cottony white spots were observed throughout the bronchial tree (Fig. 1B). The patient subsequently decompensated and required intubation. A direct Gram stain of the tracheal aspirate collected immediately after intubation revealed the fruiting bodies of a fungus (Fig. 1C). Fungal elements are uncommonly seen on direct Gram stains of clinical specimens, and it is very rare to see fruiting bodies, as shown in Fig. 1C. The tapering phialides with phialoconidia observed on Gram staining resembled those of Penicillium or Paecilomyces species. The infectious diseases fellow taking care of the patient was notified of the positive Gram stain and was provided a differential diagnosis of Penicillium versus Paecilomyces species on the basis of the Gram stain. Within 3 days of incubation, a yellow-tan mold was recovered from both mycology cultures and aerobic bacteriology cultures (Fig. 1D). A lactophenol cotton blue scotch tape prep revealed long, slender, tapering phialides characteristic of Paecilomyces species. At the time of isolation, the standard of care in the laboratory was to perform genus-level identification based on morphology. On the basis of the macroscopic and microscopic morphology, an identification of Paecilomyces sp. was reported. Unfortunately, after the specimen was obtained, the patient continued to decompensate and expired shortly thereafter.
FIG 1.
(A) CT of the patient's chest showing nodular infiltrates highly suggestive of fungal infection. (B) Bronchoscopy with a fungal growth (yellow arrow) on the carina, next to the right main stem bronchus (blue arrow). (C) Direct Gram stain of patient's tracheal aspirate showing tapering phialides with phialoconidia suggestive of Penicillium or Paecilomyces species. (D) Yellow-tan colonies on Sabouraud dextrose agar characteristic of Paecilomyces species.
DISCUSSION
Paecilomyces species are emerging pathogens. Isolates were previously considered contaminants when recovered in culture because of the ubiquitous nature of the genus, but because of the growing number of immunocompromised patients, Paecilomyces is increasingly associated with disease (1). Paecilomyces species have also been reported to cause infections in immunocompetent hosts, but this is much less common (1). Cases of endocarditis, nephritis, pulmonary infections, and catheter-related fungemia, as well as nail, ocular, cutaneous, and subcutaneous infections, have been attributed to Paecilomyces species (2). Infection occurs through inhalation, through breakdown of the skin, and through indwelling catheters. Paecilomyces variotii and Paecilomyces lilacinus are the two species most commonly associated with human infections. Other species, such as Paecilomyces marquandii and Paecilomyces javanicus, have also been reported, although less frequently (3). Of note, P. lilacinus was recently reassigned to a novel genus, Purpureocillium, and renamed Purpureocillium lilacinum. However, it will be included in the discussion below since it has historically been included in studies and reviews of Paecilomyces species.
Fungal pneumonia is a relatively common disease in bone marrow transplant patients, especially during the profound immunosuppression of graft-versus-host disease. The most common causative agents are Aspergillus species, Fusarium species, members of the order Mucorales, and, less frequently, other molds. Including this case, there have been five cases of Paecilomyces pneumonia reported to date (3). Of those, four patients were immunocompromised, including two patients with hematologic malignancies, similar to the present case (3). Patient presentations consisted of fever, pleuritic pain, productive cough, and dyspnea. Chest imaging demonstrated nodules, hilar lymphadenopathy, cavitary lesions, and opacities. Fungal structures were seen on direct smear in three of the five cases, although in one case, fungal structures were only noted on a second specimen submitted 10 days after the initial smear-negative specimen. In the other two cases, fungal forms were noted on histology of biopsy and autopsy specimens, respectively. In the limited reported cases, the mortality rate was 40% (3).
Paecilomyces species are members of the traditionally described hyaline hyphomycetes and are found ubiquitously in the environment, primarily in soil, decaying plants, and food products. Paecilomyces species are known to feed on agricultural pests, leading to their use as a bionematicide where the mold is liberally applied on farms in order to provide enduring protection against pests. Paecilomyces species have been used throughout the world and may have been used around the patient's home town, where he worked as a farmer. However, no human infection has ever been conclusively associated with such practices.
Growth in culture is rapid; maturity is reached within 3 days. Colonies are usually flat and powdery or velvety. The surface color can be variable, ranging from yellow-tan, pink-mauve, and white to yellow-green. Notably, P. lilacinum colonies are lilac in color and typically grow more slowly than Paecilomyces species. However, the color of Paecilomyces colonies is never bright green or blue-green, as seen with Penicillium species. The reverse is off-white, pinkish, yellow, or pale brown (2). The difference in surface color between the two genera is important for identification, as the microscopic morphologies of Paecilomyces and Penicillium species show a high degree of similarity. It is important to note that some Penicillium species may not produce a characteristic blue-green pigment and may resemble Paecilomyces species, potentially complicating correct identification.
The microscopic morphology of both genera, as demonstrated by lactophenol cotton blue scotch tape prep, reveals septate hyphae with unbranched conidiophores. Flask-shaped phialides with long, unbranched chains of round or oval single-celled phialoconidia are present. The major factors distinguishing the two genera, in addition to colony color, are the length and shape of the phialides. While the phialides of both genera are flask shaped, the phialides of Paecilomyces species are more elongated and taper to a long, slender neck, while the phialides of Penicillium species tend to be shorter and more compact (2). Identification to the species level on the basis of morphology alone is difficult.
Because of the difficulty in identification to the species level on the basis of morphology, molecular methods are more frequently being used for the identification of Paecilomyces and related genera. In one study, sequencing of internal transcribed spacer regions 1 and 2 and a portion of the beta-tubulin gene was performed for 34 isolates previously identified by morphological methods as P. variotti or P. lilacinus (4). Of 31 isolates morphologically identified as P. variotii, 5 (16%) were identified as either Talaromyces eburneus or Hamigera avellanea by molecular methods.
Clinical management of Paecilomyces infection consists of antifungal therapy, surgery, or a combination of the two (5). Different species of Paecilomyces show various degrees of antifungal resistance. Of the two species most frequently isolated from human infections, P. lilacinum is often highly resistant to amphotericin B in vitro, while P. variottii is typically susceptible (5). In contrast, P. variottii complex isolates appear to be resistant to voriconazole, while P. lilacinum isolates are susceptible (4). Resistance to voriconazole has been reported both in vitro and in vivo (1, 6). This includes a new diagnosis of voriconazole-resistant Paecilomyces infection in a patient on voriconazole prophylaxis (6). While no optimal antifungal treatment has been established, posaconazole is emerging as an effective treatment for Paecilomyces because there are no known cases of resistance (4, 5). Antifungal susceptibility testing of Paecilomyces species is not routinely performed in clinical microbiology laboratories, and CLSI guidelines for performance or interpretation do not exist. In vitro susceptibility data are available primarily from research studies. A recent in vitro analysis found that posaconazole and terbinafine were the only antifungals to achieve uniform in vitro susceptibility among all of the clinical isolates tested (4). Because of the differences in in vitro susceptibility profiles, accurate identification to the species level is important to help guide appropriate therapy for severe infections, although the correlation between in vitro and in vivo results remains to be elucidated.
In conclusion, pneumonia due to Paecilomyces species is rare and is diagnosed primarily in immunocompromised hosts. However, despite the infrequency of Paecilomyces pneumonia, the presence of molds other than Aspergillus and members of the order the Mucorales should be maintained in the differential diagnosis of fungal pneumonias in immunocompromised patients. Early and aggressive microbiologic diagnosis should be sought in cases without rapid improvement.
SELF-ASSESSMENT QUESTIONS
- Paecilomyces species may display various pigmentation; however, isolates never appear:
-
(a)Yellow-tan
-
(b)Yellow-green
-
(c)Blue-green
-
(d)Pink-mauve
-
(a)
- Paecilomyces species have not been associated with which of the following infection(s):
-
(a)Endocarditis
-
(b)Catheter-related fungemia
-
(c)Skin infections
-
(d)Cholangitis
-
(a)
- Which antifungal is emerging as the most effective treatment for Paecilomyces species?
-
(a)Amphotericin B
-
(b)Posaconazole
-
(c)Voriconazole
-
(d)Flucytosine
-
(a)
(For answers to the self-assessment questions and take-home points, see page 2628 in this issue [doi:10.1128/JCM.00050-16].)
REFERENCES
- 1.Pastor FJ, Guarro J. 2006. Clinical manifestations, treatment and outcome of Paecilomyces lilacinus infections. Clin Microbiol Infect 12:948–960. doi: 10.1111/j.1469-0691.2006.01481.x. [DOI] [PubMed] [Google Scholar]
- 2.Larone DH. 2011. Medically important fungi: a guide to identification, 5th ed ASM Press, Washington, DC. [Google Scholar]
- 3.Steiner B, Aquino VR, Paz AA, Silla LM, Zavascki A, Goldani LZ. 2013. Paecilomyces variotii as an emergent pathogenic agent of pneumonia. Case Rep Infect Dis 2013:273848. doi: 10.1155/2013/273848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houbraken J, Verweij PE, Rijs AJ, Borman AM, Samson RA. 2010. Identification of Paecilomyces variotii in clinical samples and settings. J Clin Microbiol 48:2754–2761. doi: 10.1128/JCM.00764-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tortorano AM, Richardson M, Roilides E, van Diepeningen A, Caira M, Munoz P, Johnson E, Meletiadis J, Pana ZD, Lackner M, Verweij P, Freiberger T, Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, Lanternier F, Pagano L, Skiada A, Akova M, Arendrup MC, Boekhout T, Chowdhary A, Cuenca-Estrella M, Guinea J, Guarro J, de Hoog S, Hope W, Kathuria S, Lortholary O, Meis JF, Ullmann AJ, Petrikkos G, Lass-Florl C, European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group, European Confederation of Medical Mycology. 2014. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect 20(Suppl 3):S27–S46. doi: 10.1111/1469-0691.12465. [DOI] [PubMed] [Google Scholar]
- 6.Chamilos G, Kontoyiannis DP. 2005. Voriconazole-resistant disseminated Paecilomyces variotii infection in a neutropenic patient with leukaemia on voriconazole prophylaxis. J Infect 51:e225–e228. doi: 10.1016/j.jinf.2005.02.005. [DOI] [PubMed] [Google Scholar]