Abstract
Background
Evaluation of glioblastoma disease status may be complicated by treatment-induced changes and discordance between enhancing and nonenhancing MRI. Exploratory analyses are presented (prospectively assessed pseudoprogression and therapy-related tumor pattern changes) from the AVAglio trial (bevacizumab or placebo plus radiotherapy/temozolomide for newly diagnosed glioblastoma).
Methods
MRI was done every 8 weeks (beginning 4 wk after chemoradiotherapy) using prespecified and standardized T1 and T2 protocols. Progressive disease (PD) at 10 weeks was reconfirmed at 18 weeks to distinguish pseudoprogression. Progression-free survival (PFS), excluding cases of confirmed pseudoprogression, was assessed (post-hoc/exploratory). Tumor progression patterns were determined at each disease assessment/PD (prespecified/exploratory).
Results
Of patients with PD in the bevacizumab and placebo arms, 143/354 (40.4%) and 155/387 (40.1%), respectively, had PD due to contrast-enhancing lesions, and 51/354 (14.4%) and 53/387 (13.7%) had PD due to nonenhancing lesions. Of all patients in the bevacizumab arm (n = 458), 2.2% had confirmed pseudoprogression versus 9.3% in the placebo arm (n = 463). Baseline characteristics did not differ between patients with/without pseudoprogression (including for MGMT status). Excluding confirmed pseudoprogression, PFS (hazard ratio: 0.65, 95% CI: 0.56–0.75; P < .0001, bevacizumab vs placebo) was comparable to the intent-to-treat population. At PD, most patients had the same tumor focus (local/multifocal, >84%) and infiltrative profile (>88%) as at baseline; no shift to a diffuse or multifocal phenotype was observed.
Conclusions
Pseudoprogression complicated progression assessment in a small but relevant number of patients but had negligible impact on PFS. Bevacizumab did not appear to adversely impact tumor progression patterns.
Keywords: glioblastoma, pseudoprogression, response, tumor pattern
Glioblastoma is an aggressive and invasive brain malignancy with poor prognosis.1 Standard-of-care therapy for newly diagnosed glioblastoma comprises concurrent radiotherapy and temozolomide (RT/TMZ), followed by adjuvant TMZ.2 In the randomized, phase III AVAglio (Avastin in Glioblastoma) study, the addition of bevacizumab to RT/TMZ significantly prolonged progression-free survival (PFS) versus placebo plus RT/TMZ (hazard ratio [HR]: 0.64; 95% CI: 0.55, 0.74; P < .0001; median 10.6 vs 6.2 mo),3 but no improvement was observed in overall survival (OS). The Radiation Therapy Oncology Group 0825 trial reported similar efficacy data, although PFS did not reach the predetermined significance level.4
Despite the lack of OS benefit observed with first-line bevacizumab, prolonged PFS is clinically meaningful for patients with glioblastoma. When tumors stop responding to treatment and increase in size (disease progression), patients often experience a neurologic and symptomatic decline and reduced quality of life.5 Disease progression can be established clinically (ie, worsening symptoms/neurologic deterioration) or radiologically (by MRI of tumor size). However, radiologic determination of disease progression has a major limitation: nontumoral factors can also influence contrast media enhancement, resulting in MRI scans that do not accurately reflect changes in tumor size or morphology, thereby clouding interpretation of tumor response to treatment. Therapies that increase blood–brain barrier permeability (eg, RT) can cause leakage of contrast media in the brain, producing a large area of enhancement on MRI, which mimics tumor growth (pseudoprogression).6 Pseudoprogression has been reported in up to 31% of patients with RT/TMZ-treated gliomas7–10 and may be prevalent in patients with methylated O6-methylguanine-DNA methyltransferase (MGMT).7 By contrast, agents that normalize blood–brain barrier permeability/blood flow (eg, bevacizumab) may result in a pseudoresponse (decrease in enhancement without a real antitumor effect) shortly after initiation of anti-angiogenic treatment.11 Pseudoprogression and pseudoresponse are most likely to occur during the first 3 months of treatment; particular care is needed when interpreting scans during this period.
In 2010, the Response Assessment in Neuro-Oncology (RANO) working group recommended that assessment of non-contrast-enhancing tumor components should be a key additional component of response criteria.12,13 Although AVAglio was initiated before the RANO criteria were published, its study design stipulated the use of T2-weighted (T2-w) or fluid-attenuated inversion recovery (FLAIR) MRI sequences to measure non-contrast-enhancing tumor growth.12,13 A decision tree was also prospectively implemented in AVAglio (at wk 10–18 of treatment) to help investigators ensure that patients with increased enhancement at the first post-RT scan (potential pseudoprogression) were not excluded from continuing study treatment. Although pseudoresponse could not be prospectively assessed, further exploratory and post-hoc analyses to understand the potential impact of pseudoimaging phenomena on the efficacy results were carried out and are reported here.
Beyond pseudoprogression and pseudoresponse, AVAglio also examined whether bevacizumab treatment influenced the way in which tumors progressed, that is, whether tumors changed during treatment. Preclinical data in transgenic tumors and uncontrolled/retrospective trials have suggested that bevacizumab-treated tumors could become more infiltrative or recur at distant points from the original tumor.14–18 Small prospective studies have reported relatively high rates of infiltrative disease at progression,19,20 but a case-matched study did not demonstrate an increased risk of distant or diffuse relapse.21 It is not known whether changes in tumor pattern during the course of treatment may influence OS or PFS. Analyses examining whether bevacizumab influenced the pattern of tumor progression (prespecified and exploratory) and whether tumor pattern affected survival (post-hoc and exploratory) were carried out in AVAglio. In this manuscript we report the details of pseudoprogression and pseudoresponse as assessed by the investigator, and patterns of tumor growth as assessed by an independent review facility (IRF; or central review), as well as the impact of these imaging phenomena on patient outcomes.
Methods
Patients
Full details have been published previously.3 Briefly, patients (≥18 y) had newly diagnosed supratentorial glioblastoma (confirmed from resection/biopsy), World Health Organization performance status ≤2, and stable/decreasing corticosteroid dose during the 5 days prior to randomization. The protocol was approved by local ethics committees. The study was conducted in accordance with the Declaration of Helsinki. Patients provided written informed consent.
Study Design
AVAglio (BO21990/NCT00943826)22 was a randomized, double-blind, placebo-controlled, multicenter, phase III trial (Supplementary Fig. S1). After debulking surgery, patients received RT (6 wk) in combination with daily TMZ and bevacizumab or placebo every 2 weeks. Following a 28-day treatment break, patients then received TMZ and bevacizumab or placebo (for six 4-wk cycles) and then single-agent bevacizumab or placebo until progressive disease (PD) or unacceptable toxicity. Co-primary endpoints were investigator-assessed PFS and OS.3 Assessment of patterns of tumor progression by an IRF was a protocol-defined exploratory endpoint.
Patients were randomized (1:1) centrally with region (western Europe/eastern Europe/Asia/USA/other) and recursive partitioning analysis class (3/4/5) as stratification factors. The study sponsor, investigators, and patients were blinded to treatment. Unblinding was allowed for safety reasons, or at PD, if deemed critical by the investigator for deciding on subsequent treatment.
Determination of Progression/Response
Disease assessments were scheduled at baseline, at the end of the treatment break, every 8 weeks during adjuvant TMZ therapy, and then every 9 weeks and at PD. An IRF reviewed all MRI scans for response as a prospectively defined secondary endpoint. Response/progression was determined according to modified Macdonald criteria, which incorporated radiologic assessment (prespecified, dedicated protocol), neurologic assessment, and corticosteroid use. Radiologic assessments were performed using MRI: changes in the sum of the products of the diameters (SPD) of index lesions (contrast-enhanced T1-w sequences with assessment of measurable contrast-enhancing lesions ≥10 mm up to a maximum of 10 lesions), and qualitative assessment of non-index lesions (defined as nonenhancing lesions on FLAIR or T2-w sequences, and enhancing nonmeasurable lesions on contrast-enhanced T1-w sequences [one diameter <10 mm and/or groups of >10 index lesions]). The specific modifications to the Macdonald criteria were intended to include contemporaneous expert recommendations to include assessment of nonenhancing tumor in a qualitative fashion, as was later adopted as part of the RANO criteria.12,13 Evaluation of neurologic function was based on the investigator's assessment of the patient's neurologic state compared with the previous assessment. Corticosteroid use was based on intake at the time of each disease assessment compared with the previous assessment.
PD was defined as 25% increase in index lesions, unequivocal progression of existing non-index lesions (qualitative assessment by the investigator), new lesions, or worsened neurologic symptoms (if corticosteroid dose was stable or increased).
Response was defined as complete response (disappearance of all index lesions [sustained ≥4 wk], no worsening of non-index lesions [sustained ≥4 wk], no new lesions, corticosteroid dose not exceeding physiologic levels, improved neurologic symptoms) or partial response (≥50% decrease in SPD of index lesions [sustained ≥4 wk], no progression of non-index lesions, no new lesions, stable/reduced corticosteroids, improved/stable neurologic symptoms).
Evaluation of Potential Imaging Phenomena
A prospectively designed algorithm was used to assess pseudoprogression during the study (Supplementary Fig. S2). The algorithm was implemented after the treatment break, when most pseudoprogression occurs, with pseudoprogression-related changes reducing over time. Briefly, at week 10, if there was a ≥25% increase in index lesions or progression of non-index lesions, but no new lesions or clinical worsening (ie, potential pseudoprogression), the algorithm allowed the patient to continue study treatment until the next assessment (wk 18). At that time, if pseudoprogression was confirmed, patients continued treatment per protocol. If the patient had confirmed PD, the previous assessment time was considered to be the real time of progression.
Sensitivity analyses were conducted to address the impact of imaging phenomena on PFS: (i) PFS excluding patients with confirmed pseudoprogression; (ii) PFS excluding patients with potential pseudoprogression; and (iii) PFS excluding patients with a PFS event during the first 92 days of treatment. The 92-day limit was chosen to minimize the possibility that any early post-RT and postoperative changes were influencing the determination of PFS.
Pseudoresponse could not be assessed directly, but a theoretical impact of pseudoresponse on PFS assessment in the bevacizumab arm would introduce a differential bias in the tumor evaluation (ie, if bevacizumab produced a pseudoresponse on MRI radiologic imaging, proportionally more patients in the bevacizumab arm would be diagnosed with PD by non-MRI methods [by clinical deterioration], and at investigator-defined PD the size of tumors would differ between arms). The method by which PD was confirmed and the increase in tumor SPD were compared between treatment arms.
Tumor Pattern
Evaluation of tumor pattern was performed by the IRF at all disease assessments, including at investigator-assessed PD. Tumor pattern definitions were adapted from Pope.13 “Local” tumors were defined as focus of enhancement or nonenhancing tumor with mostly distinct or well-defined borders. “Multifocal” was used to describe more than one enhancing or nonenhancing tumor with intervening areas of normal brain signal. “Distant” tumors had a single new focus of enhancement or nonenhancing tumor centered outside a 30 mm margin around the primary site or margin of the resection cavity. In addition to describing tumor location, tumors were categorized by the presence and extent of nonenhancing abnormalities at each time point. The pattern would be designated as “diffuse (infiltrative)” if there was extensive hypersignal/hyperintensity on T2-w or FLAIR MRIs or “nondiffuse” if the bulk of the tumor abnormality was enhancing.
Statistical Analyses
PFS/OS analyses used Kaplan–Meier methodology to generate median values. A log-rank test generated nonconfirmatory P-values. Tumor patterns were planned exploratory analyses, but the study was not powered for this.
Results
Pseudoprogression and Pseudoresponse
Pseudoprogression was more frequent in the placebo arm than in the bevacizumab arm (Table 1). Baseline characteristics of patients with confirmed pseudoprogression in the placebo arm were similar to those of the intent-to-treat (ITT) population, including MGMT status (Supplementary Table S1).
Table 1.
Confirmed and potential pseudoprogression
| Patients, n (%) |
||
|---|---|---|
| BEV + RT/TMZ (n = 458) |
Plb + RT/TMZ (n = 463) |
|
| End of treatment break | ||
| Potential pseudoprogression | 12 (2.6) | 84 (18.1) |
| End of second maintenance cycle | ||
| Confirmed pseudoprogression | 10 (2.2) | 43 (9.3) |
| Confirmed progression | 1 (0.2) | 35 (7.6) |
| Missing | 1 (0.2) | 6 (1.3) |
Abbreviations: BEV, bevacizumab; Plb, placebo.
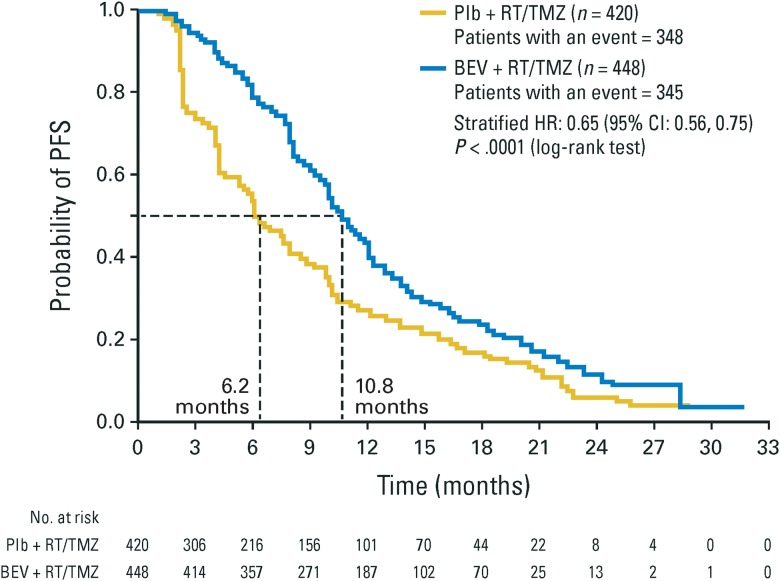
Sensitivity analyses showed that when patients with confirmed pseudoprogression (either arm) were excluded from the overall population, the median PFS (Fig. 1) (bevacizumab vs placebo, median 10.8 vs 6.2 mo, respectively, HR: 0.65; 95% CI: 0.56, 0.75; P < .0001, n = 448 and n = 420) was comparable to the ITT population. Similar results were observed for PFS when patients with potential pseudoprogression were excluded from the overall population (bevacizumab vs placebo, median 10.8 vs 7.4 mo, respectively, HR: 0.70; 95% CI: 0.60, 0.82; P < .0001, n = 446 and n = 379) and when all patients with a PFS event within the first 92 days of treatment were excluded (bevacizumab vs placebo, 11.1 vs 8.8 mo, respectively, HR: 0.78; 95% CI: 0.66, 0.92; P = .0026, n = 424 and n = 349) (Supplementary Fig. S3).
Fig. 1.
Sensitivity analyses of PFS excluding patients with confirmed pseudoprogression. Confirmed pseudoprogression means that pseudoprogression was suspected and confirmed in follow-up. BEV, bevacizumab; Plb, placebo.
As mentioned previously, pseudoresponse could theoretically introduce a differential bias in the tumor evaluation. At time of progression, there was an equivalent distribution of the proportion of progression determined primarily by radiologic means, and the proportional change in tumor size was comparable between arms (Table 2). There was no evidence of a bias toward clinical determination of PD (ie, potential pseudoresponse).
Table 2.
Method by which PD was documented and the type of MRI lesion involved in PD event
| BEV + RT/TMZ (n = 354) |
Plb + RT/TMZ (n = 387) |
|
|---|---|---|
| Method by which PD was documented, n (%) | ||
| MRI alonea | 197 (55.6) | 212 (54.8) |
| MRI with neurologic worseningb | 94 (26.6) | 110 (28.4) |
| Neurologic worseningc | 22 (6.2) | 23 (5.9) |
| Otherd | 41 (11.6) | 42 (10.9) |
| Type of MRI lesion involved in PD event,en (%) | ||
| Index lesion | 143 (40.4) | 155 (40.1) |
| Patients with SPD increase from nadir, n (%) | ||
| <25%f | 92 (41.2)g | 99 (36.7)h |
| 25–50% | 27 (12.1)g | 44 (16.3)h |
| >50–100% | 53 (23.8)g | 70 (25.9)h |
| >100% | 51 (22.9)g | 57 (21.1)h |
| Non-index lesion | 51 (14.4) | 53 (13.7) |
| New lesion(s) | 148 (41.8) | 147 (38.0) |
Note. Index lesions = enhancing post-gadolinium T1 sequence, measurable contrast enhancing ≥10 mm up to a maximum of 10 lesions. Non-index lesions = nonenhancing lesions by FLAIR or T2 weighted sequences and enhancing nonmeasurable lesions (1 diameter <10 mm and/or groups of >10 index lesions) PD was assessed by the investigator.
Abbreviations: BEV, bevacizumab; Plb, placebo.
aRadiologic assessment only.
bCorticosteroid use assessment plus neurologic assessment plus radiologic assessment.
cNeurologic assessment plus corticosteroid use assessment.
dAssessment in absence of PD by neurologic/radiologic assessment, or death (ie, includes patients who died prior to PD or patients for whom PD assessment could not be physically confirmed by the investigator, eg, a patient sent to hospice by a local doctor).
ePD may be based on more than one category.
fCases where a patient had an index lesion at baseline but had a PFS event based on another source (ie, a new lesion or non-index lesion).
gOf 223 patients with index lesions at baseline.
hOf 270 patients with index lesions at baseline.
Tumor Pattern
Baseline characteristics of patients with diffuse and nondiffuse tumors were similar and comparable to the ITT population (Supplementary Table S2), with the exception that a Mini-Mental State Examination score of ≥27 was more frequent in patients with nondiffuse tumors at baseline compared with patients who had diffuse tumors and the ITT population. MGMT status was similar between subgroups.
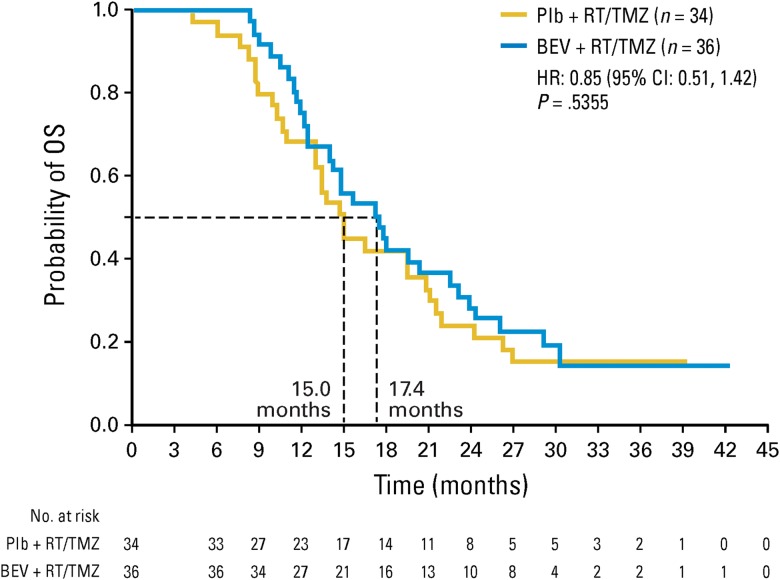
At progression, most patients (>84%) had the same tumor focus (ie, local or multifocal tumors) that they had at baseline (Table 3). There was no trend for a change from local to multifocal or distant tumors with bevacizumab treatment (Table 3). Similarly, the majority of patients (>88%) presented with the same infiltrative profile at PD as they had at baseline (Table 3), noting a similar change from a nondiffuse to diffuse tumor pattern at progression of 12.0% in the bevacizumab arm and 10.2% in the placebo arm. For the few patients in both arms whose disease changed from nondiffuse at baseline to diffuse at PD, no clinically significant difference in OS was observed between arms (Fig. 2), nor was there any notable difference compared with the median OS of the ITT population.
Table 3.
Tumor location and invasiveness at baseline and at investigator-assessed progression (patterns were assessed by an IRF)
| Patients with Baseline and PD Information |
n = 299 |
n = 333 |
|
|---|---|---|---|
| BEV + RT/TMZ (n = 458) |
Plb + RT/TMZ (n = 463) |
||
| Tumor focus | |||
| Patients with no changes from baseline, n (%) | |||
| Baseline tumor | Tumor at progression | ||
| Local | Local | 198/299 (66.2) | 215/333 (64.6) |
| Multifocal | Multifocal | 57/299 (19.1) | 63/333 (18.9) |
| Patients with changes from baseline, n (%) | |||
| Baseline tumor | Tumor at progression | ||
| Local | Multifocal | 19/299 (6.4) | 30/333 (9.0) |
| Distant | 11/299 (3.7) | 15/333 (4.5) | |
| Multifocal | Local | 9/299 (3.01) | 2/333 (2.4) |
| Distant | 4/299 (1.3) | 2/333 (2.4) | |
| No pattern | Local | 1/299 (0.3) | 0/0 (0.0) |
| Tumor infiltration | |||
| Patients with no changes from baseline, n (%) | |||
| Baseline tumor | Tumor at progression | ||
| Nondiffuse | Nondiffuse | 64/299 (21.4) | 104/333 (31.2) |
| Diffuse | Diffuse | 197/299 (65.9) | 194/333 (58.3) |
| Patients with changes from baseline, n (%) | |||
| Baseline tumor | Tumor at progression | ||
| Nondiffuse | Diffuse | 36/299 (12.0) | 34/333 (10.2) |
| Diffuse | Nondiffuse | 2/299 (0.7) | 1/333 (0.3) |
Abbreviations: BEV, bevacizumab; Plb, placebo.
Fig. 2.
OS of patients who changed from having a nondiffuse tumor at baseline to a diffuse tumor at PD. BEV, bevacizumab; Plb, placebo.
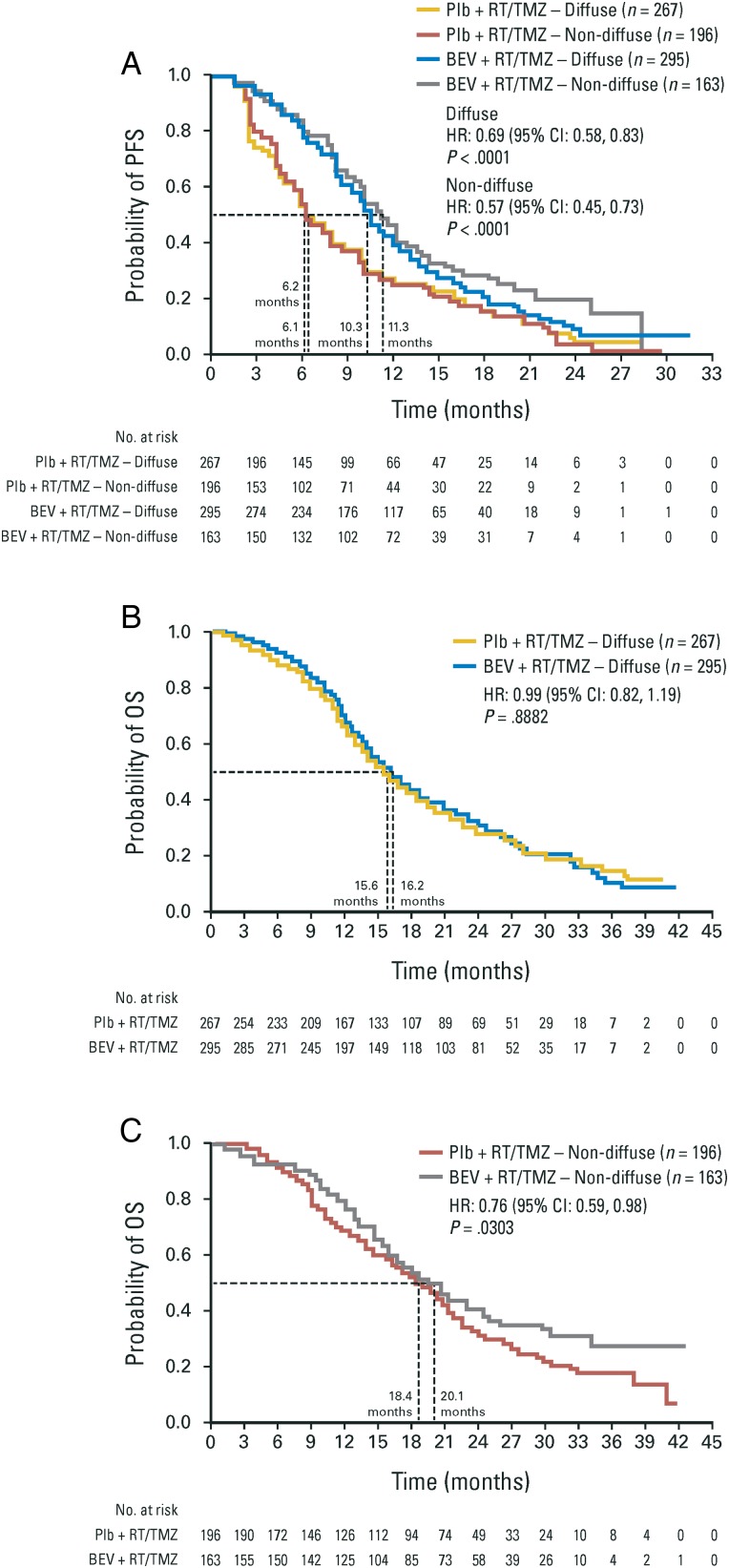
The OS of patients (either arm) with diffuse disease at baseline was shorter compared with patients who had nondiffuse tumors at baseline (Fig. 3). The magnitude of PFS benefit in patients treated with bevacizumab was generally similar for patients with diffuse or nondiffuse baseline tumors and comparable to the ITT population (Fig. 3, and Supplementary Table S3). Patients with multifocal disease at baseline had shorter median PFS and OS, compared with patients who had local tumors. In each subgroup, the magnitude of benefit between arms was similar to that seen in the ITT population (Supplementary Table S3 and Supplementary Fig. S4A and S4B).
Fig. 3.
(A) PFS in patients with diffuse or nondiffuse tumors at baseline. (B) OS in patients with diffuse tumors at baseline. (C) OS in patients with nondiffuse tumors at baseline. BEV, bevacizumab; Plb, placebo.
A summary of treatment after progression by patterns of tumor progression and treatment group is reported in Supplementary Table S4.
Discussion
AVAglio was the first large study to systematically assess pseudoprogression prospectively, by delaying the decision on progression at week 10 to week 18 in cases of suspected pseudoprogression. This approach, intended to standardize assessment and limit the likelihood of discontinuing effective therapy (TMZ) in affected patients, resulted in an observation of lower pseudoprogression rates than previously reported.7–10 However, direct comparisons with previous studies are difficult due to differences in study baselines; for example, treatment start in AVAglio was 28–49 days postsurgery, which may be later in the disease course than in other studies. Patients in AVAglio also had a new baseline scan at study entry (within 4 wk before treatment start), which may have limited the likelihood of determining pseudoprogression. Previous assessments have also typically been single-center, retrospective, single-arm, or small cohort studies using less stringent criteria for the definition and resolution of pseudoprogression. A previous study has suggested a relationship between MGMT status and pseudoprogression,7 which was not demonstrated here despite a large sample size with balanced MGMT status.
The slightly higher rates of pseudoprogression reported in the placebo arm could have theoretically influenced the measurement of PFS. We excluded patients with potential or confirmed pseudoprogression from PFS analysis, and indeed all patients with a PFS event in the first 3 months of the study (when imaging misinterpretations are most likely); results for each of these analyses were comparable to the PFS seen in the ITT population, suggesting that pseudoprogression did not significantly influence PFS measurement in our study.
Pseudoresponse is more difficult to ascertain and could only be measured indirectly in AVAglio. Previously, high response rates for bevacizumab-treated tumors were nominally attributed to decreased contrast enhancement due to modified vessel permeability rather than real tumor shrinkage.23 A theoretical impact of such pseudoresponse on PFS in the bevacizumab arm would have introduced a differential bias to tumor evaluation at progression. Had bevacizumab influenced progression, a higher proportion of progression based on clinical deterioration/non-index lesions and larger enhancing tumors (at time of investigator-defined PD) would be expected in the bevacizumab arm, which was not the case. The distribution of the overall and radiologic source of progression assessment and change in SPD of index lesions was equivalent between arms.
The measurement of patterns of progression, carried out in a consistent manner, provides additional prospective data to address the concerns of potential changes in tumor invasiveness. Most patients' tumor pattern type remained identical at the time of PD compared with baseline. In those patients whose tumor type did change from nondiffuse to diffuse during treatment, OS did not seem to be adversely affected (however, P-values were nonconfirmatory due to the exploratory nature, small sample size, and multiple testing). Baseline infiltrative status was more influential for survival than the shift in pattern during treatment, with diffuse or multifocal disease at baseline being associated with poorer survival.
There are limitations to the analyses presented. For example, some reported cases of pseudoprogression might have occurred at delayed time points or occurred in association with clinical symptoms that would not have been captured by our algorithm. Therefore, the stringency of our criteria/algorithm might have resulted in a small proportion of patients being diagnosed with PD when in fact they had pseudoprogression, particularly in the control arm. In this study, there was a small subset of patients (n < 10) who had early PD and a long OS; as there was a small risk that some instances of delayed pseudoprogression were not identified by the pseudoprogression algorithm at weeks 10 and 18, it is possible that some or all of these patients might have had pseudoprogression rather than PD.24 In addition, the non-ITT PFS analyses and measurement of patterns of progression were exploratory, and in some cases the number of patients was small or baseline characteristics were unbalanced (probably due to the breaking of randomization by the subgroup analysis).
Despite the limitations, our results contribute to a significant improvement in understanding and assessing the response to anti-angiogenic therapy for glioblastoma, although further advances are needed, particularly for interpretation of recent studies on recurrent glioblastoma.25 For example, further development of the RANO criteria to include baseline measurement of nonenhancing components and definition of “significant changes” in T2-w or FLAIR MRI sequences (including volumetric analysis), and precise guidelines on how to address pseudoprogression, would be valuable. An algorithm, as used in the present study, could be useful and should be tested in other prospective studies. Mandating confirmatory MRIs for suspected pseudoprogression in clinical trials would be effective, but the development of tools to distinguish pseudoprogression without having to wait for a follow-up scan would be preferable. Several techniques are under investigation, including T1-w subtraction maps, diffusion-weighted and perfusion-weighted MRI,26 the apparent diffusion coefficient,27 cerebral blood volume measurement by MRI perfusion,28 18F-fluorodeoxyglucose PET,6 and multiparametric histogram analysis of MR images.29
In summary, the rate of confirmed pseudoprogression was low for patients with newly diagnosed glioblastoma treated with bevacizumab plus RT/TMZ and had a negligible effect on the assessment of PFS. We also inferred that pseudoresponse had minimal impact on PFS, based on a lack of differential bias in how tumor progression was confirmed. Finally, in the small proportion of patients whose tumor changed from nondiffuse to diffuse during treatment, OS did not seem to be adversely affected.
Supplementary Material
Funding
The study was funded by F. Hoffmann-La Roche Ltd.
Supplementary Material
Acknowledgments
The authors thank Lauren Abrey, MD, Josep Garcia, PhD, and Marcela Oancea, PhD for their critical review of the manuscript and valuable contributions to the interpretation of the data. Third party medical writing support, under the direction of the authors, was provided by Emma L. McConnell, PhD (Gardiner-Caldwell Communications) and was funded by F. Hoffmann-La Roche Ltd.
Conflict of interest statement. W.W. has received research grant support and personal fees from F. Hoffmann-La Roche, research grant support from MSD, personal fees from Prime Oncology, and nonfinancial support from Eli Lilly and Apogenix. O.L.C. has received personal fees and nonfinancial support from F. Hoffmann-La Roche and personal fees from AstraZeneca and has a patent issued related to a plasmatic biomarker of bevacizumab efficacy (Europe 12305565.9). M.B. has received personal fees from F. Hoffmann-La Roche, Codman, Vascular Dynamics, Guerbet, and Novartis, and research grant support from Guerbet, Siemens, and DFG. W.M. and R.H. have received personal fees from F. Hoffmann-La Roche. F.S. has received personal fees and nonfinancial support from F. Hoffmann-La Roche. R.N. has received personal fees from Eisai, MSD, F. Hoffmann-La Roche, and Chugai. C.R. and Y.K. have received personal fees from F. Hoffmann-La Roche. T.C. has received research grant support and personal fees from F. Hoffmann-La Roche and Genentech.
References
- 1. Central Brain Tumor Registry of the United States. Available at http://www.cbtrus.org/ Accessed November 25, 2015.
- 2. Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352 (10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Chinot OL, Wick W, Mason W et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370 (8):709–722. [DOI] [PubMed] [Google Scholar]
- 4. Gilbert MR, Dignam JJ, Armstrong TS et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370 (8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Galanis E, Wu W, Cloughesy T et al. Phase 2 trial design in neuro-oncology revisited: a report from the RANO group. Lancet Oncol. 2012;13 (5):e196–e204. [DOI] [PubMed] [Google Scholar]
- 6. Hygino da Cruz LC, Rodriguez I, Domingues RC et al. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32 (11):1978–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brandes AA, Franceschi E, Tosoni A et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26 (13):2192–2197. [DOI] [PubMed] [Google Scholar]
- 8. Gerstner ER, McNamara MB, Norden AD et al. Effect of adding temozolomide to radiation therapy on the incidence of pseudo-progression. J Neurooncol. 2009;94 (1):97–101. [DOI] [PubMed] [Google Scholar]
- 9. Sanghera P, Perry J, Sahgal A et al. Pseudoprogression following chemoradiotherapy for glioblastoma multiforme. Can J Neurol Sci. 2010;37 (1):36–42. [DOI] [PubMed] [Google Scholar]
- 10. Taal W, Brandsma D, de Bruin HG et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113 (2):405–410. [DOI] [PubMed] [Google Scholar]
- 11. Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22 (6):633–638. [DOI] [PubMed] [Google Scholar]
- 12. Chinot OL, Macdonald DR, Abrey LE et al. Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci Rep. 2013;13 (5):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wen PY, Macdonald DR, Reardon DA et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28 (11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 14. Norden AD, Young GS, Setayesh K et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70 (10):779–787. [DOI] [PubMed] [Google Scholar]
- 15. Pope WB, Xia Q, Paton VE et al. Patterns of progression in patients with recurrent glioblastoma treated with bevacizumab. Neurology. 2011;76 (5):432–437. [DOI] [PubMed] [Google Scholar]
- 16. Zuniga RM, Torcuator R, Jain R et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91 (3):329–336. [DOI] [PubMed] [Google Scholar]
- 17. Iwamoto FM, Abrey LE, Beal K et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73 (15):1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Groot JF, Fuller G, Kumar AJ et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12 (3):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Narayana A, Kelly P, Golfinos J et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg. 2009;110 (1):173–180. [DOI] [PubMed] [Google Scholar]
- 20. Narayana A, Gruber D, Kunnakkat S et al. A clinical trial of bevacizumab, temozolomide, and radiation for newly diagnosed glioblastoma. J Neurosurg. 2012;116 (2):341–345. [DOI] [PubMed] [Google Scholar]
- 21. Wick A, Dörner N, Schäfer N et al. Bevacizumab does not increase the risk of remote relapse in malignant glioma. Ann Neurol. 2011;69 (3):586–592. [DOI] [PubMed] [Google Scholar]
- 22. Clinicaltrials.gov: A study of Avastin® (bevacizumab) in combination with temozolomide and radiotherapy in patients with newly diagnosed glioblastoma. Available at https://clinicaltrials.gov/ct2/show/NCT00943826 Accessed November 25, 2015.
- 23. Clarke JL, Chang S. Pseudoprogression and pseudoresponse: Challenges in brain tumor imaging. Curr Neurol Neurosci Rep. 2009;9 (3):241–246. [DOI] [PubMed] [Google Scholar]
- 24. Radbruch A, Fladt J, Kickingereder P et al. Pseudoprogression in patients with glioblastoma: clinical relevance despite low incidence. Neuro Oncol. 2015;17 (1):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taal W, Oosterkamp HM, Walenkamp AM et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15 (9):943–953. [DOI] [PubMed] [Google Scholar]
- 26. Nasseri M, Gahramanov S, Netto JP et al. Evaluation of pseudoprogression in patients with glioblastoma multiforme using dynamic magnetic resonance imaging with ferumoxytol calls RANO criteria into question. Neuro Oncol. 2014;16 (8):1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brynolfsson P, Nilsson D, Henriksson R et al. ADC texture–an imaging biomarker for high-grade glioma? Med Phys. 2014;41 (10):101903. [DOI] [PubMed] [Google Scholar]
- 28. Kong DS, Kim ST, Kim EH et al. Diagnostic dilemma of pseudoprogression in the treatment of newly diagnosed glioblastomas: the role of assessing relative cerebral blood flow volume and oxygen-6-methylguanine-DNA methyltransferase promoter methylation status. AJNR Am J Neuroradiol. 2011;32 (2):382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cha J, Kim ST, Kim HJ et al. Differentiation of tumor progression from pseudoprogression in patients with posttreatment glioblastoma using multiparametric histogram analysis. AJNR Am J Neuroradiol. 2014;35 (7):1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.