Abstract
Background
The self-controlled case series (SCCS) is a useful design for investigating associations between outcomes and transient exposures. The SCCS design controls for all fixed covariates, but effect modification can still occur. This can be evaluated by including interaction terms in the model which, when exponentiated, can be interpreted as a relative incidence ratio (RIR): the change in relative incidence (RI) for a unit change in an effect modifier.
Methods
We conducted a scoping review to investigate the use of RIRs in published primary SCCS studies, and conducted a case-study in one of our own primary SCCS studies to illustrate the use of RIRs within an SCCS analysis to investigate subgroup effects in the context of comparing whole cell (wcp) and acellular (acp) pertussis vaccines. Using this case study, we also illustrated the potential utility of RIRs in addressing the healthy vaccinee effect (HVE) in vaccine safety surveillance studies.
Results
Our scoping review identified 122 primary studies reporting an SCCS analysis. Of these, 24 described the use of interaction terms to test for effect modification. 21 of 24 studies reported stratum specific RIs, 22 of 24 reported the p-value for interaction, and less than half (10 of 24) reported the estimate of the interaction term/RIR, the stratum specific RIs and interaction p-values. Our case-study demonstrated that there was a nearly two-fold greater RI of ER visits and admissions following wcp vaccination relative to acp vaccination (RIR = 1.82, 95 % CI 1.64–2.01), where RI estimates in each subgroup were clearly impacted by a strong healthy vaccinee effect.
Conclusions
We demonstrated in our scoping review that calculating RIRs is not a widely utilized strategy. We showed that calculating RIRs across time periods is useful for the detection of relative changes in adverse event rates that might otherwise be missed due to the HVE. Many published studies of vaccine-associated adverse events could have missed/underestimated important safety signals masked by the HVE. With further development, our application of RIRs could be an important tool to address the HVE, particularly in the context of self-controlled study designs.
Keywords: Epidemiologic research design, Self-controlled case series, Vaccination, Vaccine safety
Key messages
The self-controlled case series design (SCCS) is a case-only design, and as such the within-subject SCCS analysis controls for all fixed baseline covariates and has become a method of choice for studies of adverse events following vaccination.
Despite adjustment for baseline covariates, effect modification can still occur in SCCS analyses and can be tested by including interaction terms in the SCCS model. When exponentiated, these interaction terms can be interpreted as relative incidence ratios (RIR).
In this paper we present the results of our scoping review investigating the use of RIRs in published SCCS analyses, and also a case-study using one of our primary SCCS studies applying RIRs to investigate comparative subgroup effects, and as a mechanism to improve the detection and quantification of safety signals in the presence of the healthy vaccinee effect (HVE) in vaccine safety surveillance studies.
Many published studies of adverse events immediately following vaccinating using SCCS could have underestimated or failed to detect important safety signals by not recognizing and addressing the impact of the HVE.
Background
Post-marketing surveillance is important for ongoing evaluation of the safety of vaccines, and is typically based on observational data, for which conventional study designs (eg. case-control, cohort) are particularly vulnerable to confounding. This is because many factors that are associated with avoidance or delay of vaccination are also associated with the health outcomes of interest [1–4].
The self-controlled case series design (SCCS) was developed to address a number of challenges associated with studying the association between adverse health outcomes and transient exposures, such as vaccination, in observational data. The SCCS is a case-only design where inference is based on disease cases and their exposures, in which each individual serves as his or her own control, implicitly adjusting for all fixed covariates (eg. sex, socio-economic status) [5–7]. The SCCS is fit with a conditional Poisson regression model, for which general use SAS macros and R functions have been made available by authors of the SCCS methodology in addition to extensive reference material and examples (http://statistics.open.ac.uk/sccs) [6].
The fitted SCCS conditional Poisson model provides estimates of relative incidence (RI) of adverse events, comparing incidence in exposed periods (eg. immediately following a vaccination) to unexposed periods, within individuals. The SCCS has important advantages: 1) it addresses confounding resulting from differences between the vaccinated and unvaccinated individuals in non-randomized study settings; 2) traditional cohort and case-control designs may not be feasible for studying vaccines with coverage approaching 100 % as it would be difficult to recruit unvaccinated controls; and 3) safety surveillance systems typically only collect data for individuals who reported an adverse event thought to be related to vaccination.
Despite the built-in control of time-invariant confounders in an SCCS model, it is possible that effect modification (interaction) exists such that the magnitude and/or direction of the RI differs according to one or more (fixed) covariates (e.g. age, sex, socio-demographic factors, co-morbidities). Within the framework of an SCCS model it is possible to address interactions between exposure (e.g. vaccination) and one or more fixed covariates with respect to the outcome of interest by including interaction terms [6]. The exponentiated parameter estimate for the interaction term can be interpreted as a “relative incidence ratio” (RIR) as it is exactly equivalent to the ratio of the RI in one group compared to the RI in the designated reference group (if the interacting variable is categorical) or the change in the relative incidence given a one unit increase in the covariate (if the interacting variable is continuous). We have previously published a number of studies comparing RIs for adverse events following immunization (AEFI) among important subpopulations using RIRs. We have reported that rates of ER visits and admissions vary according to: quintiles of birth weight [8], birth order [9], quintiles of neighborhood income [10], sex [11] and gestational age at birth (prematurity) [12]. We have also used RIRs to compare the safety of influenza vaccination in patients with inflammatory bowel disease to that in healthy controls [13].
Despite its numerous strengths, the SCCS is not immune to a critical but perhaps under-recognized phenomenon that can influence the interpretation of an SCCS analysis: the healthy vaccinee effect (HVE) [14–16]. If an individual has been ill, recently hospitalized, or otherwise unwell, vaccination may be deferred by the provider/patient/parent/guardian until the health of the individual improves. This is especially true for vaccinations in early infancy. For this reason, when observing the health status of an individual with a completed vaccination, this individual is more likely to be in a healthy state immediately before and after their vaccination. The HVE has the effect of reducing event rates in the immediate pre- and post-vaccination periods. The impact of the HVE is particularly evident in studies that utilize non-specific outcome measures, for example health service utilization (hospital admissions, ER visits, physician visits), as a metric for evaluating AEFIs. Figure 1 illustrates the healthy vaccinee effect for ER visits and admissions relative to date of vaccination for routine pediatric vaccination at age 6 months in Ontario, Canada.
Fig. 1.
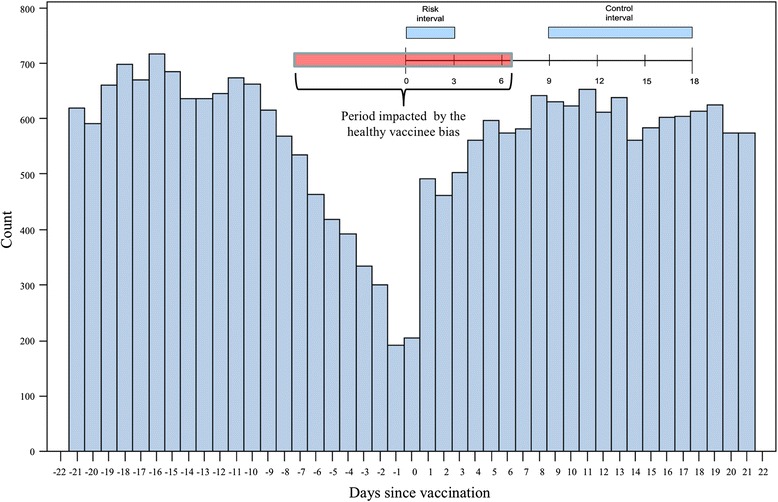
Frequency of ER visits and hospital admissions in the 3 weeks before and after vaccination (DTaP-IPV-Hib at 6 months of age): Illustration of the impact of the healthy vaccinee effect: Data from Ontario, Canada [22]. Count = number of combined endpoints of emergency room visit, hospitalization and death. Days since vaccination = number of days before or after vaccination, day 0 being the day of vaccination
The impact of the HVE may be much harder to quantify for less common events such as convulsions, and nearly impossible for extremely rare events (e.g. encephalitis, hypotonic hypo-responsive episodes (HHE)). For these rare and more serious outcomes, the HVE may also not have enough of an impact to be relevant, but there is not enough data available to confirm this. It is possible that studies reporting no increased risk of adverse events following vaccination may in fact have missed clinically important safety signals that were distorted by the HVE. Although the HVE is acknowledged in the literature, its potential impact on the detection of adverse events in the first few days following a completed vaccination is not as well recognized.
Our study sought to: 1) Investigate the use of RIRs among all published primary SCCS studies through a comprehensive scoping review, and 2) Present our case-study applying relative incidence ratios (RIRs) in the analysis of one of our own primary SCCS studies of adverse events following vaccination to investigate subgroup effects in the context of comparing whole cell (wcp) and acellular (acp) pertussis vaccines. Using this case study, we also sought to demonstrate the potential utility of RIRs in addressing the healthy vaccinee effect (HVE) that could mask important safety signals in many vaccine safety surveillance studies.
Methods
Scoping review of use of RIRs in primary SCCS studies
We searched the PubMed and Scopus databases for all papers published between January 1st, 1995 and April 30th, 2014 with the keywords “self-controlled case series” OR “self-controlled risk interval” OR “self-controlled cohort”, and also all papers that cited any of the main methods papers describing the SCCS [6, 7, 17–21]. The titles and abstracts were then reviewed and all studies reporting an original analysis of observational data using the SCCS methodology were retained for full review. The full texts of the retained manuscripts were then reviewed to determine if interaction tests were performed in the SCCS model, how they were applied and how the results were reported, as well as searching the references cited in the reviewed papers for additional studies that may have been missed. Detailed information about each study was extracted and reported in the study results.
Case-study of the application of relative incidence ratios (RIRs) in an SCCS analysis
The diphtheria, tetanus, acellular pertussis, inactivated polio and Haemophilus influenzae b (DTaP-IPV-Hib) component vaccine is given at 2, 4 and 6 months of age in Ontario, Canada. For component vaccines, adverse reactions typically occur immediately following a vaccination (0–72 h post-injection) because they contain no live replicating virus. Under these conditions, the risk period overlaps with the period in which reduced rates of adverse events are observed due to the HVE. Therefore, when observing the incidence of adverse events in the most likely risk period (from 0 to 72 h post-injection for DTaP-IPV-Hib) versus a control period farther removed from vaccination, the HVE may mask increased adverse event risk associated with vaccine administration.
We have previously conducted a study of emergency room (ER) visits and admissions following DTaP-IPV-Hib vaccine recommended at ages 2, 4 and 6 months in which we found no evidence of increased risk of events in the first 72 h following vaccination (compared to the control period: days 9 to 18), but we observed a strong HVE, which is described by Fig. 1 [22]. In the studies we have conducted using the SCCS, our implementation of the design was somewhat atypical in that we defined very short control periods (e.g. 9 days) and only included exposure time in the post-vaccination period [8–12, 22–24]. Our rationale for the selected control periods was two-fold. Firstly, when studying vaccinations in early infancy, background rates of ER visits and hospitalizations change very rapidly, especially in the first few months following birth. Therefore, careful age stratification is required to control for changing background event rates when longer control intervals are used, which would further complicate the analysis. Secondly, as vaccinations in first year after birth are closely spaced (e.g. 2, 4, 6 and 12 months), tight control intervals were required in order to investigate associations involving individual vaccinations so that the control intervals did not overlap with risk periods associated with subsequent vaccinations. Although we used an atypical implementation of unexposed control periods in our case-study, the methods applied in our case study are broadly applicable to SCCS models in general.
Results
Scoping review of use of RIRs in primary SCCS studies
Our electronic database search returned 334 articles. Titles and abstracts were reviewed to eliminate duplicates, and to identify those studies that reported an original analysis of observational data using the SCCS methodology, leaving 122 studies. After retrieval and full text review of the 122 remaining studies, 24 described the use of interaction terms in the SCCS model to test for effect modification. No additional studies were identified through references listed by the reviewed papers. Details of the 24 included studies are given in Table 1. For the final subset of qualifying manuscripts, 10 of 24 studies reported the estimate of the interaction term/RIR in addition to the stratum specific relative incidence estimates and interaction p-values. Twenty-one of 24 studies reported the stratum specific RIs, and 22 of 24 reported the p-value for the test for interaction. Two of 24 studies reported no details of the interaction tests, other than that they were performed, and did not achieve statistical significance (Table 1).
Table 1.
Studies that reported testing for interactions, and/or comparing subgroups in an SCCS modeling context
| Study a | Exposure | Outcomes | Interaction Tested | Estimates/RIR/int. p-value reported |
|---|---|---|---|---|
| Wilson et al. [11] | 12 month MMR vaccination | ER visits + admissions | Sex | yes/yes/yes |
| Kwong et al. [26] | Influenza illness and influenza immunization | Guillain-Barré syndrome | Age, sex, month of vaccination | yes/no/yes |
| Benchimol et al. [13] | Influenza vaccination | ER visits + admissions + physician visits IBD flares | IBD versus healthy controls | yes/yes/yes |
| Wilson et al. [10] | 2, 4, 6 (DTaP) and 12 month (MMR) vaccination | ER visits + admissions | SES (Neighborhood income quintiles) | yes/yes/yes |
| Hawken et al. [23] | Acellular/whole cell pertussis vaccine | ER visits + admissions | Whole cell (1994–1996) versus acellular pertussis vaccine (1998–2000) | yes/yes/yes |
| Wilson et al. [12] | 2 month vaccination | ER visits + admissions | Preterm versus full term infants | yes/yes/yes |
| Wilson et al. [8] | 2, 4, 6 and 12 month vaccination | ER visits + admissions | Quintiles of birthweight | yes/yes/yes |
| Connolly-Anderson et al. [27] | Hemorrhagic fever with renal syndrome | Acute myocardial infarction and stroke | Sex | yes/no/yes |
| Langan et al. [28] | Herpes Zoster infection | Stroke | Antiviral Therapy | yes/no/yes |
| Dodd et al. [29] | H1N1 vaccination | Guillain-Barré syndrome | Age, sex, adjuvanted vs. non-adjuvanted vaccine, concomitant seasonal flu vaccine | yes/no/yes |
| Butt et al. [30] | Antihypertensives | Falls | Sex | yes/no/yes |
| Andrews et al. [31] | MMR vaccination | Thrombocytopenic purpura | Country | yes/no/yes |
| Tokars et al. [32] | H1N1 and seasonal influenza vaccination | Guillain-Barré syndrome | age, sex, vaccine type, received season flu vaccine, site | yes/no/yes |
| Pariente et al. [33] | Antipsychotic use | Myocardial infarction | Previous history of cardiovascular disease | no/no/nob |
| Warren-Gash et al. [34] | Influenza vaccination | Acute MI | Age group, sex, type of infarction, and history of vascular disease | yes/no/yes |
| Tse et al. [35] | Influenza vaccination | Febrile seizures | concomitant 13-valent pneumococcal conjugate vaccine (PCV13), age | yes/yes/yes |
| Gwini et al. [36] | Influenza vaccination | Acute myocardial infarction | Age, sex | yes/no/yes |
| Pattenden et al. [37] | Heat Exposure | Mortality | Ozone levels | yes/yes/yes |
| Andrews et al. [38] | Acellular pertussis/whole cell pertussis vaccine | Convulsions, | Whole cell period vs. acellular period | yes/yes/yes |
| Douglas et al. [39] | Thiazolidinediones | Fractures | Rosiglitazone versus pioglitazone | yes/no/yes |
| Miller et al. [40] | MMR | Convulsions and aseptic meningitis | Vaccine Manufacturer, Concomitant MCC vaccination vs. separate | yes/no/yes |
| Game et al. [41] | Initiation of dialysis | Foot ulceration | Haemodialysis vs. ambulatory peritoneal dialysis | no/no/noa |
| Miller et al. [42] | MMR vaccination | Gait Disturbance | doses of thimerisol containing vaccines by 4 months, \ mercury exposure intensity by 6 months | yes/no/yes |
| Sardinas et al. [43] | Oral polio vaccine | Intussusception | age | no/no/yes |
aStudies reported above the gray dividing bar are studies published by the authors and their collaboratorsbReported that interaction was tested and did not reach statistical significance
Case-study of the application of RIRs in an SCCS analysis
Table 2 presents a comparison of relative incidence of ER visits and admissions observed among children in Ontario, Canada in the 3 days following the 2-month diphtheria-whole-cell-pertussis-tetanus (DPT) vaccination (risk period) vs. days 9 to 18 (control period). We compared the RI during the period 1994 to 1996 when the more reactogenic whole cell pertussis vaccine was being administered with the RI during the period from 1998 to 2000 when the less reactogenic diphtheria-tetanus-acellular pertussis vaccine (DTaP) was being administered. The RI during the whole cell period was 1.08 (95 % CI 1.02–1.15) and the RI during the acellular period was 0.60 (95 % CI 0.55–0.65). The RIR for the whole cell versus acellular period was calculated to be 1.82 (95 % CI 1.64–2.01). This provides evidence that the relative incidence of events was nearly two-fold higher in the whole-cell versus acellular vaccine usage periods.
Table 2.
ER Visits and Admissions in the first 72 h following vaccination versus days 9 to 18 for the time period when whole cell pertussis vaccine was used compared to the time period when acellular pertussis vaccine was used [23]
| Risk events (first 72 h) | Control events (Day 9–18) | Relative incidence | 95 % CI | Relative incidence ratio | 95 % CI | P-valuea | |
|---|---|---|---|---|---|---|---|
| Apr 94–Mar96 (whole-cell) | 1323 | 3663 | 1.08 | 1.02–1.15 | 1.82 | 1.64–2.01 | P < 0.0001 |
| Apr98–Mar00 (acellular) | 697 | 3508 | 0.60 | 0.55–0.65 | 1 (ref) | - | - |
a p-value for interaction of time period (whole cell or acellular periods) with risk
Employing the parameter estimates generated from the fitted conditional Poisson model, the RI in each time period as well as the RIR comparing the whole cell versus acellular period can be expressed as:
where βrisk is the parameter estimate of log (RI) in the reference group (acellular period) and βperiod is the parameter estimate of the interaction term of period (whole cell vs. acellular period) with risk. Since this is a dichotomous interaction variable, the period variable would take the value 1 for the whole cell period and 0 for the acellular period. For a categorical interaction with m categories, period would be a vector of (m-1) dummy variables indicating subgroup membership, or period may be a continuous variable. The main effect terms for the fixed covariates interacting with exposure cancel out (eg. biological sex does not change between an individual’s exposed and unexposed periods) leaving just the interaction terms. A likelihood ratio test is employed to compare the model with interaction terms included to the model that excludes these terms. This tests the hypothesis that the relative incidence of outcomes with respect to exposure (i.e. vaccination) depends on the value of the covariate involved in the interaction [6, 7].
Visual inspection of the daily frequency of events relative to day of vaccination in both periods strongly suggest that the RI estimates in both the whole cell and acellular periods were impacted by the healthy vaccinee effect (Fig. 2). The effect is very similar in the week preceding vaccination in both the whole cell and the acellular pertussis periods, but in the days following the whole cell vaccinations (1994–1996), there is clearly a spike in events on the first day following vaccination which is largely washed out by the HVE (RI 1.08 = 95 % CI 1.02–1.15) but is still statistically significant with p < 0.0001 due to high statistical power. However, in the acellular period, there appears to be no spike following vaccination, but rather in the days following vaccination there is an approximate mirror image of the decrease in event rate seen before vaccination (RI = 0.60, 95 % CI 0.55–0.65). In both periods, the daily frequency of events is nearly halved by the day before vaccination with very similar relative incidence for the week before vaccination compared to the control period (days 9–18) (Fig. 2).
Fig. 2.
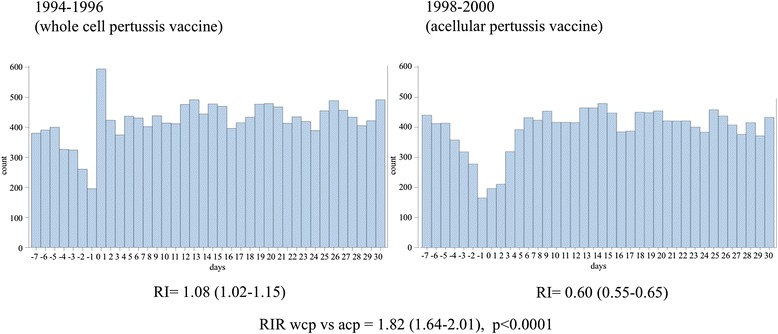
Emergency room visits and admissions before and after 2-month vaccination for whole cell pertussis combination vaccine (1994–1996) versus acellular pertussis vaccine (1998–2000). Count = number of combined endpoints of emergency room visit, hospitalization and death. Days = number of days before or after vaccination, day 0 being the day of vaccination
When we calculated the RIR for the whole cell versus acellular periods, the similar HVE in both periods is essentially cancelled out and the higher relative incidence of adverse events associated with the whole cell combination vaccine becomes clear (RIR = 1.82, 95 % CI 1.64–2.01) (Fig. 2). This suggests that RIRs provide a useful effect estimate that can be constructed in such a way to potentially overcome the healthy vaccinee effect to detect safety signals (or relative changes in safety signals) that might otherwise be missed.
Discussion
In this manuscript, we presented the results of our scoping review of primary SCCS studies and the use of relative incidence ratios therein, as well as case-study from one of our primary SCCS studies providing a worked example of the utility, strengths and limitations of RIRs for describing subgroup effects as well as effect modification by continuous covariates.
In the context of vaccine safety surveillance, we are often interested in detecting increases or decreases in incidence of serious adverse event and changes in healthcare utilization patterns related to vaccine reactions following introduction of a new vaccine formulation, manufacturer, or other modification. In this case, interest may be focused on detecting changes in relative incidence over time or in important subgroups. Using relative incidence ratios (RIR), the change in relative incidence across time or physical subgroups of interest can be estimated and formally tested. For example, if a different formulation of a vaccine is introduced at a known point in time, then an SCCS model can be fit, with common risk and control periods across the entire population, but an interaction term is then included in the model, which estimates the RIR comparing the period after the new vaccine is introduced versus the period in which the old formulation was used.
In our case-study we have illustrated how, in some cases, the HVE can bias the observed relative incidence of adverse events that occur within the first few days following a vaccination. Calculating the RIR across groups to be compared (or in our case-study, time periods to be compared) similarly affected by the HVE would, in effect, cancel out the HVE and provide a potentially less biased estimate of the change in RI across the subgroups. Therefore, this could present a useful strategy for overcoming the healthy vaccinee effect, when relative changes in rates of adverse events across time periods, jurisdictions or subgroups of vaccines are of primary interest.
In the SCCS modeling context, RIRs are not afforded the same protection from confounding as the subgroup specific RI estimates, which is a potential limitation of our proposed applications of RIRs. For example, if we suspect that the relative incidence of adverse events depends on sex (i.e. females are more susceptible to adverse events following vaccination than males who are similarly exposed) we would test this hypothesis by including an interaction term between risk period and sex. This term, if statistically significant, provides evidence that sex is an effect modifier. However, since we would now be estimating an interaction effect across levels of a fixed baseline covariate, this could no longer be considered a within-individual effect estimate, and hence, any observed interaction effect could be the result of confounding. This issue can be addressed in the same way it is addressed in other modeling situations, by statistically controlling for other potential confounders in the SCCS model in order to assess whether the interaction effect of interest persists after statistical adjustment. This further adjustment is implemented by introducing additional interaction terms for the potential confounder(s) of interest and then observing whether the parameter estimate of the target effect modifier changes substantively. Stratified analysis is also a useful strategy to observe whether the interaction of interest is consistent across subgroups of known potential confounders of interest. These remedies afford less reassurance than the basic SCCS model, which provides main effect estimates that are implicitly controlled for all known and unknown fixed covariates. In generating RIR estimates we are limited to adjusting for known confounders, for which data are available. Vanderweele and Knol [25] point out that, if the point of the subgroup analysis is to identify vulnerable subsets of individuals for possible intervention, then confounding in interaction effects is of much less importance. If the aim is to make causal inference with respect to the source of the interaction, then the confounding would be important to account for [25].
Conclusions
In this review we have demonstrated the potential utility of RIRs, which are based upon a test of interaction in the SCCS conditional Poisson model. We have discussed the strengths and limitations of RIRs for describing subgroup effects as well as effect modification by continuous covariates. We have also conducted a scoping review that demonstrated that very few primary SCCS studies are making use of RIRs to evaluate relative subgroup effects.
We have proposed that calculating RIRs across time periods (year over year for example) is very useful for detecting relative changes in rates of adverse events, which could constitute safety signals that might otherwise be missed due to the HVE. We emphasize many published studies of adverse events immediately following vaccination could have underestimated or failed to detect potentially important safety signals masked by the HVE. Our proposed application of RIRs could be an important tool in future studies to address the HVE. Further study is needed, including simulation studies and case studies in real data to assess the impact of different patterns of healthy vaccinee effect, the impact of increasing severity of confounding, as well as other violations of assumptions, on the reliability of inference using RIRs in post-marketing surveillance using the SCCS study design.
Acknowledgments
This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions and statements expressed herein are those of the author, and not necessarily those of CIHI.
Funding
SH received support for this work through a Canadian Institutes for Health Research (CIHR) Doctoral Research Award. EIB was supported by a New Investigator Award from the Canadian Institutes of Health Research (CIHR), Canadian Association of Gastroenterology and Crohn’s and Colitis Canada.
Availability of data and materials
Details on the article extraction and review process for the scoping review are available upon request. All other results and findings presented were based on previously published data about which more information can be furnished upon request to the corresponding author.
Authors’ contributions
SH conceptualized and designed the study, performed all data analyses, drafted the manuscript and approved the final version. BP, EIB, JL, RD and SM reviewed the manuscript, gave feedback on important intellectual content and approved the final version. KM contributed to the conceptualization and design of the study, and contributed to writing the manuscript, reviewing and revising it for important intellectual content and approved the final version. All the authors accept responsibility for the reported research.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- DTaP
Diphtheria-tetanus-acellular pertussis vaccine
- DTaP-IPV-Hib
Diphtheria, tetanus, acellular pertussis, inactivated polio and Haemophilus influenzae b combination vaccine
- DPT
Diphtheria-whole-cell pertussis tetanus vaccine
- ER
Emergency room
- HHE
Hypotonic hypo-responsive episodes
- HVE
Healthy vaccinee effect
- MMR
Measles mumps and rubella vaccine
- RI
Relative incidence
- RIR
Relative incidence ratio
- SCCS
Self-controlled case series
Contributor Information
Steven Hawken, Phone: +1-613-798-5555, Email: shawken@ohri.ca.
Beth K. Potter, Email: bpotter@uottawa.ca
Julian Little, Email: jlittle@uottawa.ca.
Eric I. Benchimol, Email: ebenchimol@cheo.on.ca
Salah Mahmud, Email: salah.mahmud@umanitoba.ca.
Robin Ducharme, Email: roducharme@ohri.ca.
Kumanan Wilson, Email: kwilson@ohri.ca.
References
- 1.Christenson B, Lundburgh P. Comparison between cohorts vaccinated and unvaccinated against influenza and pneumococcal infection. Epidemiol Infect. 2003;129(03):515. doi: 10.1017/S095026880200780X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jessop LJ, Kelleher CC, Murrin C, Lotya J, Clarke AT, O’Mahony D, et al. Determinants of partial or no primary immunisations. Arch Dis Child. 2010;95(8):603–605. doi: 10.1136/adc.2009.161810. [DOI] [PubMed] [Google Scholar]
- 3.Smith P. Children Who Have Received No Vaccines: Who Are They and Where Do They Live? Pediatrics. 2004;114(1):187–195. doi: 10.1542/peds.114.1.187. [DOI] [PubMed] [Google Scholar]
- 4.Wei F, Mullooly JP, Goodman M, McCarty MC, Hanson AM, Crane B, et al. Identification and characteristics of vaccine refusers. BMC Pediatr. 2009;9(1):18. doi: 10.1186/1471-2431-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrington P, Pugh S, Colville A, Flower A, Nash J, Morgan-Capner P, et al. A new method for active surveillance of adverse events from diphtheria/tetanus/pertussis and measles/mumps/rubella vaccines. Lancet. 1995;345(8949):567–569. doi: 10.1016/S0140-6736(95)90471-9. [DOI] [PubMed] [Google Scholar]
- 6.Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25(10):1768–1797. doi: 10.1002/sim.2302. [DOI] [PubMed] [Google Scholar]
- 7.Whitaker H, Hocine M, Farrington C. The methodology of self-controlled case series studies. Stat Methods Med Res. 2009;18(1):7. doi: 10.1177/0962280208092342. [DOI] [PubMed] [Google Scholar]
- 8.Wilson K, Hawken S, Kwong JC, Deeks SL, Manuel DG, Henningsen KH, et al. Impact of birth weight at term on rates of emergency room visits and hospital admissions following vaccination at 2 months of age. Vaccine. 2011;29(46):8267–8274. doi: 10.1016/j.vaccine.2011.08.107. [DOI] [PubMed] [Google Scholar]
- 9.Hawken S, Kwong JC, Deeks SL, Crowcroft NS, Ducharme R, Manuel DG, et al. Association between birth order and emergency room visits and acute hospital admissions following pediatric vaccination: a self-controlled study. PLoS One. 2013;8(12):e81070. doi: 10.1371/journal.pone.0081070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson K, Ducharme R, Hawken S. Association between socioeconomic status and adverse events following immunization at 2, 4, 6 and 12 months. Hum Vaccin Immunother. 2013;9(5):1153–1157. doi: 10.4161/hv.23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson K, Ducharme R, Ward B, Hawken S. Increased emergency room visits or hospital admissions in females after 12-month MMR vaccination, but no difference after vaccinations given at a younger age. Vaccine. 2014;32(10):1153–1159. doi: 10.1016/j.vaccine.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Wilson K, Hawken S. Incidence of adverse events in premature children following 2-month vaccination. Hum Vaccin Immunother. 2012;8(5):592–595. doi: 10.4161/hv.19377. [DOI] [PubMed] [Google Scholar]
- 13.Benchimol EI, Hawken S, Kwong JC, Wilson K. Safety and utilization of influenza immunization in children with inflammatory bowel disease. Pediatrics. 2013;131(6):e1811–e1820. doi: 10.1542/peds.2012-3567. [DOI] [PubMed] [Google Scholar]
- 14.Virtanen M, Peltola H, Paunio M, Heinonen OP. Day-to-day reactogenicity and the healthy vaccinee effect of measles-mumps-rubella vaccination. Pediatrics. 2000;106(5):E62. doi: 10.1542/peds.106.5.e62. [DOI] [PubMed] [Google Scholar]
- 15.Davis RL, Marcuse E, Black S, Shinefield H, Givens B, Schwalbe J, et al. MMR2 immunization at 4 to 5 years and 10 to 12 years of age: a comparison of adverse clinical events after immunization in the Vaccine Safety Datalink project. The Vaccine Safety Datalink Team. Pediatrics. 1997;100(5):767–771. doi: 10.1542/peds.100.5.767. [DOI] [PubMed] [Google Scholar]
- 16.Fine PE, Chen RT. Confounding in studies of adverse reactions to vaccines. Am J Epidemiol. 1992;136(2):121–135. doi: 10.1093/oxfordjournals.aje.a116479. [DOI] [PubMed] [Google Scholar]
- 17.Farrington C. Control without separate controls: evaluation of vaccine safety using case-only methods. Vaccine. 2004;22(15–16):2064–2070. doi: 10.1016/j.vaccine.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Farrington C, Nash J, Miller E. Case series analysis of adverse reactions to vaccines: a comparative evaluation. Am J Epidemiol. 1996;143(11):1165. doi: 10.1093/oxfordjournals.aje.a008695. [DOI] [PubMed] [Google Scholar]
- 19.Farrington C, Whitaker H. Semiparametric analysis of case series data. J R Stat Soc: Ser C: Appl Stat. 2006;55(5):553–594. doi: 10.1111/j.1467-9876.2006.00554.x. [DOI] [Google Scholar]
- 20.Farrington CP. Relative incidence estimation from case series for vaccine safety evaluation. Biometrics. 1995;51(1):228–235. doi: 10.2307/2533328. [DOI] [PubMed] [Google Scholar]
- 21.Andrews NJ. Statistical assessment of the association between vaccination and rare adverse events post-licensure. Vaccine. 2001;20(Suppl 1):S49–S53. doi: 10.1016/S0264-410X(01)00280-8. [DOI] [PubMed] [Google Scholar]
- 22.Wilson K, Hawken S, Potter BK, Chakraborty P, Kwong J, Crowcroft N, et al. Patterns of emergency room visits, admissions and death following recommended pediatric vaccinations–a population based study of 969,519 vaccination events. Vaccine. 2011;29(21):3746–3752. doi: 10.1016/j.vaccine.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 23.Hawken S, Manuel DG, Deeks SL, Kwong JC, Crowcroft NS, Wilson K. Underestimating the Safety Benefits of a New Vaccine: The Impact of Acellular Pertussis Vaccine Versus Whole-Cell Pertussis Vaccine on Health Services Utilization. Am J Epidemiol. 2012;176(11):1035–1042. doi: 10.1093/aje/kws167. [DOI] [PubMed] [Google Scholar]
- 24.Wilson K, Hawken S, Kwong JC, Deeks S, Crowcroft NS, Van Walraven C, et al. Adverse Events following 12 and 18 Month Vaccinations: a Population-Based, Self-Controlled Case Series Analysis. PLoS ONE. 2011;6(12):e27897. doi: 10.1371/journal.pone.0027897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanderweele TJ, Knol MJ. Interpretation of subgroup analyses in randomized trials: heterogeneity versus secondary interventions. Ann Intern Med. 2011;154(10):680–683. doi: 10.7326/0003-4819-154-10-201105170-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwong JC, Vasa P, Campitelli MA, Hawken S, Wilson K, Rosella LC, et al. Risk of Guillain-Barr syndrome after seasonal influenza vaccination and influenza health-care encounters: a self controlled study. The Lancet Infectious Diseases. 2013;13(9):769–76. doi: 10.1016/S1473-3099(13)70104-X. [DOI] [PubMed] [Google Scholar]
- 27.Connolly-Andersen AM, Hammargren E, Whitaker H, Eliasson M, Holmgren L, Klingström J, et al. Increased risk of acute myocardial infarction and stroke during hemorrhagic fever with renal syndrome: A self-controlled case series study. Circulation. 2014;129(12):1295–1302. doi: 10.1161/CIRCULATIONAHA.113.001870. [DOI] [PubMed] [Google Scholar]
- 28.Langan SM, Minassian C, Smeeth L, Thomas SL. Risk of stroke following herpes zoster: A self-controlled case-series study. Clin Infect Dis. 2014;58(11):1497–1503. doi: 10.1093/cid/ciu098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodd CN, Romio SA, Black S, Vellozzi C, Andrews N, Sturkenboom M, et al. International collaboration to assess the risk of Guillain Barré Syndrome following Influenza A (H1N1) 2009 monovalent vaccines. Vaccine. 2013;31(40):4448–4458. doi: 10.1016/j.vaccine.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 30.Butt DA, Mamdani M, Austin PC, Tu K, Gomes T, Glazier RH. The risk of falls on initiation of antihypertensive drugs in the elderly. Osteoporos Int. 2013;24(10):2649–2657. doi: 10.1007/s00198-013-2369-7. [DOI] [PubMed] [Google Scholar]
- 31.Andrews N, Stowe J, Miller E, Svanström H, Johansen K, Bonhoeffer J, et al. A collaborative approach to investigating the risk of thrombocytopenic purpura after measles–mumps–rubella vaccination in England and Denmark. Vaccine. 2012;30(19):3042–3046. doi: 10.1016/j.vaccine.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Tokars JI, Lewis P, Destefano F, Wise M, Viray M, Morgan O, et al. The Risk of Guillain-Barré Syndrome Associated with Influenza A (H1N1) 2009 Monovalent Vaccine and 2009–2010 Seasonal Influenza Vaccines: Results from Self-Controlled Analyses. Pharmacoepidemiol Drug Saf. 2012;21(5):546–552. doi: 10.1002/pds.3220. [DOI] [PubMed] [Google Scholar]
- 33.Pariente A, Fourrier-Reglat A, Ducruet T, Farrington P, Beland SG, Dartigues JF, et al. Antipsychotic use and myocardial infarction in older patients with treated dementia. Arch Intern Med. 2012;172(8):648–653. doi: 10.1001/archinternmed.2012.28. [DOI] [PubMed] [Google Scholar]
- 34.Warren-Gash C, Hayward AC, Hemingway H, Denaxas S, Thomas SL, Timmis AD, et al. Influenza infection and risk of acute myocardial infarction in England and Wales: a CALIBER self-controlled case series study. J Infect Dis. 2012;206(11):1652–1659. doi: 10.1093/infdis/jis597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tse A, Tseng HF, Greene SK, Vellozzi C, Lee GM. Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the Vaccine Safety Datalink Project, 2010–2011. Vaccine. 2012;30(11):2024–2031. doi: 10.1016/j.vaccine.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 36.Gwini SM, Coupland CAC, Siriwardena AN. The effect of influenza vaccination on risk of acute myocardial infarction: Self-controlled case-series study. Vaccine. 2011;29(6):1145–1149. doi: 10.1016/j.vaccine.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 37.Pattenden S, Armstrong B, Milojevic A, Heal MR, Chalabi Z, Doherty R, et al. Ozone, heat and mortality: Acute effects in 15 British conurbations. Occup Environ Med. 2010;67(10):699–707. doi: 10.1136/oem.2009.051714. [DOI] [PubMed] [Google Scholar]
- 38.Andrews N, Stowe J, Wise L, Miller E. Post-licensure comparison of the safety profile of diphtheria/tetanus/whole cell pertussis/haemophilus influenza type b vaccine and a 5-in-1 diphtheria/tetanus/acellular pertussis/haemophilus influenza type b/polio vaccine in the United Kingdom. Vaccine. 2010;28(44):7215–7220. doi: 10.1016/j.vaccine.2010.08.062. [DOI] [PubMed] [Google Scholar]
- 39.Douglas IJ, Evans SJ, Pocock S, Smeeth L. The risk of fractures associated with thiazolidinediones: a self-controlled case-series study. PLoS Med. 2009;6(9):e1000154. doi: 10.1371/journal.pmed.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller E, Andrews N, Stowe J, Grant A, Waight P, Taylor B. Risks of convulsion and aseptic meningitis following measles-mumps-rubella vaccination in the United Kingdom. Am J Epidemiol. 2007;165(6):704–709. doi: 10.1093/aje/kwk045. [DOI] [PubMed] [Google Scholar]
- 41.Game FL, Chipchase SY, Hubbard R, Burden RP, Jeffcoate WJ. Temporal association between the incidence of foot ulceration and the start of dialysis in diabetes mellitus. Nephrol Dial Transplant. 2006;21(11):3207–3210. doi: 10.1093/ndt/gfl427. [DOI] [PubMed] [Google Scholar]
- 42.Miller E, Andrews N, Grant A, Stowe J, Taylor B. No evidence of an association between MMR vaccine and gait disturbance. Arch Dis Child. 2005;90(3):292–296. doi: 10.1136/adc.2003.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sardiñas MAG, Cárdenas AZ, Marie GC, Peña MS, Santiago MA, Sanchez MV, et al. Lack of association between intussusception and oral polio vaccine in Cuban children. Eur J Epidemiol. 2001;17(8):783–787. doi: 10.1023/A:1015675932509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Details on the article extraction and review process for the scoping review are available upon request. All other results and findings presented were based on previously published data about which more information can be furnished upon request to the corresponding author.


