Abstract
Unmanned aerial vehicles (UAVs) could potentially be used to transport microbiological specimens. To examine the impact of UAVs on microbiological specimens, blood and sputum culture specimens were seeded with usual pathogens and flown in a UAV for 30 ± 2 min. Times to recovery, colony counts, morphologies, and matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS)-based identifications of the flown and stationary specimens were similar for all microbes studied.
TEXT
One of the factors that worsened the West African Ebola outbreak was the poor roads that hindered the transport of biological samples (1, 2). While the problem of poor road access is not new or unique to West Africa, there is now a relatively inexpensive solution with a relatively low barrier to implementation, unmanned aerial vehicles (UAVs). Because of this low barrier, the use of UAVs in industries such as the film, mining, and agriculture industries is expanding rapidly. These industries represent the majority of the 5,292 exemptions that the U.S. Federal Aviation Authority has granted so far (as of June 2016) for the use of UAVs (http://www.faa.gov/uas/beyond_the_basics/section_333/). One reason the use of UAVs in health care lags behind that in other industries is that transporting biological specimens requires additional specimen-specific validation (3–7). UAVs are a viable way to transport laboratory specimens only if the UAVs do not adversely affect the results for those specimens (3–7). For example, pneumatic tubes commonly used for in-house hospital transportation cause damage to various types of specimens (6, 7). The forces applied on a sample transported by a UAV include sudden accelerations and decelerations, exposure to ambient temperatures, and other impacts which cannot be predicted a priori. In addition, the work presented here will help to provide a template for future validation experiments using other UAVs, organisms, sample types, or environments. In this report, we examine the impact of drone transport on blood and sputum specimens (Fig. 1).
FIG 1.
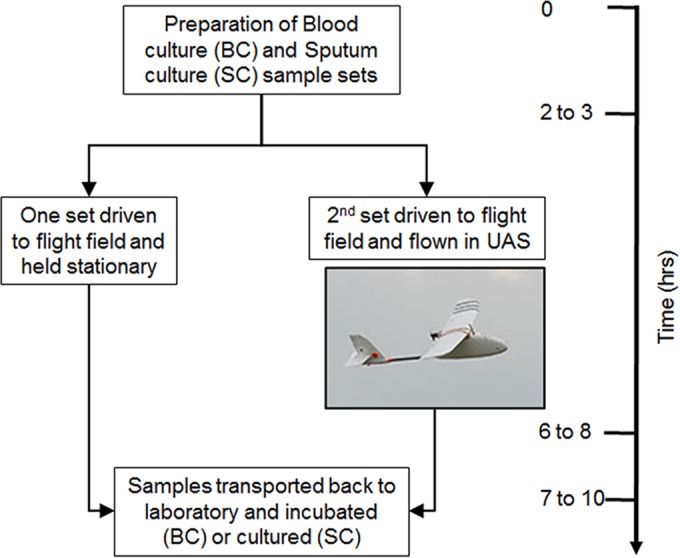
Timeline and schematic for blood and sputum drone flights.
Six sets of paired aerobic and anaerobic Bactec blood culture bottles were inoculated with 10 ml of whole blood from a commercial blood bank and spiked with one of four organisms (Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, or Bacteroides fragilis), for a total of 12 bottles per organism. S. aureus and E. coli strains were clinical isolates. The S. pneumoniae (ATCC 49619) and B. fragilis (ATCC 25285) strains were standard type strains. Dilutions were made in Mueller-Hinton broth to achieve final concentrations of 10 CFU/ml of whole blood added (see Data Set S1 in the supplemental material). Plate counts were performed to verify anticipated spike levels.
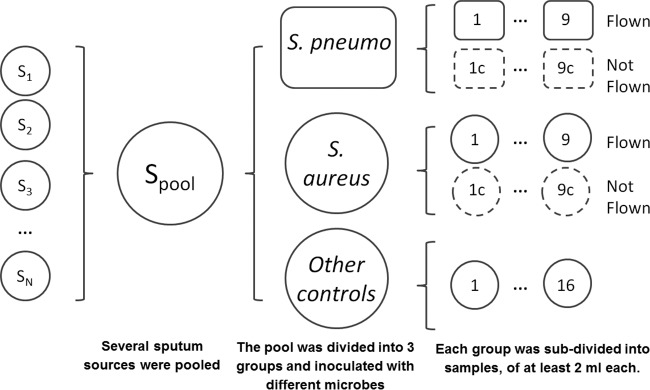
Pathogen-free (containing only normal flora), nonmucoidal sputa (such as those recovered from patients without cystic fibrosis) were collected from the Johns Hopkins Hospital clinical microbiology laboratory over a 10-day period. Samples from patients with a history of acid-fast bacillus (AFB) positivity were excluded. Sputa collected prior to 5 days preflight were stored at −80°C; subsequent samples were stored at 4°C. Sample collection stopped at t minus 2 days to allow verification of the pathogen-free status as defined above. The sputa were pooled and diluted ∼35% (vol/vol) with sterile saline to afford a large enough pool for the experiment. The sputa were eventually split into 52 vials, with each containing 2 ml of pooled sputum. Thirty-six of these 52 vials were inoculated with 50 μl of S. aureus or S. pneumoniae from initial concentrations of 1 McFarland, 1/10 McFarland, and 1/100 McFarland standards (Data Set S1 and Fig. 2). The remaining 16 sputum specimens were additional controls for background, transit, etc. (Data Set S1 and Fig. 2). The initial 36 sputum specimens as well as all the blood specimens were packed in individual biohazard bags and driven to the flight field under ambient conditions. Half of the samples were flown in the UAV for 30 ± 2 min, and the rest were held stationary. Based on our flight speeds, this 30-min flight was the equivalent of a 20- to 25-km distance. Samples were flown in a small fixed-wing aircraft (Aero, 3D Robotics, Berkeley, CA). A fixed-wing aircraft was selected over other aircraft types, such as a helicopter or multirotor aircraft, because it has the best range capability for a given takeoff weight, is least expensive, and is mechanically simple. The aircraft was launched by a hand toss and landed by sliding to a stop on its belly (https://vimeo.com/150113435). The aircraft was marked with an IATA label designating the contents a class 6.2 infectious substance. The maximum ambient temperatures on the first and second flight days were 45°F and 47°F, respectively. The minimum temperatures during the experimental window were 38°F and 39°F, respectively.
FIG 2.
Preparation of sputum specimens.
After the flight operations were concluded, all of the samples (flown and stationary) were transported back to the Johns Hopkins Hospital microbiology laboratory. Blood culture bottles were placed directly onto the BD Bactec FX instrument (Becton Dickinson, NJ, USA). The sputum samples were plated on standard media (blood agar plate [BAP; Remel R01202], MacConkey [MAC; Remel R01552], MRSA Select [Bio-Rad 63747], chocolate [CHOC; Remel R060482], chocolate with bacitracin [CHOCB; Hardy E11], anaerobic CDC agar plates [ANA-BAP; Remel R01036], and Columbia CNA agar with 5% sheep blood [CNA; Remel R01322]). Organisms in positive blood cultures and sputum samples were identified using standard microbiological methods and matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Bruker Corp., MA, USA).
The times to growth (Fig. 3) for the blood specimens, as well as the colony counts for the sputum specimens, were similar for the flown and stationary sample sets. In addition, there was no significant difference in the conditions of growth (media, O2 dependence, etc.) for flown versus stationary blood or sputum specimens (see Data Set S1 and Fig. S1 in the supplemental material). There were small differences in the amounts of recovered S. pneumoniae between the 1-McFarland-standard flown and stationary sample sets (Data Set S1) because the stationary samples had higher levels of background alpha-hemolytic bacteria present. But these differences were not large or consistent.
FIG 3.
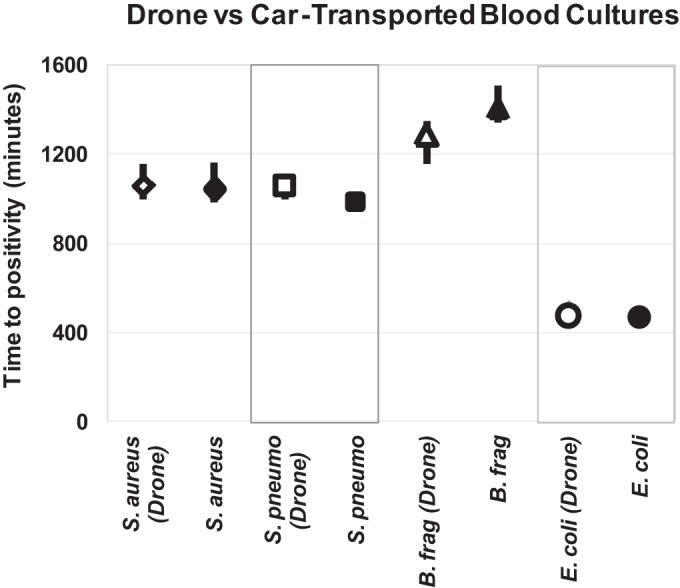
Times to positivity for drone-transported versus stationary samples. Each marker represents the average time to positivity for all bottles whose contents are positive for each type of organism. The bars represent the range (highest and lowest) of times to positivity for each set of samples. Of note, there are bars in the S. pneumoniae and E. coli panels. However, they are small and are obscured by the markers.
At the inception of this work, there was no precedent for packaging microbiological samples for UAV transport. Our approach to address this was described earlier (8). Briefly, we considered environmental variables that might be relevant for this mode of transportation, including temperature, atmospheric pressure, and acceleration. Changes in temperature and atmospheric pressure with altitude are small (0.6°C/100 m and 0.012 atm/100 m) for the environment in which the aircraft were operating (9). Therefore, we reasoned that no specific measures would be needed to stabilize temperature or pressure when ambient conditions were not extreme. However, because the UAV was launched by a hand toss and landed by sliding to a stop on its belly (https://vimeo.com/150113435), we anticipated that acceleration might be a significant environmental factor. To mitigate these effects, we lined the fuselage with custom-cut vibration-absorbing foam. The fuselage itself is made of impact-absorbing expanded polystyrene (EPS) foam (i.e., Styrofoam).
To the best of our knowledge, this is the first report of the impact of UAV transportation on microbiological specimens. Previous work on UAV transport of laboratory specimens focused on routine chemistry, hematology, and coagulation tests (8). The results of this small study are consistent with the possibility of using of UAVs for the transport of microbiological samples. However, the use of drones for medical sample transport is not yet settled. While there are models showing that the use of drone networks can increase the reach and decrease the cost of vaccines (10), there is very limited real-world data either supporting or opposing drone transport of medical specimens. In addition, there are other factors to consider, including regulations, cost, and safety. We will address these issues briefly below.
The exact number of UAVs sold in 2015 is not known; however, estimates based on market reports suggest that the number is between 1 million and 2 million. In spite of this, only 57 of the 174 countries in the world have regulations regarding civilian drone use (http://uavcoach.com/drone-laws/). Most of these regulations are de facto bans rather than a list of requirements for safe and legal UAV use. In the United States, there is a two-tiered regulatory approach to drones. The first tier comprises the broad restrictions covering all drone use, and the second tier comprises the exemptions granted for specific-use cases (https://www.faa.gov/uas/). Looking forward, the list of countries with regulations governing drone use is increasing quickly in response to the rapid adoption of drone technology by the public. Costs for drones differ by several logs, depending on the grade (military versus civilian) and technological endowment (flight controller, sense-and-avoid ability, etc.) of the vehicle. The drone system (airframe, flight controller, etc.) used for this study cost <$2,000.
Regarding safety, commercial air carriers in the United States in 2015 had 0.1 fatalities per 100 million passengers on board, and general aviation (i.e., mostly small personal aircraft) had 1.03 fatal accidents per 100,000 flight hours (11). No one can currently answer the question “How safe are drones?”, because the analogous data for drones are unavailable. In keeping with the nascent nature of this field, clear requirements for safe operation of drones are still being determined by relevant groups such as the FAA. However, comparing the current state of UAV safety to that for manned flight is instructive, as the eventual framework for drone safety will likely mirror that currently in place for other forms of air traffic. There are three components of the framework that are key in ensuring the safety of air traffic. These are vehicular (aircraft), operator (pilot), and operational (weather) safety. We will examine each of these components briefly for current manned aircraft compared to drones.
The vehicles used for manned aircraft are certified in the design phase, and each individual aircraft is registered and regularly inspected. In contrast, only a few types of drones are currently individually registered and inspected. It is expected that as the use of commercial drones increases, regulatory requirements for UAVs will approach the stringency of those for manned aircraft. Nevertheless, drones are not an inherently less safe option, as they can have certain safety features, such as automatic sense-and-avoid technology, that are not present in manned aircraft (12, 13).
The operators of manned aircraft, such as pilots and maintenance technicians, are trained and licensed to FAA standards. In addition, their skill, health, and knowledge are regularly verified. On the other hand, the FAA recently proposed a new licensing procedure adapted to drone pilots (14). Finally, aviation operations account for routes, weather conditions, backup airports, and fuel reserves, among other factors. While drones do not require the same infrastructure that manned aircraft do, the lack of operational planning is currently the biggest area of difference between manned aviation and drones. Nevertheless, as with vehicular and pilot requirements, operational planning will become standardized as drones become part of the default airspace. To address operational issues in the short term, the FAA has confined drone operations to 400 feet of altitude within the pilot's visual range. The current study was performed in keeping with the operational criteria outlined above and currently indicated for the use of small drones in the United States. The vehicle was under the control of a ground-based pilot, flew within the pilot's visual range, was conducted away from populated areas, and flew at an altitude of less than 100 m. In addition, the flights were conducted in keeping with regulations governing the packaging and transport by air of potentially hazardous materials (Advisory Circular 91-57 [AC 91-57; Model Aircraft Operating Standards] [15] and IATA guidelines for the packaging of potentially infectious liquid biological materials [REF 6.1] [16]). Briefly, each sample was enclosed by three layers of packaging and enough STP absorbent material (Saf-T-Pak, Hanover, MD) to absorb twice the full volume of all the samples in the payload. The primary receptacles were the original sample containers, the secondary receptacles were the biohazard bags, and the tertiary receptacle was the rigid aircraft fuselage, made of impact-absorbent EPS foam.
The purpose of these initial experiments was to examine the feasibility of drone transportation of microbiological samples as well as to provide a template for future experiments. The findings are that the drone transport system tested herein had no adverse impact on the times to growth or the other phenotypes of the sample types or microbes that were tested. The microbes in this initial study of drone transportation of microbiological samples were selected to reflect major medically relevant microbiological groups (anaerobes, aerobes, and fastidious organisms). However, this study did not address the full range of organisms that are clinically relevant. Full adoption of UAV transport of diagnostic specimens will require similar studies for other types of organisms, specimens, and environmental conditions.
Supplementary Material
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01204-16.
REFERENCES
- 1.World Health Organization. 2015. Factors that contributed to undetected spread of the Ebola virus and impeded rapid containment. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/disease/ebola/one-year-report/factors/en/. [Google Scholar]
- 2.Fayia EM., III 26 August 2014. Bad roads hamper deliveries of needed Ebola emergency materials | The Liberian Observer, Monrovia, Liberia. http://www.liberianobserver.com/news/bad-roads-hamper-deliveries-needed-ebola-emergency-materials.
- 3.Poznanski W, Smith F, Bodley F. 1978. Implementation of a pneumatic-tube system for transport of blood specimens. Am J Clin Pathol 70:291–295. [DOI] [PubMed] [Google Scholar]
- 4.Weaver DK, Miller D, Leventhal EA, Tropeano V. 1978. Evaluation of a computer-directed pneumatic-tube system for pneumatic transport of blood specimens. Am J Clin Pathol 70:400–405. [DOI] [PubMed] [Google Scholar]
- 5.Keshgegian AA, Bull GE. 1992. Evaluation of a soft-handling computerized pneumatic tube specimen delivery system. Effects on analytical results and turnaround time. Am J Clin Pathol 97:535–540. [DOI] [PubMed] [Google Scholar]
- 6.Sodi R, Darn SM, Stott A. 2004. Pneumatic tube system induced haemolysis: assessing sample type susceptibility to haemolysis. Ann Clin Biochem 41:237–240. [DOI] [PubMed] [Google Scholar]
- 7.Bruner KW Jr, Kissling CW. 1980. Evaluation of a pneumatic-tube system for delivery of blood specimens to the blood bank. Am J Clin Pathol 73:593–596. [DOI] [PubMed] [Google Scholar]
- 8.Amukele TK, Sokoll LJ, Pepper D, Howard DP, Street J. 2015. Can unmanned aerial systems (drones) be used for the routine transport of chemistry, hematology, and coagulation laboratory specimens? PLoS One 10:e0134020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biot J-B. 1811. De la mesure des hauteurs par les observations du baromètre. In Tables barométriques partatives, donnant les difféerences de niveau par une simple soustraction Klostermann, Paris, France: http://books.google.com/books?id=U-ETAAAAQAAJ. [Google Scholar]
- 10.Haidari LA, Brown ST, Ferguson M, Bancroft E, Spiker M, Wilcox A, Ambikapathi R, Sampath V, Connor DL, Lee BY. 2016. The economic and operational value of using drones to transport vaccines. Vaccine 34:4062–4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Federal Aviation Administration. 2015. FAA fiscal year 2015 performance and accountability report. Federal Aviation Administration, Washington DC: http://www.faa.gov/about/plans_reports/media/2015-FAA-PAR.pdf. [Google Scholar]
- 12.Utt J, McCalmont J, Deschenes M. 2005. Development of a sense and avoid system. American Institute of Aeronautics and Astronautics, Reston, VA: http://arc.aiaa.org/doi/abs/10.2514/6.2005-7177. [Google Scholar]
- 13.Yu X, Zhang Y. 2015. Sense and avoid technologies with applications to unmanned aircraft systems: review and prospects. Prog Aerosp Sci 74:152–166. [Google Scholar]
- 14.Federal Register. 2015. Operation and certification of small unmanned aircraft systems. Fed Regist 80:9543–9590. https://www.federalregister.gov/articles/2015/02/23/2015-03544/operation-and-certification-of-small-unmanned-aircraft-systems. [Google Scholar]
- 15.Federal Aviation Administration. AC 91-57. Model aircraft operating standards. Federal Aviation Administration, Washington, DC: http://www.faa.gov/documentLibrary/media/Advisory_Circular/91-57.pdf. [Google Scholar]
- 16.International Air Transport Association. 2013. Dangerous goods regulations (IATA—resolution 618 attachment “A”). International Air Transport Association, Montreal, Canada. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.