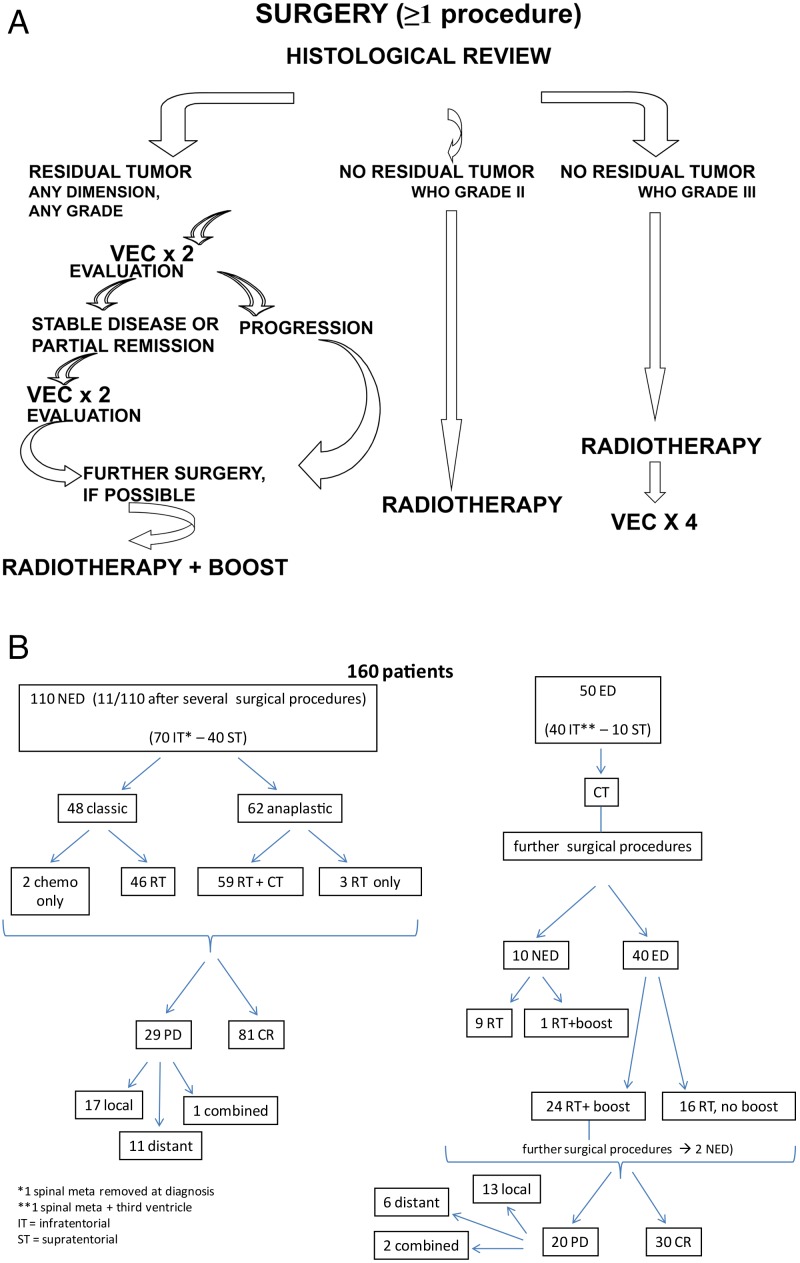
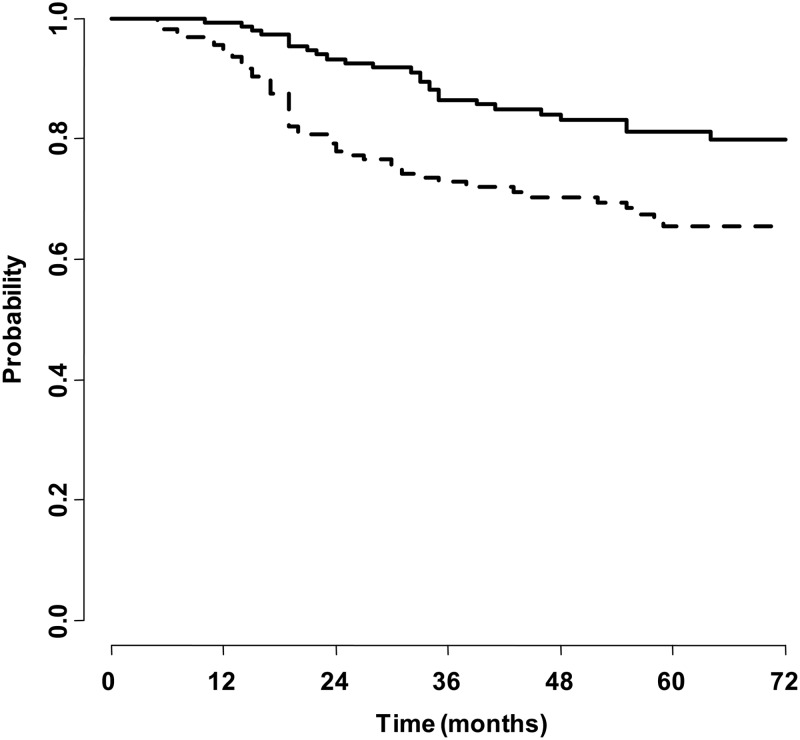
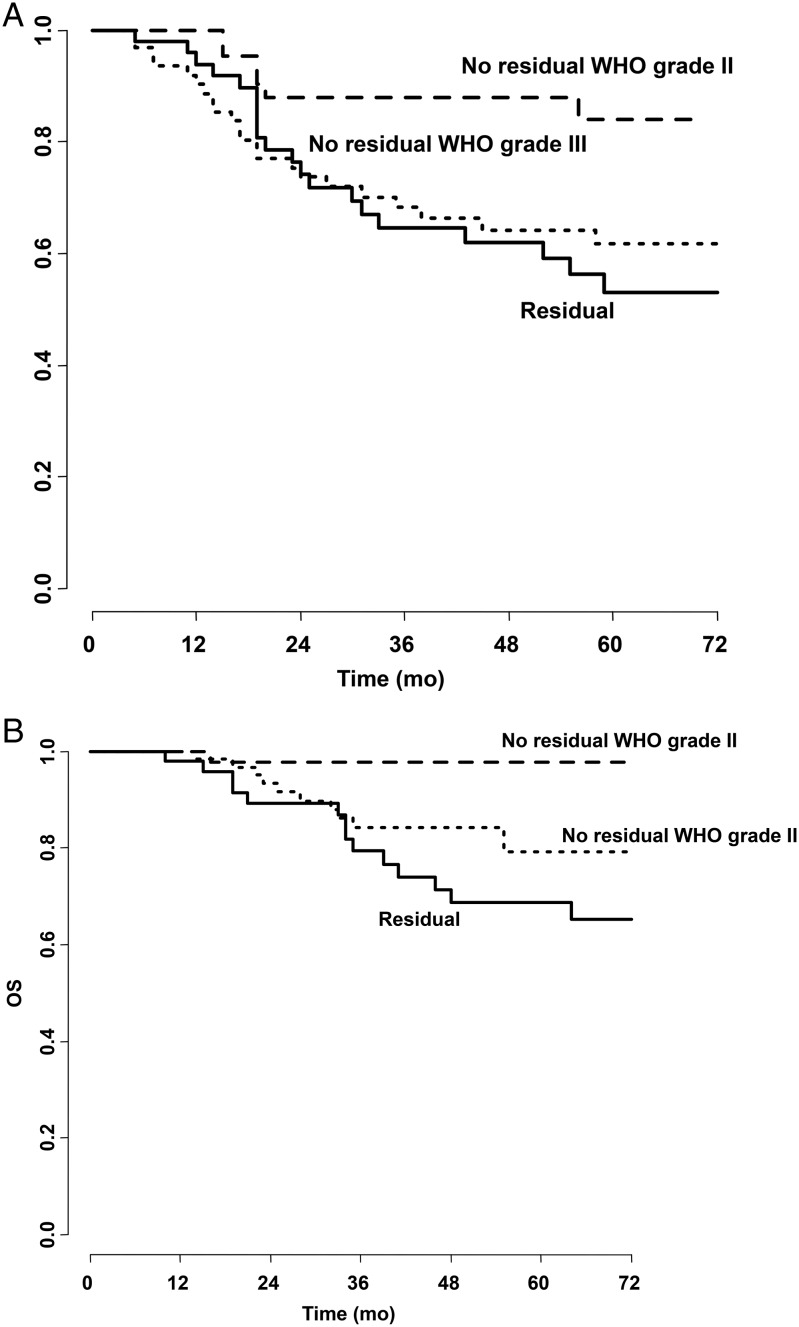
Maura Massimino
Maura Massimino
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,✉,
Rosalba Miceli
Rosalba Miceli
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Felice Giangaspero
Felice Giangaspero
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Luna Boschetti
Luna Boschetti
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Piergiorgio Modena
Piergiorgio Modena
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Manila Antonelli
Manila Antonelli
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Paolo Ferroli
Paolo Ferroli
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Daniele Bertin
Daniele Bertin
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Emilia Pecori
Emilia Pecori
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Laura Valentini
Laura Valentini
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Veronica Biassoni
Veronica Biassoni
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Maria Luisa Garrè
Maria Luisa Garrè
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Elisabetta Schiavello
Elisabetta Schiavello
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Iacopo Sardi
Iacopo Sardi
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Armando Cama
Armando Cama
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Elisabetta Viscardi
Elisabetta Viscardi
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Giovanni Scarzello
Giovanni Scarzello
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Silvia Scoccianti
Silvia Scoccianti
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Maurizio Mascarin
Maurizio Mascarin
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Lucia Quaglietta
Lucia Quaglietta
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Giuseppe Cinalli
Giuseppe Cinalli
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Barbara Diletto
Barbara Diletto
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Lorenzo Genitori
Lorenzo Genitori
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Paola Peretta
Paola Peretta
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Anna Mussano
Anna Mussano
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Annamaria Buccoliero
Annamaria Buccoliero
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Giuseppina Calareso
Giuseppina Calareso
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Salvina Barra
Salvina Barra
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Angela Mastronuzzi
Angela Mastronuzzi
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Carlo Giussani
Carlo Giussani
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Carlo Efisio Marras
Carlo Efisio Marras
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Rita Balter
Rita Balter
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Patrizia Bertolini
Patrizia Bertolini
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Ermanno Giombelli
Ermanno Giombelli
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Milena La Spina
Milena La Spina
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Francesca R Buttarelli
Francesca R Buttarelli
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Bianca Pollo
Bianca Pollo
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1,
Lorenza Gandola
Lorenza Gandola
1Department of Pediatrics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (M.M., L.B., V.B., E.S.); Department of Medical Statistics, Biometry, and Bioinformatics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (R.M.); Pediatric Radiotherapy, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (L.G., E.P., B.D.); Department of Radiology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (G.C.); Department of Radiological, Oncological, and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy (F.G., M.A.); Department of Neurology and Psychiatry, Sapienza University, Rome, Italy (F.R.B.); IRCCS Neuromed, Pozzilli, Italy (F.G.); Laboratory of Genetics, Pathology Unit, S. Anna General Hospital, Como, Italy (P.M.); Neurosurgery Department, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (P.F., L.V.); Pathology Unit, IRCCS Istituto Neurologico Carlo Besta, Milan, Italy (B.P.); Department of Pediatric Onco-Hematology, Regina Margherita Children's Hospital, Torino, Italy (D.B.); Department of Neurosurgery, Regina Margherita Children's Hospital, Torino, Italy (P.P.); Radiotherapy Unit, Regina Margherita Children's Hospital, Torino, Italy (A.M.); Istituto Giannina Gaslini, Genova, Italy (M.L.G., A.C.); Department of Neuro-oncology, Ospedale Pediatrico Meyer, Firenze, Italy (I.S.); Neurosurgery Unit, Ospedale Pediatrico Meyer, Firenze, Italy (L.G.); Pediatric Oncology Unit, Padova University, Padova, Italy (E.V.); Radiotherapy Department, IOV-IRCCS, Padova, Italy (G.S.); Department of Radiation Oncology, Careggy Hospital, Firenze, Italy (S.S.); Pathology Unit, Careggy Hospital, Firenze, Italy (A.B.); Department of Pediatric Radiotherapy, CRO, Aviano, Italy (M.M.); Department of Pediatric Oncology, Ospedale Santobono-Pausillipon, Napoli, Italy (L.Q.); Neurosurgery Unit, Ospedale Santobono-Pausillipon, Napoli, Italy (G.C.); Department of Radiation Oncology, IRCCS San Martino IST, National Cancer Research Institute, Genoa, Italy (S.B.); Pediatric Hematology and Oncology Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (A.M.); Neurosurgery Unit, Ospedale Pediatrico Bambino Gesù, Rome, Italy (C.E.M.); Neurosurgery Unit, University of Milano-Bicocca, Ospedale San Gerardo, Monza, Italy (C.G.); Pediatric Oncology Unit, Policlinico GB Rossi, Verona, Italy (R.B.); Department of Pediatric Oncology, University Hospital of Parma, Catania, Italy (P.B.); Neurosurgery Unit, University Hospital of Parma, Catania, Italy (E.G.); Center of Pediatric Hematology Oncology, Catania, Italy (M.L.S.)
1