Abstract
Pituitary adenomas are benign intracranial neoplasms that are frequently well-controlled with standard treatments that include surgical resection, radiotherapy, and agents that modulate hormonal excess. Unfortunately, a subset of patients remains uncontrolled or develops complications from these interventions. For these patients, chemotherapy is an additional treatment option that could improve outcomes. Temozolomide is an oral chemotherapy with a favorable side-effect profile that has shown activity against pituitary adenomas. Its non-overlapping toxicity and ability to induce rapid tumor regression renders it a potentially important adjunctive treatment. In patients with tumors that cannot be optimally addressed with standard treatments, there may be a role for early initiation of temozolomide.
Keywords: chemotherapy, neuro-oncology, pituitary adenoma, pituitary carcinoma, temozolomide
Pituitary adenomas are common intracranial neoplasms with a prevalence of 15% in autopsy studies and 20% in radiologic studies.1 The majority of adenomas lack clinical significance and are less than 5 mm in diameter. The pituitary adenomas that cause significant morbidity and mortality are the macroadenomas, which are greater than 1 cm in size, and the functional adenomas. Macroadenomas account for ∼50% of tumors and are frequently nonfunctional. Greater than 50% of tumors are functional and are frequently microadenomas; of these, 60%–80% secrete prolactin (PRL), 15%–20% secrete growth hormone (GH), and 5%–10% secrete adrenocorticotropic hormone (ACTH).2–4 While most pituitary adenomas are well-controlled with current treatments including surgery, radiotherapy, and agents that modulate hormonal excess, some adenomas grow or continue to oversecrete despite maximal intervention. Patients may exhaust conventional options, or they may be poor candidates or unable to tolerate these treatments. For some people with pituitary adenomas, chemotherapy may be worthy of early consideration to decrease morbidity and improve outcomes.
Chemotherapy in the Treatment of Pituitary Adenomas
It has become evident from case literature that pituitary adenomas and carcinomas (adenomas that have developed distant metastases) are chemotherapy-responsive tumors. The literature primarily supports the use of chemotherapy as salvage therapy for heavily pretreated adenomas and carcinomas that exhibit progressive growth despite multiple efforts at resection and irradiation.
Many chemotherapies have been tried, including temozolomide, lomustine, 5-flurouracil, cisplatin, carboplatin, and etoposide, with variable success (Table 1).5–11 Temozolomide has the most literature supporting its use because of its high response rate and favorable side-effect profile. At this time, there are more than100 patients with an aggressive pituitary adenoma or carcinoma who received treatment with temozolomide and are reported in the literature.
Table 1.
Chemotherapy regimens and their outcomes
| Regimen | Patients | Course |
|---|---|---|
| Temozolomide | >100 patients | Response rate of 55% for aggressive adenomas and 58% for pituitary carcinomas.13 |
| Lomustine + 5FU |
|
|
| Methotrexate + 5FU | 1 GH-secreting carcinoma | The tumor was stable for 2 years after the commencement of chemotherapy.7 |
| Etoposide + cisplatin + tamoxifen | 1 PRL-secreting carcinoma | No response.6 |
| Etoposide + cisplatin | 1 ACTH-secreting carcinoma | Interval decrease in tumor extension, subsequently, the patient was changed to etoposide and carboplatin due to renal failure, and then he developed leptomeningeal dissemination.8 |
| Etoposide + carboplatin | 1 PRL-secreting carcinoma | No response.9 |
| 5FU + cyclophosphamide + doxorubicin | 1 TSH-secreting carcinoma | Marked decrease in her lesions.10 |
| Cisplatin + vincristine + bleomycin | 1 GH carcinoma | No response.11 |
Abbreviations: 5FU, 5-fluorouracil; ACTH, adrenocorticotropic hormone; GH, growth hormone; PRL, prolactin; TSH, thyroid stimulating hormone.
Temozolomide is frequently the treatment of last resort for locally aggressive and metastatic pituitary tumors; consequently, a response generally refers to a reduction in tumor size. One challenge in drawing conclusions from case reports and case series is the absence of standardized response criteria. Meaningful responses have been defined as a greater than 20%–80% reduction in size, with a reduction in tumor size sometimes referring to a reduction in tumor volume and occasionally to a reduction in greatest diameter.12 With this caveat, the response rate may be as high as 55% for aggressive adenomas and as high as 58% for pituitary carcinomas.13
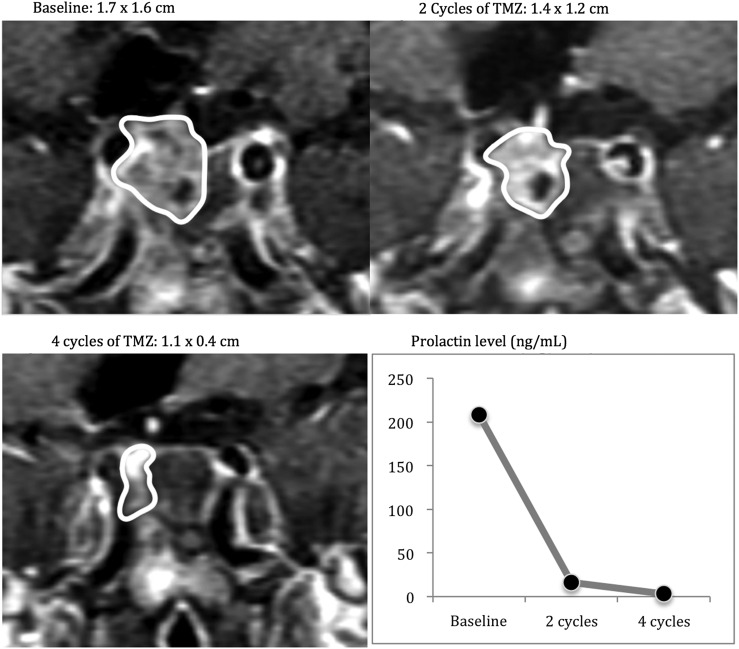
The largest case series reported the outcome of treatment with temozolomide on 24 consecutive patients at 7 participating institutions, including 16 patients with locally aggressive pituitary adenomas and 8 with pituitary carcinomas. Of these 24 patients, 21 were considered evaluable, and 16 of these 21 had hormone-secreting tumors. The response rate for this unselected cohort was 48% after a median of 6 cycles of temozolomide dosed at 150–200 mg/m2/day for 5 days every 4 weeks.12 In addition to the high rate of radiographic response, there were also dramatic reductions in prolactin (PRL), adrenocorticotropic hormone (ACTH), and growth hormone (GH) secretion.12 The functional tumors that responded radiographically also tended to respond biochemically. Among the 16 functional tumors, hormone levels in all subtypes (PRL-, GH-, and ACTH-secreting tumors) decreased by 53%–98% with normalization of hormone levels in 3 patients. In this and other case series, it has been observed that a response to temozolomide generally occurs after a median of three 4-week cycles (Figure 1).12,14,15 The durability of temozolomide response in this highly pretreated cohort was variable; notably, 2 of the 8 carcinomas had a complete response and did not recur after 48 and 91 months of follow-up after completing 6 and 23 cycles of treatment, respectively.12 In treatment-naïve tumors, which are typically smaller and less invasive, durable responses may occur at a higher rate.
Fig. 1.
A patient with an enlarging prolactinoma on cabergoline, status post resection and fractionated radiotherapy who received treatment with temozolomide.
While a radiographic response rate of 50% is exciting, the response rate may approach 100% in patients whose pituitary adenomas have low O6-methylguanine DNA methyltransferase (MGMT) expression as determined semiquantitatively by immunohistochemistry.12 MGMT is a DNA repair enzyme that reverses the methylated DNA adducts induced by temozolomide. In glioblastoma (GBM), low expression of MGMT as determined by immunohistochemistry has been associated with responsiveness to temozolomide;16 however, only MGMT promoter methylation, which results in silencing of the MGMT gene, has been validated as a predictive and prognostic marker.17 In pituitary adenomas, an inverse relationship between MGMT promoter methylation and MGMT expression has been reported by some,18,19 but not all, investigators.20,21 At this time, the evidence primarily supports a relationship between treatment response and low MGMT expression by immunohistochemistry as opposed to MGMT promoter methylation, which is technically more reliable.12,22
Risks of Temozolomide Compared With Transsphenoidal Surgery and Radiotherapy
Temozolomide is a well-tolerated alkylating agent; the immediate hematologic toxicities of temozolomide are typically short-lived and easily managed with dose reduction.23 The other common side effects are nausea, constipation, and fatigue and are relatively mild compared with other chemotherapies. The more worrisome potential complications are delayed and permanent, such as treatment-related myelodysplastic syndrome and treatment-related acute myelogenous leukemia. The incidence is unknown given its rarity; however, it likely occurs in fewer than 0.1% of patients treated with temozolomide. The risk may be related to the duration of treatment, exposure to other alkylating agents, and the length of patient survival, which will be greater in patients with pituitary adenoma than those with GBM.24,25 Because of its DNA-damaging effects, temozolomide is teratogenic, and it may reduce fertility (by causing azoospermia and premature ovarian failure) to a greater degree than radiotherapy ( RT), which can cause hypogonadotropic hypogonadism.26 Fertility preservation efforts may be considered in young patients prior to the start of temozolomide.
The benefits of treating pituitary adenomas with chemotherapy may outweigh the risks, particularly when balancing the risks against the potential hazards of alternative treatments. Transsphenoidal surgery has a relatively low morbidity and an estimated mortality of ∼0.6% in the general population. A subset of patients who are at high risk of surgical complications and who may benefit from consideration of chemotherapy are those with giant pituitary macroadenomas (macroadenomas greater than 4 cms). These tumors often require staged resection and are often resected transcranially. Surgical resection results in high rates of mortality and morbidity: 4.4% and 14%, respectively. Common complications include CSF leak, visual deterioration, massive middle cerebral artery infarcts, hypothalamic failure, and severe diabetes insipidus with potential adipsia.27 Treatment with temozolomide prior to surgery to decrease tumor volume or following partial resection warrants further study as it may improve outcomes.
With regard to RT, the risk of complications may be particularly high for young patients, given their expected longevity. In addition to causing optic neuropathy and radiation necrosis (typically within the first year of treatment), RT increases the risk for cerebrovascular disease over the long term due to exposure of both carotid arteries and the increased risk of focal accelerated atherosclerosis.28 In a large study including 331 patients with a pituitary adenoma treated with postoperative RT to a median dose of 45 Gy in 20 to 30 fractions, patients had a stroke rate of 4% at 5 years, 11% at 10 years, and 21% at 20 years.29 Moreover, RT has been associated with secondary malignancies with a 10-year cumulative risk of 2.0% and a 20-year risk of 2.4% (the most common malignancies being meningiomas, sarcomas, gliomas, and primitive neuroectodermal tumors),30,31 which is substantially greater than the risk of acute myelogenous leukemia in patients treated with temozolomide. RT is a common cause of hypopituitarism; in a recent meta-analysis including 19,153 adults with hypopituitarism, hypopituitarism was associated with excess mortality due to vasculopathy from pituitary irradiation, metabolic syndrome, and increased prevalence of atherosclerosis related to pituitary insufficiency.32
Some patients have a relative contraindication to RT, e.g., patients with cancer predisposition syndromes such as neurofibromatosis, who have an increased risk of developing secondary malignancies after treatment with RT, and patients with multiple sclerosis, who are at risk of developing further demyelination from that treatment. These patients may be optimal candidates for early chemotherapy instead of RT.33–35
Recurrent Nonfunctional Pituitary Adenoma
Ready access to MRIs makes it easy to monitor for recurrence or progression and to treat at that time before the tumor causes symptoms. For this reason, postoperative RT is no longer a standard practice, although it may still be justified in high-risk patients. Identifying the tumors that will behave aggressively remains a challenge, given that the World Health Organization grading system provides limited prognostic information.36
For patients with nonfunctional pituitary adenomas that have recurred or progressed, RT is a standard second-line treatment. Tumor control following treatment of a nonfunctional pituitary adenoma with RT is approximately 90% based on retrospective series.37 In patients with pituitary adenomas that are within 5 mm of the optic nerve and chiasm or greater than 1 cm, stereotactic radiosurgery often cannot be performed safely, and fractionated RT may be suboptimal.38,39 When an optimal outcome cannot be achieved with RT, an initial trial of temozolomide may be reasonable. Tumors that respond to temozolomide may not require further treatment, and a partial response to temozolomide may permit a complete resection or make RT safer and possibly more effective.
Recurrent or Refractory Functional Pituitary Adenomas
Functional pituitary adenomas can cause morbidity through continued oversecretion and by causing complications due to mass effect.
Adrenocorticotropic Hormone and Growth Hormone-secreting Pituitary Adenomas
ACTH-secreting pituitary adenomas are responsible for Cushing disease, whereas GH-secreting adenomas give rise to acromegaly. These 2 conditions have high morbidity and mortality, making it imperative that these patients achieve a biochemical response to treatment. Cushing disease patients have an increased cardiovascular risk (even more so when hormonal hypersecretion is uncontrolled) with a 5-year mortality rate as high as 50%.40 Acromegalics have an excess mortality of approximately 2-fold compared with the general population; again, much of this excess risk is associated with uncontrolled hypersecretion.41 For acromegaly, the main causes of death are vascular and respiratory, although there may also be an increased risk of malignancy.42 Normalizing cortisol levels or the action at the glucocorticoid receptor in patients with Cushing disease and a GH level < 1 µg/L and normalized IGF-1 levels in acromegalics have been associated with improved outcomes.43–45
In Cushing disease, it is estimated that only 9%–37% are macroadenomas.2,46 First-line therapy in the treatment of ACTH-secreting and GH-secreting pituitary adenomas is transsphenoidal surgery. For ACTH-secreting microadenomas, surgery leads to remission in 65%–90% of patients.47 For GH-secreting microadenomas, the success rate is equally high with remission rates exceeding 85%,43 but unlike ACTH-secreting tumors, GH-secreting adenomas are predominantly macroadenomas.2,3 The success rate of transsphenoidal surgery in achieving a biochemical response in macroadenomas is low: the remission rate for ACTH-secreting macroadenomas is less than 65% (and just 12.5% by the most stringent criteria),39,48 while the remission rate for GH-secreting pituitary macroadenomas is around 40%–50%.43 Besides tumor size, other factors that negatively impact remission rates include involvement of the cavernous sinus, younger age, and dural invasion.49–51 Because of its tolerability and relatively limited toxicity, it may be reasonable in certain cases to consider temozolomide in the neoadjuvant (preoperative) setting for patients deemed incurable by surgery alone due to local invasiveness. Treated tumors are often necrotic, which may facilitate tumor resection52 and possibly result in improved outcomes.
Given the high morbidity and mortality of patients with Cushing disease or acromegaly and the unsatisfactory response to surgery in a subset of these patients, postoperative adjuvant therapy may be indicated. The clearest indication for postoperative adjuvant treatment is in patients with continued hormonal hypersecretion.53 Cushing disease patients without postoperative adrenal insufficiency and acromegalics who do not achieve low levels of GH are at high risk for recurrence.54–56 Treatment with temozolomide is a potential option that may have a better risk-benefit profile than RT and may be an alternative to observation.
Patients with Cushing disease with a recurrent ACTH-secreting adenoma—who are poor candidates for, refractory to, or intolerant of medical therapies that modulate hormonal excess and are treated with fractionated RT or stereotactic radiosurgery—achieve biochemical control in approximately 50%–60% of cases within 3–5 years.44 For GH-secreting pituitary adenomas, response rates are lower at 10%–60%.43 In addition to the long-term complications of RT, the high failure rate and delayed response ranging from several months to several years, are significant drawbacks to this approach.57 A subset of patients (particularly individuals with low MGMT expression and a large amount of residual tumor) who are predicted to have a worse response to RT may benefit from a trial of temozolomide prior to RT. Response typically occurs after a few cycles. Again, if adequate control is achieved with temozolomide as a single agent, then no further treatment may be needed; however, if the patient only has a partial response, treatment may then be consolidated with RT.
Finally, in patients with recurrent or metastatic ACTH-secreting tumors who are poor surgical candidates, temozolomide may prevent or postpone the need for bilateral adrenalectomy. While bilateral adrenalectomy remains a potentially life-saving procedure in severe cases of ACTH-secreting tumors, it causes permanent adrenal insufficiency and places patients at risk for Nelson syndrome (ie, progression of the pituitary adenoma from loss of negative feedback) and does not decrease tumor burden—an important consideration given that the pituitary is in a high-risk anatomic location.58
Prolactinomas
Prolactinomas are much more responsive to medical therapy than ACTH- and GH-secreting pituitary adenomas. Dopamine agonists frequently result in dramatic reductions in tumor size: a 50% decrease in size is achieved in 64% of patients receiving bromocriptine and in 96% receiving cabergoline.59 Hence, dopamine agonists are first-line agents even when patients have a visual field deficit due to compression of the optic nerves/chiasm from the tumor. Enlargement of a prolactinoma in spite of treatment or development of complications from dopamine agonists, such as valvular disease from high-dose cabergoline, are the main indications for second-line therapy.
Serum PRL is not normalized in 10%–20% of patients with prolactinomas who are treated with cabergoline.60 An elevated PRL level is not an immediate indication for a change in treatment. Hyperprolactinemia only needs to be treated when it is preventing pregnancy or causing complications such as osteoporosis, hypogonadism, bothersome hirsutism, or galactorrhea.
When dopamine agonist therapy fails to control the tumor, transsphenoidal surgery is highly effective. It cures 80%–90% of PRL-secreting microadenomas but only 50% of PRL-secreting macroadenomas.61 RT may control further tumor growth in resistant or malignant prolactinomas, but it normalizes PRL levels in only 25% of cases and may require up to 20 years for the maximal effect to be achieved.62 Hence, it is possible that chemotherapy is the best treatment option when patients are having complications from hyperprolactinemia, the patient is refractory to a dopamine agonist, and total resection cannot be achieved.
Uncertainties and Future Directions
The literature provides limited guidance on the expected response rate of treatment-naïve pituitary adenomas to temozolomide. The high prevalence of low MGMT expression in nonrecurrent, noninvasive pituitary adenomas suggests early temozolomide may be an option, but unfortunately the reports addressing this issue are conflicting.18,63–65
At this time, it is unclear whether temozolomide is more effective for pituitary adenomas when used as monotherapy or used in combination with other treatments. Prior to the randomized phase 3 trial that established concurrent RT and temozolomide followed by 6 cycles of adjuvant temozolomide as the standard of care in GBM, preclinical work suggested that temozolomide acted as a radiosensitizer.66 It is possible that the most effective use of temozolomide in pituitary adenomas is in conjunction with fractionated RT. Preclinical work also suggests that capecitabine augments the cytotoxicity of temozolomide when it is administered 7–9 days before temozolomide; this finding led to a phase 2 trial (NCT00869050) that investigated this combination in neuroendocrine tumors. Four patients with ACTH-secreting pituitary tumors were treated with temozolomide and capecitabine: 2 patients achieved a complete response, one had a 75% response, and one had stable disease. All 4 patients were found to have low MGMT expression by immunohistochemistry, so the high rate of response may be due to the temozolomide alone.67
The optimal dosing schedule for temozolomide when used as monotherapy for pituitary adenoma remains unanswered. The most common dosing schedule for temozolomide is 150–200 mg/m2/day for 5 days every 4 weeks. The optimal duration of treatment is unknown. In the landmark trial that resulted in FDA approval for use in GBM, patients were treated with 6 cycles of adjuvant temozolomide;23 however, in practice, patients with GBM generally receive between 6 and 24 cycles. The rationale for a shorter course of treatment, particularly in patients with pituitary adenomas, is that temozolomide causes cumulative bone marrow toxicity, and individuals with pituitary tumors are more likely to be long-term survivors.
Whole-exome sequencing of pituitary adenomas may identify additional molecular markers (other than MGMT expression) that may predict treatment response. It has already identified potentially targetable mutations in ACTH-secreting pituitary adenomas that have the potential to change clinical practice. A USP8 deubiquitinase mutation has been found in a subset of ACTH-secreting pituitary adenomas.68 USP8 mutations result in upregulation of the epithelial growth factor receptor (EGFR), suggesting that such patients may be sensitive to EGFR tyrosine kinase inhibitors such as lapatinib, although this remains untested.
Conclusion
The use of temozolomide in the treatment of malignant pituitary adenomas has begun to enter treatment guidelines based on case reports (the lowest quality of evidence).60 While most pituitary adenomas can be controlled with current standard treatments, some tumors remain uncontrolled despite maximal intervention, and some patients have complications from surgery and RT. These patients should be considered for chemotherapy, particularly temozolomide, but may also warrant the exploration of tyrosine kinase inhibitors and other targeted therapies. Temozolomide is a highly effective treatment in an appropriately selected population and may be superior to the current standard of care for some patients. Clinical trials that define the best predictors of response to temozolomide, the expected response rate, and indications for use are urgently needed.
Funding
This research was funded in part through the NIH /NCI Cancer Center Support Grant P30 CA008748.
Acknowledgments
We would like to thank Sharon Wardlaw, MD, for her input on this manuscript.
Conflict of interests statement. Dr. Lin has no conflict of interest. Dr. Sum has no conflict of interest. Dr. DeAngelis serves on the scientific advisory board for Juno Therapeutics.
References
- 1. Ezzat S, Asa SL, Couldwell WT et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101:613–619. [DOI] [PubMed] [Google Scholar]
- 2. Raappana A, Koivukangas J, Ebeling T, Pirila T. Incidence of pituitary adenomas in Northern Finland in 1992-2007. J Clin Endocrinol Metab. 2010;95:4268–4275. [DOI] [PubMed] [Google Scholar]
- 3. Agustsson TT, Baldvinsdottir T, Jonasson JG et al. The epidemiology of pituitary adenomas in Iceland, 1955-2012: a nationwide population-based study. Eur J Endocrinol. 2015;173:655–664. [DOI] [PubMed] [Google Scholar]
- 4. Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab. 2006;91:4769–4775. [DOI] [PubMed] [Google Scholar]
- 5. Kaltsas GA, Mukherjee JJ, Plowman PN, Monson JP, Grossman AB, Besser GM. The role of cytotoxic chemotherapy in the management of aggressive and malignant pituitary tumors. J Clin Endocrinol Metab. 1998;83:4233–4238. [DOI] [PubMed] [Google Scholar]
- 6. Walker JD, Grossman A, Anderson JV et al. Malignant prolactinoma with extracranial metastases: a report of three cases. Clin Endocrinol (Oxf). 1993;38:411–419. [DOI] [PubMed] [Google Scholar]
- 7. Asai A, Matsutani M, Funada N, Takakura K. Malignant growth hormone-secreting pituitary adenoma with hematogenous dural metastasis: case report. Neurosurgery. 1988;22:1091–1094. [DOI] [PubMed] [Google Scholar]
- 8. Cornell RF, Kelly DF, Bordo G et al. Chemotherapy-induced regression of an adrenocorticotropin-secreting pituitary carcinoma accompanied by secondary adrenal insufficiency. Case Rep Endocrinol. 2013;2013:675298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hurel SJ, Harris PE, McNicol AM, Foster S, Kelly WF, Baylis PH. Metastatic prolactinoma: effect of octreotide, cabergoline, carboplatin and etoposide; immunocytochemical analysis of proto-oncogene expression. J Clin Endocrinol Metab. 1997;82:2962–2965. [DOI] [PubMed] [Google Scholar]
- 10. Mixson AJ, Friedman TC, Katz DA et al. Thyrotropin-secreting pituitary carcinoma. J Clin Endocrinol Metab. 1993;76:529–533. [DOI] [PubMed] [Google Scholar]
- 11. Hashimoto N, Handa H, Nishi S. Intracranial and intraspinal dissemination from a growth hormone-secreting pituitary tumor. Case report. J Neurosurg. 1986;64:140–144. [DOI] [PubMed] [Google Scholar]
- 12. Bengtsson D, Schroder HD, Andersen M et al. Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide. J Clin Endocrinol Metab. 2015;100:1689–1698. [DOI] [PubMed] [Google Scholar]
- 13. Bruno OD, Juarez-Allen L, Christiansen SB et al. Temozolomide Therapy for Aggressive Pituitary Tumors: Results in a Small Series of Patients from Argentina. Int J Endocrinol. 2015;2015:587893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raverot G, Sturm N, de Fraipont F et al. Temozolomide treatment in aggressive pituitary tumors and pituitary carcinomas: a French multicenter experience. J Clin Endocrinol Metab. 2010;95:4592–4599. [DOI] [PubMed] [Google Scholar]
- 15. Losa M, Bogazzi F, Cannavo S et al. Temozolomide therapy in patients with aggressive pituitary adenomas or carcinomas. J Neurooncol. 2016;126:519–525. [DOI] [PubMed] [Google Scholar]
- 16. Capper D, Mittelbronn M, Goeppert B, Meyermann R, Schittenhelm J. Secreted protein, acidic and rich in cysteine (SPARC) expression in astrocytic tumour cells negatively correlates with proliferation, while vascular SPARC expression is associated with patient survival. Neuropathol Appl Neurobiol. 2010;36:183–197. [DOI] [PubMed] [Google Scholar]
- 17. Hegi ME, Diserens AC, Gorlia T et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. [DOI] [PubMed] [Google Scholar]
- 18. McCormack AI, McDonald KL, Gill AJ et al. Low O6-methylguanine-DNA methyltransferase (MGMT) expression and response to temozolomide in aggressive pituitary tumours. Clin Endocrinol (Oxf). 2009;71:226–233. [DOI] [PubMed] [Google Scholar]
- 19. Jiang XB, Hu B, He DS et al. Expression profiling of O(6) methylguanine-DNA-methyl transferase in prolactinomas: a correlative study of promoter methylation and pathological features in 136 cases. BMC Cancer. 2015;15:644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salehi F, Scheithauer BW, Kros JM et al. MGMT promoter methylation and immunoexpression in aggressive pituitary adenomas and carcinomas. J Neurooncol. 2011;104:647–657. [DOI] [PubMed] [Google Scholar]
- 21. Arya S, Majaid MA, Shwetha SD et al. Implications of MGMT methylation status in pituitary adenoma. Pathol Res Pract. 2014;210:407–411. [DOI] [PubMed] [Google Scholar]
- 22. McCormack AI, Wass JA, Grossman AB. Aggressive pituitary tumours: the role of temozolomide and the assessment of MGMT status. Eur J Clin Invest. 2011;41:1133–1148. [DOI] [PubMed] [Google Scholar]
- 23. Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 24. Chamberlain MC, Raizer J. Extended exposure to alkylator chemotherapy: delayed appearance of myelodysplasia. J Neurooncol. 2009;93:229–232. [DOI] [PubMed] [Google Scholar]
- 25. Momota H, Narita Y, Miyakita Y, Shibui S. Secondary hematological malignancies associated with temozolomide in patients with glioma. Neuro Oncol. 2013;15:1445–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Strowd RE, Blackwood R, Brown M et al. Impact of temozolomide on gonadal function in patients with primary malignant brain tumors. J Oncol Pharm Pract. 2013;19:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sinha S, Sharma BS. Giant pituitary adenomas--an enigma revisited. Microsurgical treatment strategies and outcome in a series of 250 patients. Br J Neurosurg. 2010;24:31–39. [DOI] [PubMed] [Google Scholar]
- 28. Tomlinson JW, Holden N, Hills RK et al. Association between premature mortality and hypopituitarism.: West Midlands Prospective Hypopituitary Study Group. Lancet. 2001;357:425–431. [DOI] [PubMed] [Google Scholar]
- 29. Brada M, Burchell L, Ashley S, Traish D. The incidence of cerebrovascular accidents in patients with pituitary adenoma. Int J Radiat Oncol Biol Phys. 1999;45:693–698. [DOI] [PubMed] [Google Scholar]
- 30. Minniti G, Traish D, Ashley S, Gonsalves A, Brada M. Risk of second brain tumor after conservative surgery and radiotherapy for pituitary adenoma: update after an additional 10 years. J Clin Endocrinol Metab. 2005;90:800–804. [DOI] [PubMed] [Google Scholar]
- 31. Loeffler JS, Niemierko A, Chapman PH. Second tumors after radiosurgery: tip of the iceberg or a bump in the road? Neurosurgery. 2003;52:1436–1440. [DOI] [PubMed] [Google Scholar]
- 32. Pappachan JM, Raskauskiene D, Kutty VR, Clayton RN. Excess mortality associated with hypopituitarism in adults: a meta-analysis of observational studies. J Clin Endocrinol Metab. 2015;100:1405–1411. [DOI] [PubMed] [Google Scholar]
- 33. Murphy CB, Hashimoto SA, Graeb D, Thiessen BA. Clinical exacerbation of multiple sclerosis following radiotherapy. Arch Neurol. 2003;60:273–275. [DOI] [PubMed] [Google Scholar]
- 34. Peterson K, Rosenblum MK, Powers JM, Alvord E, Walker RW, Posner JB. Effect of brain irradiation on demyelinating lesions. Neurology. 1993;43:2105–2112. [DOI] [PubMed] [Google Scholar]
- 35. Lin AL, Gutmann DH. Advances in the treatment of neurofibromatosis-associated tumours. Nat Rev Clin Oncol. 2013;10:616–624. [DOI] [PubMed] [Google Scholar]
- 36. Mete O, Asa SL. Therapeutic implications of accurate classification of pituitary adenomas. Semin Diagn Pathol. 2013;30:158–164. [DOI] [PubMed] [Google Scholar]
- 37. Ding D, Starke RM, Sheehan JP. Treatment paradigms for pituitary adenomas: defining the roles of radiosurgery and radiation therapy. J Neurooncol. 2014;117:445–457. [DOI] [PubMed] [Google Scholar]
- 38. Minniti G, Scaringi C, Amelio D, Maurizi Enrici R. Stereotactic Irradiation of GH-Secreting Pituitary Adenomas. Int J Endocrinol. 2012;2012:482861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pivonello R, De Leo M, Cozzolino A, Colao A. The Treatment of Cushing's Disease. Endocr Rev. 2015;36:385–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Plotz CM, Knowlton AI, Ragan C. Natural course of Cushing's syndrome as compared with the course of rheumatoid arthritis treated by hormones. Ann Rheum Dis. 1952;11:308–309. [PubMed] [Google Scholar]
- 41. Bates AS, Van't Hoff W, Jones JM, Clayton RN. An audit of outcome of treatment in acromegaly. Q J Med. 1993;86:293–299. [PubMed] [Google Scholar]
- 42. Orme SM, McNally RJ, Cartwright RA, Belchetz PE. Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab. 1998;83:2730–2734. [DOI] [PubMed] [Google Scholar]
- 43. Katznelson L, Laws ER Jr., Melmed S et al. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3933–3951. [DOI] [PubMed] [Google Scholar]
- 44. Biller BM, Grossman AB, Stewart PM et al. Treatment of adrenocorticotropin-dependent Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab. 2008;93:2454–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Holdaway IM, Rajasoorya RC, Gamble GD. Factors influencing mortality in acromegaly. J Clin Endocrinol Metab. 2004;89:667–674. [DOI] [PubMed] [Google Scholar]
- 46. Katznelson L, Bogan JS, Trob JR et al. Biochemical assessment of Cushing's disease in patients with corticotroph macroadenomas. J Clin Endocrinol Metab. 1998;83:1619–1623. [DOI] [PubMed] [Google Scholar]
- 47. Fleseriu M, Petersenn S. Medical management of Cushing's disease: what is the future? Pituitary. 2012;15:330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Woo YS, Isidori AM, Wat WZ et al. Clinical and biochemical characteristics of adrenocorticotropin-secreting macroadenomas. J Clin Endocrinol Metab. 2005;90:4963–4969. [DOI] [PubMed] [Google Scholar]
- 49. Karavitaki N, Turner HE, Adams CB et al. Surgical debulking of pituitary macroadenomas causing acromegaly improves control by lanreotide. Clin Endocrinol (Oxf). 2008;68:970–975. [DOI] [PubMed] [Google Scholar]
- 50. Lonser RR, Ksendzovsky A, Wind JJ, Vortmeyer AO, Oldfield EH. Prospective evaluation of the characteristics and incidence of adenoma-associated dural invasion in Cushing disease. J Neurosurg. 2012;116:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tanaka Y, Hongo K, Tada T, Sakai K, Kakizawa Y, Kobayashi S. Growth pattern and rate in residual nonfunctioning pituitary adenomas: correlations among tumor volume doubling time, patient age, and MIB-1 index. J Neurosurg. 2003;98:359–365. [DOI] [PubMed] [Google Scholar]
- 52. Syro LV, Ortiz LD, Scheithauer BW et al. Treatment of pituitary neoplasms with temozolomide: a review. Cancer. 2011;117:454–462. [DOI] [PubMed] [Google Scholar]
- 53. Tsang RW, Brierley JD, Panzarella T, Gospodarowicz MK, Sutcliffe SB, Simpson WJ. Radiation therapy for pituitary adenoma: treatment outcome and prognostic factors. Int J Radiat Oncol Biol Phys. 1994;30:557–565. [DOI] [PubMed] [Google Scholar]
- 54. Costenaro F, Rodrigues TC, Rollin GA, Ferreira NP, Czepielewski MA. Evaluation of Cushing's disease remission after transsphenoidal surgery based on early serum cortisol dynamics. Clin Endocrinol (Oxf). 2014;80:411–418. [DOI] [PubMed] [Google Scholar]
- 55. Pendharkar AV, Sussman ES, Ho AL, Hayden Gephart MG, Katznelson L. Cushing's disease: predicting long-term remission after surgical treatment. Neurosurg Focus. 2015;38:E13. [DOI] [PubMed] [Google Scholar]
- 56. Kinoshita Y, Tominaga A, Usui S et al. Clinical features and natural course of acromegaly in patients with discordance in the nadir GH level on the oral glucose test and the IGF-1 value at 3 months after adenomectomy. Neurosurg Rev. 2016;39:313–319. [DOI] [PubMed] [Google Scholar]
- 57. Sheehan JP, Niranjan A, Sheehan JM et al. Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy, and role in the neurosurgical treatment armamentarium. J Neurosurg. 2005;102:678–691. [DOI] [PubMed] [Google Scholar]
- 58. Patel J, Eloy JA, Liu JK. Nelson's syndrome: a review of the clinical manifestations, pathophysiology, and treatment strategies. Neurosurg Focus. 2015;38:E14. [DOI] [PubMed] [Google Scholar]
- 59. Molitch ME. Management of medically refractory prolactinoma. J Neurooncol. 2014;117:421–428. [DOI] [PubMed] [Google Scholar]
- 60. Melmed S, Casanueva FF, Hoffman AR et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:273–288. [DOI] [PubMed] [Google Scholar]
- 61. Klibanski A. Clinical practice. Prolactinomas. N Engl J Med. 2010;362:1219–1226. [DOI] [PubMed] [Google Scholar]
- 62. Gillam MP, Molitch ME, Lombardi G, Colao A. Advances in the treatment of prolactinomas. Endocr Rev. 2006;27:485–534. [DOI] [PubMed] [Google Scholar]
- 63. Lau Q, Scheithauer B, Kovacs K, Horvath E, Syro LV, Lloyd R. MGMT immunoexpression in aggressive pituitary adenoma and carcinoma. Pituitary. 2010;13:367–379. [DOI] [PubMed] [Google Scholar]
- 64. Salehi F, Scheithauer BW, Kovacs K et al. O-6-methylguanine-DNA methyltransferase (MGMT) immunohistochemical expression in pituitary corticotroph adenomas. Neurosurgery. 2012;70:491–496. [DOI] [PubMed] [Google Scholar]
- 65. Wang Y, Li J, Tohti M et al. The expression profile of Dopamine D2 receptor, MGMT and VEGF in different histological subtypes of pituitary adenomas: a study of 197 cases and indications for the medical therapy. J Exp Clin Cancer Res. 2014;33:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. van Rijn J, Heimans JJ, van den Berg J, van der Valk P, Slotman BJ. Survival of human glioma cells treated with various combination of temozolomide and X-rays. Int J Radiat Oncol Biol Phys. 2000;47:779–784. [DOI] [PubMed] [Google Scholar]
- 67. Zacharia BE, Gulati AP, Bruce JN et al. High response rates and prolonged survival in patients with corticotroph pituitary tumors and refractory Cushing disease from capecitabine and temozolomide (CAPTEM): a case series. Neurosurgery. 2014;74:E447–455; discussion E455. [DOI] [PubMed] [Google Scholar]
- 68. Reincke M, Sbiera S, Hayakawa A et al. Mutations in the deubiquitinase gene USP8 cause Cushing's disease. Nat Genet. 2015;47:31–38. [DOI] [PubMed] [Google Scholar]