Abstract
Background
The prognosis for children with malignant glioma is poor. This study was designed to determine whether lomustine and temozolomide following radiotherapy and concurrent temozolomide improves event-free survival (EFS) compared with historical controls with anaplastic astrocytoma (AA) or glioblastoma (GBM) and whether survival is influenced by the expression of O6-methylguanine-DNA-methyltransferase (MGMT).
Methods
Following maximal surgical resection, newly diagnosed children with nonmetastatic high-grade glioma underwent involved field radiotherapy with concurrent temozolomide. Adjuvant chemotherapy consisted of up to 6 cycles of lomustine 90 mg/m2 on day 1 and temozolomide 160 mg/m2/day ×5 every 6 weeks.
Results
Among the 108 eligible patients with AA or GBM, 1-year EFS was 0.49 (95% CI, 0.39–0.58), similar to the original CCG-945-based design model. However, EFS and OS were significantly improved in ACNS0423 compared with the 86 AA or GBM participants treated with adjuvant temozolomide alone in the recent ACNS0126 study (1-sided log-rank P = .019 and .019, respectively). For example, 3-year EFS was 0.22 (95% CI, 0.14–0.30) in ACNS0423 compared with 0.11 (95% CI, 0.05–0.18) in ACNS0126. Stratifying the comparison by resection extent, the addition of lomustine resulted in significantly better EFS and OS in participants without gross-total resection (P = .019 and .00085 respectively). The difference in EFS and OS was most pronounced for participants with GBM (P = .059 and 0.051, respectively), and those with MGMT overexpression (P = .00036 and .00038, respectively).
Conclusion
The addition of lomustine to temozolomide as adjuvant therapy in ACNS0423 was associated with significantly improved outcome compared with the preceding COG ACNS0126 HGG study in which participants received temozolomide alone.
Keywords: astrocytoma, glioblastoma, lomustine, pediatric high-grade glioma, temozolomide
Children with high-grade gliomas (HGGs) have a poor prognosis despite use of multimodality therapy including surgery, radiotherapy, and chemotherapy.1–3 No significant improvement in outcome for children with HGG has occurred in the last 25 years, when radiotherapy plus chemotherapy were shown to achieve longer survival than radiotherapy alone.4 Currently, radiation with adjuvant and concurrent temozolomide is widely used, based on adult data showing an improvement in outcome compared with radiation alone,5 although a single-arm pediatric study did not show improvement over historical nitrosourea-based therapies.1
Before the advent of temozolomide, nitrosoureas were considered the most active chemotherapeutic agents against HGGs. Synergistic effects of temozolomide and nitrosoureas have been seen in mice with human HGG xenografts,6 and the combination of lomustine/temozolomide as adjuvant therapy in adults with HGGs yielded encouraging long-term survival data.7 A pediatric phase 1 study determined the maximal tolerated dose for the temozolomide/lomustine combination in newly diagnosed patients with HGG with a median overall survival (OS) of 17.6 months.8
The current trial sought to determine whether the combination of temozolomide and lomustine as adjuvant therapy after administration of temozolomide during radiotherapy would improve event-free survival (EFS) compared with historical controls.
Methods
Eligibility
Patients aged ≥3 and <22 years at the time of institutional diagnosis of a nondisseminated AA, GBM, or gliosarcoma were eligible. Specimens were centrally reviewed by the primary neuropathologist (P.B.). If the central review diagnosis differed from the institutional diagnosis, the specimen was reviewed by 2 additional study neuropathologists (D.B. and M.R.) to establish a consensus central review diagnosis.
Participants could not have received prior treatment other than surgery or corticosteroids. Other eligibility criteria included enrollment within 31 days of surgical resection; Karnofsky or Lansky score ≥50%; and adequate bone marrow, renal, hepatic, and pulmonary functions. Phenobarbital and cimetidine were prohibited because of potential interactions with lomustine. Informed consent was obtained at enrollment. The protocol was approved by the institutional review board of the participating institutions.
Treatment
Intracranial tumors received a radiotherapy dose of 54.0 Gy to the preoperative tumor volume plus a 2 cm margin in 1.8 Gy fractions if a gross-total resection (GTR) was performed. For incomplete resections, residual disease was boosted with 3 additional fractions to a total dose of 59.4 Gy. Primary spinal cord tumors received a dose of 50.4–54.0 Gy in 1.8 Gy fractions regardless of resection extent. During radiation, participants received temozolomide 90 mg/m2/day for 42 days. Pneumocystis jiroveci pneumonia prophylaxis with monthly pentamidine was used during radiotherapy; Trimethoprim-sulfamethoxazole prophylaxis could be substituted during adjuvant therapy. Four weeks after completion of radiotherapy, participants started adjuvant therapy with lomustine 90 mg/m2 on day 1 and temozolomide 160 mg/m2/day × 5. Cycles were repeated every 42 days or when counts recovered, for a total of 6 cycles.
Study Evaluations and Dose Modifications for Toxicity
Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria, version 3.0. Counts were done weekly during chemoradiotherapy. Temozolomide was held for grade 4 hematologic toxicity and restarted at 75 mg/m2 after counts recovered (absolute neutrophil count (ANC) > 1000/μL; platelets >100 000/μL). For grade 3 nonhematologic toxicity (NHT), temozolomide was held and restarted at 60 mg/m2 if the toxicity resolved to ≤grade 1 within 7 days. If the toxicity recurred or did not resolve within 7 days, temozolomide was not restarted. Temozolomide was discontinued and not restarted for grade 4 NHT.
History and physical examinations and chemistries were done before each course, and counts were done weekly during adjuvant chemotherapy. MRI scans were done before every other course. Both temozolomide and lomustine doses were reduced by 25% if platelets did not recover to ≥100 000/μL or ANC to ≥1000/μL by day 49. If counts did not recover by day 49 on the reduced dose, lomustine was discontinued; temozolomide in subsequent courses was given at full dose, and the cycles were administered every 4 weeks (instead of every 6 weeks) provided counts recovered before starting each course. If counts did not recover by day 49 of any course without lomustine, chemotherapy was discontinued. Prophylactic granulocyte-colony stimulating factor (G-CSF) was not allowed. Lomustine and temozolomide doses were reduced by 25% for ≥grade 3 NHT (other than nausea, vomiting, infection, or fever) that returned to ≤grade 1 by day 49. If the toxicity recurred and did not improve to meet the pretreatment eligibility criteria by day 49, no further chemotherapy was given.
Participants were removed from therapy for progressive disease, which was defined as ≥25% increase in the largest cross-sectional area of the target lesion or the appearance of new lesions.
Expression of O6-methylguanine-DNA-methyltransferase
MGMT expression was analyzed as previously described1 using mouse anti-O6-methylguanine-DNA-methyltransferase (MGMT) antibody (mT23.2, Zymed Laboratories, 1:100).9 Tumors were categorized as exhibiting little or no expression (0/1) or scattered positive cells (2) similar to normal brain versus overexpression, in which staining was observed in most or nearly all cells (3/4) as previously described.2 This approach allowed direct measurement of MGMT protein expression and demonstrated robust correlations with alkylator response in prior studies.1,2
The study also included analysis of p53 expression and MIB labeling, based on their association with outcome in prior high-grade glioma cohorts,10,11 and mismatch repair (MMR), an alternative mechanism for alkylator resistance. Methods for p53,10 MIB-1,11 and MMR12 testing have previously been reported.
Statistical Considerations
The study was conceived as the second in a series of trials designed to assess whether an experimental therapy improved outcome compared with a statistical model developed from COG historical experience, specifically a cohort of patients from the Children's Cancer Group (CCG) 945 study (carried out in the 1980s) and the more recent ACNS0126 study. The data from CCG-945 were represented by the nonmixture parametric cure model for the probability distribution function of , where Φ is the standard normal distribution, and one year EFS is 47.3%. The experimental therapy was considered to represent a significant improvement versus this model if the 1-sided test of the null hypothesis was rejected at the 0.05 level. The P value for the likelihood ratio test of the fit of the parametric model described was calculated using a reference distribution.
Both ACNS0126 and ACNS0423 involved the use of temozolomide during radiotherapy. Participants on ACNS0126 received temozolomide alone after radiotherapy, whereas ACNS0423 administered temozolomide and lomustine after radiotherapy. During the course of evaluating the results from ACNS01261 (by which time ACNS0423 had completed accrual), it became apparent that there were substantial differences between the ACNS0126 and CCG-945 cohorts, particularly in the frequency of diagnostic discordance on consensus neuropathological review, reflecting the widespread application of more stringent WHO classification criteria.13 In addition, CCG-945 included “other eligible high-grade gliomas,” which had a dramatically more favorable prognosis than AAs and GBMs in that study,3 in part because they included a mixture of less aggressive tumor types13 and were excluded from the ACNS0126 and ACNS0423 cohorts. It was therefore recognized that a more valid comparator for the ACNS0423 study data was the ACNS0126 cohort.
Because the published ACNS0126 cohort included only participants whose diagnosis was confirmed by central review,1 comparisons between that cohort and ACNS0423 were based on central review diagnosis. The same individuals who conducted central neuropathological review for ACNS0126 also performed this function for ACNS0423. EFS was defined as the time from study enrollment to disease progression, second malignant neoplasm, death, or last follow-up. OS was the time from enrollment to death or last follow-up. Participants without an event were censored at last contact. EFS and OS were estimated by Kaplan-Meier methods.14 Confidence intervals were calculated using the log-log transformation of the estimate. Except for the primary analysis, the log-rank test was used to compare risk for EFS event and death across groups defined by participant characteristics such as histology and resection extent.15 Tests for risk of EFS and death associated with treatment effect were 1-sided, as specified in the protocol. Tests for other exploratory analyses were 2-sided.
Data were finalized as of September 30, 2012, and censored 6 months prior (March 31, 2012). Participants in ACNS0126 with follow-up longer than 77.44 months (6.5 y) were censored at 6.5 years (near the maximum follow-up among non-failures in ACNS0423, 6.45 years).
Results
Participant Characteristics
Between March 21, 2005, and August 3, 2007, 118 participants were enrolled (Table 1 and Supplementary Material, Fig. S1-CONSORT Diagram). Three patients were ineligible. On central review, 7 patients were found not to have HGGs and are not evaluated herein. Table 1 summarizes characteristics of the 108 eligible and evaluated participants. Median age was 12.0 years (range, 3–21 y), 54% of participants were male, and 24% had GTR.
Table 1.
Baseline patient characteristics in the ACNS0423 cohort, compared with the preceding ACNS0126 anaplastic astrocytoma and glioblastoma cohorts
| Patient Characteristic | ACNS0423 |
ACNS0126 |
||
|---|---|---|---|---|
| Frequency | Percent | Frequency | Percent | |
| Sex | ||||
| Male | 58 | 53.7 | 44 | 51.2 |
| Female | 50 | 46.3 | 42 | 48.8 |
| Age (y) | ||||
| <5 | 8 | 7.4 | 3 | 3.5 |
| 5–9 | 25 | 23.1 | 27 | 31.4 |
| 10–14 | 38 | 35.2 | 31 | 36.0 |
| ≥15 | 37 | 34.3 | 25 | 29.1 |
| Race | ||||
| White | 83 | 76.9 | 60 | 69.8 |
| Black | 10 | 9.3 | 15 | 17.4 |
| Other/unknown | 15 | 13.9 | 11 | 12.8 |
| Extent of resection | ||||
| Biopsy onlya | 34 | 31.5 | 26 | 30.2 |
| Partial (10%–49%) | 7 | 6.5 | 8 | 9.3 |
| Subtotal (50%–95%)b | 17 | 15.7 | 15 | 17.4 |
| Radical subtotal (96%–99%)b | 19 | 17.6 | 12 | 14.0 |
| Gross total | 26 | 24.1 | 22 | 25.6 |
| Unknown/not available | 5 | 4.6 | 3 | 3.5 |
| Central Pathologic Diagnosis | ||||
| Anaplastic astrocytoma | 46 | 42.6 | 31 | 36.0 |
| Glioblastoma | 62 | 57.4 | 55 | 64.0 |
| Primary site | ||||
| Cerebral hemisphere | 55 | 50.9 | 45 | 52.3 |
| Basal ganglia-diencephalon | 33 | 30.6 | 25 | 29.1 |
| Brainstem | 5 | 4.6 | 2 | 2.3 |
| Cerebellar | 9 | 8.3 | 5 | 5.8 |
| Spinal Cord | 6 | 5.6 | 9 | 10.5 |
aThere were 6 of the 26 ACNS0126 participants with biopsy only that had missing extent of resection. However, there was an indication of surgery (with tissue sampling) and so these were coded as “biopsy-only” in the ACNS0126 analysis.
bThe 2 extent of resection categories differed slightly in ACNS0126 compared with ACNS0423 by “5%”. Subtotal was considered 50%–90% in ACNS0126 versus 50%–95% in ACNS0423, and extensive/radical subtotal was considered 96%–99% in ACNS0423 versus 90%–99% in ACNS0126. The relative percentages do not differ significantly between studies.
Pathologic Concordance
Similar to ACNS0126 (where only 6.5% of participants had non-HGG histologies on consensus central review) but in contrast to CCG-945 (in which almost 30% of tumors were non-HGGs), only 6.1% of participants enrolled in ACNS0423 (7 of 115) had non-HGG diagnoses on central review (6 low-grade gliomas and 1 medulloblastoma). Four tumors institutionally diagnosed as AA were reclassified as GBM on central review, 8 GBMs were reclassified as AAs, and one gliosarcoma was reclassified as GBM.
Toxicity
Grade 3 or 4 NHTs were minimal and usually not chemotherapy-related. Toxicities with overall incidence of at least 5% are shown in Table 2. Hematological toxicity during maintenance was substantial with 63.3% of participants experiencing grade 3 or 4 neutropenia and 52.2% experiencing at least grade 3 thrombocytopenia, which compared with 38% and 15% during maintenance in ACNS0126.1 However, only 4 participants had febrile neutropenia (4.4%). Anemia was experienced by 24.4% of participants in ACNS0423 versus only 2% in ACNS0126. No deaths due to toxicity were encountered. In addition, 4 participants developed grade 2 hyperbilirubinemia during maintenance, one of whom had protocol therapy stopped because of prolonged time to resolution.
Table 2.
Grade 3 and 4 toxicities occurring in at least 5% of patients or involving hepatotoxicity during chemoradiotherapy and maintenance cycles 1–6 (as one group)
| Toxicity Type | Reporting Period |
|||
|---|---|---|---|---|
| Chemoradiotherapy (N = 106a) |
Maintenance (N = 90) |
|||
| N | % | N | % | |
| Hemoglobin | 1 | 0.9 | 22 | 24.4 |
| Leukocytes (total WBC) | 8 | 7.5 | 46 | 51.1 |
| Lymphopenia | 20 | 18.9 | 27 | 30 |
| Neutrophils/granulocytes (ANC/AGC) | 12 | 11.3 | 57 | 63.3 |
| Platelets | 15 | 14.2 | 47 | 52.2 |
| Nausea | 1 | 0.9 | 5 | 5.6 |
| ALT, SGPT (serum glutamic pyruvic transaminase) | 0 | 0 | 2 | 2.2 |
| AST, SGOT(serum glutamic oxaloacetic transaminase) | 0 | 0 | 3 | 3.3 |
| Bilirubin (hyperbilirubinemia) | 0 | 0 | 1 | 1.1 |
aTwo patients did not begin protocol therapy and are therefore excluded from toxicity calculations.
Myelosuppression during maintenance was cumulative. Table 3 shows participants who underwent dose modifications based upon protocol-specified toxicity guidelines, almost exclusively reflecting hematological toxicity. G-CSF was not permitted as per the protocol. Thirty-four percent (31 of 90 participants) had no dose modifications during any course, but (as noted in the Consort Diagram, Supplementary Material, Fig. S1) 31 participants experienced disease progression during maintenance, and a number of others stopped protocol therapy because of noncompliance, physician recommendation, death, or refusal of further therapy (in addition to 6 participants whose counts did not recover during the specified interval). Accordingly, there was attrition in the number of participants who began each course, in parallel with a declining percentage of participants who completed the course without dose reductions. Ultimately, only 44 participants received all 6 courses, and only 14 completed the final course without dose modification. Six deaths occurred during treatment or within 31 days after the end of the treatment, one due to ventricular pleural shunt infection and the remainder to disease progression.
Table 3.
ACNS0423 CCNU dose modification during maintenance period
| No Dose Modification |
Dose Modification |
Total | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| 1–6 | 31 | 34 | 59 | 66 | 90 |
| 1 | 88 | 98 | 2 | 2 | 90 |
| 2 | 46 | 57 | 35 | 43 | 81 |
| 3 | 31 | 46 | 36 | 54 | 67 |
| 4 | 26 | 48 | 28 | 52 | 54 |
| 5 | 19 | 40 | 29 | 60 | 48 |
| 6 | 14 | 32 | 30 | 68 | 44 |
Of the 108 eligible and evaluated participants, 89 experienced recurrence or progression (n = 85) or death from other events (n = 4). There were 79 deaths (75 following documented progression/relapse of the malignant glioma). Three participants died without a report of progression (one from ventricular pleural shunt infection, one with residual disease but no reported date of progression, and one lost to follow-up after the second maintenance course whose death had no cause recorded). One other participant died of acute myeloid leukemia approximately 16 months after completing therapy. The median follow-up duration was 4.96 years for the n = 19 participants alive without events.
Event-free and Overall Survival
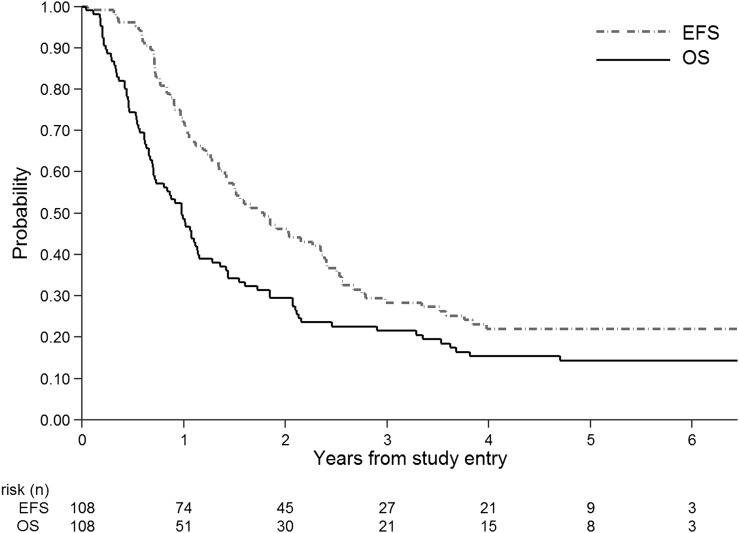
For ACNS0423, 1-year EFS in the cohort of 108 central review-confirmed AA and GBM participants was 0.49 (95% confidence interval (CI): 0.39–0.58) (Fig. 1), not significantly different from the CCG-945-based design model (P = .76). One-year overall survival (OS) was 0.72 (0.62, 0.80); an estimate of the median time to death from relapse was 5.6 months (4.3, 7.2 mo). Not unexpectedly in an intent-to-treat analysis, ACNS0423 participants who completed maintenance (n = 43) had a significantly better EFS than those who did not (n = 65) (P = .0000004, data not shown).
Fig. 1.
ACNS0423 EFS (event-free survival) and OS (overall survival) for all participants (n = 108).
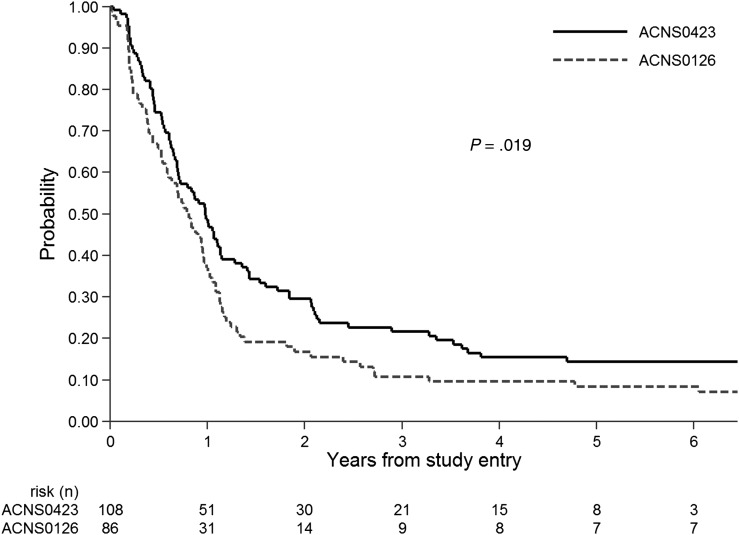
A significant outcome improvement in ACNS0423 compared with the more contemporaneous and clinically relevant ACNS0126 cohort was apparent on secondary analysis. As shown in Fig. 2, EFS in ACNS0423 was significantly greater than EFS in the 86 AA or GBM participants treated with adjuvant temozolomide alone in ACNS0126 (P = .019); one-year EFS was 0.49 (95% CI: 0.039, 0.58) versus 0.37 (0.27, 0.47), and 3-year EFS was 0.22 (0.14, 0.30) versus 0.11 (0.05, 0.18). OS in ACNS0423 was also significantly different from ACNS0126 (P = .019): 3-year OS was 0.28 (0.20, 0.37) in ACNS0423 compared with 0.19 (0.12, 0.29) in ACNS0126.
Fig. 2.
Event-free survival of AA and GBM patients in ACNS0423 (n = 108) versus ACNS0126 (n = 86), 1-sided log-rank P = .019.
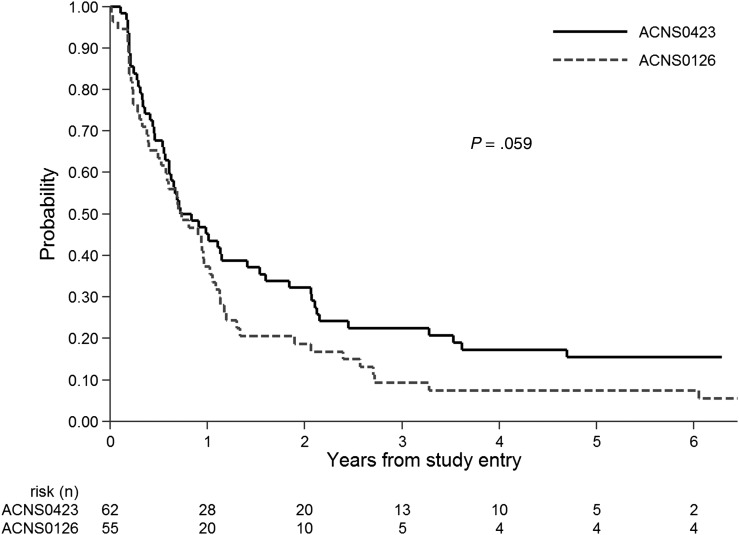
When comparing treatment efficacy within groups defined by histology, participants on ACNS0423 with GBM had nominally reduced risk for EFS (Fig. 3) and OS events when compared with ACNS0126 (P = .059 and 0.051, respectively). A weaker trend in favor of ACNS0423 was noted for AA participants (P = .095 and .082, respectively). The proportion of participants with GBM was similar in ACNS0423 (57%) and ACNS0126 (64%). Within the ACNS0423 cohort, there was no difference in EFS between the GBM and AA subsets of participants (2-sided log-rank P = .78). The outcome advantage of ACNS0423 versus ACNS0126 was observed consistently across groups defined by site of primary tumor (eg, hemispheric, basal ganglia/diencephalon, other; results not shown).
Fig. 3.
Event-free survival in ACNS0423 compared with ACNS0126 for patients with glioblastoma, 1-sided log-rank P = .059.
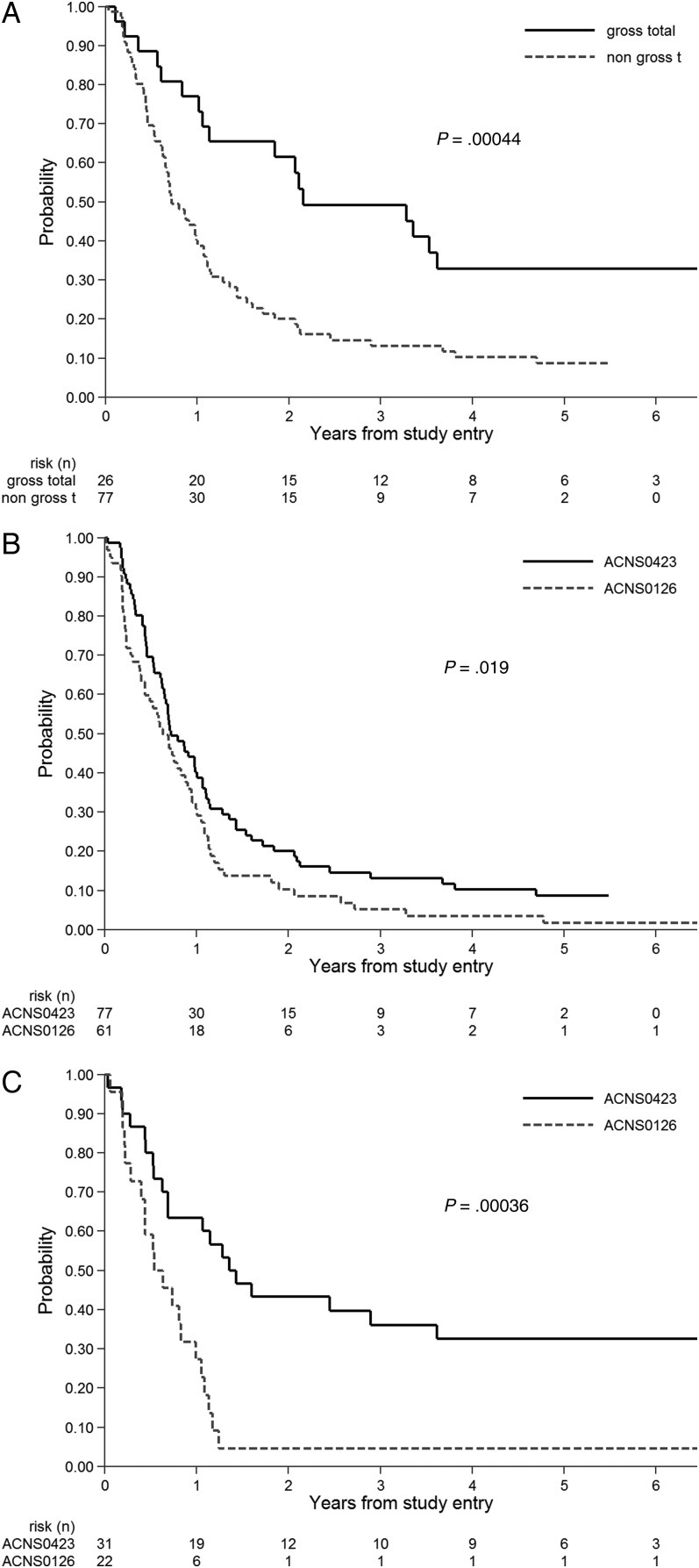
Extent of resection had a significant association with outcome in ACNS0423. Participants who had undergone GTR had dramatically better EFS (Fig. 4A) and OS than those who had not (P = .00044 and P = .0015, respectively). Given the strong association between resection extent and outcome, the comparison between ACNS0423 and ACNS0126 was stratified by extent of resection. This effect was most pronounced in children without GTR in ACNS0423, who had significantly better EFS (Fig. 4B) and OS than those in ACNS0126 (P = .019 and .00085, respectively).
Fig. 4.
A. Prognostic significance of extent of resection in ACNS0423 in terms of event-free survival (EFS) . B. EFS in ACNS0423 compared with ACNS0126 for patients who had not undergone gross total resection. One-sided log-rank P = .019. C. EFS in ACNS0423 compared with ACNS0126 for patients who had MGMT overexpression. One-sided log-rank P = .00036.
Prognostic Significance of O6-methylguanine-DNA-methyltransferase
Paraffin-embedded specimens were available for assessment of MGMT expression in 65 eligible patients. These 65 participants had significantly better EFS than the 43 for whom MGMT expression could not be determined (2-sided log-rank P = .00055), suggesting that the participants with available tissue generally had undergone more extensive resections with ample tissue for correlative analysis. Thirty-one tumors overexpressed MGMT, whereas 34 did not. There was no significant difference in EFS or OS comparing MGMT expression status within the ACNS0423 cohort: 2-sided log-rank P = .18 and 0.40, respectively). However, participants with MGMT overexpression in ACNS0423 had a significantly better EFS (Fig 4C) and OS (P = .00036 and .00038, respectively) than those in ACNS0126; the trend in favor of ACNS0423 in non–overexpressing tumors did not reach statistical significance (P = .072 and 0.10, respectively). MMR status was determined in 44 participants (2 MMR positive, 42 MMR negative); the small number of MMR-positive participants precluded comparison of EFS across groups.
Other Biological Markers
Other biological markers that were examined in participants providing adequate tissue were p53 expression status and MIB proliferation indices. P53 expression could be assessed in 61 ACNS0423 participants (29 overexpressed, 32 without overexpression); no significant association between either EFS or OS and expression status was apparent (2-sided log-rank P = .275 and .0835, respectively). There were 59 participants for whom MIB indices could be determined (17 had indices <18, 20 between 18 and 36, and 22 > 36); there was no difference in EFS or OS across these subgroups (P = .264 and .844, respectively).
Discussion
The Stupp trial5 showed that addition of concurrent and adjuvant temozolomide to radiotherapy significantly increased survival in adults with HGG. The Children's Oncology Group undertook a single arm phase 2 trial (ACNS0126) evaluating a similar regimen in pediatrics,1 using a higher dose of temozolomide (90 vs 75 mg/m2/day) during radiotherapy. The study found similar rates of 1-year EFS and OS as the adult study. However, the results were nominally lower than those from the CCG-945 study, which may have related to the limitations of using an historical group from the 1980s as a control for a cohort treated almost 20 years later, during which time significant changes occurred in the stringency of histological classification.1,13 This evolution is exemplified by the dramatic differences in the frequency of discordant diagnoses between the CCG-945 cohort3,13 and the ACNS01261 and ACNS0423 cohorts. Moreover, CCG-945 included “other eligible high-grade gliomas” (eg, oligodendroglial lesions) which are now recognized to represent a more favorable subset of tumors3,16 and were therefore excluded from more recent cohorts. Because the ACNS0423 study was developed before these issues became apparent and before the ACNS0126 data were available to use as a contemporary control group, the original design used a model based upon the CCG-945 cohort. In comparison with that benchmark, ACNS0423 therapy—although slightly more favorable in terms of 1-year EFS—did not achieve a significant improvement in outcome.
Given that the ACNS0423 study was designed as the immediate follow-up to ACNS0126—seeking to intensify treatment by using a second active alkylating agent during adjuvant therapy—the secondary comparison in outcomes between these 2 sequential studies is far more meaningful. This comparison is of particular clinical relevance because radiation and temozolomide, rather than regimens from CCG-945, are often used as the standard therapy for pediatric HGG. Moreover, the difference in MGMT expression between CCG-945 (12 of 97) and ACNS0423 (31 of 65) was highly significant (P < .001, Fisher exact test), whereas the difference between ACNS0423 and ACNS0126 was not (P = .08, Fisher exact test), which reinforces the observation that the ACNS0126 study is a more appropriate comparator for ACNS0423. To ensure comparability between the ACNS0126 and ACNS0423 trials, the same central review pathologists were included to minimize variations in histological inclusion criteria.
We hypothesized that the dual-alkylator regimen might help to overcome MGMT-mediated resistance by depleting MGMT. Since MGMT, unlike a true enzyme, is degraded following alkyl group transfer, treatment with one alkylator may diminish MGMT levels and thereby enhance activity of the second alkylator. This strategy has been previously evaluated with encouraging results. A phase 1 study using the combination of BCNU and temozolomide documented prolonged stable disease or partial responses in 3 of 7 adults with recurrent high-grade gliomas.17 In a phase 1 study, lomustine and temozolomide produced a median OS of 17.6 months.8 A phase 2 study of lomustine and temozolomide in adults with glioblastomas18 showed a median OS of 22.6 months with a 2-year survival rate of 44.7%. In an expanded study with longer follow-up,7 the median OS was 23.1 months; 47.4% survived for 2 years, and 18.5% survived for 4 years, which compared favorably with historical results using adjuvant nitrosoureas alone.19,20
The combination of adjuvant temozolomide and lomustine in the current study was associated with a significant improvement in OS and EFS compared with adjuvant temozolomide alone in the ACNS0126 study. This effect was most apparent in patients whose tumors had MGMT overexpression, as well as those who did not undergo GTR (a particularly poor prognostic group)3,13 and in those with glioblastomas, but this should not be construed as indicating that the benefit is predominantly for certain subgroups since the data derived from these subset analyses do not support that inference. It is impossible to know if the improved results in ACNS0423 versus ACNS0126 reflected differences in molecular subsets of HGGs such as IDH-mutated tumors21,22 because of the limited amounts of tumor specimens available from ACNS0126 and the fact that this analysis was not a randomized comparison. Outcome in ACNS0423 was strongly influenced by resection status, in agreement with other pediatric high-grade glioma studies.3,13 However, neither EFS nor OS was associated with MGMT expression status, in contrast with the ACNS0126 and CCG-945 studies1,2 and adult studies using temozolomide alone.23,24
The lack of an adverse association between MGMT status and outcome in the ACNS0423 study and the survival benefit seen in ACNS0423 versus ACNS0126 in MGMT-overexpressing tumors suggests that the dual alkylator approach may counteract the impact of MGMT expression on treatment resistance in pediatric malignant gliomas, an effect not noted in a small series of adult GBM patients.7 Prior trials in adults have used alternate approaches for assessing MGMT status such as promoter methylation,23,24 although we intentionally used a protein expression assay that demonstrated prognostic significance in the ACNS0126 and CCG-945 studies1,2 to ensure comparability of the interpretations.
In considering these data, it is important to note the potential pitfalls of historical comparisons in pediatric oncology.25 Although this study provides evidence in a relatively rare tumor type of an apparent improvement in the treatment of patients that is consistent with the hypothesis underlying the study, confirmation of this finding in a randomized trial would provide the strongest evidence for this therapeutic approach. This study provides intriguing evidence within the limits that a single-therapy, historically controlled study can. Indeed, the outcome should provide motivation for promotion of the doublet treatment to a randomized comparison.
In this context, a question for future consideration is how best to incorporate these observations in subsequent studies. The combination of lomustine and temozolomide as maintenance therapy did increase hematological toxicity, particularly in terms of neutropenia and thrombocytopenia compared with temozolomide alone.1 Building upon this regimen would require the addition of agents that would not intensify this toxicity. Alternatively, given the improved results of this regimen compared with temozolomide alone, it may be considered as a replacement for temolozomide alone as the comparative benchmark in future randomized trials.
Funding
This work was supported in part by National Institutes of Health grants R01NS37704 (to I.F.P.) and U10CA98543 and U10CA180899 to the Children's Oncology Group.
Conflict of interest statement. Presented in part at the International Society of Pediatric Neuro-Oncology, 2010, Vienna, Austria. Regina I. Jakacki is currently employed by Astra-Zeneca and has been a consultant for OSI Pharmaceuticals (now Astellas); Robert S. Lavey has intellectual property for a radiation immobilization device (Medical Intelligence). Neither of these relate to the subject of this submission. The other authors have no conflicts or disclosures.
Supplementary Material
References
- 1. Cohen KJ, Pollack IF, Zhou T et al. Temozolomide in the treatment of high-grade gliomas in children: a report from the Children's Oncology Group. Neuro Oncol. 2011;13 (3):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pollack IF, Hamilton RL, Sobol RW et al. O6-methylguanine-DNA methyltransferase expression strongly correlates with outcome in childhood malignant gliomas: results from the CCG-945 Cohort. J Clin Oncol. 2006;24 (21):3431–3437. [DOI] [PubMed] [Google Scholar]
- 3. Finlay JL, Boyett JM, Yates AJ et al. Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. Childrens Cancer Group. J Clin Oncol. 1995;13 (1):112–123. [DOI] [PubMed] [Google Scholar]
- 4. Sposto R, Ertel IJ, Jenkin RD et al. The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: results of a randomized trial. A report from the Childrens Cancer Study Group. J Neurooncol. 1989;7 (2):165–177. [DOI] [PubMed] [Google Scholar]
- 5. Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352 (10):987–996. [DOI] [PubMed] [Google Scholar]
- 6. Plowman J, Waud WR, Koutsoukos AD, Rubinstein LV, Moore TD, Grever MR. Preclinical antitumor activity of temozolomide in mice: efficacy against human brain tumor xenografts and synergism with 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res. 1994;54 (14):3793–3799. [PubMed] [Google Scholar]
- 7. Glas M, Happold C, Rieger J et al. Long-term survival of patients with glioblastoma treated with radiotherapy and lomustine plus temozolomide. J Clin Oncol. 2009;27 (8):1257–1261. [DOI] [PubMed] [Google Scholar]
- 8. Jakacki RI, Yates A, Blaney SM et al. A phase I trial of temozolomide and lomustine in newly diagnosed high-grade gliomas of childhood. Neuro Oncol. 2008;10 (4):569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McLendon RE, Cleveland L, Pegram C, Bigner SH, Bigner DD, Friedman HS. Immunohistochemical detection of the DNA repair enzyme O6-methylguanine-DNA methyltransferase in formalin-fixed, paraffin-embedded astrocytomas. Lab Invest. 1998;78 (5):643–644. [PubMed] [Google Scholar]
- 10. Pollack IF, Finkelstein SD, Woods J et al. Expression of p53 and prognosis in children with malignant gliomas. N Engl J Med. 2002;346 (6):420–427. [DOI] [PubMed] [Google Scholar]
- 11. Pollack IF, Hamilton RL, Burnham J et al. Impact of proliferation index on outcome in childhood malignant gliomas: results in a multi-institutional cohort. Neurosurgery. 2002;50 (6):1238–1244; discussion 1244–1235. [DOI] [PubMed] [Google Scholar]
- 12. Pollack IF, Hamilton RL, Sobol RW et al. Mismatch repair deficiency is an uncommon mechanism of alkylator resistance in pediatric malignant gliomas: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2010;55 (6):1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pollack IF, Boyett JM, Yates AJ et al. The influence of central review on outcome associations in childhood malignant gliomas: results from the CCG-945 experience. Neuro Oncol. 2003;5 (3):197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaplan EL, Meier P. Nonparametric estimate from incomplete observations. J Amer Statist Assoc. 1958;53:457–481. [Google Scholar]
- 15. Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2nd ed New York: John Wiley and Sons; 2002. [Google Scholar]
- 16. van den Bent M, Chinot OL, Cairncross JG. Recent developments in the molecular characterization and treatment of oligodendroglial tumors. Neuro Oncol. 2003;5 (2):128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hammond LA, Eckardt JR, Kuhn JG et al. A randomized phase I and pharmacological trial of sequences of 1,3-bis(2-chloroethyl)-1-nitrosourea and temozolomide in patients with advanced solid neoplasms. Clin Cancer Res. 2004;10 (5):1645–1656. [DOI] [PubMed] [Google Scholar]
- 18. Herrlinger U, Rieger J, Koch D et al. Phase II trial of lomustine plus temozolomide chemotherapy in addition to radiotherapy in newly diagnosed glioblastoma: UKT-03. J Clin Oncol. 2006;24 (27):4412–4417. [DOI] [PubMed] [Google Scholar]
- 19. Fine HA, Dear KB, Loeffler JS, Black PM, Canellos GP. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71 (8):2585–2597. [DOI] [PubMed] [Google Scholar]
- 20. Walker MD, Green SB, Byar DP et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303 (23):1323–1329. [DOI] [PubMed] [Google Scholar]
- 21. Korshunov A, Ryzhova M, Hovestadt V et al. Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol. 2015;129 (5):669–678. [DOI] [PubMed] [Google Scholar]
- 22. Pollack IF, Hamilton RL, Sobol RW et al. IDH1 mutations are common in malignant gliomas arising in adolescents: a report from the Children's Oncology Group. Childs Nerv Syst. 2011;27 (1):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hegi ME, Diserens AC, Gorlia T et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352 (10):997–1003. [DOI] [PubMed] [Google Scholar]
- 24. Hegi ME, Liu L, Herman JG et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26 (25):4189–4199. [DOI] [PubMed] [Google Scholar]
- 25. Farewell VT, D'Angio GJ. A simulated study of historical controls using real data. Biometrics. 1981;37 (1):169–176. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.