Abstract
Endothelial cells (ECs) line the lumen of all blood vessels and play a critical role in maintaining the barrier function of the vasculature. Sealing of the vessel wall between adjacent ECs is facilitated by interactions involving junctionally expressed transmembrane proteins including tight junctional molecules, such as members of the junctional adhesion molecule (JAM) family, components of adherence junctions, such as VE-Cadherin, and other molecules such as platelet endothelial cell adhesion molecule (PECAM). Of importance, a growing body of evidence indicates that the expression of these molecules is regulated in a spatiotemporal manner during inflammation; responses that have significant implications for the barrier function of blood vessels against blood-borne macromolecules and transmigrating leukocytes. This review summarises key aspects of our current understanding of the dynamics and mechanisms that regulate the expression of EC junctional molecules during inflammation, and discusses the associated functional implications of such events in acute and chronic scenarios.
Introduction
Endothelial cells (ECs) line the inner wall of all blood vessels and are critical in maintaining the barrier function of the vasculature. Under inflammatory conditions, penetration of ECs by macromolecules and immune cells can be achieved via both transcellular mechanisms; possibly involving intracellular structures such as vesiculo-vacuolar organelles (VVOs), and paracellular mechanisms; involving breaching of tightly connected junctions between adjacent ECs1–3. With respect to the latter, it is now well accepted that strict regulation of expression, distribution and function of EC junctional proteins is pivotal for maintaining steady-state stability, integrity and barrier properties of vessel walls. Furthermore, in response to injury or infection, controlled opening/loosening of EC junctions plays a critical role in supporting an effective inflammatory response. This occurs through increased vascular permeability to macromolecules, facilitating leakage of essential blood born immunoregulatory and pro-inflammatory proteins to the extravascular tissue (eg immunoglobulins and components of the complement cascade), and also enabling breaching of venular walls by transmigrating immune cells1–3. Due to their essential role in such biological functions, there is immense interest in the signalling properties of EC junctional molecules under both physiological and pathological conditions1–3. In addition, there is increasing awareness and indeed evidence for altered cell surface expression of EC junctional molecules in inflammation; phenomena that are currently under investigation in terms of their associated mechanisms and biological implications. This review summarises the key findings related to this topic.
Expression and function of EC junctional molecules
The single cell EC layer of blood vessels is held together via complex structures comprising numerous transmembrane proteins that interact both with binding ligands on adjacent cells and with associated intracellular partners1–4. Two key junctional structures are tight junctions that incorporate members of the junctional adhesion molecule (JAM) family, endothelial cell-selective adhesion molecule (ESAM) and claudins, and adherens junctions that include VE-Cadherin1–5. Numerous other adhesion molecules are also present at EC contacts, such as PECAM-1, CD99, CD47, activated leukocyte cell adhesion molecule-1 (ALCAM-1) and ICAM-2, molecules that contribute to junction formation and properties1–6 (Figure 1). A growing body of evidence also indicates the expression of EC junctional molecules in a variety of EC intracellular compartments such as the membranous lateral border recycling compartment (LBRC), endosomes and vesicle-type structures (Figure 1). Recent developments strongly support the concept that intracellular stores of EC junctional molecules contribute to maintaining the integrity and function of the endothelium (discussed below) 4.
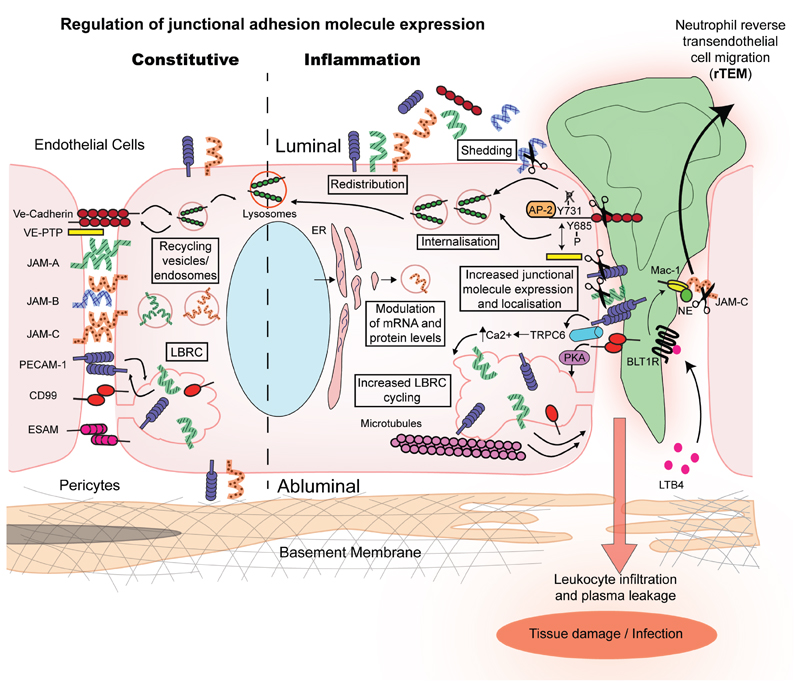
Figure 1. Mechanisms associated with regulation of expression of key endothelial cell junctional adhesion molecules.
Constitutive junctional adhesion molecule regulation involves protein internalisation in endosomes and vesicular structures, their recycling back to the cell surface and/or degradation (e.g. via lysosomes). Certain proteins such as PECAM-1, JAM-A and CD99 can also cycle between the cell membrane and the LBRC under basal conditions. Under inflammatory conditions the expression of adhesion molecules at endothelial cell junctions is regulated through additional mechanisms. Namely, increased gene transcription and mRNA translation leading to up-regulation of total junctional molecule expression at protein level in endothelial cells. JAM-A, JAM-C and PECAM-1 can be redistributed away from cell junctions to non-junctional membranes, whilst internalisation from the plasma membrane of certain molecules such as VE-Cadherin is increased. Cycling of the LBRC increases during inflammation allowing the recruitment of additional PECAM-1 and CD99 molecules to junctional sites. Enzymatic cleavage and shedding from the cell surface reduces the expression of junctional adhesion molecules at endothelial cell junctions. The mechanisms illustrated in the diagram can regulate inflammatory responses such as vascular leakage, and the magnitude and profile of leukocyte TEM. For example with respect to the latter, NE cleavage of JAM-C is known to promote neutrophil rTEM.
The two key principal roles of EC junctions during inflammation are regulation of leukocyte migration out of the vascular lumen and regulation of vascular permeability to macromolecules. With respect to the former, ECs are critical in attracting and facilitating the transmigration of immune cells, both for tissue surveillance and in direct response to sterile and infectious insults. Specifically, at sites of inflammation leukocytes exhibit a number of luminal interactions with ECs, initiating with leukocyte rolling along the endothelium, followed by leukocyte arrest and crawling. These events are mediated by a cascade of intricate molecular and cellular interactions between immune cells and ECs as described by the “leukocyte adhesion cascade”7. During leukocyte crawling, leukocytes engage with EC junctions and begin breaching venular walls2, 6, 7. Migration through the endothelial cell barrier (transendothelial cell migration; TEM) can occur via both transcellular and paracellular modes. Whilst significant use of the transcellular route has been reported across the blood-brain and blood-retinal barriers during inflammatory pathologies8, paracellular diapedesis appears to be the most prevalent mode of breaching ECs both in vitro and in vivo (~70-90%)3, 6. This was directly demonstrated in vivo through the application of high resolution 3D intravital imaging of inflamed mouse cremaster muscle venules where paracellular TEM was found to account for ~90% of all observed neutrophil TEM events as induced by multiple inflammatory stimuli9. Furthermore, a mouse strain in which the EC junctions were stabilised through expression of a VE-Cadherin-α-catenin fusion complex (replacing endogenous VE-Cadherin), exhibited reduced leukocyte (neutrophil and lymphocyte) infiltration in several models of inflammation, providing strong supportive evidence for the involvement of EC junctions in leukocyte trafficking10. Paracellular leukocyte TEM is mediated by an elaborate series of interactions between leukocytes and EC junctional adhesion molecules including PECAM-1, JAMs, CD47, ALCAM-1, ESAM, ICAM-2 and CD99 (for reviews on this topic see references2–4, 6, 11) and there exists some evidence to suggest that EC junctional molecules can also support leukocyte transcellular TEM4, 6. In addition, there is strong evidence to indicate distinct and/or sequential roles for these molecules in different stages of leukocyte movement through venular walls2, 4, 6. However care needs to be exercised in formulating general concepts as roles of different molecules in different stages of leukocyte transmigration appear to be governed to a large extent by the nature of the inflammatory reaction being studied (eg the tissue and genetic background of animals in which it is elicited) and the leukocyte sub-type under observation 3, 4, 6, 7, 11. In addition, EC phenotype, morphology and junctional composition can vary between different vascular beds, differences that may well impact the profile and dynamics of vascular permeability and leukocyte-EC interactions12.
Vascular permeability to plasma proteins, and the subsequent formation of tissue oedema, is another important protective physiological reaction to tissue injury. Whilst it is well-established that neutrophils can mediate vascular permeability13, and numerous neutrophil-derived factors have been implicated in this response13, 14, the precise molecular basis of this reaction remains unclear. Of importance, recent developments in this area have provided compelling evidence for the existence of distinct molecular pathways in induction of vascular permeability and leukocyte TEM1,15, 16. Specifically, the works of Vestweber and colleagues has shed new insights into the mechanism through which VE-Cadherin can mediate these responses 3, 10, 16–18. Briefly, through generation of knock-in mice in which specific tyrosine residues of VE-Cadherin were mutated to phenylalanine, evidence was obtained for the phosphorylation state of VE-Cadherin in maintaining functional EC junctions16. Importantly, this study showed that the phosphorylation status of two distinct tyrosine residues of VE-Cadherin can selectively and exclusively regulate either vascular permeability or leukocyte diapedesis16.
Other junctional molecules involved in regulation of vascular permeability include JAM-A and JAM-C. Despite the high percentage of homology between the two proteins, these molecules appear to have opposing roles in regulating barrier function of ECs. For example, whilst genetic deletion and/or blockade of JAM-A generally results in increased EC permeability19, knocking down JAM-C decreases EC permeability in vitro20. Of note, as opposed to cultured macrovascular ECs (eg HUVECs) that constitutively express JAM-C at cell-cell contacts, quiescent microvascular ECs in culture express JAM-C predominantly within intracellular stores that can be mobilised to junctions following cellular stimulation. The latter appears to provide a mechanism through which endothelial cell permeability is regulated20. However, the role of EC JAM-C in vascular permeability in vivo remains unclear and may depend on the nature of the inflammatory model. Although treatment of wild type mice with soluble recombinant JAM-C (used as a competitive blocker of JAM-C interactions) reduces vascular permeability in response to histamine or VEGF20, specific antibody blockade of endothelial JAM-C leads to increased vascular leakage in a model of cutaneous infection by Leishmania major21. Of note, genetic inactivation of the related tight junction-associated protein ESAM, attenuates the induction of vascular permeability and delays leukocyte diapedesis in vivo22.
Collectively, as there is undisputed evidence for the involvement of EC junctional molecules in regulation of endothelial barrier function both at rest and during tissue injury, better understanding of the mechanisms that regulate remodelling of EC junctions, and the associated biological impact, could shed light on the intricacies of many vascular and inflammatory processes.
Mechanisms regulating the surface expression of EC junctional molecules in inflammation
A number of studies have reported on altered expression of EC junctional molecules in vitro and in vivo, with some studies associating such changes with altered functional read-outs1, 4. At present investigations into regulation of EC junctional molecule expression are at an early stage but to date several diverse mechanisms have been suggested as discussed below (see also Tables 1 and 2).
Table 1.
Key reported changes in expression of EC junctional molecules post inflammation, excluding cleavage-induced changes (listed in Table 2).
|
| |||||
|---|---|---|---|---|---|
| Molecule | Inflammatory stimuli/model | Change in expression | Proposed mechanism | Functional implications | Refs |
|
| |||||
| In vitro | |||||
| PECAM-1 | TNF+IFNγ (HUVECs) | - ↓ mRNA - ↓ total and junctional expression |
- mRNA de-stabilisation - Protein internalisation & degradation - Redistribution to the cell surface |
- ↓ leukocyte TEM - No effect on leukocyte TEM |
43, 72–74 |
| VE-Cadherin | TNF (HUVECs/Primary lung EC) | - ↓ junctional expression - Gap formation “curtain effect” |
- Tyrosine phosphorylation |
- ↑ permeability - Facilitate neutrophil TEM |
16, 33, 38, 39 |
| VEGF | - ↑ internalisation | - Endocytosis through a VEGFR-2-Src-Vav2-Rac-PAK signalling axis | - ↑ permeability | 37 | |
| TNF (Primary lung EC) | - ↓ junctional expression | - Leukocyte induced SHP2 mediated Y731 de-phosphorylation and endocytosis through AP-2 | - ↑ leukocyte TEM | 16 | |
| JAM-A | bFGF | - Redistribution to cell membrane | - Dissociation from integrin αvβ3 | - ↑ angiogenesis | 75 |
| OxLDL (Human and mouse aortic ECs) | - Redistribution to apical membrane | - statin dependent | - ↑ monocyte adhesion and TEM | 45 | |
| JAM-C | OxLDL | - ↑ total protein expression - ↑ expression at non junctional sites |
- None described | - ↑monocyte adhesion & TEM | 47 |
| Thrombin | - Re-localisation to junctions | - None described | - Binding and modulation of β3 integrin activity - ↑ permeability |
76 | |
| VEGF, Histamine | - Re-localisation to junctions | - None described | - Regulates actomyosin contractility - Stabilisation of VE-Cadherin - ↑ permeability |
20 | |
| In vivo | |||||
| JAM-A | Murine model of atherosclerosis | - ↑ mRNA and protein in arterial ECs & atherosclerotic plaques - luminal redistribution |
- Disturbed flow and upregulation of miR-145 | -↑monocyte recruitment into the arterial wall | 67, 69,46 |
| Murine model of hepatic I-R injury | - Protein upregulation | - None described | - Supports neutrophil TEM | 77 | |
| JAM-C | Murine model of atherosclerosis | - ↑ protein in atherosclerotic plaques | - None described | - None described | 47 |
| Human rheumatoid arthritis & osteoarthritis | - ↑ protein in synovial tissue | - None described | - None described | 70 | |
| Murine cremaster muscle I-R injury | - ↓ junctional expression- Redistribution to non junctional membrane | - ROS-mediated loss of JAM-C | - Polarised neutrophil TEM - Regulation of systemic inflammation |
9, 48, 50 | |
| Mice injected i.v. with anti-JAM-C mAb | - Redistribution to non-junctional membrane | - Loss of JAM-C/JAM-B interaction | - ↑ monocyte adhesion to lymph nodes | 78 | |
Table 2.
Cleavage of EC junctional molecules during inflammation
| Molecule | Model | Mechanism | Functional implications | Refs |
|---|---|---|---|---|
| PECAM-1 | Serum starvation (HUVECs, EOMA, BAECs) | Cleavage by caspases & shedding by MMPs | - None described | 54 |
| VE-Cadherin | fMLP stimulated neutrophils (HUVECs) | Cleavage by neutrophil elastase & Cathepsin G | - ↑ permeability - Support neutrophil TEM |
56 |
| Thrombin stimulation (HUVECs) | Cleavage by ADAM-10 | - ↑ permeability - Support T-cell migration |
55 | |
| TNF stimulation (HUVECs) | Cleavage dependent on tyrosine kinases, Src kinase & MMPs | - None described | 30 | |
| JAM-A | PMA, TNFα+ IFNγ, PAF (HUVECs) | Cleavage by ADAM-10, ADAM-17 | - ↓ EC migration - ↓ neutrophil TEM |
57 |
| JAM-B | HBMEC incubation with tumour-cell secreted supernatant | Cleavage by Cathepsin S | - ↑ TEM of brain metastatic tumour cells | 58 |
| JAM-C | LPS, IL1-β, IL-18, MIF, IL-17, TNF, PMA (HUVECs) | Cleavage by ADAM-10, ADAM-17 | - Supports angiogenesis | 65 |
| Murine cremaster muscle I-R and local LTB4 | Cleavage by NE as supported by presentation of NE to JAM-C via neutrophil Mac-1 | - ↑ neutrophil rTEM | 50 | |
Internalisation and redistribution
Internalisation and/or redistribution of molecules to intracellular compartments are commonly reported means through which the expression of a wide range of cell surface presented transmembrane proteins are regulated in many cell types. Such responses are also emerging as important mechanisms in control of EC barrier functions. Several EC junctional proteins, such as PECAM and VE-Cadherin, under-go some form of intracellular recycling or degradation3, 4. In addition, many others such as JAM-C, JAM-A and CD99 are also present in intracellular stores and are therefore susceptible to mobilisation to and from EC junctions post stimulation (Figure 1)4. In this context, ECs show a unique array of intracellular compartments that are associated with junctional molecule internalisation, storage and re-cycling. These include the membrane invagination structure termed LBRC (commonly found in close proximity of EC junctional lateral borders), components of the vesicular system, VVO’s and caveolae, and endosomes which are additionally associated with delivery of proteins to lysosomes for destruction. Current evidence suggests that the nature of the carrier system is relatively specific to the cargo molecule, with some molecules showing intracellular re-cycling both at rest and also during acute inflammation, as discussed below.
The LBRC represents an internalisation organelle, apparently unique to ECs and containing key junctional molecules including PECAM-1, JAM-A, CD99 and the poliovirus receptor (PVR; CD155), but not VE-Cadherin, which is actively excluded from this domain 4, 23, 24. This compartment is believed to provide a means through which adhesion molecules and additional membrane can be efficiently recruited to sites of leukocyte diapedesis on demand4. In support of this concept, changes in the intracellular pool of PECAM-1 have been located to sites where the LBRC is re-directed towards the cell border to support paracellular migration25, or towards the cell body during transcellular migration26. Whilst there is now ample evidence to support a role for LBRC in regulation of leukocyte TEM, there remain many un-answered question regarding the trafficking of this structure, an area that has been developed in recent studies by Muller and colleagues4. For example, vesicles of the LBRC compartment are moved by kinesin molecular motors along microtubules 4, 23 25. Furthermore, whilst it has been known for more than 20 years that a transient increase in EC cytosolic free calcium is required for TEM, Weber et al have recently demonstrated that the Ca2+ channel, transient receptor potential canonical 6 (TRPC6), mediates this response and have linked this reaction with LBRC trafficking27. Specifically, homophilic interaction of leukocyte and EC PECAM triggered the activation of TRPC6 and promoted its co-localization with PECAM-1 during TEM and trafficking of the LBRC. Of note, selective inactivation of EC TRPC6 blocked neutrophil TEM whilst its activation rescued TEM during conditions of PECAM blockade, suggesting that TRPC6 acts down-stream of PECAM-1 ligation. The importance of this pathway in regulation of leukocyte transmigration was also demonstrated in vivo where chimeric mice deficient in EC TRPC6 exhibited defective neutrophil TEM in a model of acute inflammation27. Down-stream of the pathway triggered by PECAM engagement, trafficking of the LBRC is also regulated by EC CD99 through activation of PKA, a mechanism involving ezrin and soluble adenylyl cyclase28. Collectively such studies have shed much light on the mechanism through which LBRC is recruited to sites of leukocyte TEM and have strengthened the evidence for the functional importance of this trafficking towards and away from EC junctions4.
Changes in VE-Cadherin junctional expression can also have profound impact on EC barrier function5. VE-Cadherin displays constitutive endosomal internalisation from the EC surface via a clathrin-dependent pathway29 and can then be lysosomally degraded30 or recycled back to the EC surface through Rab11a mediated-trafficking in order to recover EC barrier properties after junctional challenge31. VE-Cadherin maintenance at EC junctions is regulated by different cytoplasmic binding partners of this molecule such as p120-catenin and Src kinase. Briefly, p120 dissociation from VE-Cadherin intracellular tail regulates cadherin levels preventing its endocytosis and degradation29, 30. In addition, more recent works identified a specific motif of VE-Cadherin intracellular tail that is required for p120 binding and VE-Cadherin internalisation. Mutations in this region strongly affect EC migration, although the impact of such effects on EC barrier function remains to be elucidated32. On the other hand, changes in VE-Cadherin phosphorylation status destabilise EC contacts, a response that supports vascular leakage and leukocyte migration3, 5, 16, 33, 34. VE-Cadherin phosphorylation can be regulated by p12033 and the kinases Src and Pyk2 in response to ICAM-1 ligation34. This is necessary for leukocyte TEM but does not imply internalisation of the molecule and so will not be discussed further. Phosphorylation of residues Y658 and Y685 have however been shown to be responsible for internalisation and ubiquitinisation of the protein following bradykinin and histamine-induced permeability35. This phenomenon occurs specifically in veins and not arteries, possibly due to the shear-stress dependent activation of junctional Src in the former, and regulates loosening of venular EC junctions and leakage35. More recently, heterotrimeric G protein Gα13 binding to VE-Cadherin has been shown to regulate Src-mediated phosphorylation of VE-Cadherin and its internalisation, identifying a unique role for Gα13 in mediating EC barrier disruption in vivo in response to vascular permeability factors such as histamine and bradykinin36. Internalisation of the molecule can also be regulated by phosphorylation of S665 through the VEGFR-2–Src–Vav2–Rac–PAK signalling axis following VEGF stimulation37. This promotes VE-Cadherin association with β-arrestin2 and its internalisation through clathrin-coated vesicles.
As well as responding to inflammatory mediators, VE-Cadherin is also transiently displaced from EC junctions by transmigrating leukocytes, a reaction described as the “curtain effect” since it rapidly reseals behind emigrating cells38, 39. In addition, VE-Cadherin can be internalized in response to leukocyte-EC interactions16. New findings have identified the molecular basis of this phenomenon by demonstrating how leukocyte-EC interactions trigger SHP2 mediated dephosphorylation of Y731 and further endocytosis of VE-Cadherin through binding of adaptin AP-216. Although Y731 dephosphorylation appeared essential for leukocyte TEM in vivo, it was not necessary for inflammation-induced vascular permeability. Conversely, phosphorylation of Y685 was required for junction destabilization but was dispensable for leukocyte diapedesis, indicating the intricacies of regulating VE-Cadherin phosphorylation and its functional implications16. Under resting conditions VE-Cadherin binds to VE-PTP, a phosphatase that maintains VE-Cadherin and its associated catenins in a non-phosphorylated state critical for optimal adhesive functions of VE-Cadherin and EC contact integrity. Following engagement of leukocytes with ECs or stimulation with VEGF, VE-PTP dissociates from VE-Cadherin, promoting its phosphorylation, subsequent loss of VE-Cadherin interactions and hence loosening of EC junctions18. Of note, VE-PTP has also recently been described to regulate EC junctional stability by a VE-Cadherin independent mechanism involving its interaction with Tie-2. Briefly, VE-PTP-Tie 2 interaction can dampen the tyrosine kinase activity of this receptor and hence its ability to stabilize EC junctions. Pharmacological or genetic ablation of VE-PTP leads to increased EC junctional stability in vivo via Tie-2, counteracting vascular leakage and leukocyte transmigration induced by inflammatory mediators. Thus, activation of Tie-2 via inhibition of VE-PTP protects endothelial junctions against inflammation-induced destabilization and overrides the negative effect of VE-PTP inhibition on the adhesive function of VE-cadherin40. VE-Cadherin association with one or another partner is reversible and can be spatially and temporally regulated. Of note, the actin-binding protein EPS8 has recently been identified as a binding partner of VE-Cadherin. EPS8 promotes VE-Cadherin ubiquitination and phosphorylation, leading to increased internalisation and enhanced cell surface turnover of the molecule41. This interaction mediates transduction of signals impinging on the regulation of the transcriptional cofactor Yes-associated protein (YAP) and as a result modulates vascular permeability41.
Similar to internalisation, redistribution of adhesion molecules away from EC junctions and onto the cell body may represent a means through which an inflammatory reaction is regulated. Such a response may facilitate the development of an inflammatory event through promotion of leukocyte adhesion to the EC surface (via increased expression of adhesion molecules on the EC apical membrane) or its termination through inhibiting TEM (via reduced expression of molecules at junctions between adjacent cells). Redistribution of EC junctional molecules has been reported within in vitro and in vivo models of acute inflammation with respect to several proteins (Figure 1 and Table 1). For example, expressions of PECAM-1 and JAM-A are reduced from junctions of HUVECs treated with the cytokine combination IFNγ and TNF with no apparent reduction in total cellular protein levels42, 43. A similar phenomenon was noted for JAM-A in brain ECs stimulated with CCL2 and LPS, a response that was associated with increased adhesion of monocytes and neutrophils44. This occurred via internalization of the molecule by macropinocytosis and its transient storage in recycling endosomes before being recruited back to the apical side of ECs. These findings indicate that redistribution may be supported by internalization pathways. JAM-A redistribution to the apical membrane of aortic ECs also occurs in response to pro-atherogenic oxidized lipoproteins in vitro44, 45 and in vivo in murine models of atherosclerosis in regions of disturbed flow46. The latter response was associated with increased monocyte recruitment into the arterial wall and enhanced atherosclerotic lesion formation46.
A significant body of work has investigated the regulation of expression of JAM-C and its functional implications. In vitro, HUVECs treated with oxidised LDL, but not TNF, IL1β, VEGF or histamine, showed redistribution of JAM-C from junctions to the cell surface20, 47. This redistribution resulted in the ability of JAM-C to mediate both leukocyte adhesion and TEM as compared to JAM-C on unstimulated ECs that only supported leukocyte diapedesis20, 47. As previously mentioned, in quiescent microvascular ECs JAM-C is mainly intracellularly expressed and is recruited to junctions following short-term stimulation with stimuli such as VEGF or histamine. This induced expression of JAM-C at EC junctions was shown to support vascular permeability, a response mediated through modulation of actomyosin-based endothelial contractility and regulation of VE-Cadherin–mediated cell–cell contacts in a Rap1-dependent manner20. In contrast to in vitro studies, in vivo JAM-C is expressed at EC junctions, as indicated through analysis of numerous murine tissues48. However in line with in vitro works, there is evidence for the presence of JAM-C in intracellular vesicles within microvascular ECs in vivo48. This intracellular store of JAM-C appeared to be available for mobilisation under inflammatory conditions in that re-distribution of JAM-C from intracellular vesicles and EC junctions to EC non-junctional plasma membrane regions was noted in a murine model of ischemia-reperfusion injury. The in vivo redistribution of EC JAM-C was associated with enhanced luminal neutrophil-venular wall interactions9, 48. As well as mediating leukocyte adhesion and diapedesis, there is also evidence from both in vitro and in vivo works for the ability of EC JAM-C to mediate polarised migration of leukocytes through endothelial cell monolayers9, 49. Specifically, Bradfield and colleagues showed that inhibition of EC JAM-C can lead to enhanced monocyte reverse TEM (rTEM) through TNF-stimulated HUVECs, ie increased frequency of monocyte movement in an abluminal-to-luminal direction49. In vivo, our studies provided the first direct evidence for the occurrence of neutrophil rTEM in a mammalian model (inflamed mouse cremaster muscle), a phenomenon that was significantly enhanced under conditions of reduced EC junctional expression or functionality of JAM-C. This was achieved through the use of EC JAM-C deficient mice, antibody blockade of JAM-C or following induction of inflammatory reactions, such as ischemia-reperfusion injury, that cause reduced expression of junctional EC JAM-C9, 50. The underlying mechanism through which EC JAM-C supports luminal-to-abluminal TEM is at present unclear but maybe related to the role of JAM-C in maintaining EC polarity51. Although the pathophysiological relevance of neutrophil rTEM requires further investigations, our current data suggests that rTEM neutrophils stemming from a primary site of injury may contribute to dissemination of systemic inflammation and second organ damage9 50.
Overall it is becoming increasingly clear that altered surface localization and/or expression of EC junctional molecules, as mediated via multiple different modes, can lead to altered functional properties of molecules with respect to both leukocyte trafficking and regulation of vascular permeability to macromolecules.
Enzymatic cleavage and shedding from the cell surface
A number of EC junctional molecules have been reported to be enzymatically cleaved by leukocyte and/or EC derived proteases. Although such events have been implicated to several vascular responses, including permeability, immune cell recruitment, vascular repair and angiogenesis (Table 2)52, 53, this aspect of the field requires further exploration and critical assessment. Of importance, caution is required when linking the shedding of a certain cell surface protein to a specific functional read-out(s) as commonly the study cannot rule out the possibility that the observed effects was mediated via the shedding of other cell surface proteins that were not analysed. Thus, much of the studies cited below are correlations that do not necessarily link a defined shedding phenomenon with the reported biological observation.
To date, three families of proteases have been associated with such responses, namely ADAMs, MMPs and serine proteases. For example, PECAM-1 is shed by MMPs from the cell surface during EC apoptosis54 and VE-Cadherin is reportedly cleaved by ADAM1055, neutrophil elastase (NE) and Cathepsin G56. Although the latter studies have associated enzymatic cleavage of VE-Cadherin with its role as a regulator of vascular permeability and leukocyte TEM,additional investigations are needed here
Numerous studies have investigated enzymatic cleavage of members of the JAM family. Specifically, JAM-A can be cleaved by ADAM17, and to a lesser extent, by ADAM10 post stimulation of ECs by certain inflammatory stimuli57. Functionally, soluble JAM-A blocked migration of cultured ECs and reduced neutrophil TEM in vitro and decreased neutrophil infiltration in a murine air pouch model in vivo57. Hence, generation of soluble JAM-A, as mediated through ADAM17/10, may regulate JAM-A-mediated functions through destabilisation of JAM-A homophilic interactions at sites of inflammation. More recently, Sevenich and colleagues proposed that Cathepsin S-mediated cleavage of endothelial JAM-B promotes transmigration of metastatic cells across brain microvascular ECs58. In addition, genetic or pharmacological targeting of Cathepsin B impaired brain metastasis in a model of breast cancer, suggesting proteolytic processing of JAM-B at the blood-brain barrier can modulate site-specific metastasis58, although the cleavage of endothelial JAM-B at specific sites of tumour cell TEM was not addressed. Findings from our laboratory have demonstrated that NE can cleave JAM-C50. In line with our previous works showing that loss of EC JAM-C can promote neutrophil reverse TEM (rTEM)9, NE cleavage of EC JAM-C promoted neutrophil rTEM50. Under conditions of ischemia-reperfusion injury, this response was driven by endogenously generated LTB4 and exogenous LTB4 was highly efficacious at causing specific loss of venular JAM-C without affecting the expression of other EC junctional molecules50. The impact of LTB4-NE axis on JAM-C cleavage was totally neutrophil dependent, with NE governing the cleavage of EC JAM-C at sites of intense neutrophil infiltration50. Collectively, our findings demonstrated that NE is presented to EC JAM-C via activated neutrophil Mac-1, and since the latter is a ligand for JAM-C59, Mac-1 appears to act as a molecular “bridge” between NE and JAM-C (Figure 1). Finally, since the activation of local LTB4-NE axis could drive remote organ damage, the findings of this study provided additional evidence for the involvement of neutrophil rTEM in propagation of a local sterile inflammatory response towards a systemic multi-organ phenomena50.
Following enzymatic cleavage, junctional molecule ectodomains can be shed into the bloodstream. A number of such soluble forms have been quantified in plasma of patients with inflammatory conditions including trauma, atherosclerosis and rheumatoid arthritis, with the levels commonly correlating with the severity of the disease50,60–63. In our studies elevated concentrations of sJAM-C were detected in plasma of trauma patients as compared to healthy controls, a parameter that further increased in patients that developed acute respiratory distress syndrome (ARDS) post-admission50. As increased plasma content of sJAM-C is associated with trauma-induced organ failure 50, and is also elevated in serum or synovial fluid from rheumatoid arthritis, psoriatic arthritis, osteoarthritis and systemic sclerosis patients64, 65, sJAM-C may be a useful vascular-derived biomarker for assessing the extent of a systemic inflammatory response.
Apart from their potential role as biomarkers, relatively little is known about the biological consequences of released soluble ectodomains in patho-physiological scenarios, and there is evidence for both pro- and anti-inflammatory roles. Of note, it has been reported that generation of sVE-Cadherin contributes to inflammation-induced breakdown of endothelial barrier function through inhibition of VE-Cadherin binding66 and as such promotes leukocyte TEM via increased vascular permeability55. In contrast, exogenous administration of soluble forms of PECAM-1, JAM-A and JAM-C suppress leukocyte transmigration in several rodent models of inflammation27, 48, 57, 67. The mechanism through which these pharmacological interventions act is at present unclear but is likely due to competitive binding of the soluble molecules with their ligands on either ECs or circulating leukocytes.
Impact of acute vs chronic inflammatory insults on EC junctional molecule expression and function
The mechanisms associated with altered expression of EC junctional adhesion molecules (discussed above) may differ between acute and chronic inflammatory scenarios. Numerous in vitro and in vivo studies have investigated the impact of short-term acute inflammatory insults on expression of EC regulatory molecules. In such scenarios, the expression of molecules on the apical side of ECs that facilitate leukocyte adhesion to the endothelium, such as E-selectin, P-selectin, ICAM-1 and VCAM-1, is generally elevated. This supports increased luminal leukocyte-EC interactions (eg rolling, crawling and/or firm adhesion) and overall capture of leukocytes from the blood stream7,68. Conversely, the expression of EC adhesion molecules at cell-cell junctions is commonly reduced under such conditions (Tables 1 and 2), potentially leading to decreased barrier properties of EC junctions. One manner in which this occurs is re-distribution of adhesion molecules away from the junctions to the luminal side of the venule, a response that may provide a means through which leukocytes are guided to EC junctions in a haptotactic manner. Although the highly regulated changes in junctional molecule expression are necessary for the appropriate development of the acute inflammatory response, and may indeed play a role in its outcome, evidence suggests that they can also have pathogenic implications, as discussed above for JAM-C and its role in modulating rTEM and second organ damage.
As opposed to acute inflammatory responses, chronic inflammatory states allow time for further molecular pathways to become activated, such as up-regulation of junctional protein expression at transcriptional and translational level47, 67, 69, 70. This can result in increased junctional protein expression levels, compared to resting states, as noted for example in the context of EC JAM-C in atherosclerosis47, rheumatoid arthritis and osteoarthritis70 and EC JAM-A in atherosclerotic vessels46, 67, 69. In line with this, increased concentrations of soluble junctional molecules have been found in plasma from a number of chronic inflammatory pathologies such as stroke-induced ischemia62, rheumatoid arthritis61, 65, atherosclerosis and hypertension60, 71. Enhanced expression of EC junctional molecules may account for increased or prolonged recruitment of leukocytes and/or their retention during chronic inflammation.
Conclusion
The endothelial cell barrier allows regulated and selective passage of appropriate solutes and immune cells during resting and inflammatory conditions. This vital function is mediated by interactions between ECs through junctional molecules such as VE-Cadherin, JAMs and PECAM-1. Remodelling of the EC membrane during inflammation includes reorganisation of junctional molecules, a response that is pivotal for regulation of vascular permeability and leukocyte extravasation. Changes in expression levels of junctional molecules can also be temporally and spatially regulated by inflammatory mediators and leukocyte TEM. These mechanisms include cell surface redistribution and internalisation of key cell border structures, the recycling of intracellular pools of molecules and their enzymatic cleavage. Such changes may also have a role in orchestrating the inflammatory response under chronic conditions and impacting its resolution. Collectively, better understanding of the molecular mechanisms that mediate the spatiotemporal expression and trafficking of EC junctional molecules could identify novel means of targeting both acute and chronic inflammatory pathologies.
Acknowledgments
None
Sources of Funding
The authors are funded by the Wellcome Trust (Investigator Award to SN Ref:098291/Z/12/Z). NR is additionally supported by funding from the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme (FP7/2007-2013) under REA grant agreement no [608765].
Abbreviations
- LBRC
Lateral Border Recycling Compartment
- ER
endoplasmic Reticulum
- NE
Neutrophil Elastase
- HUVECs
Human Umbilical Vein Endothelial Cells
- BAECs
Bovine Aortic Endothelial Cells
- HBMEC
Human Brain Microvascular Endothelial Cells
- EOMA
Mouse Hemangioendothelioma Endothelial Cells
- bFGF
Basic Fibroblast Growth Factor
- OxLDL
Oxidised Low Density Lipoprotein
- i.v.
intravenously
- mAb
monoclonal antibody
Footnotes
Disclosure
None
References
- 1.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: Molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: Leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 3.Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol. 2015;15:692–704. doi: 10.1038/nri3908. [DOI] [PubMed] [Google Scholar]
- 4.Muller WA. Localized signals that regulate transendothelial migration. Current opinion in immunology. 2015;38:24–29. doi: 10.1016/j.coi.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannotta M, Trani M, Dejana E. Ve-Cadherin and endothelial adherens junctions: Active guardians of vascular integrity. Dev Cell. 2013;26:441–454. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41:694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 8.Engelhardt B, Ransohoff RM. Capture, crawl, cross: The T Cell code to breach the blood-brain barriers. Trends in immunology. 2012;33:579–589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, Meda P, et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nature immunology. 2011;12:761–769. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulte D, Kuppers V, Dartsch N, Broermann A, Li H, Zarbock A, Kamenyeva O, Kiefer F, Khandoga A, Massberg S, Vestweber D. Stabilizing the Ve-Cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J. 2011;30:4157–4170. doi: 10.1038/emboj.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerhardt T, Ley K. Monocyte trafficking across the vessel wall. Cardiovascular research. 2015;107:321–330. doi: 10.1093/cvr/cvv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hickey MJ, Westhorpe CL. Imaging inflammatory leukocyte recruitment in kidney, lung and liver-3challenges to the multi-step paradigm. Immunology and cell biology. 2013;91:281–289. doi: 10.1038/icb.2012.83. [DOI] [PubMed] [Google Scholar]
- 13.DiStasi MR, Ley K. Opening the flood-gates: How neutrophil-endothelial interactions regulate permeability. Trends in immunology. 2009;30:547–556. doi: 10.1016/j.it.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finsterbusch M, Voisin MB, Beyrau M, Williams TJ, Nourshargh S. Neutrophils recruited by chemoattractants in vivo induce microvascular plasma protein leakage through secretion of TNF. J Exp Med. 2014;211:1307–1314. doi: 10.1084/jem.20132413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnoor M, Lai FP, Zarbock A, Klaver R, Polaschegg C, Schulte D, Weich HA, Oelkers JM, Rottner K, Vestweber D. Cortactin deficiency is associated with reduced neutrophil recruitment but increased vascular permeability in vivo. J Exp Med. 2011;208:1721–1735. doi: 10.1084/jem.20101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wessel F, Winderlich M, Holm M, Frye M, Rivera-Galdos R, Vockel M, Linnepe R, Ipe U, Stadtmann A, Zarbock A, Nottebaum AF, et al. Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of Ve-Cadherin. Nature immunology. 2014;15:223–230. doi: 10.1038/ni.2824. [DOI] [PubMed] [Google Scholar]
- 17.Vestweber D. Relevance of endothelial junctions in leukocyte extravasation and vascular permeability. Ann N Y Acad Sci. 2012;1257:184–192. doi: 10.1111/j.1749-6632.2012.06558.x. [DOI] [PubMed] [Google Scholar]
- 18.Broermann A, Winderlich M, Block H, Frye M, Rossaint J, Zarbock A, Cagna G, Linnepe R, Schulte D, Nottebaum AF, Vestweber D. Dissociation of VE-PTP from Ve-Cadherin is required for leukocyte extravasation and for vegf-induced vascular permeability in vivo. J Exp Med. 2011;208:2393–2401. doi: 10.1084/jem.20110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luissint AC, Nusrat A, Parkos CA. JAM-related proteins in mucosal homeostasis and inflammation. Seminars in immunopathology. 2014;36:211–226. doi: 10.1007/s00281-014-0421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orlova VV, Economopoulou M, Lupu F, Santoso S, Chavakis T. Junctional adhesion molecule-c regulates vascular endothelial permeability by modulating Ve-Cadherin-mediated cell-cell contacts. J Exp Med. 2006;203:2703–2714. doi: 10.1084/jem.20051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballet R, Emre Y, Jemelin S, Charmoy M, Tacchini-Cottier F, Imhof BA. Blocking junctional adhesion molecule c enhances dendritic cell migration and boosts the immune responses against leishmania major. PLoS pathogens. 2014;10:e1004550. doi: 10.1371/journal.ppat.1004550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wegmann F, Petri B, Khandoga AG, Moser C, Khandoga A, Volkery S, Li H, Nasdala I, Brandau O, Fassler R, Butz S, et al. Esam supports neutrophil extravasation, activation of rho, and vegf-induced vascular permeability. J Exp Med. 2006;203:1671–1677. doi: 10.1084/jem.20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 2003;421:748–753. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 24.Feng G, Sullivan DP, Han F, Muller WA. Segregation of Ve-Cadherin from the lbrc depends on the ectodomain sequence required for homophilic adhesion. Journal of cell science. 2015;128:576–588. doi: 10.1242/jcs.159053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mamdouh Z, Kreitzer GE, Muller WA. Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. J Exp Med. 2008;205:951–966. doi: 10.1084/jem.20072328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mamdouh Z, Mikhailov A, Muller WA. Transcellular migration of leukocytes is mediated by the endothelial lateral border recycling compartment. J Exp Med. 2009;206:2795–2808. doi: 10.1084/jem.20082745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber EW, Han F, Tauseef M, Birnbaumer L, Mehta D, Muller WA. Trpc6 is the endothelial calcium channel that regulates leukocyte transendothelial migration during the inflammatory response. J Exp Med. 2015;212:1883–1899. doi: 10.1084/jem.20150353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson RL, Buck J, Levin LR, Winger RC, Wang J, Arase H, Muller WA. Endothelial cd99 signals through soluble adenylyl cyclase and pka to regulate leukocyte transendothelial migration. J Exp Med. 2015;212:1021–1041. doi: 10.1084/jem.20150354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao K, Garner J, Buckley KM, Vincent PA, Chiasson CM, Dejana E, Faundez V, Kowalczyk AP. P120-catenin regulates clathrin-dependent endocytosis of Ve-Cadherin. Mol Biol Cell. 2005;16:5141–5151. doi: 10.1091/mbc.E05-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol. 2003;163:535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan Z, Wang ZG, Segev N, Hu S, Minshall RD, Dull RO, Zhang M, Malik AB, Hu G. Rab11a mediates vascular endothelial-cadherin recycling and controls endothelial barrier function. Arterioscler Thromb Vasc Biol. 2016;36:339–349. doi: 10.1161/ATVBAHA.115.306549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nanes BA, Chiasson-MacKenzie C, Lowery AM, Ishiyama N, Faundez V, Ikura M, Vincent PA, Kowalczyk AP. P120-catenin binding masks an endocytic signal conserved in classical cadherins. J Cell Biol. 2012;199:365–380. doi: 10.1083/jcb.201205029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alcaide P, Newton G, Auerbach S, Sehrawat S, Mayadas TN, Golan DE, Yacono P, Vincent P, Kowalczyk A, Luscinskas FW. P120-catenin regulates leukocyte transmigration through an effect on Ve-Cadherin phosphorylation. Blood. 2008;112:2770–2779. doi: 10.1182/blood-2008-03-147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, src- and pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 35.Orsenigo F, Giampietro C, Ferrari A, et al. Phosphorylation of Ve-Cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun. 2012;3:1208. doi: 10.1038/ncomms2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong H, Gao X, Feng S, Siddiqui MR, Garcia A, Bonini MG, Komarova Y, Vogel SM, Mehta D, Malik AB. Evidence of a common mechanism of disassembly of adherens junctions through galpha13 targeting of Ve-Cadherin. J Exp Med. 2014;211:579–591. doi: 10.1084/jem.20131190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gavard J, Gutkind JS. Vegf controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of Ve-Cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 38.Allport JR, Muller WA, Luscinskas FW. Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J Cell Biol. 2000;148:203–216. doi: 10.1083/jcb.148.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001;167:2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 40.Frye M, Dierkes M, Kuppers V, Vockel M, Tomm J, Zeuschner D, Rossaint J, Zarbock A, Koh GY, Peters K, Nottebaum AF, et al. Interfering with VE-PTP stabilizes endothelial junctions in vivo via tie-2 in the absence of Ve-Cadherin. J Exp Med. 2015;212:2267–2287. doi: 10.1084/jem.20150718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giampietro C, Disanza A, Bravi L, Barrios-Rodiles M, Corada M, Frittoli E, Savorani C, Lampugnani MG, Boggetti B, Niessen C, Wrana JL, et al. The actin-binding protein eps8 binds Ve-Cadherin and modulates yap localization and signaling. J Cell Biol. 2015;211:1177–1192. doi: 10.1083/jcb.201501089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romer LH, McLean NV, Yan HC, Daise M, Sun J, DeLisser HM. IFN-gamma and TNF-alpha induce redistribution of PECAM-1 (cd31) on human endothelial cells. J Immunol. 1995;154:6582–6592. [PubMed] [Google Scholar]
- 43.Ozaki H, Ishii K, Horiuchi H, Arai H, Kawamoto T, Okawa K, Iwamatsu A, Kita T. Cutting edge: Combined treatment of TNF-alpha and IFN-gamma causes redistribution of junctional adhesion molecule in human endothelial cells. J Immunol. 1999;163:553–557. [PubMed] [Google Scholar]
- 44.Stamatovic SM, Sladojevic N, Keep RF, Andjelkovic AV. Relocalization of junctional adhesion molecule a during inflammatory stimulation of brain endothelial cells. Molecular and cellular biology. 2012;32:3414–3427. doi: 10.1128/MCB.06678-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitt MM, Fraemohs L, Hackeng TM, Weber C, Koenen RR. Atherogenic mononuclear cell recruitment is facilitated by oxidized lipoprotein-induced endothelial junctional adhesion molecule-a redistribution. Atherosclerosis. 2014;234:254–264. doi: 10.1016/j.atherosclerosis.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Schmitt MM, Megens RT, Zernecke A, Bidzhekov K, van den Akker NM, Rademakers T, van Zandvoort MA, Hackeng TM, Koenen RR, Weber C. Endothelial junctional adhesion molecule-a guides monocytes into flow-dependent predilection sites of atherosclerosis. Circulation. 2014;129:66–76. doi: 10.1161/CIRCULATIONAHA.113.004149. [DOI] [PubMed] [Google Scholar]
- 47.Keiper T, Al-Fakhri N, Chavakis E, Athanasopoulos AN, Isermann B, Herzog S, Saffrich R, Hersemeyer K, Bohle RM, Haendeler J, Preissner KT, et al. The role of junctional adhesion molecule-c (JAM-C) in oxidized ldl-mediated leukocyte recruitment. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:2078–2080. doi: 10.1096/fj.05-4196fje. [DOI] [PubMed] [Google Scholar]
- 48.Scheiermann C, Colom B, Meda P, Patel NS, Voisin MB, Marrelli A, Woodfin A, Pitzalis C, Thiemermann C, Aurrand-Lions M, Imhof BA, et al. Junctional adhesion molecule-c mediates leukocyte infiltration in response to ischemia reperfusion injury. Arterioscler Thromb Vasc Biol. 2009;29:1509–1515. doi: 10.1161/ATVBAHA.109.187559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bradfield PF, Scheiermann C, Nourshargh S, Ody C, Luscinskas FW, Rainger GE, Nash GB, Miljkovic-Licina M, Aurrand-Lions M, Imhof BA. JAM-C regulates unidirectional monocyte transendothelial migration in inflammation. Blood. 2007;110:2545–2555. doi: 10.1182/blood-2007-03-078733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colom B, Bodkin JV, Beyrau M, Woodfin A, Ody C, Rourke C, Chavakis T, Brohi K, Imhof BA, Nourshargh S. Leukotriene b4-neutrophil elastase axis drives neutrophil reverse transendothelial cell migration in vivo. Immunity. 2015;42:1075–1086. doi: 10.1016/j.immuni.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ebnet K, Aurrand-Lions M, Kuhn A, Kiefer F, Butz S, Zander K, Meyer zu Brickwedde MK, Suzuki A, Imhof BA, Vestweber D. The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein par-3: A possible role for JAMs in endothelial cell polarity. Journal of cell science. 2003;116:3879–3891. doi: 10.1242/jcs.00704. [DOI] [PubMed] [Google Scholar]
- 52.Dreymueller D, Pruessmeyer J, Groth E, Ludwig A. The role of adam-mediated shedding in vascular biology. Eur J Cell Biol. 2012;91:472–485. doi: 10.1016/j.ejcb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Garton KJ, Gough PJ, Raines EW. Emerging roles for ectodomain shedding in the regulation of inflammatory responses. J Leukoc Biol. 2006;79:1105–1116. doi: 10.1189/jlb.0106038. [DOI] [PubMed] [Google Scholar]
- 54.Ilan N, Mohsenin A, Cheung L, Madri JA. PECAM-1 shedding during apoptosis generates a membrane-anchored truncated molecule with unique signaling characteristics. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:362–372. doi: 10.1096/fj.00-0372com. [DOI] [PubMed] [Google Scholar]
- 55.Schulz B, Pruessmeyer J, Maretzky T, Ludwig A, Blobel CP, Saftig P, Reiss K. Adam10 regulates endothelial permeability and t-cell transmigration by proteolysis of vascular endothelial cadherin. Circ Res. 2008;102:1192–1201. doi: 10.1161/CIRCRESAHA.107.169805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hermant B, Bibert S, Concord E, Dublet B, Weidenhaupt M, Vernet T, Gulino-Debrac D. Identification of proteases involved in the proteolysis of vascular endothelium cadherin during neutrophil transmigration. J Biol Chem. 2003;278:14002–14012. doi: 10.1074/jbc.M300351200. [DOI] [PubMed] [Google Scholar]
- 57.Koenen RR, Pruessmeyer J, Soehnlein O, Fraemohs L, Zernecke A, Schwarz N, Reiss K, Sarabi A, Lindbom L, Hackeng TM, Weber C, et al. Regulated release and functional modulation of junctional adhesion molecule a by disintegrin metalloproteinases. Blood. 2009;113:4799–4809. doi: 10.1182/blood-2008-04-152330. [DOI] [PubMed] [Google Scholar]
- 58.Sevenich L, Bowman RL, Mason SD, Quail DF, Rapaport F, Elie BT, Brogi E, Brastianos PK, Hahn WC, Holsinger LJ, Massague J, et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin s. Nat Cell Biol. 2014;16:876–888. doi: 10.1038/ncb3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santoso S, Sachs UJ, Kroll H, Linder M, Ruf A, Preissner KT, Chavakis T. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin mac-1. J Exp Med. 2002;196:679–691. doi: 10.1084/jem.20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cavusoglu E, Kornecki E, Sobocka MB, Babinska A, Ehrlich YH, Chopra V, Yanamadala S, Ruwende C, Salifu MO, Clark LT, Eng C, et al. Association of plasma levels of f11 receptor/junctional adhesion molecule-a (f11r/JAM-A) with human atherosclerosis. J Am Coll Cardiol. 2007;50:1768–1776. doi: 10.1016/j.jacc.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 61.Sidibe A, Mannic T, Arboleas M, Subileau M, Gulino-Debrac D, Bouillet L, Jan M, Vandhuick T, Le Loet X, Vittecoq O, Vilgrain I. Soluble Ve-Cadherin in rheumatoid arthritis patients correlates with disease activity: Evidence for tumor necrosis factor alpha-induced Ve-Cadherin cleavage. Arthritis Rheum. 2012;64:77–87. doi: 10.1002/art.33336. [DOI] [PubMed] [Google Scholar]
- 62.Zaremba J, Losy J. sPECAM-1 in serum and csf of acute ischaemic stroke patients. Acta Neurol Scand. 2002;106:292–298. doi: 10.1034/j.1600-0404.2002.01339.x. [DOI] [PubMed] [Google Scholar]
- 63.Zhang RY, Liu YY, Li L, Cui W, Zhao KJ, Huang WC, Gu XW, Liu W, Wu J, Min D, Mao EQ, et al. Increased levels of soluble vascular endothelial cadherin are associated with poor outcome in severe sepsis. J Int Med Res. 2010;38:1497–1506. doi: 10.1177/147323001003800433. [DOI] [PubMed] [Google Scholar]
- 64.Manetti M, Guiducci S, Romano E, Rosa I, Ceccarelli C, Mello T, Milia AF, Conforti ML, Ibba-Manneschi L, Matucci-Cerinic M. Differential expression of junctional adhesion molecules in different stages of systemic sclerosis. Arthritis Rheum. 2013;65:247–257. doi: 10.1002/art.37712. [DOI] [PubMed] [Google Scholar]
- 65.Rabquer BJ, Amin MA, Teegala N, Shaheen MK, Tsou PS, Ruth JH, Lesch CA, Imhof BA, Koch AE. Junctional adhesion molecule-c is a soluble mediator of angiogenesis. J Immunol. 2010;185:1777–1785. doi: 10.4049/jimmunol.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flemming S, Burkard N, Renschler M, Vielmuth F, Meir M, Schick MA, Wunder C, Germer CT, Spindler V, Waschke J, Schlegel N. Soluble Ve-Cadherin is involved in endothelial barrier breakdown in systemic inflammation and sepsis. Cardiovascular research. 2015;107:32–44. doi: 10.1093/cvr/cvv144. [DOI] [PubMed] [Google Scholar]
- 67.Ostermann G, Fraemohs L, Baltus T, Schober A, Lietz M, Zernecke A, Liehn EA, Weber C. Involvement of JAM-A in mononuclear cell recruitment on inflamed or atherosclerotic endothelium: Inhibition by soluble JAM-A. Arterioscler Thromb Vasc Biol. 2005;25:729–735. doi: 10.1161/01.ATV.0000157154.14474.3b. [DOI] [PubMed] [Google Scholar]
- 68.Reglero-Real N, Marcos-Ramiro B, Millan J. Endothelial membrane reorganization during leukocyte extravasation. Cell Mol Life Sci. 2012;69:3079–3099. doi: 10.1007/s00018-012-0987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Babinska A, Azari BM, Salifu MO, Liu R, Jiang XC, Sobocka MB, Boo D, Al Khoury G, Deitch JS, Marmur JD, Ehrlich YH, et al. The f11 receptor (f11r/JAM-A) in atherothrombosis: Overexpression of f11r in atherosclerotic plaques. Thromb Haemost. 2007;97:272–281. [PubMed] [Google Scholar]
- 70.Rabquer BJ, Pakozdi A, Michel JE, Gujar BS, Haines GK, 3rd, Imhof BA, Koch AE. Junctional adhesion molecule c mediates leukocyte adhesion to rheumatoid arthritis synovium. Arthritis Rheum. 2008;58:3020–3029. doi: 10.1002/art.23867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ong KL, Leung RY, Babinska A, Salifu MO, Ehrlich YH, Kornecki E, Wong LY, Tso AW, Cherny SS, Sham PC, Lam TH, et al. Elevated plasma level of soluble f11 receptor/junctional adhesion molecule-a (f11r/JAM-A) in hypertension. Am J Hypertens. 2009;22:500–505. doi: 10.1038/ajh.2009.23. [DOI] [PubMed] [Google Scholar]
- 72.Rival Y, Del Maschio A, Rabiet MJ, Dejana E, Duperray A. Inhibition of platelet endothelial cell adhesion molecule-1 synthesis and leukocyte transmigration in endothelial cells by the combined action of TNF-alpha and IFN-gamma. J Immunol. 1996;157:1233–1241. [PubMed] [Google Scholar]
- 73.Shaw SK, Perkins BN, Lim YC, Liu Y, Nusrat A, Schnell FJ, Parkos CA, Luscinskas FW. Reduced expression of junctional adhesion molecule and platelet/endothelial cell adhesion molecule-1 (CD31) at human vascular endothelial junctions by cytokines tumor necrosis factor-alpha plus interferon-gamma does not reduce leukocyte transmigration under flow. Am J Pathol. 2001;159:2281–2291. doi: 10.1016/s0002-9440(10)63078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stewart RJ, Kashour TS, Marsden PA. Vascular endothelial platelet endothelial adhesion molecule-1 (PECAM-1) expression is decreased by TNF-alpha and IFN-gamma. Evidence for cytokine-induced destabilization of messenger ribonucleic acid transcripts in bovine endothelial cells. J Immunol. 1996;156:1221–1228. [PubMed] [Google Scholar]
- 75.Naik MU, Mousa SA, Parkos CA, Naik UP. Signaling through JAM-1 and alphavbeta3 is required for the angiogenic action of bfgf: Dissociation of the JAM-1 and alphavbeta3 complex. Blood. 2003;102:2108–2114. doi: 10.1182/blood-2003-04-1114. [DOI] [PubMed] [Google Scholar]
- 76.Li X, Stankovic M, Lee BP, Aurrand-Lions M, Hahn CN, Lu Y, Imhof BA, Vadas MA, Gamble JR. JAM-C induces endothelial cell permeability through its association and regulation of beta3 integrins. Arterioscler Thromb Vasc Biol. 2009;29:1200–1206. doi: 10.1161/ATVBAHA.109.189217. [DOI] [PubMed] [Google Scholar]
- 77.Khandoga A, Kessler JS, Meissner H, Hanschen M, Corada M, Motoike T, Enders G, Dejana E, Krombach F. Junctional adhesion molecule-a deficiency increases hepatic ischemia-reperfusion injury despite reduction of neutrophil transendothelial migration. Blood. 2005;106:725–733. doi: 10.1182/blood-2004-11-4416. [DOI] [PubMed] [Google Scholar]
- 78.Lamagna C, Meda P, Mandicourt G, Brown J, Gilbert RJ, Jones EY, Kiefer F, Ruga P, Imhof BA, Aurrand-Lions M. Dual interaction of JAM-C with JAM-B and alpha(m)beta2 integrin: Function in junctional complexes and leukocyte adhesion. Mol Biol Cell. 2005;16:4992–5003. doi: 10.1091/mbc.E05-04-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]