Abstract
Introduction:
Treatment of severe hemorrhagic shock due to acute blood loss from traumatic injuries in a Jehovah’s witness (JW) trauma patient is very challenging since hemostatic blood product resuscitation is limited by refusal of the transfusion of allogeneic blood products.
Case Presentation:
We describe a multifaceted approach to the clinical care of a severely anemic JW trauma patient including the early administration of a bovine hemoglobin-based oxygen carrier (HBOC) as a bridge to resolution of critical anemia (nadir hemoglobin 3.9 g/dL). Hemoglobin-based oxygen carrier infusions were used to supplement oxygen delivery until endogenous erythropoiesis could restore adequate red blood cell mass. Subsequent endogenous bone marrow recovery was supported by early administration of high-dose erythropoiesis-stimulating agents and iron supplementation.
Conclusions:
Early HBOC administration can be used in the treatment of severe hemorrhagic shock in trauma patients who refuse allogeneic blood.
Keywords: Anemia, Erythropoietin-Stimulating Agents, Hemoglobin-Based Oxygen Carrier, Hepcidin, Iron, Jehovah’s Witness
1. Introduction
Hemorrhage is the leading cause of preventable death in the early phase of trauma. Prevention of death from hemorrhage requires rapid hemorrhage control and appropriate hemostatic resuscitation. In Jehovah’s witness (JW) patients with severe traumatic injuries, hemostatic resuscitation is limited by refusal of the transfusion of allogeneic blood products.
Nontraditional methods of reducing blood loss and optimizing erythropoiesis have been used to support anemic JW patients including exogenous erythropoietin administration, folic acid, ascorbic acid and iron supplementation (1, 2). Using these methods, survival has been reported following high volume blood loss in JW patients including ruptured aortic dissection (3), splenic laceration (4), poly-trauma (5, 6) and subtotal colectomy for bleeding (7). However, death following surgical interventions for traumatic injuries in JW patients has also been reported (8, 9).
To prevent both ischemic complications and death due to critical anemia and lack of oxygen delivery, hemoglobin-based oxygen carriers (HBOC) have been used to supplement oxygen delivery until endogenous erythropoiesis can restore adequate red blood cell mass (10, 11).This case report describes a multifaceted approach to care of a severely anemic JW trauma patient including the administration of a bovine HBOC as a bridge to resolution of critical anemia with endogenous bone marrow recovery.
2. Case Presentation
A 23-year-old male pedestrian was struck and crushed between two cars. In the trauma bay he had an intact airway, clear breath sounds, normal mentation, heart rate 99 and systolic blood pressure 118/65 mmHg. Physical exam confirmed diffuse abdominal tenderness, severe pelvic tenderness and right flank and left lower back contusions. He complained of abdominal, back and right groin pain. Admission hemoglobin was 13.4 g/dL (hematocrit 42.8%).
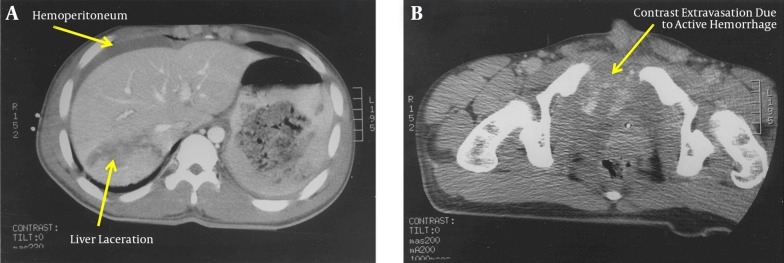
CT scan of the abdomen/pelvis revealed a Grade III liver laceration in the posterior segment of the right hepatic lobe, extending to the right hepatic and portal veins, with associated hemoperitoneum (Figure 1 A) and a pelvic hematoma with active hemorrhage in the space of Retzius and periurethral region (Figure 1 B). Angiography confirmed segmental extravasation from branches of both right and left internal iliac arteries and bleeding from branches of the right hepatic artery which required selective embolization. An inferior vena cava filter was placed.
Figure 1. A, CT scan of the abdomen/pelvis confirming a Grade III liver laceration in the posterior segment of the right hepatic lobe, extending to the right hepatic vein and portal system, Associated hemoperitoneum is noted; B, CT scan of the abdomen/pelvis confirming a severe open-book pelvic fracture with pelvic hematoma with active hemorrhage in the space of Retzius and peri-urethral region.
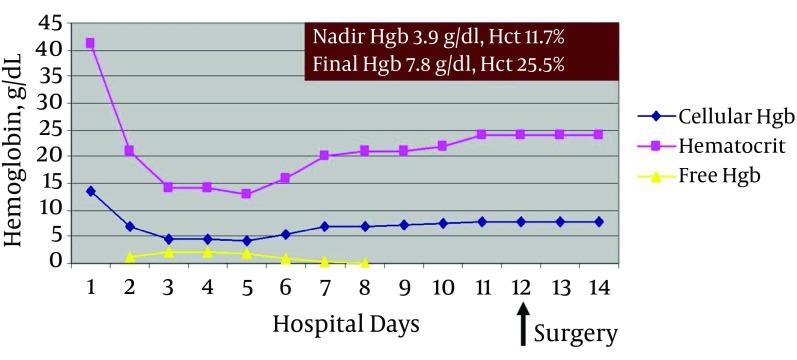
Repeat hematocrit following angiography was 15.1% and decreased further to 11.7% (hemoglobin 3.9 g/dL). Coagulation profile was normal. He developed worsening tachycardia and hypotension. Both patient and family declined allogeneic red blood cell (RBC) transfusion. Compassionate use Hemopure (3 units/90 g hemoglobin/250 mL, HBOC-201 bovine Hb glutamer-250, OPK Biotech, Cambridge, MA) was administered via FDA compassionate use program approximately 30 hours after admission. Daily high-dose recombinant erythropoietin and IV iron sucrose were initiated. Additional Hemopure transfusions (4 units) were administered over the next 6 days (Figure 2).
Figure 2. Hematocrit Cellular Hemoglobin and Free Hemoglobin Levels During the JW Trauma Patient’s Hospital Course.
Hemopure administration is represented by the free hemoglobin level.
Phlebotomy was minimized with pediatric tube use. When hematocrit was stable (24%) on post-trauma day 12, he underwent surgery for percutaneous pinning of pelvic fractures. He was discharged home the next day with outpatient physical therapy with hemoglobin 7.7 g/dL (hematocrit 25.5%). Renal function was unchanged by Hemopure administration (total 7 units transfused) as serum creatinine did not rise above 1.0 mg/dL throughout his hospital course.
3. Discussion
This case highlights several important features of trauma management of a JW patient who refuses transfusion of allogeneic blood. First, HBOCs can temporarily supply enough hemoglobin to support oxygen delivery in the patient with critical anemia. Second, endogenous production of erythrocytes can be facilitated by exogenous erythropoietin and iron supplementation. Third, the combination of a HBOC, erythropoietin and iron can enhance hemoglobin levels over several days in preparation for safe, non-emergent surgery. Finally, it is critical to minimize unnecessary iatrogenic diagnostic blood loss that may exacerbate anemia.
HBOCs, developed via polymerization or human or bovine hemoglobin (12), are not approved by the FDA (require compassionate use approval) (13), but are approved for human clinical use in South Africa and Russia. Most JW patients will accept Hemopure (derived from bovine hemoglobin). HBOCs underwent multiple clinical trials and were not FDA-approved due to higher rates of myocardial infarction and death most likely related to hypertension and vasoconstriction from the nitric oxide scavenging properties of free hemoglobin, but this meta-analysis was flawed in that it included multiple HBOC products (14). HBOC-201 eliminated allogeneic transfusion in the majority of subjects in a multicenter Phase III trial in elective orthopedic surgery (15). Although HBOCs have failed to show significant benefit over allogeneic human RBC transfusion, this case highlights a scenario in which allogeneic RBC transfusion is not an option and HBOCs are an acceptable alternative, similar to potential use in military or rural areas where RBC transfusion is not available.
The optimal hemoglobin level at which to consider HBOC administration is unclear, but 5 - 6 g/dL is commonly used. Increased mortality has been documented with hemoglobin less than 5 g/dL (16, 17) and the odds ratio for death in patients who refuse blood transfusion increases 2.5 for every 1 g less than 8 g/dL (18). In a prior study of HBOC use in trauma, myocardial ischemia as evidence by ST changes on electrocardiogram and elevated troponin levels developed when hemoglobin decreased below 4 g/dL (10) at which time HBOCs were transfused. The long-term cognitive effects of critical anemia are unclear. Therefore, consideration for HBOC administration should occur once hemoglobin is 5 - 6 g/dL.
Immobilized trauma patients have a high rate of deep vein thrombosis. High dose erythropoietin may further increase thrombotic risk in trauma patients (19, 20). This patient pre-emptively had an IVC filter placed during angiography which should be considered in similar patients.
In conclusion, this case demonstrates that a multifaceted approach to hemorrhagic shock management in a trauma patient who refuses allogeneic blood transfusion can ensure adequate oxygen delivery with concomitant early administration of HBOCs for acute blood loss and erythropoietin and iron to augment endogenous erythropoiesis.
Footnotes
Authors’ Contributions:Both authors contributed equally to manuscript preparation.
References
- 1.Berend K, Levi M. Management of adult Jehovah's Witness patients with acute bleeding. Am J Med. 2009;122(12):1071–6. doi: 10.1016/j.amjmed.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 2.Beliaev AM. High-risk anaemic Jehovah's Witness patients should be managed in the intensive care unit. Blood Transfus. 2013;11(3):330–2. doi: 10.2450/2013.0043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto H, Yamamoto F, Yamaura G, Motokawa M, Tanaka F, Sato H, et al. Rupture of chronic type B aortic dissection in a Jehovah's Witness: successful surgical repair without blood transfusion. Ann Vasc Surg. 2012;26(4):571 e11–6. doi: 10.1016/j.avsg.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Ayiomamitis GD, Alkari B, Owera A, Ammori BJ. Emergency laparoscopic splenectomy for splenic trauma in a Jehovah's Witness patient. Surg Laparosc Endosc Percutan Tech. 2008;18(6):626–30. doi: 10.1097/SLE.0b013e31818133c6. [DOI] [PubMed] [Google Scholar]
- 5.Lorentzen K, Kjaer B, Jorgensen J. Supportive treatment of severe anaemia in a Jehovah's Witness with severe trauma. Blood Transfus. 2013;11(3):452–3. doi: 10.2450/2013.0263-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaziri K, Roland JC, Robinson LL, Reines HD, Fakhry SM. Extreme anemia in an injured Jehovah's Witness: a test of our understanding of the physiology of severe anemia and the threshold for blood transfusion. J Trauma. 2009;67(1):E11–3. doi: 10.1097/TA.0b013e318047bfc8. [DOI] [PubMed] [Google Scholar]
- 7.Raman SR, Parithivel VS, Cosgrove JM. Emergency subtotal colectomy in a Jehovah's Witness with massive lower gastrointestinal bleeding: challenges encountered and lessons learned. Am J Crit Care. 2011;20(2):179. doi: 10.4037/ajcc2011498. [DOI] [PubMed] [Google Scholar]
- 8.Lundy JB, Lewis CJ, Cancio LC, Cap AP. Experience with the use of Hemopure in the care of a massively burned adult. Int J Burns Trauma. 2014;4(1):45–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Streubel PN, Arndt S, Armitage MS, Wilson CH, Gladden PB. Fatal knee dislocation in a morbidly obese Jehovah's Witness. A case report. Obes Surg. 2010;20(9):1316–8. doi: 10.1007/s11695-008-9733-2. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald MC, Chan JY, Ross AW, Liew SM, Butt WW, Baguley D, et al. A synthetic haemoglobin-based oxygen carrier and the reversal of cardiac hypoxia secondary to severe anaemia following trauma. Med J Aust. 2011;194(9):471–3. doi: 10.5694/j.1326-5377.2011.tb03064.x. [DOI] [PubMed] [Google Scholar]
- 11.Marinaro J, Smith J, Tawil I, Billstrand M, Crookston KP. HBOC-201 use in traumatic brain injury: case report and review of literature. Transfusion. 2009;49(10):2054–9. doi: 10.1111/j.1537-2995.2009.02235.x. [DOI] [PubMed] [Google Scholar]
- 12.Napolitano LM. Hemoglobin-based oxygen carriers: first, second or third generation? Human or bovine? Where are we now? Crit Care Clin. 2009;25(2):279–301. doi: 10.1016/j.ccc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Weiskopf RB. Hemoglobin-based oxygen carriers: compassionate use and compassionate clinical trials. Anesth Analg. 2010;110(3):659–62. doi: 10.1213/ANE.0b013e3181c85255. [DOI] [PubMed] [Google Scholar]
- 14.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299(19):2304–12. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jahr JS, Mackenzie C, Pearce LB, Pitman A, Greenburg AG. HBOC-201 as an alternative to blood transfusion: efficacy and safety evaluation in a multicenter phase III trial in elective orthopedic surgery. J Trauma. 2008;64(6):1484–97. doi: 10.1097/TA.0b013e318173a93f. [DOI] [PubMed] [Google Scholar]
- 16.Weiskopf RB, Silverman TA. Balancing potential risks and benefits of hemoglobin-based oxygen carriers. Transfusion. 2013;53(10):2327–33. doi: 10.1111/trf.12339. [DOI] [PubMed] [Google Scholar]
- 17.Viele MK, Weiskopf RB. What can we learn about the need for transfusion from patients who refuse blood? The experience with Jehovah's Witnesses. Transfusion. 1994;34(5):396–401. doi: 10.1046/j.1537-2995.1994.34594249050.x. [DOI] [PubMed] [Google Scholar]
- 18.Carson JL, Noveck H, Berlin JA, Gould SA. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42(7):812–8. doi: 10.1046/j.1537-2995.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 19.Napolitano LM, Fabian TC, Kelly KM, Bailey JA, Block EF, Langholff W, et al. Improved survival of critically ill trauma patients treated with recombinant human erythropoietin. J Trauma. 2008;65(2):285–97. doi: 10.1097/TA.0b013e31817f2c6e. [DOI] [PubMed] [Google Scholar]
- 20.Corwin HL, Gettinger A, Fabian TC, May A, Pearl RG, Heard S, et al. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med. 2007;357(10):965–76. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]