Abstract
Background
Toca 511, a gamma retroviral replicating vector encoding cytosine deaminase, used in combination with 5-fluorocytosine (5-FC) kills tumor by local production of 5-fluorouracil (5-FU), inducing local and systemic immunotherapeutic response resulting in long-term survival after cessation of 5-FC. Toca 511 and Toca FC (oral extended-release 5-FC) are under investigation in patients with recurrent high-grade glioma. Lomustine is a treatment option for patients with high-grade glioma.
Methods
We investigated the effects of lomustine combined with Toca 511 + 5-FC in syngeneic orthotopic glioma models. Safety and survival were evaluated in immune-competent rat F98 and mouse Tu-2449 models comparing Toca 511 + 5-FC to lomustine + 5-FC or the combination of Toca 511 + 5-FC + lomustine. After intracranial implantation of tumor, Toca 511 was delivered transcranially followed by cycles of intraperitoneal 5-FC with or without lomustine at the first or fourth cycle.
Results
Coadministration of 5-FC with lomustine was well tolerated. In F98, combination Toca 511 + 5-FC and lomustine increased median survival, but “cures” were not achieved. In Tu-2449, combination Toca 511 + 5-FC and lomustine increased median survival and resulted in high numbers of cure. Rejection of tumor rechallenge occurred after treatment with Toca 511 + 5-FC or combined with lomustine, but not with lomustine + 5-FC. Mixed lymphocyte–tumor cell reactions using splenocytes from cured animals showed robust killing of target cells in an effector:target ratio–dependent manner with Toca 511 + 5-FC and Toca 511 + 5-FC + lomustine day 10.
Conclusion
The combination of Toca 511 + 5-FC and lomustine shows promising efficacy with no additive toxicity in murine glioma models. Immunotherapeutic responses resulting in long-term survival were preserved despite lomustine-related myelosuppression.
Keywords: 5-FC, high-grade gliomas, immunotherapy, lomustine (CCNU), Toca 511
High-grade gliomas (HGGs), including glioblastoma, are the most common forms of primary brain cancer and typically recur following treatment with standard of care. Despite recent advances in treatment, the prognosis for patients with HGG remains poor, with comparatively short overall survival (OS) and a profound impact on quality of life.1 The combination of Toca 511 (vocimagene amiretrorepvec),2 a retroviral replicating vector (RRV) carrying a prodrug activating gene, and Toca FC (extended-release 5-FC) is being investigated for the treatment of HGG. Toca 511 can successfully and safely deliver an optimized yeast cytosine deaminase gene to tumors after either intratumoral3,4 or intravenous administration in orthotopic glioma models.5 Toca 511, in conjunction with subsequent Toca FC, provides both direct killing of tumor cells by local production of 5-fluorouracil (5-FU) and induction of a local and systemic immunotherapeutic response resulting in long-term survival after cessation of 5-FC treatment. The combination of Toca 511 and Toca FC is under investigation, with Toca 511 delivered intratumorally (NCT01156584), into the wall of the resection cavity (NCT01470794, NCT02414165) or via intravenous infusion followed by injection into the wall of the resection cavity (NCT01985256) in patients with recurrent HGG.
Nitrosoureas (eg, lomustine, carmustine) are alkylating agents that have shown modest benefit in some clinical trials for the treatment of newly diagnosed6 and recurrent gliomas.7–9 Lomustine (1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea, CCNU) is used alone or in combination with other antineoplastic agents, including procarbazine, vincristine, and bevacizumab. Lomustine is usually given in combination as a single oral dose of 90–130 mg/m2 in cycles every 6 to 8 weeks but commonly produces dose-limiting toxicity.8,10 A recent phase III randomized study comparing lomustine with or without bevacizumab in patients with recurrent glioblastoma did not show a survival advantage for the combination.11 Nitrosoureas alkylate DNA and RNA and may also inhibit several key enzymatic processes by carbamoylation of amino acids in proteins. Nitrosoureas such as lomustine exhibit some action even against noncycling cells but specifically cause delays at S and G2 of the cell cycle. They are known to synergize with agents that are active in S phase such as 5-FU.12,13
5-FU is myelosuppressive when delivered systemically and has an extremely narrow therapeutic window, precluding its use for treatment of brain tumor.14 This is overcome by the use of Toca 511 and 5-FC to produce 5-FU locally within the tumor microenvironment, avoiding systemic immune suppression. Lomustine is also myelosuppressive. Dose-related, delayed myelosuppression is the most frequent and serious toxicity of lomustine, usually occurring 4 to 6 weeks after drug administration.6 Therefore, it is important to confirm that any advantages of lomustine plus 5-FU synergistic chemotoxicity are not outweighed by any detrimental effects on Toca 511 and 5-FC induced antitumor immune response. The ability to combine active chemotherapeutic drugs with nontoxic or low toxicity anticancer agents may represent advancement in the treatment of HGG. We aimed to assess the therapeutic potential of Toca 511 and 5-FC with lomustine and define any potential additive toxicity in preclinical models of glioma.
We determined the effects of lomustine in combination with Toca 511 and 5-FC treatment using 2 syngeneic orthotopic glioma models. Survival was evaluated in immune-competent murine models comparing Toca 511 and 5-FC treatment versus lomustine and 5-FC without vector or the combination of Toca 511 and 5-FC plus lomustine. The rat F98 syngeneic glioma model is commonly used for assessment of chemotherapeutic agents but is only minimally immunogenic and is relatively resistant to RRV infection and is therefore a good model to isolate possible synergistic chemotoxicity to tumors.15,16 The Tu-2449 syngeneic glioma model has been used to show antitumor immune activity generated by Toca 511 and 5-FC treatment.5 It is an ideal model to test for the impact of potentially immunosuppressive drugs like lomustine on antitumor immunity. The results presented here provided support for the investigation of Toca 511 and Toca FC with lomustine in patients with HGG, and a phase I study is now in progress (NCT01470794).
Materials and Methods
Drugs and Reagents
Lomustine was purchased from Sigma-Aldrich. We contracted a chemical supplier to synthesize 5-FC to order for in vivo assays.
Toca 511
Details of Toca 511 design, modification, and production have been previously described.2,3
Cell Culture
The rat glioma cell line F98 (American Type Culture Collection) and the mouse glioma cell line Tu-2449, which is a well-established orthotopic glioma model in immunocompetent mice,17,18 were cultured as described.3–5
Animals and In-life Observations
Male Fischer rats (weight ∼200–250 g) were purchased from Charles River Laboratories. Female B6C3F1 mice (age ∼8 wk) were purchased from Harlan. Animals were acclimated for 7 days after arrival. Routine general health, in-life observations, and body weights were collected throughout the course of the study (Supplementary Tables S1–S3 and S5). All animal protocols and experiments were approved by the Institutional Animal Care and Use Committee.
Lomustine Treatment
Lomustine was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 50 mg/mL and administered i.p. in the appropriate volume to yield a dose of 30 mg/kg in mice19 (human equivalent dose 90 mg/m2) and rats20 (human equivalent dose 180 mg/m2). The appropriate volume of DMSO was administered to controls.
Intracranial Surgeries
Animals underwent stereotactic placement of an indwelling guide cannula with a 3.0 mm (B6C3F1 mice)3,4 or 5.5 mm (Fischer rats) projection implanted into the right striatum, fitted with a cap. The stereotaxic coordinates for mouse studies were anterior-posterior = 0.5 mm, medial-lateral = 1.8 mm, and dorsal-ventral = 3.5 mm (from bregma). The stereotaxic coordinates for rat studies were anterior-posterior = 1.5 mm, medial-lateral = 3.5 mm, dorsal-ventral = 5.5 mm (from bregma).
In vivo Survival Studies
The syngeneic cell line F98 was used as an orthotopic brain tumor model in Fischer rats. The syngeneic cell line Tu-2449 was used as an orthotopic brain tumor model in B6C3F1 mice as described3,4 (Supplementary material).
Tumor Challenge Assays
Tumor challenge was performed on all “cured” mice from B6C3F1/Tu-2449 tumor long-term survival studies by subcutaneous implantation of 5 × 105 Tu-2449SC (subcutaneous growth adapted subline) cells in 100 µL volume into each mouse on day 90 post implantation of intracranial (i.c.) cells. As a control, Tu-2449SC cells were implanted into age-matched naïve B6C3F1 mice. Tumor measurements were performed 3 times a week.
xCELLigence Analysis
Cytotoxicity of splenocytes derived from cured mice from intracranial Tu-2449 tumor long-term survival studies was determined by xCELLigence real-time cell analysis21 (Acea Biosciences) (Supplementary material). Unstimulated lymphocytes from non-tumor-bearing mice were used in the tumor cell killing assay as control.
Toxicity Studies in Naïve Animals
The potential toxicity of the combination of lomustine with cyclic 5-FC was assessed in non-tumor-bearing animals. Fischer rats or B6C3F1 mice were assessed 3 times a week after drug administration and blood samples were collected for hematology analysis at designated time points (Supplementary material).
Statistical Analyses
Survival data were plotted using the Kaplan–Meier method and were compared by the log-rank test or Student's t-test as noted. P-values of <.05 were considered statistically significant in all analyses, which were done with GraphPad Prism 5 statistical software.
Results
Combination Treatment with Toca 511 + 5-FC and Lomustine Prolonged Survival in the F98 Glioma Model
The effect of combining Toca 511 + 5-FC with lomustine was tested in the F98 rat orthotopic glioma model. This model has an infiltrative pattern of growth, necrosis, and other attributes associated with human glioblastoma.22 This cell line is O6-DNA methylguanine-methyltransferase nonmethylated6,15,23 and is relatively sensitive to lomustine (half-maximal inhibitory concentration [IC50]= 20.8 µM; data not shown).
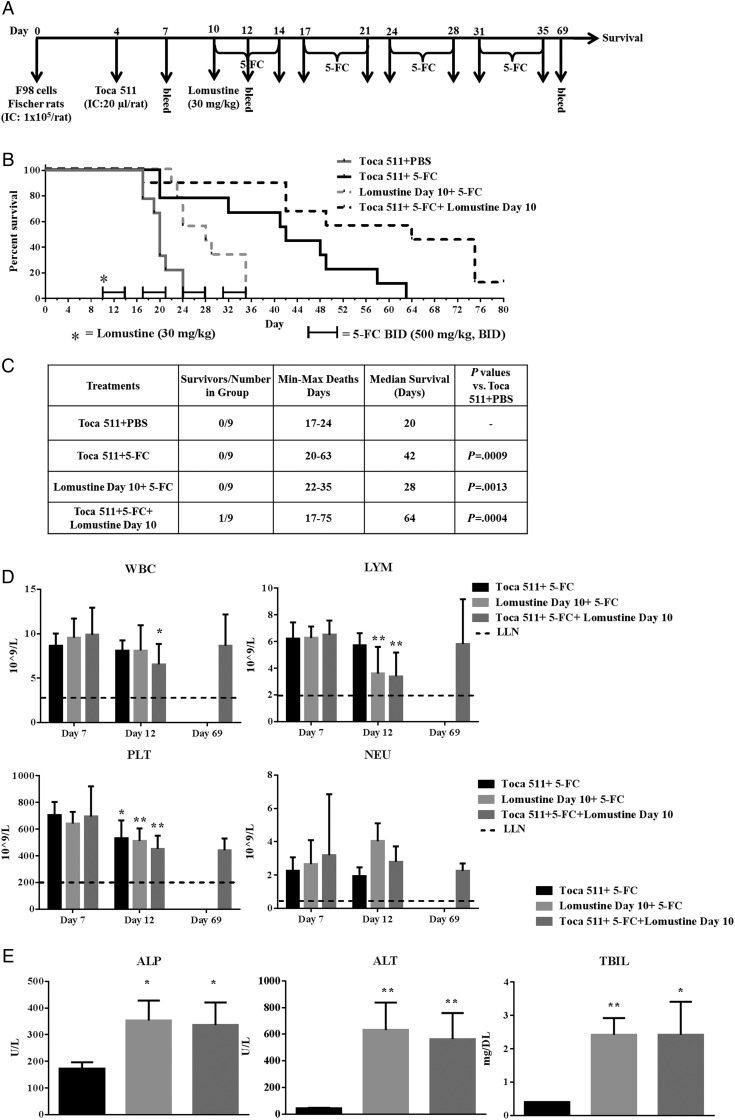
F98 tumor-bearing Fischer rats were treated with either lomustine or 5-FC alone or in combination at indicated time points for a total of four 5-FC treatment cycles (Fig. 1A). Treatment of rats with Toca 511 + 5-FC or lomustine day 10 + 5-FC or in combination Toca 511 + 5-FC + lomustine day 10 resulted in statistically prolonged survival compared with Toca 511 + phosphate buffered saline (PBS) control (P = .0009, P = .0013, P = .0004, respectively). Moreover, Toca 511 + 5-FC alone significantly prolonged survival compared with lomustine day 10 + 5-FC (P = .018) (Fig. 1B). The relative order of median survival was Toca 511 + 5-FC + lomustine day 10 > Toca 511 + 5-FC > lomustine day 10 + 5-FC > Toca 511 + PBS (Fig. 1C). Treatment with Toca 511 + 5-FC alone (Supplementary Fig. S1A) or in combination with lomustine did not result in a high level of “cured” animals after 5-FC treatment. Therefore, the presence of cellular immunity against the parental tumor cells in Toca 511 + 5-FC alone or in combination with lomustine-treated animals was not determined.
Fig. 1.
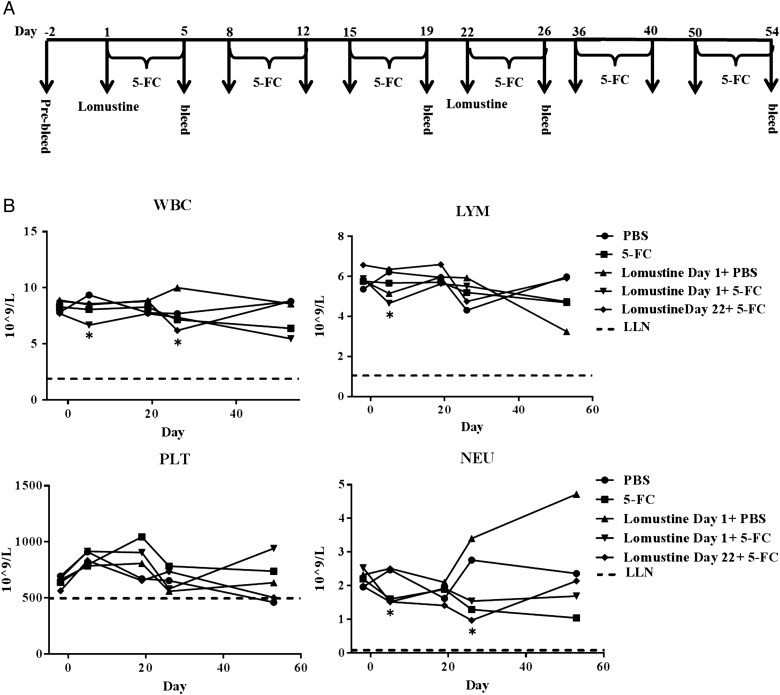
Lomustine alone or in combination with Toca 511 + 5-FC survival in F98 tumor-bearing Fischer rats. (A) Schematic representation of the schedule of drug administration and blood collections. (B) Kaplan–Meier survival analysis. Groups of male Fischer rats were implanted i.c. with F98 cells then dosed i.c. with vehicle control or i.c. with Toca 511. Lomustine was administered at day 10. PBS or 5-FC started at day 10. Four cycles of PBS or 5-FC treatment were given to rats and survival was examined out to 80 days. (C) Summary of the survival analysis. (D) Blood samples were collected 7, 12, and 69 (survivors) days after tumor cell inoculation. WBC, LYM, PLT, and NEU were determined. Dotted line represents LLN for age-matched rats (*P < .05, **P < .01; day 7 vs day 12). (E) Alkaline phosphatase (ALP), alanine aminotransferase (ALT), and total bilirubin (TBIL) were determined (*P < .05, **P < .01; groups vs Toca 511 + 5-FC).
Combination of Toca-511 + 5-FC with Lomustine Had Promising Efficacy and Therapeutic Activity in the Tu-2449 Glioma Model
The Tu-2449 mouse glioma cell line was used to examine the compatibility of lomustine with Toca 511 + 5-FC. Previous studies have shown that this model displays several of the features of human glioblastoma and is highly angiogenic.17,24 We have shown that antitumor immunity is generated in this model after treatment with Toca 511 + 5-FC4. This cell line is relatively sensitive to lomustine (IC50= 18.6 µM; data not shown).
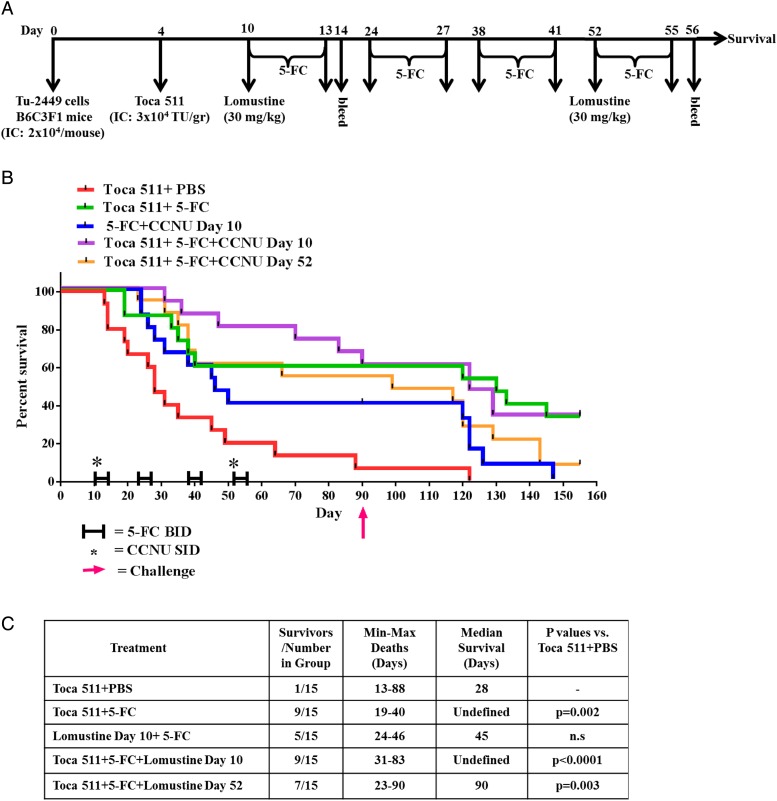
Tu-2449 tumor-bearing B6C3F1 mice were treated with either lomustine or 5-FC alone or in combination at indicated time points for a total of four 5-FC treatment cycles (Fig. 2A). Treatment of mice with Toca 511 + 5-FC alone or in combination with lomustine at day 10 or lomustine at day 52 resulted in statistically prolonged survival compared with Toca 511 + PBS control (P = .002, P < .0001, P = .003, respectively) (Fig. 2B). The median survivals in Toca 511 + 5-FC and Toca 511 + 5-FC + lomustine day 10 treatment groups were not determined, as more than 50% of the animals survived until day 90. The relative order of median survival was Toca 511 + 5-FC = Toca 511 + 5-FC + lomustine day 10 > Toca 511 + 5-FC + lomustine day 52 > lomustine day 10 + 5-FC > Toca 511 + PBS (Fig. 2C). Treatment with Toca 511 + 5-FC (Supplementary Fig. S1B) or lomustine alone or in combination resulted in a high number of “cures” after cycles of 5-FC. Importantly, lomustine in combination with Toca 511 + 5-FC did not negatively impact the immunotherapeutic effect of Toca 511 + 5-FC leading to long-term survival after cessation of 5-FC.
Fig. 2.
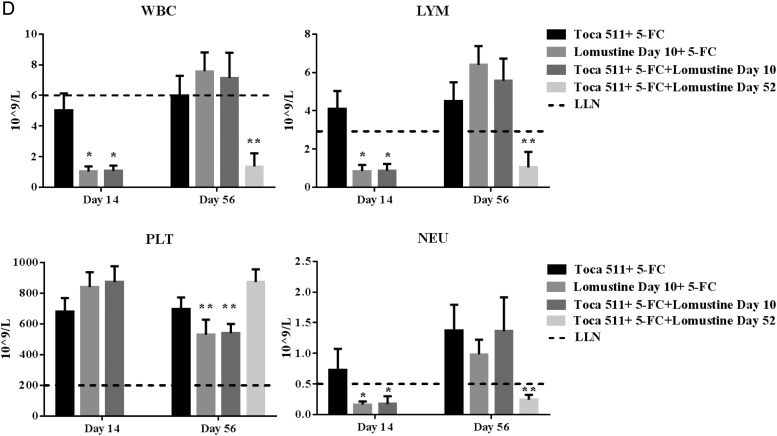
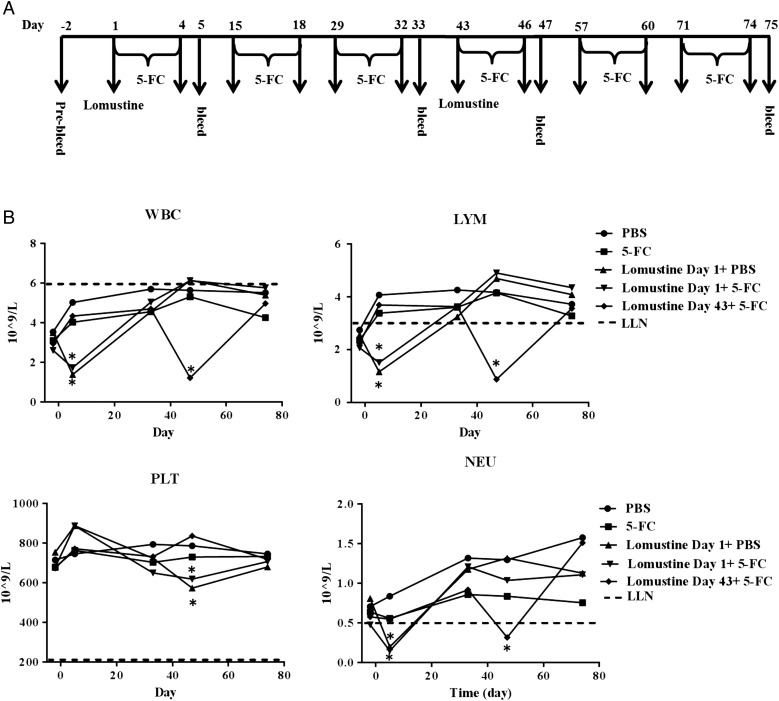
Lomustine alone or in combination with Toca 511 + 5-FC induced antitumor immunological memory in Tu-2449 glioma-bearing mice. (A) Schematic representation of the schedule of drug administration and blood collections. (B) Kaplan–Meier survival analysis. Groups of female B6C3F1 mice were implanted i.c. with Tu-2449 then dosed i.c. with vehicle (control) or i.c. with Toca 511. Lomustine was administered either 10 or 52 days after tumor cell inoculation. Four cycles of 5-FC or PBS were given to mice and survival was examined out to 90 days. Cured mice were subcutaneously challenged at day 90. Tumor growth was monitored out to 158 days. (C) Summary of the survival analysis out to 90 days post intracranial tumor implantations (D) WBC, LYM, PLT, and NEU were determined. Dotted line represents LLN for age-matched mice (*P < .0001 vs Toca 511 + 5-FC day 14, **P < .0001 vs Toca 511 + 5-FC day 56).
Antitumor Immunological Memory Is Induced in Long-Term Survivors after Toca 511 + 5-FC Alone or in Combination with Lomustine Treatments in the Tu-2449 Glioma Model
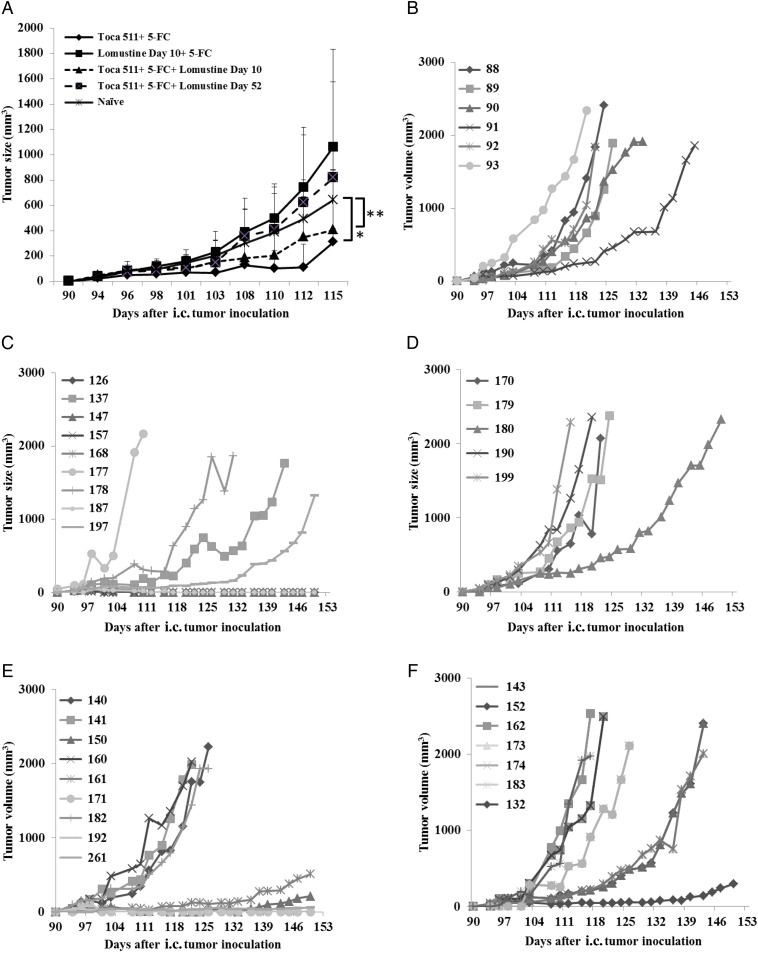
We reported earlier that Toca 511 + 5-FC treatment induces an immunological memory.4 However, lomustine can weaken the immune response and have long-lasting effects after the treatment.25 To further evaluate the effect of lomustine on the Toca 511 + 5-FC induced immune response, Tu-2449SC cells were implanted into the right flanks of cured mice on day 90 after the original i.c. tumor implantation. Tumors engrafted and grew in all naïve animals challenged with Tu-2449SC cells (Fig. 3A). Although subcutaneous tumors were observed initially during the first week, some of these tumors implanted in the previously cured mice that were treated with either Toca 511 + 5-FC or Toca 511 + 5-FC + lomustine day 10 spontaneously disappeared in 2 weeks without any recurrence (5/9 each treatment vs 0/5 control), indicating the induction of an antitumor immune response. In contrast, tumors in long-term survivors from lomustine day 10 + 5-FC and Toca 511 + 5-FC + lomustine day 52 treatments engrafted and did not shrink during the experiment (0/5 and 0/7, respectively) (Fig. 3B-F). This suggests that lomustine day 10 + 5-FC without Toca 511 did not induce antitumor immune memory. The late addition of lomustine in the Toca 511 + 5-FC + lomustine day 52 group may have interfered with long-term immune memory, even though there was initially sufficient antitumor effect on the intracranial mass to prolong survival compared with the control.
Fig. 3.
Tumor challenge assays in cured mice from Tu-2449 tumor long-term survival. Cured mice from Tu-2449 intracranial tumor long-term survival were subcutaneously challenged with Tu-2449SC cells 90 days after initial tumor establishment. As a control, Tu-2449SC cells were implanted into naïve B6C3F1 mice. (A) Tumor growth was significantly reduced in mice cured of brain tumor with Toca 511 + 5-FC and Toca 511 + 5-FC + lomustine day 10 treatment compared with naïve controls (*P = .009, **P = .02, respectively). Tumor measurements, performed 3 times a week, to monitor growth of subcutaneous tumors after challenge in naïve controls (B) vs cured animals from (C) Toca 511 + 5-FC, (D) lomustine day 10 + 5-FC, (E) Toca 511 + 5-FC + lomustine day 10, and (F) Toca 511 + 5-FC + lomustine day 52 groups.
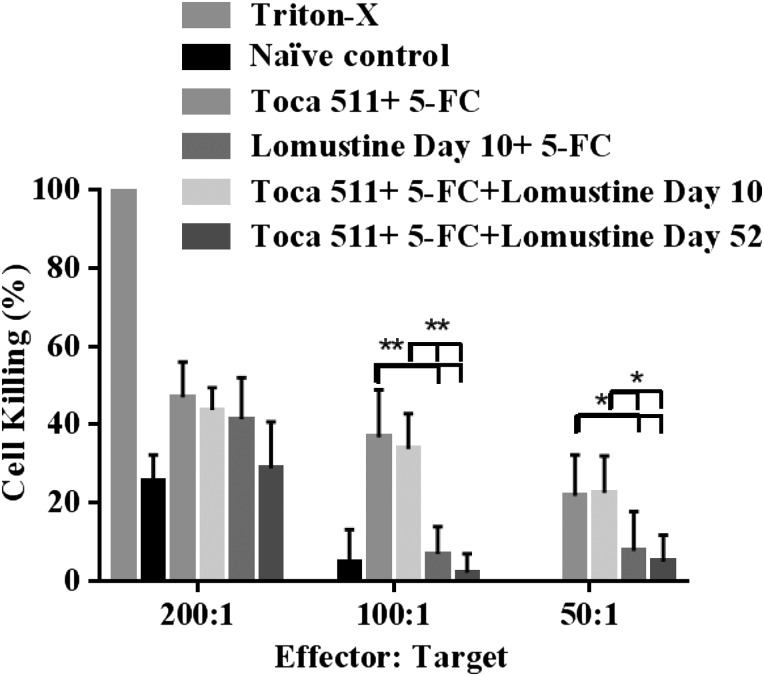
To measure immunological reactivity or recognition of tumor antigens, mixed lymphocyte–tumor cell reactions using splenocytes from cured animals were monitored in real time by xCELLigence electrical impedance analysis and showed robust killing of Tu-2449 target cells over time in an effector:target ratio–dependent manner in Toca 511 + 5-FC and Toca 511 + 5-FC + lomustine day 10 treatment groups. There were improved responses in the Toca 511 + 5-FC, Toca 511 + 5-FC + lomustine day 10, and Toca 511 + 5-FC + lomustine day 52 treated animals compared with lomustine day 10 + 5-FC treated animals in an effector:target ratio–dependent manner (P < .05) (Fig. 4). Importantly, the response was better in Toca 511 + 5-FC and Toca 511 + 5-FC + lomustine day 10 treated animals compared with the responses from Toca 511 + 5-FC + lomustine day 52 treated animals in all the effector:target ratios tested (P < .05).
Fig. 4.
Analysis of mixed lymphocyte-tumor cells mediated antitumor immune response. Cellular immunity was evaluated in splenocytes from cured mice from experiment shown in Fig. 2B. xCELLigence real-time cell impedance analysis monitoring mixed lymphocyte-tumor cells mediated antitumor immune response shows splenocytes from cured mice activated against Tu-2449 cells mediate effector:target cell ratio–dependent killing. (*P < .05, **P < .0001).
Combination of Toca 511 + 5-FC with Lomustine Had No Additive Hematological Toxicity in Tumor-Bearing Rats
Toca 511 + 5-FC is designed to generate high levels of 5-FU locally in the tumor but is not expected to generate high levels of systemic 5-FU. We have shown that Toca 511 + 5-FC produces higher 5-FU levels in the tumor compared with plasma in tumor-bearing animals.3,26 Animals that received Toca 511 + 5-FC also did not exhibit signs of local toxicity (Supplementary Fig. S1). Both systemic 5-FU and lomustine are known to cause hematological toxicity, and therefore it is important to examine any potential drug interaction. Complete blood counts and blood chemistry were evaluated in the tumor-bearing rats at day 7 (before treatment) and day 12 (2 days post lomustine or 5-FC treatment) (Fig. 1D). The blood counts were within the lower limit of normal (LLN) during the study. Lymphocytes (LYM) and platelets (PLT) significantly decreased in the lomustine day 10 + 5-FC and Toca 511 + 5-FC + lomustine day 10 combination treatment at day 12 compared with the values at day 7 (P< .01). The decrease in white blood cells (WBC) was significant in the Toca 511 + 5-FC + lomustine day 10 combination treatment at day 12 compared with the values at day 7 (P < .05). Coadministration of 5-FC with lomustine did not enhance hematological toxicity in tumor-bearing rats.
Combination of Toca 511 + 5-FC with Lomustine Had No Additive Hepatotoxicity in Tumor-Bearing Rats
Lomustine is also associated with minor transient serum enzyme elevations and has been linked to rare cases of clinically apparent acute hepatotoxicity. Mild and transient elevations in serum aminotransferase or alkaline phosphatase or total bilirubin levels are found in animals and human patients treated with lomustine. In this study, serum levels of these enzymes were also significantly higher in groups receiving lomustine alone or in combination (P < .05) (Fig. 1E). Results indicate that Toca 511 + 5-FC in combination with lomustine treatment does not have any additive hepatotoxicity.
5-FC with Lomustine at 30 mg/kg Was Well Tolerated in Non-Tumor-Bearing Rats
Naïve Fischer rats were treated with either lomustine or 5-FC alone or in combination at indicated time points for a total of 6 treatment cycles of 5-FC (Fig. 5A). Complete blood counts were collected at baseline and 4 days after initiating treatment with lomustine or 5-FC. Lomustine or 5-FC alone or in combination (lomustine + 5-FC) caused transient decreases from baseline in WBC, LYM, PLT, and neutrophils (NEU) after the first and fourth 5-FC cycles normalized to change in the PBS group (all P < .05); however, all values were above the LLN (Fig. 5B). Coadministration of 5-FC with lomustine was well tolerated and did not enhance hematological toxicity in naïve Fischer rats. Rats that were treated with lomustine or in combination with 5-FC had abnormalities in lungs, kidneys, and liver, consistent with known lomustine-related findings27–29 (Supplementary Table S4). There were no safety-related findings in animals treated with 5-FC alone (Supplementary Table S3, Fig. S2).
Fig. 5.
Hematological toxicology analysis of naïve Fischer rats that received 5-FC cycles with or without lomustine. (A) Schematic representation of the schedule of drug administration and blood collections. (B) WBC, LYM, PLT, and NEU were determined. Dotted line represents LLN for age-matched mice (*P < .05 vs baseline, normalized to PBS).
Combination of Toca 511 + 5-FC with Lomustine Had No Additive Hematological Toxicity in Tumor-Bearing Mice
Blood counts were within the LLN after Toca 511 + 5-FC alone. Lomustine day 10 + 5-FC or the combination treatments Toca 511 + 5-FC + lomustine day 10 or Toca 511 + 5-FC + lomustine day 52 caused significant decreases in WBC, LYM, PLT, and NEU compared with Toca 511 + 5-FC alone (all P < .0001) (Fig. 2D). However, values were within the LLN at the end of the fourth cycles of 5-FC treatment. As in the F98 tumor-bearing Fischer rats, administration of 5-FC with lomustine did not enhance hematological toxicity in the Tu-2449 tumor-bearing mice.
5-FC with Lomustine at 30 mg/kg Had No Additive Toxicity in Non-Tumor-Bearing Mice
Naïve B6C3F1 mice were also treated with either lomustine or 5-FC alone or in combination at indicated time points for a total of six 5-FC treatment cycles (Fig. 6A). Complete blood counts were collected at baseline and 4 days after initiating treatment with lomustine or 5-FC. Lomustine alone or in combination with 5-FC at day 1 (lomustine day 1 + 5-FC) and day 43 (lomustine day 43 + 5-FC) caused transient decreases from baseline in WBC, LYM, PLT, and NEU after the first and fourth 5-FC cycles normalized to change in the PBS group and were below the LLN (all P < .01) (Fig. 6B). As in the naïve Fischer rats, coadministration of 5-FC with lomustine did not enhance hematological toxicity in naïve B6C3F1 mice. There were no safety-related findings in animals treated with 5-FC alone (Supplementary Table S5).
Fig. 6.
Hematological toxicology analysis of naïve B6C3F1 mice received 5-FC cycles with or without lomustine. (A) Schematic representation of the schedule of drug administration and blood collections. (B) WBC, LYM, PLT, and NEU were determined. Dotted line represents LLN for age-matched mice (*P < .01 vs baseline, normalized to PBS).
Discussion
This is the first report demonstrating that Toca 511 + 5-FC treatment in combination with lomustine had improved chemotoxicity against tumor in a difficult to treat model, with no additive hematological toxicity, and did not adversely affect immunotherapeutic activity at clinically relevant doses. The safety and efficacy of Toca 511 + 5-FC in combination with lomustine was explored in 2 rodent glioma models. In both models, animals that received Toca 511 + 5-FC or in combination with lomustine had statistically prolonged survival compared with PBS-treated control animals. Furthermore, in the mouse Tu-2449 glioma model, where immunological memory is induced with Toca 511 + 5-FC treatment, the combination with early addition of lomustine did not adversely impact induction of antitumor immune responses, despite lomustine-mediated hematological changes.
F98 rat tumors are more aggressive and somewhat more difficult to infect compared with Tu-2449 mouse glioma cells. Therefore, the rat F98 glioma may be a good model with which to study combination therapies as a surrogate for those human HGGs that may be somewhat more resistant to RRV spread. Combination treatment with Toca 511 + 5-FC and lomustine increased median survival in this difficult to treat model. However, high levels of cure were not achieved. One reason for this lack of durable response may be the weak immunogenicity of F98 cells. Earlier studies showed that transfection with the gene encoding the B7.1 costimulatory molecule,30 or syngeneic cellular vaccination combined with granulocyte-macrophage colony-stimulating factor, did not enhance F98 immunogenicity.31 In addition, the difference between the Tu-2449 and F98 glioma models in lomustine response might be dependent on both the dose and the schedule of treatment, which can influence the induction of tumor regression and long-term immune memory. Additional combinations or regimens may be required to break the immune tolerance in this highly resistant model.
Previously, we also showed that immune components play a role in eradicating intracranial tumor in the mouse Tu-2449 glioma model after Toca 511 + 5-FC treatment4 (Hiraoka, unpublished manuscript). In the current study using the mouse Tu-2449 intracranial glioma model, long-term survival was achieved in the majority of animals treated with Toca 511 + 5-FC alone or in combination with lomustine and in at least some animals with the other treatments tested. Long-term survivors were challenged with parental tumor cells to determine induction of antitumor immunity. Tumor rejection was observed only in the Toca 511 + 5-FC alone or in combination with lomustine, but not in the lomustine + 5-FC treatment group. The strong antitumor activity, presumably directed against innate tumor antigens, was observed more frequently in Toca 511 + 5-FC alone and Toca 511 + 5-FC + lomustine day 10 treated mice compared with Toca 511 + 5-FC + lomustine day 52 treated mice.
Assays by xCELLigence confirmed the presence of cellular immunity against the parental tumor cells in Toca 511 + 5-FC alone or in combination with lomustine-treated long-term survivors. Lymphocytes were collected from animals that had cleared Toca 511-transduced Tu-2449 tumors through treatment with 5-FC. Three ratios of effector cells (sensitized lymphocytes) to target (live Tu-2449 cells) were used to show that those animals that clear Tu-2449 tumors through Toca 511 gene transfer and 5-FC therapy established a lasting local and systemic immune response with cytotoxic activity against the same tumor cells. Consistent with the negative impact of late lomustine treatment on tumor rejection after tumor challenge, Toca 511 + 5-FC and Toca 511 + 5-FC + lomustine day 10 treated animals had the highest levels of cytotoxic activity at various effector:target ratios. Lomustine + 5-FC alone or Toca 511 + 5-FC + lomustine day 52 treated animals did not show significant cytotoxic activity at low effector:target ratios compared with Toca 511 + 5-FC or Toca 511 + 5-FC + lomustine day 10 treatment.
Hiraoka (unpublished manuscript) recently demonstrated that in the Tu-2449 syngeneic glioma model, immune rejection responses directed against endogenous tumor antigens are mediated by CD4+ T cells in conjunction with CD8+ T cells and likely enable full tumor eradication and durable responses. The difference in response to rechallenge between Toca 511 + 5-FC + lomustine day 10 and Toca 511 + 5-FC + lomustine day 52 treatment might be related to the recovery time of LYM populations after lomustine. Based on our data, even though LYM significantly decreased 4 days after lomustine, they recovered and were within the LLN values 4 weeks posttreatment. However, the recovered LYM population is now considered to be immunologically naïve until such time as they are activated against tumor antigen. Hiraoka also reported that Toca 511 + 5-FC treated Tu-2449 tumors decreased rapidly shortly after 5-FC treatment started and never recurred even without further 5-FC. Complete eradication of tumor cells was confirmed in long-term surviving animals. Therefore, tumor antigen sufficient for priming of naïve T cells may not have been available after late lomustine treatment. Alternatively, even in the case of incomplete eradication of tumor, the 5-FC treatment schedule may have affected antigen availability. Repeated cycles of 5-FC might be needed for the development of immune responses against tumor antigens released by Toca 511 + 5-FC induced cell killing.
Nitrosourea-based combination chemotherapy was standard treatment for recurrent HGGs before the introduction of the alkylating agent temozolomide in 1999.9 In spite of its apparent immunosuppressive effect, temozolomide does not inhibit antitumor immunity and therefore can be combined with immunotherapeutic approaches to treat brain cancer.32 Differences between the effects on immunotherapies of alkylating agents may be related to variable sensitivity of different WBC subsets, timing of nadir, or extent of cumulative effects on the immune populations. This highlights the need to determine empirically whether potential immune-suppressive treatments can adversely affect immunotherapeutic approaches such as Toca 511 and 5-FC. Our data suggest that Toca 511 + 5-FC may help to overcome lomustine-related immunosuppression in tumors with immunogenic capacity and potentiate the antitumor effect of lomustine in some dosing regimens.
Lomustine-related myelosuppression usually occurs 4 to 6 weeks after drug administration and is dose related.27,33 The WBC nadir occurs 3–4 weeks and the PLT nadir 4–5 weeks after administration. Cell counts return to normal 6–7 weeks after administration.34 As reported here, after Toca 511 + 5-FC or lomustine + 5-FC or in combination treatment, the blood counts were within the LLN in tumor-bearing rats. In the mouse glioma model, groups treated with 5-FC + lomustine alone or in combination with Toca 511 + 5-FC had transient but significant lower WBC, LYM, PLT, and NEU counts compared with the Toca 511 + PBS control or Toca 511 + 5-FC treatment. Plasma concentrations of 5-FC at above 100 µg/mL for extended periods of time (6 wk) or systemic 5-FU dosing is also known to cause myelosuppression.35 However, Toca 511 + 5-FC produces locally high levels of 5-FU and does not result in myelosuppression. As expected, the hematological changes in the Toca 511 + 5-FC or in combination with lomustine groups were not significantly higher than the lomustine + 5-FC treatment group.
Lomustine and 5-FU are associated with off-target organ toxicity mostly with cumulative or high doses.28,29,36 Therefore, administration of both drugs simultaneously theoretically could be associated with an unacceptable level of toxicity. In our study, lomustine alone or in combination with 5-FC caused serum enzyme elevations that were linked to hepatotoxicity in the rat glioma model. Animals treated with 5-FC alone had no treatment-related abnormalities. No additional histological changes were observed in rats treated with lomustine in combination with 5-FC, beyond those seen in lomustine alone, suggesting that 5-FC and lomustine could be used in combination with no additive detrimental effects.
In conclusion, the combination of Toca 511 + 5-FC and lomustine shows promising efficacy with no additive toxicity in murine models of glioma. Importantly, in immunogenic tumors, the ability of Toca 511 + 5-FC to induce local and systemic immunotherapeutic responses resulting in long-term survival was preserved despite lomustine-related myelosuppression. These results support combining lomustine with Toca 511 at early cycles of 5-FC for the treatment of patients with HGG. A phase I study exploring the combination of Toca 511 and Toca FC with lomustine is in progress (NCT01470794).
Unpublished data
Hiraoka K. et al, unpublished manuscript “Retroviral replicating vector-mediated prodrug conversion achieves long-term control of tumor recurrence and leads to durable anti-cancer immunity.” Corresponding author Kasahara N. (nkasahara@med.miami.edu).
Supplementary material
Funding
We thank the Investors in Tocagen Inc., Accelerate Brain Cancer Cure-ABC2 Foundation (Washington DC), the National Brain Tumor Society (Watertown MA), the American Brain Tumor Association (Chicago IL), the Musella Foundation (Hewlett NY), and Voices Against Brain Cancer (New York NY) for financial support.
Supplementary Material
Acknowledgments
We thank Dr Nicholas A. Boyle for his critical reading of the manuscript.
Conflict of interest statement. K.Y., T.T.H., F.L.E., D.M., C.E.I., H.E.G., D.J.J., and J.M.R. are employees and/or shareholders of Tocagen Inc.
References
- 1. Le Rhun E, Taillibert S, Chamberlain MC. The future of high-grade glioma: where we are and where are we going. Surg Neurol Intern. 2015;6 (suppl 1):S9–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perez OD, Logg CR, Hiraoka K et al. . Design and selection of Toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol Ther. 2012;20 (9):1689–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ostertag D, Amundson KK, Lopez Espinoza F et al. . Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neurooncol. 2012;14 (2):145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang TT, Hlavaty J, Ostertag D et al. . Toca 511 gene transfer and 5-fluorocytosine :in combination with temozolomide demonstrates synergistic therapeutic efficacy in a temozolomide-sensitive glioblastoma model. Cancer Gene Ther. 2013;20 (10):544–551. [DOI] [PubMed] [Google Scholar]
- 5. Huang TT, Parab S, Burnett R et al. . Intravenous administration of retroviral replicating vector, Toca 511, demonstrates therapeutic efficacy in orthotopic immune-competent mouse glioma model. Hum Gene Ther. 2015;26 (2):82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wick W, Hartmann C, Engel C et al. . NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27 (35):5874–5880. [DOI] [PubMed] [Google Scholar]
- 7. Figueiredo EG, Faria JW, Teixeira MJ. Treatment of recurrent glioblastoma with intra-arterial BCNU [1, 3-bis (2-chloroethyl)-1-nitrosourea]. Arq Neuropsiquiatr. 2010;68 (5):778–782. [DOI] [PubMed] [Google Scholar]
- 8. Rahman R, Hempfling K, Norden AD et al. . Retrospective study of carmustine or lomustine with bevacizumab in recurrent glioblastoma patients who have failed prior bevacizumab. Neurooncol. 2014;16 (11):1523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brada M, Stenning S, Gabe R et al. . Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high-grade glioma. J Clin Oncol. 2010;28 (30):4601–4608. [DOI] [PubMed] [Google Scholar]
- 10. Glas M, Happold C, Rieger J et al. . Long-term survival of patients with glioblastoma treated with radiotherapy and lomustine plus temozolomide. J Clin Oncol. 2009;27 (8):1257–1261. [DOI] [PubMed] [Google Scholar]
- 11. Wick W, Brandes A, Gorlia T et al. . LB-05PHASE III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the eortc 26101 trial. Neurooncol. 2015;17 (suppl 5):v1–v230. [Google Scholar]
- 12. Moertel CG, Schutt AJ, Hahn RG et al. . Therapy of advanced colorectal cancer with a combination of 5-fluorouracil, methyl-1,3-cis(2-chlorethyl)-1-nitrosourea, and vincristine. J Natl Cancer Inst. 1975;54 (1):69–71. [DOI] [PubMed] [Google Scholar]
- 13. Tobey RA, Crissman HA. Comparative effects of three nitrosourea derivatives on mammalian cell cycle progression. Cancer Res. 1975;35 (2):460–470. [PubMed] [Google Scholar]
- 14. Miller CR, Williams CR, Buchsbaum DJ et al. . Intratumoral 5-fluorouracil produced by cytosine deaminase/5-fluorocytosine gene therapy is effective for experimental human glioblastomas. Cancer Res. 2002;62 (3):773–780. [PubMed] [Google Scholar]
- 15. Barth RF, Kaur B. Rat brain tumor models in experimental neuro-oncology: the C6, 9L, T9, RG2, F98, BT4C, RT-2 and CNS-1 gliomas. J Neurooncol. 2009;94 (3):299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Towner RA, Gillespie DL, Schwager A et al. . Regression of glioma tumor growth in F98 and U87 rat glioma models by the Nitrone OKN-007. Neurooncol. 2013;15 (3):330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pohl U, Wick W, Weissenberger J et al. . Characterization of Tu-2449, a glioma cell line derived from a spontaneous tumor in GFAP-v-src-transgenic mice: comparison with established murine glioma cell lines. Int J Oncol. 1999;15 (4):829–834. [DOI] [PubMed] [Google Scholar]
- 18. Smilowitz HM, Weissenberger J, Weis J et al. . Orthotopic transplantation of v-src-expressing glioma cell lines into immunocompetent mice: establishment of a new transplantable in vivo model for malignant glioma. J Neurosurg. 2007;106 (4):652–659. [DOI] [PubMed] [Google Scholar]
- 19. Muller PJ, Tator CH, Bloom M. The effect of phenobarbital on the toxicity and tumoricidal activity of CCNU in a murine brain tumor model. J Neurosurg 1980;52 (3):359–366. [DOI] [PubMed] [Google Scholar]
- 20. Gutin PH, Hilton J, Fein VJ et al. . DNA damage in the intracerebral rat gliosarcoma 9L treated with 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea. Cancer Res. 1977;37 (10):3761–3765. [PubMed] [Google Scholar]
- 21. Martinez-Serra J, Gutierrez A, Munoz-Capo S et al. . xCELLigence system for real-time label-free monitoring of growth and viability of cell lines from hematological malignancies. Onco Targets Ther. 2014;7:985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bryant MJ, Chuah TL, Luff J et al. . A novel rat model for glioblastoma multiforme using a bioluminescent F98 cell line. J Clin Neurosci. 2008;15 (5):545–551. [DOI] [PubMed] [Google Scholar]
- 23. Tzeng JJ, Barth RF, Orosz CG et al. . Phenotype and functional activity of tumor-infiltrating lymphocytes isolated from immunogenic and nonimmunogenic rat brain tumors. Cancer Res. 1991;51 (9):2373–2378. [PubMed] [Google Scholar]
- 24. Hlavaty J, Jandl G, Liszt M et al. . Comparative evaluation of preclinical in vivo models for the assessment of replicating retroviral vectors for the treatment of glioblastoma. J Neurooncol. 2011;102 (1):59–69. [DOI] [PubMed] [Google Scholar]
- 25. Schwartz CL. Long-Term survivors of childhood cancer: the late effects of therapy. Oncologist. 1999;4 (1):45–54. [PubMed] [Google Scholar]
- 26. Yagiz K, Rodriguez-Aguirre ME, Espinoza FL et al. . Intravenous Delivery of Toca 511 Gene Therapy in Combination with 5-Fluorocytosine for Intratumoral Production of 5-Fluorouracil in a Colon Cancer Metastasis Model. Paper presented at: Molecular Therapy2015.
- 27. Buyukcelik A, Akbulut H, Yalcin B et al. . Overdose of lomustine: report of two cases. Tumori. 2004;90 (6):628–629. [DOI] [PubMed] [Google Scholar]
- 28. Ellis ME, Weiss RB, Kuperminc M. Nephrotoxicity of lomustine. A case report and literature review. Cancer Chemother Pharmacol. 1985;15 (2):174–175. [DOI] [PubMed] [Google Scholar]
- 29. Trent KC, Myers L, Moreb J. Multiorgan failure associated with lomustine overdose. Ann Pharmacother. 1995;29 (4):384–386. [DOI] [PubMed] [Google Scholar]
- 30. Paul DB, Barth RF, Yang W et al. . B7.1 expression by the weakly immunogenic F98 rat glioma does not enhance immunogenicity. Gene Ther. 2000;7 (12):993–999. [DOI] [PubMed] [Google Scholar]
- 31. Clavreul A, Delhaye M, Jadaud E et al. . Effects of syngeneic cellular vaccinations alone or in combination with GM-CSF on the weakly immunogenic F98 glioma model. J Neurooncol. 2006;79 (1):9–17. [DOI] [PubMed] [Google Scholar]
- 32. Candolfi M, Yagiz K, Wibowo M et al. . Temozolomide does not impair gene therapy-mediated antitumor immunity in syngeneic brain tumor models. Clin Cancer Res. 2014;20 (6):1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wick W, Puduvalli VK, Chamberlain MC et al. . Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28 (7):1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weiss RB, Issell BF. The nitrosoureas: carmustine (BCNU) and lomustine (CCNU). Cancer Treat Rev. 1982;9 (4):313–330. [DOI] [PubMed] [Google Scholar]
- 35. Vermes A, Guchelaar HJ, Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother. 2000;46 (2):171–179. [DOI] [PubMed] [Google Scholar]
- 36. Yazbeck R, Lindsay R, Abbott CA et al. . Combined effects of muricid extract and 5-fluorouracil on intestinal toxicity in rats. Evid Based Complement Alternat Med. 2015;2015:170858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.